Comparative Effectiveness of Various Multi-Antigen Vaccines in Controlling Campylobacter jejuni in Broiler Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Campylobacter Culture for Experimental Challenge
2.2. Vaccine Preparation
2.2.1. CpG ODN
2.2.2. Preparation of Campylobacter OMPs
2.2.3. Preparation of the Killed Campylobacter Vaccine
2.2.4. Preparation of the Campylobacter Lysate
2.3. Egg Incubation and Chicken Housing
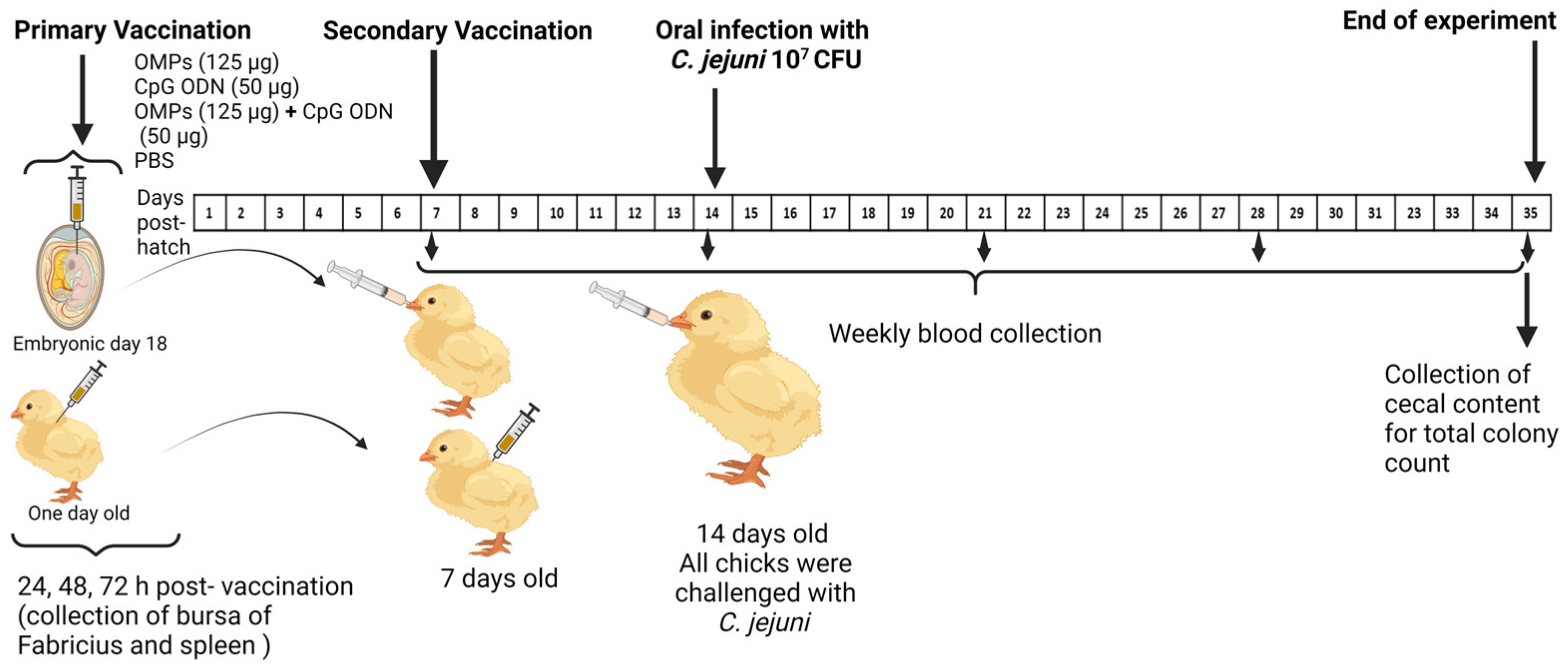
2.4. Experimental Design
2.4.1. First Trial
2.4.2. Second Trial
2.5. RNA Extraction and Complementary DNA (cDNA) Synthesis
2.6. Quantitative Real-Time PCR (RT-qPCR)
2.7. Enzyme-Linked Immunosorbent Assay (ELISA) for Measuring Serum IgY and IgM Antibody Levels
2.8. Enumeration of C. jejuni Colony Count
2.9. Statistical Analysis
3. Results
3.1. First Trial
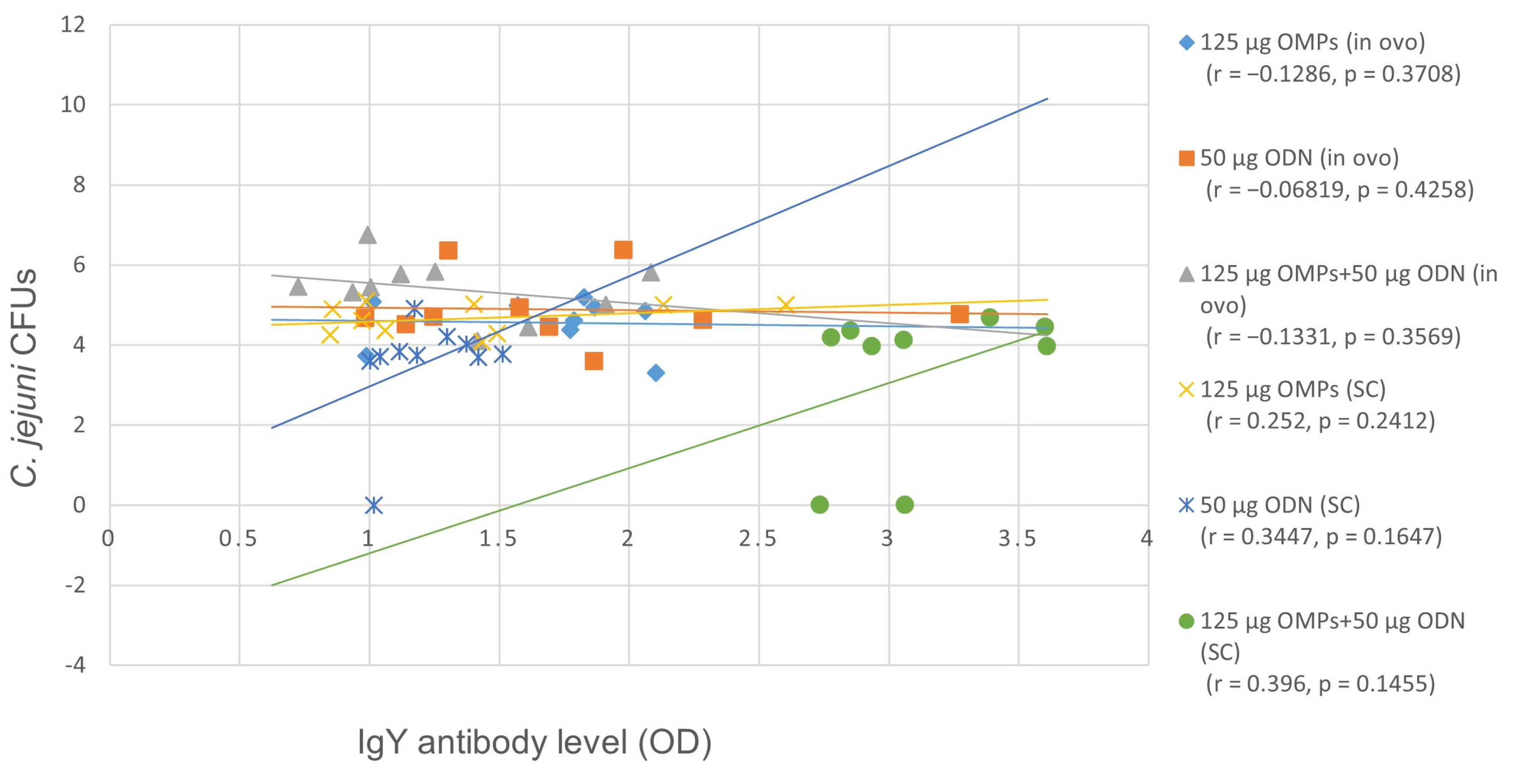
3.1.1. The Effects of in Ovo and SC Administration of C. jejuni OMPs and CpG ODN on Cecal Colonization with C. jejuni
3.1.2. The Effects of in Ovo and SC Administration of C. jejuni OMPs and CpG ODN on the Serum Ab Levels
Serum IgY Ab Levels
Serum IgM Ab Levels
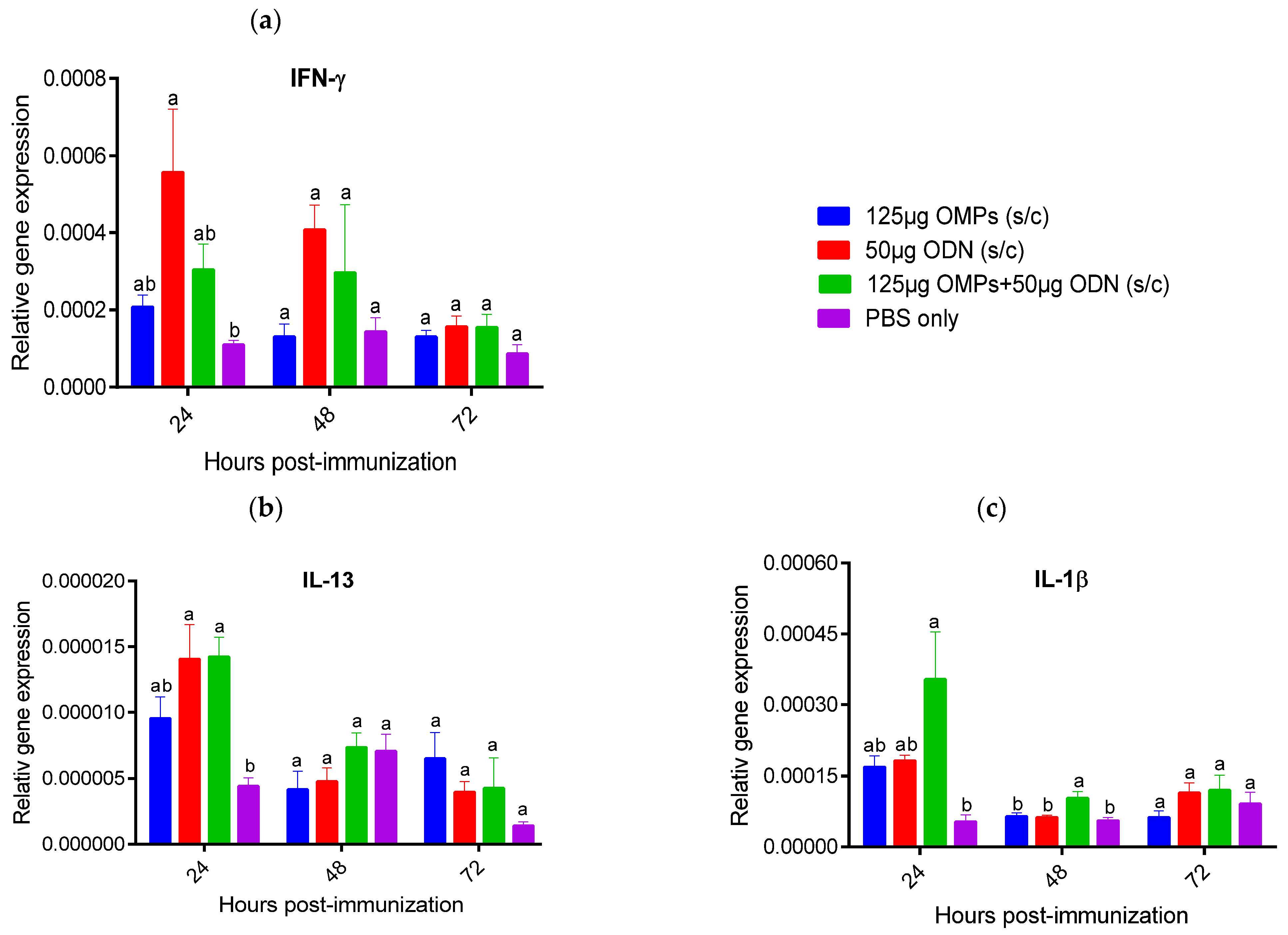
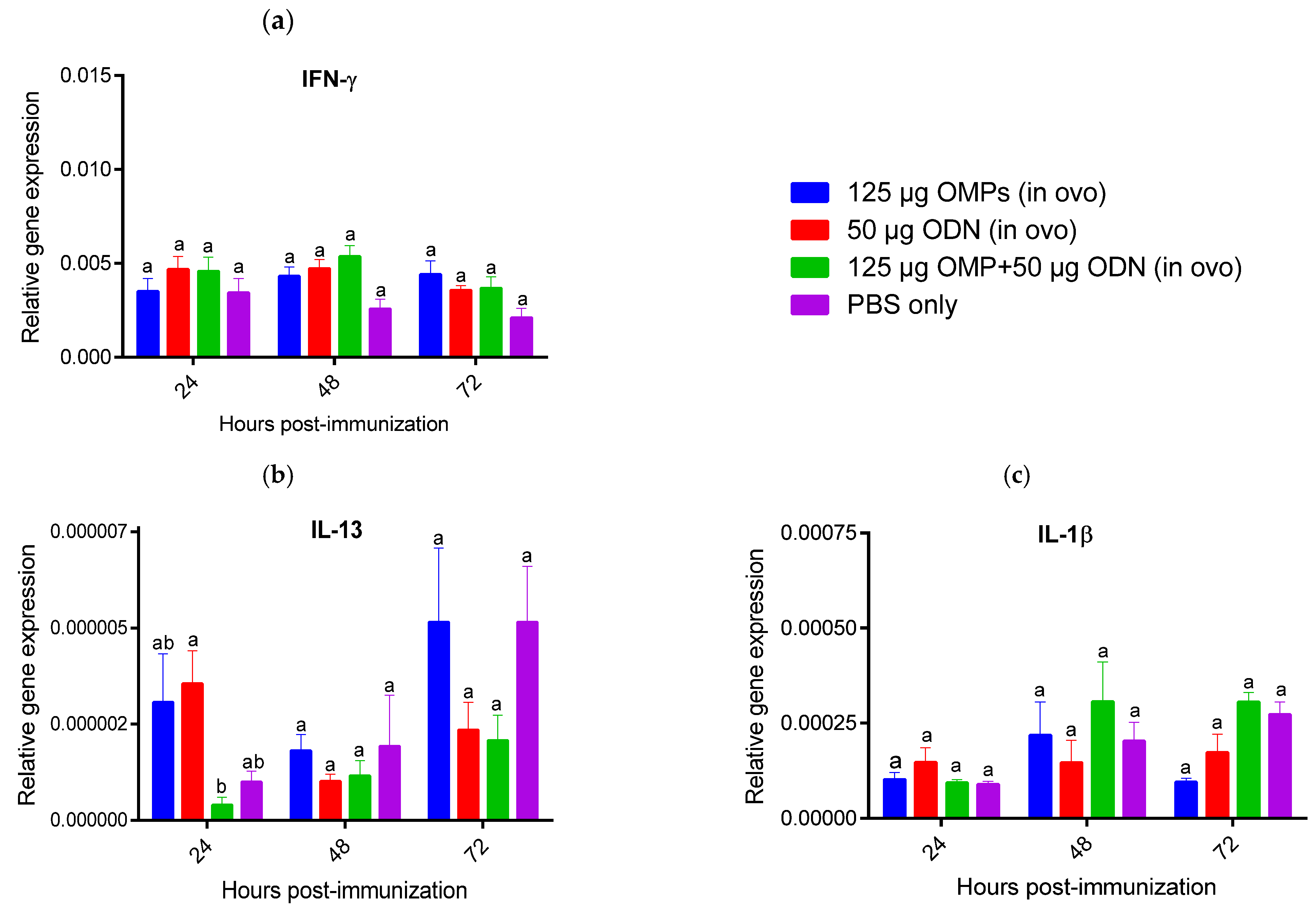
3.1.3. The Effects of in Ovo and SC Administration of C. jejuni OMPs and CpG ODN on Cytokine Gene Expression in the Spleen and Bursa of Fabricius
3.2. Second Trial
3.2.1. The Effects of SC Administration of Various Concentrations of C. jejuni OMPs, Heat-Killed and Whole Lysate Vaccines on Cecal Colonization with C. jejuni
3.2.2. The Effects of SC Administration of Various Concentrations of C. jejuni OMPs, Heat-Killed and Whole Lysate Vaccines on the Serum Ab Levels
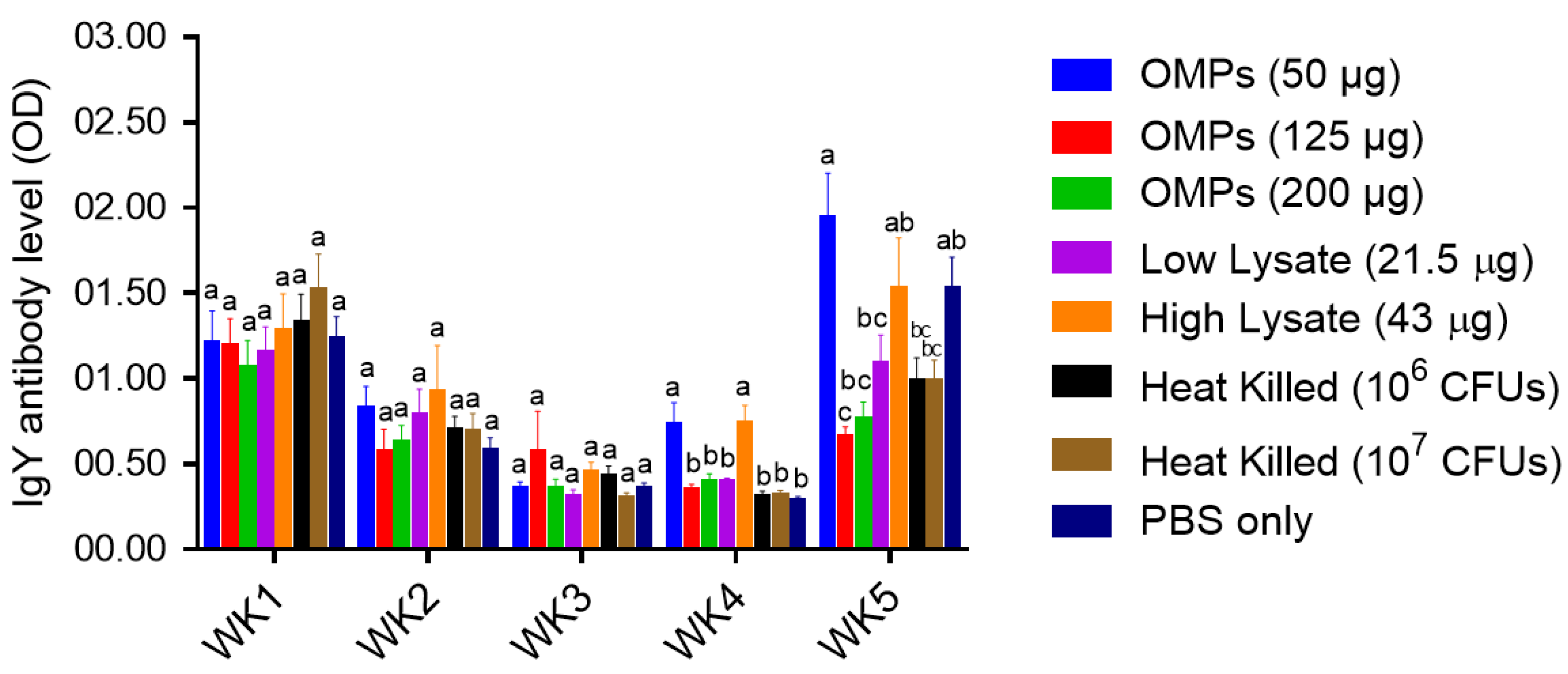
Serum IgY Ab Levels
Serum IgM Ab Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campylobacter (Campylobacteriosis). Available online: https://www.cdc.gov/campylobacter/index.html (accessed on 14 April 2024).
- Gölz, G.; Rosner, B.; Hofreuter, D.; Josenhans, C.; Kreienbrock, L.; Löwenstein, A.; Schielke, A.; Stark, K.; Suerbaum, S.; Wieler, L.H. Relevance of Campylobacter to public health—The need for a One Health approach. Int. J. Med. Microbiol. 2014, 304, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Morishita, T.Y.; Zhang, Q. Campylobacter colonization in poultry: Sources of infection and modes of transmission. Anim. Health Res. Rev. 2002, 3, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.G.; Fearnley, C. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 2003, 69, 4343–4351. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Messam, L.L.M.; Meade, J.; Gibbons, J.; McGill, K.; Bolton, D.; Whyte, P. The impact of biosecurity and partial depopulation on Campylobacter prevalence in Irish broiler flocks with differing levels of hygiene and economic performance. Infect. Ecol. Epidemiol. 2016, 6, 31454. [Google Scholar] [PubMed]
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention strategies to control Campylobacter at different stages of the food chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Ty, M.; Taha-Abdelaziz, K.; Demey, V.; Castex, M.; Sharif, S.; Parkinson, J. Performance of distinct microbial based solutions in a Campylobacter infection challenge model in poultry. Anim. Microbiome 2022, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Hermans, D.; Van Deun, K.; Martel, A.; Van Immerseel, F.; Messens, W.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. Colonization factors of Campylobacter jejuni in the chicken gut. Vet. Res. 2011, 42, 82. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.H.; Donoghue, A.M.; Venkitanarayanan, K.; Reyes-Herrera, I.; Aguiar, V.F.; Blore, P.J.; Donoghue, D.J. Water administration of the medium-chain fatty acid caprylic acid produced variable efficacy against enteric Campylobacter colonization in broilers. Poult. Sci. 2011, 90, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Meunier, M.; Guyard-Nicodème, M.; Vigouroux, E.; Poezevara, T.; Béven, V.; Quesne, S.; Amelot, M.; Parra, A.; Chemaly, M.; Dory, D. A DNA prime/protein boost vaccine protocol developed against Campylobacter jejuni for poultry. Vaccine 2018, 36, 2119–2125. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, T.; Pina-Mimbela, R.; Kumar, A.; Binjawadagi, B.; Liu, Z.; Renukaradhya, G.J.; Rajashekara, G. Evaluation of nanoparticle-encapsulated outer membrane proteins for the control of Campylobacter jejuni colonization in chickens. Poult. Sci. 2013, 92, 2201–2211. [Google Scholar] [CrossRef]
- Pumtang-On, P.; Mahony, T.J.; Hill, R.A.; Vanniasinkam, T. A systematic review of Campylobacter jejuni vaccine candidates for chickens. Microorganisms 2021, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Hodgins, D.C.; Alkie, T.N.; Quinteiro-Filho, W.; Yitbarek, A.; Astill, J.; Sharif, S. Oral administration of PLGA-encapsulated CpG ODN and Campylobacter jejuni lysate reduces cecal colonization by Campylobacter jejuni in chickens. Vaccine 2018, 36, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Vandeputte, J.; Martel, A.; Van Rysselberghe, N.; Antonissen, G.; Verlinden, M.; De Zutter, L.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F.; Garmyn, A. In ovo vaccination of broilers against Campylobacter jejuni using a bacterin and subunit vaccine. Poult. Sci. 2019, 98, 5999–6004. [Google Scholar] [CrossRef] [PubMed]
- Lin, J. Novel approaches for Campylobacter control in poultry. Foodborne Pathog. Dis. 2009, 6, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M. Antimicrobial resistance and recent alternatives to antibiotics for the control of bacterial pathogens with an emphasis on foodborne pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Renu, S.; Patil, V.; Schrock, J.; Feliciano-Ruiz, N.; Selvaraj, R.; Renukaradhya, G.J. Immune response to Salmonella enteritidis infection in broilers immunized orally with chitosan-based Salmonella subunit nanoparticle vaccine. Front. Immunol. 2020, 11, 935. [Google Scholar] [CrossRef] [PubMed]
- Ricks, C.A.; Avakian, A.; Bryan, T.; Gildersleeve, R.; Haddad, E.; Ilich, R.; King, S.; Murray, L.; Phelps, P.; Poston, R. In ovo vaccination technology. Adv. Vet. Med. 1999, 41, 495–515. [Google Scholar] [PubMed]
- Abdelaziz, K.; Helmy, Y.A.; Yitbarek, A.; Hodgins, D.C.; Sharafeldin, T.A.; Selim, M.S. Advances in Poultry Vaccines: Leveraging Biotechnology for Improving Vaccine Development, Stability, and Delivery. Vaccines 2024, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, T.; Foldvari, M.; Zachar, T.; Popowich, S.; Chow-Lockerbie, B.; Ivanova, M.V.; Tikoo, S.; Kurukulasuriya, S.; Willson, P.; Gomis, S. Protection of neonatal broiler chickens following in ovo delivery of oligodeoxynucleotides containing CpG motifs (CpG-ODN) formulated with carbon nanotubes or liposomes. Avian Dis. 2015, 59, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Gomis, S.; Babiuk, L.; Godson, D.L.; Allan, B.; Thrush, T.; Townsend, H.; Willson, P.; Waters, E.; Hecker, R.; Potter, A. Protection of chickens against Escherichia coli infections by DNA containing CpG motifs. Infect. Immun. 2003, 71, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Keestra, A.M.; de Zoete, M.R.; Bouwman, L.I.; van Putten, J.P. Chicken TLR21 is an innate CpG DNA receptor distinct from mammalian TLR. J. Immunol. 2010, 185, 460–467. [Google Scholar] [CrossRef] [PubMed]
- de Zoete, M.R.; Keestra, A.M.; Roszczenko, P.; van Putten, J.P. Activation of human and chicken toll-like receptors by Campylobacter spp. Infect. Immun. 2010, 78, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, T.; Ahmed, K.A.; Goonewardene, K.; Popowich, S.; Kurukulasuriya, S.; Karunarathna, R.; Gupta, A.; Lockerbie, B.; Foldvari, M.; Tikoo, S.K. Synthetic CpG-ODN rapidly enriches immune compartments in neonatal chicks to induce protective immunity against bacterial infections. Sci. Rep. 2019, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- McCoy, E.C.; Doyle, D.; Burda, K.; Corbeil, L.B.; Winter, A.J. Superficial antigens of Campylobacter (Vibrio) fetus: Characterization of antiphagocytic component. Infect. Immun. 1975, 11, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Alkie, T.N.; Hodgins, D.C.; Shojadoost, B.; Sharif, S. Characterization of host responses induced by Toll-like receptor ligands in chicken cecal tonsil cells. Vet. Immunol. Immunopathol. 2016, 174, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Astill, J.; Kulkarni, R.R.; Read, L.R.; Najarian, A.; Farber, J.M.; Sharif, S. In vitro assessment of immunomodulatory and anti-Campylobacter activities of probiotic lactobacilli. Sci. Rep. 2019, 9, 17903. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Alkie, T.N.; Hodgins, D.C.; Yitbarek, A.; Shojadoost, B.; Sharif, S. Gene expression profiling of chicken cecal tonsils and ileum following oral exposure to soluble and PLGA-encapsulated CpG ODN, and lysate of Campylobacter jejuni. Vet. Microbiol. 2017, 212, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, K.; Nixon, T.; Joye, A.; Hassan, H.; Alizadeh, M.; Sharif, S.; Kulkarni, R.R. Modulation of functional activity of heat-stressed chicken macrophages by poultry-derived probiotic lactobacilli. Can. J. Anim. Sci. 2022. [Google Scholar] [CrossRef]
- Paul, M.S.; Mallick, A.I.; Haq, K.; Orouji, S.; Abdul-Careem, M.F.; Sharif, S. In vivo administration of ligands for chicken toll-like receptors 4 and 21 induces the expression of immune system genes in the spleen. Vet. Immunol. Immunopathol. 2011, 144, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Sławinska, A.; Siwek, M.Z.; Bednarczyk, M.F. Effects of synbiotics injected in ovo on regulation of immune-related gene expression in adult chickens. Am. J. Vet. Res. 2014, 75, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.H.; Lee, I.K.; Kim, G.; Gu, M.J.; Kim, H.Y.; Park, B.; Park, T.S.; Han, S.H.; Yun, C. Changes in bursal B cells in chicken during embryonic development and early life after hatching. Sci. Rep. 2018, 8, 16905. [Google Scholar] [CrossRef] [PubMed]
- Brisbin, J.T.; Gong, J.; Parvizi, P.; Sharif, S. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 2010, 17, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Hodgins, D.C.; Barjesteh, N.; St. Paul, M.; Ma, Z.; Monteiro, M.A.; Sharif, S. Evaluation of a polysaccharide conjugate vaccine to reduce colonization by Campylobacter jejuni in broiler chickens. BMC Res. Notes 2015, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Nauta, M.J.; Johannessen, G.; Adame, L.L.; Williams, N.; Rosenquist, H. The effect of reducing numbers of Campylobacter in broiler intestines on human health risk. Microb. Risk Anal. 2016, 2, 68–77. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F. Update and review of control options for Campylobacter in broilers at primary production. EFSA J. 2020, 18, e06090. [Google Scholar] [PubMed]
- El-Shibiny, A.; Connerton, P.L.; Connerton, I.F. Enumeration and diversity of campylobacters and bacteriophages isolated during the rearing cycles of free-range and organic chickens. Appl. Environ. Microbiol. 2005, 71, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulkarni, R.R.; Sharif, S.; Hassan, H.; Alizadeh, M.; Pratt, S.; Abdelaziz, K. In ovo feeding of probiotic lactobacilli differentially alters expression of genes involved in the development and immunological maturation of bursa of Fabricius in pre-hatched chicks. Poult. Sci. 2024, 103, 103237. [Google Scholar] [CrossRef] [PubMed]
- Szczypka, M.; Suszko-Pawłowska, A.; Kuczkowski, M.; Gorczykowski, M.; Lis, M.; Kowalczyk, A.; Łukaszewicz, E.; Poradowski, D.; Zbyryt, I.; Bednarczyk, M. Effects of selected prebiotics or synbiotics administered in ovo on lymphocyte subsets in bursa of the fabricius, thymus, and spleen in non-immunized and immunized chicken broilers. Animals 2021, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- McGruder, E.D.; Ramirez, G.A.; Kogut, M.H.; Moore, R.W.; Corrier, D.E.; DeLoach, J.R.; Hargis, B.M. In ovo administration of Salmonella enteritidis-immune lymphokines confers protection to neonatal chicks against Salmonella enteritidis organ infectivity. Poult. Sci. 1995, 74, 18–25. [Google Scholar] [CrossRef]
- Gimeno, I.M.; Faiz, N.M.; Cortes, A.L.; Barbosa, T.; Villalobos, T.; Pandiri, A.R. In ovo vaccination with turkey herpesvirus hastens maturation of chicken embryo immune responses in specific-pathogen-free chickens. Avian Dis. 2015, 59, 375–383. [Google Scholar] [CrossRef] [PubMed]
- McCarty, J.E.; Brown, T.P.; Giambrone, J.J. Delay of infectious bursal disease virus infection by in ovo vaccination of antibody-positive chicken eggs. J. Appl. Poult. Res. 2005, 14, 136–140. [Google Scholar] [CrossRef]
- Alizadeh, M.; Shojadoost, B.; Astill, J.; Taha-Abdelaziz, K.; Karimi, S.H.; Bavananthasivam, J.; Kulkarni, R.R.; Sharif, S. Effects of in ovo inoculation of multi-Strain Lactobacilli on cytokine gene expression and antibody-mediated Immune responses in chickens. Front. Vet. Sci. 2020, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Astill, J.; Alqazlan, N.; Shojadoost, B.; Taha-Abdelaziz, K.; Bavananthasivam, J.; Doost, J.S.; Sedeghiisfahani, N.; Sharif, S. In ovo co-administration of vitamins (A and D) and probiotic lactobacilli modulates immune responses in broiler chickens. Poult. Sci. 2022, 101, 101717. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.; Vakharia, V.; Liu, M.; Song, H. In ovo vaccine against infectious bursal disease. Md. Int. J. Poult. Sci. 2007, 6, 770–775. [Google Scholar] [CrossRef]
- Taghavi, A.; Allan, B.; Mutwiri, G.; Van Kessel, A.; Willson, P.; Babiuk, L.; Potter, A.; Gomis, S. Protection of neonatal broiler chicks against Salmonella Typhimurium septicemia by DNA containing CpG motifs. Avian Dis. 2008, 52, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Villanueva, K.; Renu, S.; Gourapura, R.; Selvaraj, R. Efficacy of a nanoparticle vaccine administered in-ovo against Salmonella in broilers. PLoS ONE 2021, 16, e0247938. [Google Scholar] [CrossRef] [PubMed]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-antibiotics strategies to control Salmonella infection in poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Careem, M.F.; Hunter, D.B.; Lambourne, M.D.; Barta, J.; Sharif, S. Ontogeny of cytokine gene expression in the chicken spleen. Poult. Sci. 2007, 86, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Lebman, D.A.; Shrader, B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J. Exp. Med. 1989, 170, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Junttila, I.S. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 2018, 9, 338745. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.G.; Sutherland, A.P.; Newton, R.; Qian, F.; Cachero, T.G.; Scott, M.L.; Thompson, J.S.; Wheway, J.; Chtanova, T.; Groom, J. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J. Immunol. 2004, 173, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Hu, H. CD4 T-cell differentiation in vitro. In T-Cell Receptor Signaling: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2020; pp. 91–99. [Google Scholar]
- Beal, R.K.; Powers, C.; Wigley, P.; Barrow, P.A.; Smith, A.L. Temporal dynamics of the cellular, humoral and cytokine responses in chickens during primary and secondary infection with Salmonella enterica serovar Typhimurium. Avian Pathol. 2004, 33, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Astill, J.; Shojadoost, B.; Borrelli, S.; Monteiro, M.A.; Sharif, S. Campylobacter-derived ligands induce cytokine and chemokine expression in chicken macrophages and cecal tonsil mononuclear cells. Vet. Microbiol. 2020, 246, 108732. [Google Scholar] [CrossRef] [PubMed]
- Taha-Abdelaziz, K.; Yitbarek, A.; Alkie, T.N.; Hodgins, D.C.; Read, L.R.; Weese, J.S.; Sharif, S. PLGA-encapsulated CpG ODN and Campylobacter jejuni lysate modulate cecal microbiota composition in broiler chickens experimentally challenged with C. Jejuni. Sci. Rep. 2018, 8, 12076. [Google Scholar] [CrossRef] [PubMed]
- Glünder, G.; Spiering, N.; Hinz, K. Investigations on parenteral immunization of chickens with a Campylobacter mineral oil vaccine. In Proceedings of the International Congress of the World Veterinary Poultry Association, Budapest, Hungary, 20–22 August 1997; Nagy, B., Mulder, R., Eds.; European Commission: Budapest, Hungary, 1997; pp. 247–253. [Google Scholar]







| Treatment | Birds (n)/ Group | Primary Immunization | Booster Immunization | Challenge Age (d) | |||
|---|---|---|---|---|---|---|---|
| Day | Route | Volume (mL) | Route/Volume (mL)/Age (d) | ||||
| 1 | C. jejuni OMPs (125 µg) | 34 | 18th ED | Amniotic | 0.1 | Oral/1/7 | 14 |
| 2 | CpG ODN (50 µg) | 34 | 18th ED | Amniotic | 0.1 | Oral/1/7 | 14 |
| 3 | C. jejuni OMPs (125 µg) + CpG ODN (50 µg) | 34 | 18th ED | Amniotic | 0.1 | Oral/1/7 | 14 |
| 4 | PBS | 34 | 18th ED | Amniotic | 0.1 | Oral/1/7 | 14 |
| 5 | C. jejuni OMPs (125 µg) | 34 | 1-day old | SC | 0.2 | SC/0.2/7 | 14 |
| 6 | CpG ODN (50 µg) | 34 | 1-day old | SC | 0.2 | SC/0.2/7 | 14 |
| 7 | C. jejuni OMPs (125 µg) + CpG ODN (50 µg) | 34 | 1-day old | SC | 0.2 | SC/0.2/7 | 14 |
| 8 | PBS | 34 | 1-day old | SC | 0.2 | SC/0.2/7 | 14 |
| Group | Treatment | Birds (n)/ Group | Primary Immunization | Booster Immunization | Challenge Age (d) | ||
|---|---|---|---|---|---|---|---|
| Day | Route | Volume (mL) | Route/Volume (mL)/Age (d) | ||||
| 1 | C. jejuni OMPs (50 µg) | 10 | 1-day old | SC | 0.2 | SC/0.2/14 | 15 |
| 2 | C. jejuni OMPs (125 µg) | 10 | 1-day old | SC | 0.2 | SC/0.2/14 | 15 |
| 3 | C. jejuni OMPs (200 µg) | 10 | 1-day old | SC | 0.2 | SC/0.2/14 | 15 |
| 4 | C. jejuni heat-killed (106 CFUs/bird) | 10 | 1-day old | SC | 0.2 | SC/0.2/14 | 15 |
| 5 | C. jejuni heat-killed (107 CFUs/bird) | 10 | 1-day old | SC | 0.2 | SC/0.2/14 | 15 |
| 6 | C. jejuni lysate (21.5 µg) | 10 | 1-day old | SC | 0.2 | SC/0.2/14 | 15 |
| 7 | C. jejuni lysate (43 µg) | 10 | 1-day old | SC | 0.2 | SC/0.2/14 | 15 |
| 8 | PBS | 10 | 1-day old | SC | 0.2 | SC/0.2/14 | 15 |
| Gene | Primer Sequence (5′-3″) | Annealing Temp. (°C) | Reference |
|---|---|---|---|
| IFN-γ | F: ACACTGACAAGTCAAAGCCGC R: AGTCGTTCATCGGGAGCTTG | 60 | [28] |
| IL-10 | F: TTTGGCTGCCAGTCTGTGTC R: CTCATCCATCTTCTCGAACGTC | 64 | [29] |
| IL-1β | F: GTGAGGCTCAACATTGCGCTGTA R: TGTCCAGGCGGTAGAAGATGAAG | 64 | [30] |
| IL-13 | F: ACTTGTCCAAGCTGAAGCTGTC R: TCTTGCAGTCGGTCATGTTGTC | 60 | [31] |
| IL-4 | F: GCTCTCAGTGCCGCTGATG R: GGAAACCTCTCCCTGGATGTC | 58 | [32] |
| BAFF | F: CACGTCATCCAGCAGAAGGAT R: ACAAGAGGACAGGAGCATTGC | 55 | [33] |
| TGF-β | F: CGGCCGACGATGAGTGGCTC R: CGGGGCCCATCTCACAGGGA | 60 | [34] |
| β-actin | F: CAACACAGTGCTGTCTGGTGGTA R: ATCGTACTCCTGCTTGCTGATCC | 60 | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naguib, M.; Sharma, S.; Schneider, A.; Wehmueller, S.; Abdelaziz, K. Comparative Effectiveness of Various Multi-Antigen Vaccines in Controlling Campylobacter jejuni in Broiler Chickens. Vaccines 2024, 12, 908. https://doi.org/10.3390/vaccines12080908
Naguib M, Sharma S, Schneider A, Wehmueller S, Abdelaziz K. Comparative Effectiveness of Various Multi-Antigen Vaccines in Controlling Campylobacter jejuni in Broiler Chickens. Vaccines. 2024; 12(8):908. https://doi.org/10.3390/vaccines12080908
Chicago/Turabian StyleNaguib, Mostafa, Shreeya Sharma, Abigail Schneider, Sarah Wehmueller, and Khaled Abdelaziz. 2024. "Comparative Effectiveness of Various Multi-Antigen Vaccines in Controlling Campylobacter jejuni in Broiler Chickens" Vaccines 12, no. 8: 908. https://doi.org/10.3390/vaccines12080908






