Clinical Features and Vaccination Effects among Children with Post-Acute Sequelae of COVID-19 in Taiwan
Abstract
1. Introduction
2. Methods
2.1. Data Source and Study Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Children
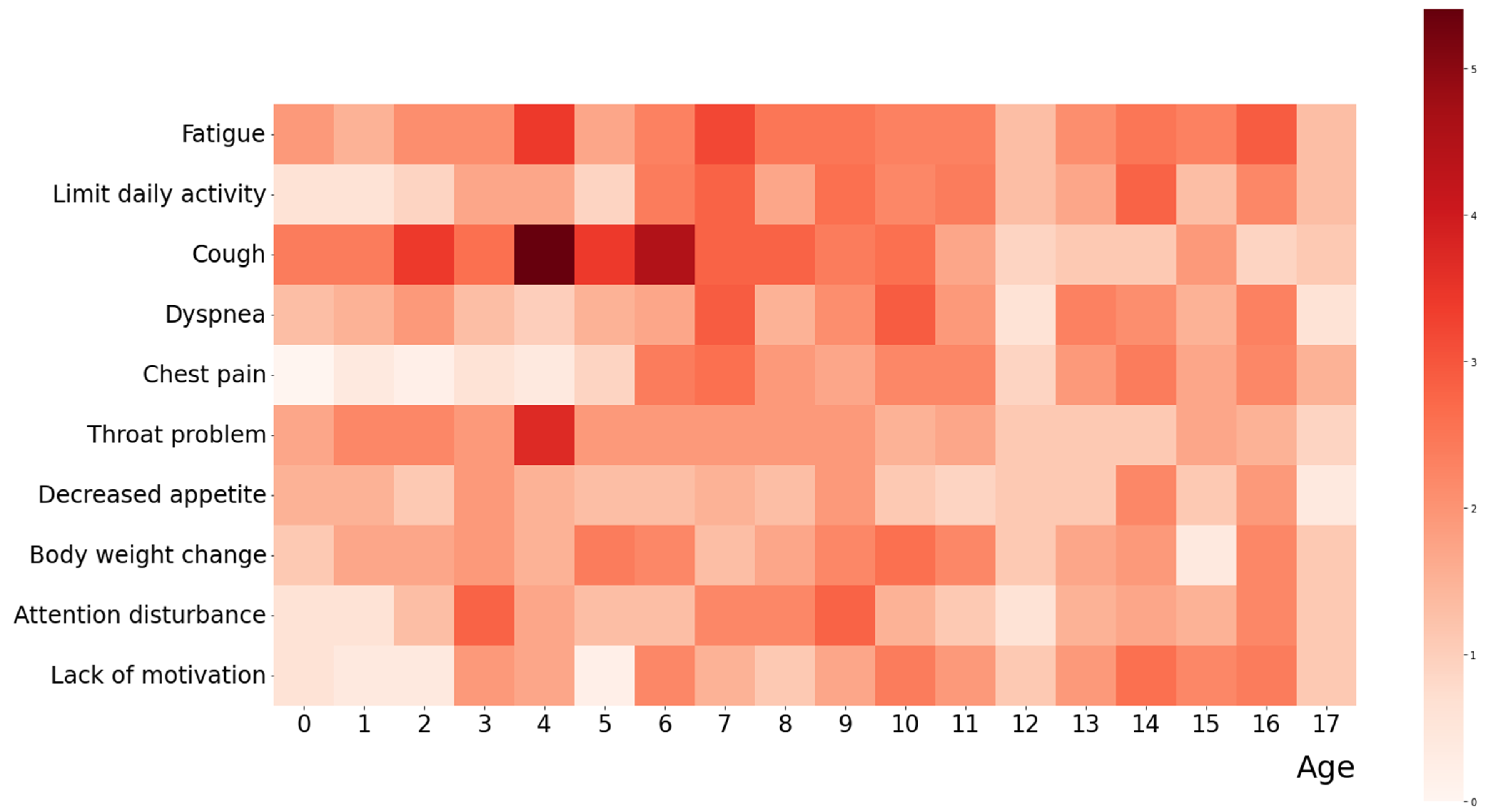
3.2. Common Clinical Symptoms during Acute and Post-COVID-19 Phases
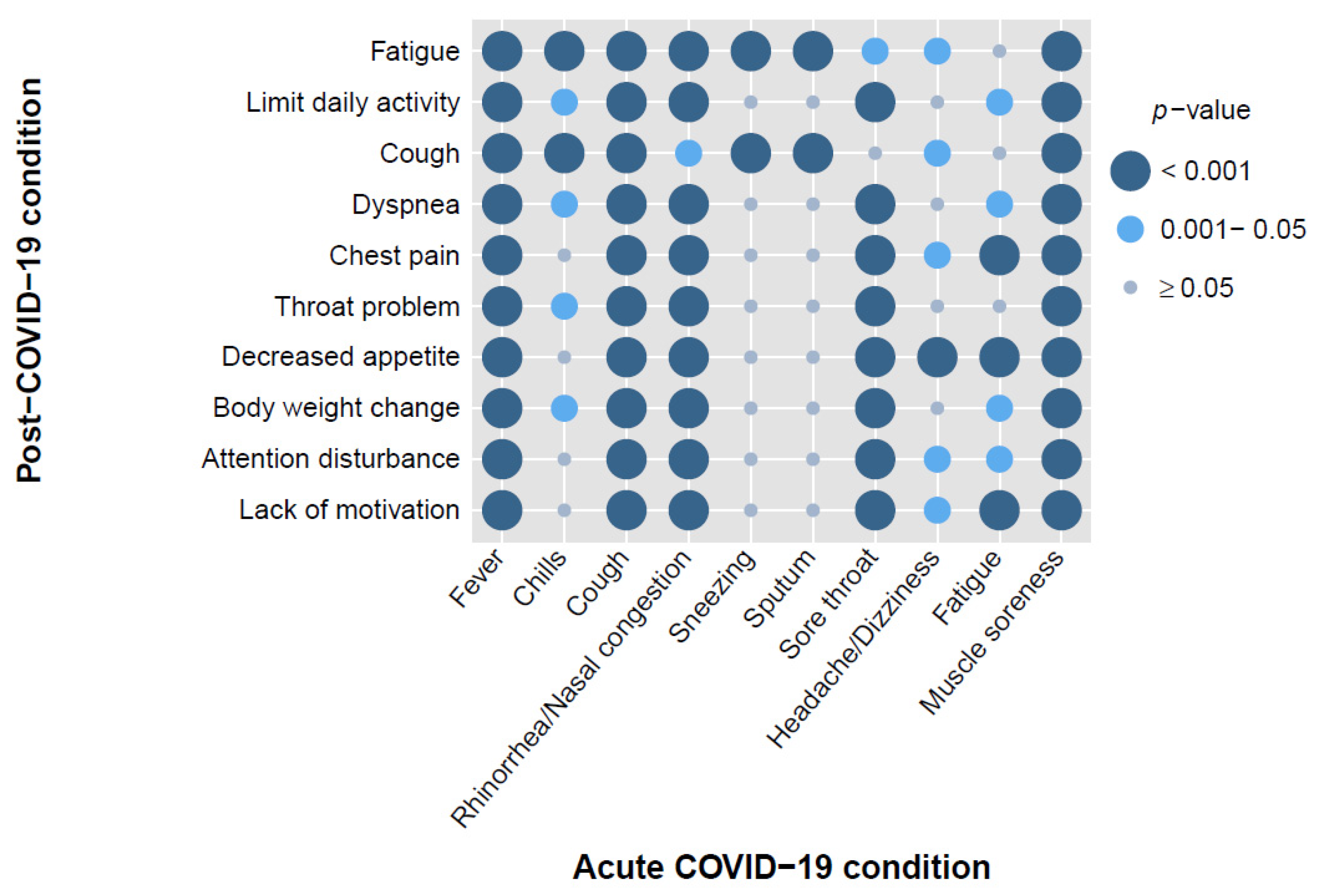
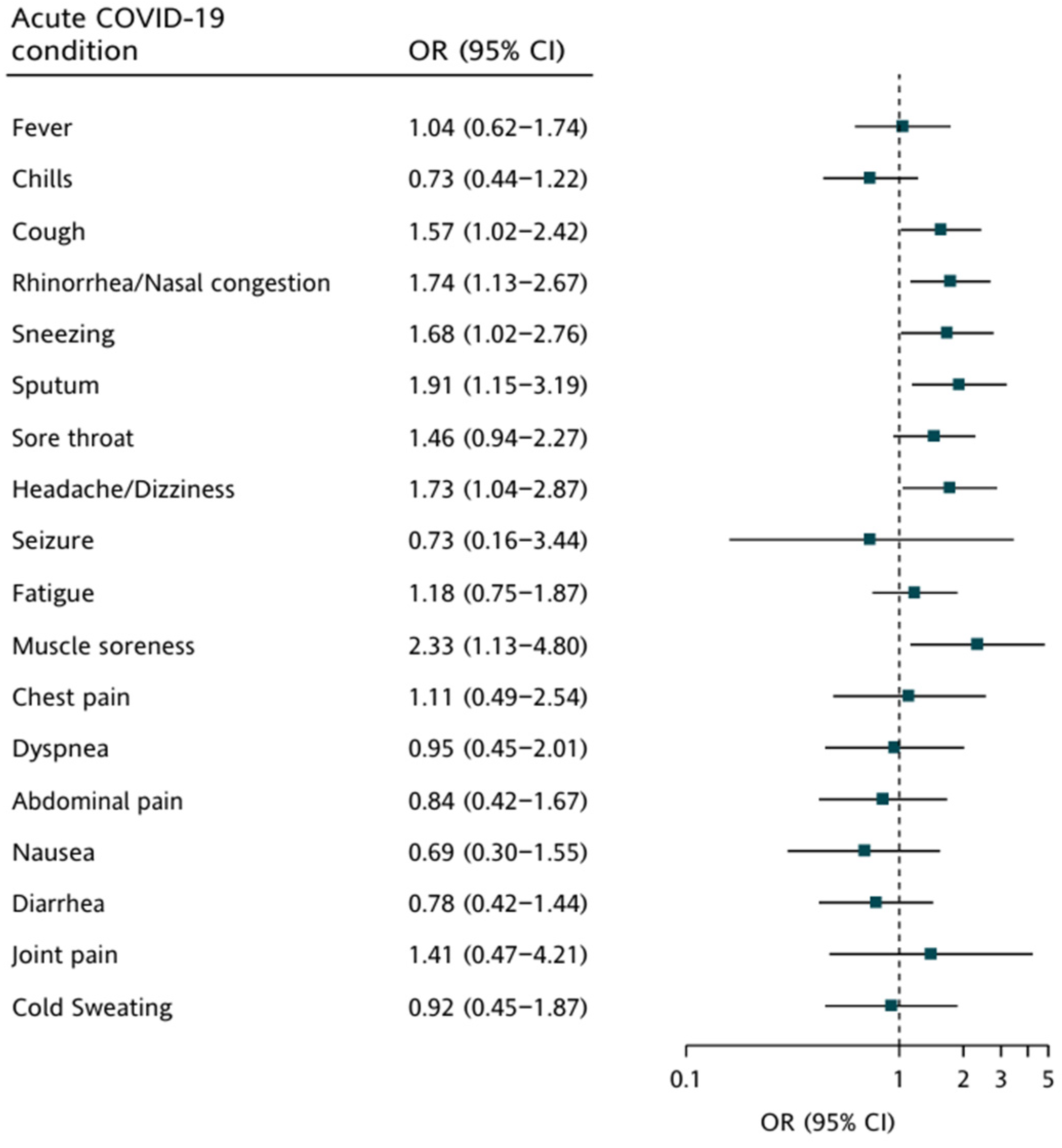
3.3. Association between Clinical Symptoms in Two Phases and Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Lee, G.M.; Razzaghi, H.; Lorman, V.; Mejias, A.; Pajor, N.M.; Thacker, D.; Webb, R.; Dickinson, K.; Bailey, L.C.; et al. Clinical Features and Burden of Postacute Sequelae of SARS-CoV-2 Infection in Children and Adolescents. JAMA Pediatr. 2022, 176, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.K.; Kim, J.H.; Han, M.Y. Long COVID in children and adolescents: Prevalence, clinical manifestations, and management strategies. Clin. Exp. Pediatr. 2023, 66, 465. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Ayuzo Del Valle, N.C.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Chiappini, E.; Licari, A.; Galli, L.; Marseglia, G.L. Prevalence and clinical presentation of long COVID in children: A systematic review. Eur. J. Pediatr. 2022, 181, 3995–4009. [Google Scholar] [CrossRef]
- Jiang, L.; Li, X.; Nie, J.; Tang, K.; Bhutta, Z.A. A Systematic Review of Persistent Clinical Features After SARS-CoV-2 in the Pediatric Population. Pediatrics 2023, 152, e2022060351. [Google Scholar] [CrossRef] [PubMed]
- Radtke, T.; Ulyte, A.; Puhan, M.A.; Kriemler, S. Long-term Symptoms After SARS-CoV-2 Infection in Children and Adolescents. JAMA 2021, 326, 869–871. [Google Scholar] [CrossRef]
- Say, D.; Crawford, N.; McNab, S.; Wurzel, D.; Steer, A.; Tosif, S. Post-acute COVID-19 outcomes in children with mild and asymptomatic disease. Lancet Child. Adolesc. Health 2021, 5, e22–e23. [Google Scholar] [CrossRef]
- Kikkenborg Berg, S.; Palm, P.; Nygaard, U.; Bundgaard, H.; Petersen, M.N.S.; Rosenkilde, S.; Thorsted, A.B.; Ersboll, A.K.; Thygesen, L.C.; Nielsen, S.D.; et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0-14 years and matched controls in Denmark (LongCOVIDKidsDK): A national, cross-sectional study. Lancet Child. Adolesc. Health 2022, 6, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Atchison, C.J.; Whitaker, M.; Donnelly, C.A.; Chadeau-Hyam, M.; Riley, S.; Darzi, A.; Ashby, D.; Barclay, W.; Cooke, G.S.; Elliott, P.; et al. Characteristics and predictors of persistent symptoms post-COVID-19 in children and young people: A large community cross-sectional study in England. Arch. Dis. Child. 2023, 108, e12. [Google Scholar] [CrossRef]
- Nugawela, M.D.; Stephenson, T.; Shafran, R.; De Stavola, B.L.; Ladhani, S.N.; Simmons, R.; McOwat, K.; Rojas, N.; Dalrymple, E.; Cheung, E.Y.; et al. Predictive model for long COVID in children 3 months after a SARS-CoV-2 PCR test. BMC Med. 2022, 20, 465. [Google Scholar] [CrossRef] [PubMed]
- Osmanov, I.M.; Spiridonova, E.; Bobkova, P.; Gamirova, A.; Shikhaleva, A.; Andreeva, M.; Blyuss, O.; El-Taravi, Y.; DunnGalvin, A.; Comberiati, P.; et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur. Respir. J. 2022, 59, 2101341. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Sherif, Z.A.; Gomez, C.R.; Connors, T.J.; Henrich, T.J.; Reeves, W.B.; Force, R.M.P.T. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023, 12, e86002. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, S.; Jagannathan, P.; Henrich, T.J.; Sherif, Z.A.; Bime, C.; Quinlan, E.; Portman, M.A.; Gennaro, M.; Rehman, J.; Force, R.M.P.T. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 2023, 12, e86014. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Johnson, A.G.; Amin, A.B.; Ali, A.R.; Hoots, B.; Cadwell, B.L.; Arora, S.; Avoundjian, T.; Awofeso, A.O.; Barnes, J.; Bayoumi, N.S.; et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence—25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 132–138. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Oliveira, M.C.L.; Simoes, E.S.A.C.; Colosimo, E.A.; Mak, R.H.; Pinhati, C.C.; Martelli, H., Jr. Association of Prior COVID-19 Vaccination With SARS-CoV-2 Infection and Death in Children and Young Persons During the Omicron Variant Period in Brazil. JAMA Pediatr. 2023, 177, 1100–1102. [Google Scholar] [CrossRef]
- Watanabe, A.; Kani, R.; Iwagami, M.; Takagi, H.; Yasuhara, J.; Kuno, T. Assessment of Efficacy and Safety of mRNA COVID-19 Vaccines in Children Aged 5 to 11 Years: A Systematic Review and Meta-analysis. JAMA Pediatr. 2023, 177, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Perrodeau, E.; Saldanha, J.; Pane, I.; Ravaud, P. Efficacy of first dose of covid-19 vaccine versus no vaccination on symptoms of patients with long covid: Target trial emulation based on ComPaRe e-cohort. BMJ Med. 2023, 2, e000229. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Boufidou, F.; Medic, S.; Lampropoulou, V.; Siafakas, N.; Tsakris, A.; Anastassopoulou, C. SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic. Int. J. Mol. Sci. 2023, 24, 12962. [Google Scholar] [CrossRef] [PubMed]
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Effect of covid-19 vaccination on long covid: Systematic review. BMJ Med. 2023, 2, e000385. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, U.; Man, K.K.C.; Chen, A.; Wong, I.C.K.; George, J.; Wilson, P.; Wei, L. Definition of Post-COVID-19 Condition Among Published Research Studies. JAMA Netw. Open 2023, 6, e235856. [Google Scholar] [CrossRef]
- Molteni, E.; Sudre, C.H.; Canas, L.S.; Bhopal, S.S.; Hughes, R.C.; Antonelli, M.; Murray, B.; Klaser, K.; Kerfoot, E.; Chen, L.; et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child. Adolesc. Health 2021, 5, 708–718. [Google Scholar] [CrossRef]
- Roge, I.; Smane, L.; Kivite-Urtane, A.; Pucuka, Z.; Racko, I.; Klavina, L.; Pavare, J. Comparison of Persistent Symptoms After COVID-19 and Other Non-SARS-CoV-2 Infections in Children. Front. Pediatr. 2021, 9, 752385. [Google Scholar] [CrossRef]
- Fainardi, V.; Meoli, A.; Chiopris, G.; Motta, M.; Skenderaj, K.; Grandinetti, R.; Bergomi, A.; Antodaro, F.; Zona, S.; Esposito, S. Long COVID in Children and Adolescents. Life 2022, 12, 285. [Google Scholar] [CrossRef]
- Patel, A.B.; Verma, A. Nasal ACE2 Levels and COVID-19 in Children. JAMA 2020, 323, 2386–2387. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2020, 106, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.; Blau, H.; Casiraghi, E.; Loomba, J.J.; Callahan, T.J.; Laraway, B.J.; Wilkins, K.J.; Antonescu, C.C.; Valentini, G.; Williams, A.E.; et al. Predictive models of long COVID. EBioMedicine 2023, 96, 104777. [Google Scholar] [CrossRef]
- Pfaff, E.R.; Girvin, A.T.; Bennett, T.D.; Bhatia, A.; Brooks, I.M.; Deer, R.R.; Dekermanjian, J.P.; Jolley, S.E.; Kahn, M.G.; Kostka, K.; et al. Identifying who has long COVID in the USA: A machine learning approach using N3C data. Lancet Digit. Health 2022, 4, e532–e541. [Google Scholar] [CrossRef]
- Arjun, M.C.; Singh, A.K.; Pal, D.; Das, K.; Alekhya, G.; Venkateshan, M.; Mishra, B.; Patro, B.K.; Mohapatra, P.R.; Subba, S.H. Characteristics and predictors of Long COVID among diagnosed cases of COVID-19. PLoS ONE 2022, 17, e0278825. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.B.; Zeng, N.; Yuan, K.; Tian, S.S.; Yang, Y.B.; Gao, N.; Chen, X.; Zhang, A.Y.; Kondratiuk, A.L.; Shi, P.P.; et al. Prevalence and risk factor for long COVID in children and adolescents: A meta-analysis and systematic review. J. Infect. Public Health 2023, 16, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, J.; Havdal, L.B.; Drevvatne, M.; Brodwall, E.M.; Lund Berven, L.; Stiansen-Sonerud, T.; Einvik, G.; Leegaard, T.M.; Tjade, T.; Michelsen, A.E.; et al. Prevalence and Characteristics Associated with Post-COVID-19 Condition Among Nonhospitalized Adolescents and Young Adults. JAMA Netw. Open 2023, 6, e235763. [Google Scholar] [CrossRef]
- Morello, R.; Mariani, F.; Mastrantoni, L.; De Rose, C.; Zampino, G.; Munblit, D.; Sigfrid, L.; Valentini, P.; Buonsenso, D. Risk factors for post-COVID-19 condition (Long Covid) in children: A prospective cohort study. EClinicalMedicine 2023, 59, 101961. [Google Scholar] [CrossRef] [PubMed]
- Vasichkina, E.; Alekseeva, D.; Kudryavtsev, I.; Glushkova, A.; Starshinova, A.Y.; Malkova, A.; Kudlay, D.; Starshinova, A. COVID-19 Heart Lesions in Children: Clinical, Diagnostic and Immunological Changes. Int. J. Mol. Sci. 2023, 24, 1147. [Google Scholar] [CrossRef] [PubMed]
- Udeh, R.; Utrero-Rico, A.; Dolja-Gore, X.; Rahmati, M.; Mc, E.M.; Kenna, T. Lactate dehydrogenase contribution to symptom persistence in long COVID: A pooled analysis. Rev. Med. Virol. 2023, 33, e2477. [Google Scholar] [CrossRef]
- Liao, B.; Liu, Z.; Tang, L.; Li, L.; Gan, Q.; Shi, H.; Jiao, Q.; Guan, Y.; Xie, M.; He, X.; et al. Longitudinal clinical and radiographic evaluation reveals interleukin-6 as an indicator of persistent pulmonary injury in COVID-19. Int. J. Med. Sci. 2021, 18, 29–41. [Google Scholar] [CrossRef]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef]
- Guven, D.; Bulus, A.D. Clinical and laboratory predictors of long-COVID in children: A single center retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 7695–7704. [Google Scholar] [CrossRef]
- Gao, P.; Liu, J.; Liu, M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12422. [Google Scholar] [CrossRef]
- Wynberg, E.; Han, A.X.; Boyd, A.; van Willigen, H.D.G.; Verveen, A.; Lebbink, R.; van der Straten, K.; Kootstra, N.; van Gils, M.J.; Russell, C.; et al. The effect of SARS-CoV-2 vaccination on post-acute sequelae of COVID-19 (PASC): A prospective cohort study. Vaccine 2022, 40, 4424–4431. [Google Scholar] [CrossRef]
- Pinto Pereira, S.M.; Nugawela, M.D.; Rojas, N.K.; Shafran, R.; McOwat, K.; Simmons, R.; Ford, T.; Heyman, I.; Ladhani, S.N.; Cheung, E.Y.; et al. Post-COVID-19 condition at 6 months and COVID-19 vaccination in non-hospitalised children and young people. Arch. Dis. Child. 2023, 108, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, C.R.; Strahm, C.; Gusewell, S.; Cusini, A.; Brucher, A.; Goppel, S.; Moller, E.; Moller, J.C.; Ortner, M.; Ruetti, M.; et al. Post-Acute Sequelae After Severe Acute Respiratory Syndrome Coronavirus 2 Infection by Viral Variant and Vaccination Status: A Multicenter Cross-Sectional Study. Clin. Infect. Dis. 2023, 77, 194–202. [Google Scholar] [CrossRef]
- Tofarides, A.G.; Christaki, E.; Milionis, H.; Nikolopoulos, G.K. Effect of Vaccination against SARS-CoV-2 on Long COVID-19: A Narrative Review. Life 2022, 12, 2057. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.T.; Milne, A.; Samms, E.; Stadon, L.; Maskell, N.A.; Hamilton, F.W. Symptoms After COVID-19 Vaccination in Patients with Persistent Symptoms After Acute Infection: A Case Series. Ann. Intern. Med. 2021, 174, 1334–1336. [Google Scholar] [CrossRef] [PubMed]
- Scherlinger, M.; Pijnenburg, L.; Chatelus, E.; Arnaud, L.; Gottenberg, J.E.; Sibilia, J.; Felten, R. Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study. Vaccines 2021, 10, 46. [Google Scholar] [CrossRef]
- Wisnivesky, J.P.; Govindarajulu, U.; Bagiella, E.; Goswami, R.; Kale, M.; Campbell, K.N.; Meliambro, K.; Chen, Z.; Aberg, J.A.; Lin, J.J. Association of Vaccination with the Persistence of Post-COVID Symptoms. J. Gen. Intern. Med. 2022, 37, 1748–1753. [Google Scholar] [CrossRef]
- Berg, S.K.; Wallach-Kildemoes, H.; Rasmussen, L.R.; Nygaard, U.; Birk, N.M.; Bundgaard, H.; Ersboll, A.K.; Thygesen, L.C.; Nielsen, S.D.; Christensen, A.V. Symptom-Specific Hospital Contacts in 12-18-Year-Olds Vaccinated against COVID-19: A Danish Register-Based Cohort Study. Vaccines 2023, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S.S.; Lerner, U.; Bivas-Benita, M.; et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: Nationwide cohort study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Dercon, Q.; Harrison, P.J. Six-month sequelae of post-vaccination SARS-CoV-2 infection: A retrospective cohort study of 10,024 breakthrough infections. Brain Behav. Immun. 2022, 103, 154–162. [Google Scholar] [CrossRef]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. EClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef] [PubMed]


| Overall (N = 500) | Vaccinated (N = 292) | Unvaccinated (N = 208) | p * | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years | ||||
| mean (SD) | 7.58 (4.60) | 9.69 (4.09) | 4.62 (3.53) | <0.0001 |
| median (IQR) | 7 (4, 11) | 9 (6.5, 13) | 4 (2, 7) | |
| Sex, n (%) | ||||
| Female | 218 (43.6) | 137 (46.9) | 81 (38.9) | 0.08 |
| Male | 282 (56.4) | 155 (53.1) | 127 (61.1) | |
| Confirmed test, n (%) | ||||
| RT-PCR | 134 (58.8) | 57 (47.9) | 77 (70.6) | <0.0001 |
| Rapid antigen test | 400 (88.1) | 243 (89.0) | 157 (86.7) | 0.46 |
| Hospitalization, n (%) | ||||
| Ward | 35 (7.1) | 16 (5.5) | 19 (9.4) | 0.10 |
| ICU | 2 (0.5) | 0 (0.0) | 2 (1.2) | 0.10 |
| Biomedical tests | ||||
| N; mean ± SD | N; mean ± SD | N; mean ± SD | p | |
| WBC (per μL) | 309; 8.30 ± 7.57 | 195; 8.21 ± 9.30 | 114; 8.45 ± 2.75 | 0.75 |
| RBC (per μL) | 309; 6.34 ± 27.66 | 195; 7.29 ± 34.82 | 114; 4.73 ± 0.43 | 0.31 |
| Neutrophils (%) | 359; 47.56 ± 13.00 | 227; 50.33 ± 11.24 | 132; 42.78 ± 14.40 | <0.0001 |
| Lymphocytes (%) | 359; 40.75 ± 13.10 | 227; 38.11 ± 10.98 | 132; 45.31 ± 15.10 | <0.0001 |
| N/L ratio | 1.17 | 1.32 | 0.94 | |
| Monocytes (%) | 359; 7.32 ± 2.90 | 227; 7.34 ± 3.11 | 132; 7.29 ± 2.52 | 0.87 |
| Eosinophils (%) | 359; 3.51 ± 3.02 | 227; 3.46 ± 2.98 | 132; 3.59 ± 3.10 | 0.71 |
| Hemoglobin (g/dL) | 308; 12.95 ± 1.49 | 194; 13.09 ± 1.48 | 114; 12.72 ± 1.47 | 0.04 |
| Platelets (×103 per μL) | 124; 322.56 ± 93.70 | 94; 301.95 ± 71.53 | 30; 387.13 ± 123.06 | 0.001 |
| ESR (mm/h) (normal range 0–20 mm/h) | 171; 7.08 ± 6.55 | 94; 6.81 ± 7.10 | 77; 7.40 ± 5.84 | 0.56 |
| hsCRP (mg/dL) (normal range 0.04–1.12 mg/dL) | 353; 0.19 ± 0.62 | 225; 0.19 ± 0.65 | 128; 0.20 ± 0.57 | 0.81 |
| LDH (U/L) (normal range 120–330 U/L) | 312; 201.21 ± 57.47 | 199; 188.90 ± 49.53 | 113; 222.88 ± 63.97 | <0.0001 |
| Ferritin (ng/mL) (normal range 10–300 ng/mL) | 297; 52.80 ± 43.14 | 191; 56.04 ± 40.17 | 106; 46.97 ± 47.67 | 0.10 |
| D-Dimer (ng/mL) (normal range ≤ 500 ng/mL) | 347; 312.24 ± 185.50 | 221; 290.78 ± 156.03 | 126; 349.88 ± 223.95 | 0.01 |
| AST (U/L) (normal range 15–45 U/L) | 322; 24.12 ± 10.65 | 207; 22.29 ± 10.35 | 115; 27.41 ± 10.42 | <0.0001 |
| ALT (U/L) (normal range 5–45 U/L) | 323; 15.92 ± 19.48 | 207; 16.29 ± 21.12 | 116; 15.27 ± 16.21 | 0.63 |
| CPK (U/L) (normal range 5–200 U/L) | 311; 117.62 ± 98.16 | 200; 115.01 ± 104.88 | 111; 122.33 ± 84.98 | 0.50 |
| Total IgE (IU/mL) (normal range < 100 IU/mL) | 269; 315.46 ± 562.40 | 178; 358.19 ± 640.76 | 91; 231.88 ± 351.76 | 0.04 |
| Total (N = 500) | 0–5 Years (N = 180) | 6–11 Years (N = 213) | 12–17 Years (N = 107) | p * | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, years | |||||
| Mean (SD) | 7.58 (4.603) | 2.76 (1.659) | 8.24 (1.706) | 14.36 (1.621) | <0.0001 |
| Median (IQR) | 7 (4, 11) | 3 (1, 4) | 8 (7, 10) | 14 (13, 16) | <0.0001 |
| Sex, n (%) | |||||
| Female | 218 (43.6) | 77 (42.8) | 88 (41.3) | 53 (49.5) | 0.36 |
| Male | 282 (56.4) | 103 (57.2) | 125 (58.7) | 54 (50.5) | |
| Confirmed test, n (%) | |||||
| RT-PCR | 134 (58.8) | 62 (70.5) | 45 (49.5) | 27 (55.1) | 0.01 |
| Rapid antigen test | 400 (88.1) | 146 (87.4) | 167 (87.4) | 87 (90.6) | 0.69 |
| Hospitalization, n (%) | |||||
| Ward | 35 (7.1) | 18 (10.1) | 12 (5.8) | 5 (4.8) | 0.15 |
| ICU | 2 (0.5) | 1 (0.7) | 1 (0.6) | 0 (0.0) | 0.76 |
| COVID-19 vaccine, n (%) | |||||
| 0 | 208 (41.6) | 140 (77.8) | 57 (26.8) | 11 (10.3) | <0.0001 |
| 1 | 139 (27.8) | 33 (18.3) | 93 (43.7) | 13 (12.1) | |
| 2 | 95 (19.0) | 6 (3.3) | 55 (25.8) | 34 (31.8) | |
| 3 | 54 (10.8) | 1 (0.6) | 8 (3.8) | 45 (42.1) | |
| 4 | 4 (0.8) | 0 (0.0) | 0 (0.0) | 4 (3.7) | |
| Biomedical tests | |||||
| N; mean ± SD | N; mean ± SD | N; mean ± SD | N; mean ± SD | p | |
| WBC (per μL) | 309; 8.30 ± 7.57 | 92; 9.67 ± 9.60 | 144; 7.46 ± 2.61 | 73; 8.24 ± 10.54 | 0.09 |
| RBC (per μL) | 309; 6.34 ± 27.66 | 92; 9.97 ± 50.70 | 144; 4.80 ± 0.52 | 73; 4.82 ± 0.53 | 0.33 |
| Neutrophils (%) | 359; 47.56 ± 13.00 | 107; 40.12 ± 15.22 | 166; 47.93 ± 10.2) | 86; 56.08 ± 8.73 | <0.0001 |
| Lymphocytes (%) | 359; 40.75 ± 13.10 | 107; 47.42 ± 16.19 | 166; 40.61 ± 9.91 | 86; 32.75 ± 9.18 | <0.0001 |
| N/L ratio | 1.17 | 0.85 | 1.18 | 1.71 | |
| Monocytes (%) | 359; 7.32 ± 2.90 | 107; 7.33 ± 2.75 | 166; 7.11 ± 3.31 | 86; 7.73 ± 2.11 | 0.27 |
| Eosinophils (%) | 359; 3.51 ± 3.02 | 107; 3.44 ± 3.25 | 166; 3.94 ± 3.20 | 86; 2.77 ± 2.08 | 0.01 |
| Hemoglobin (g/dL) | 308; 12.95 ± 1.49 | 92; 12.38 ± 1.78 | 143; 13.02 ± 1.19 | 73; 13.54 ± 1.37 | <0.0001 |
| Platelets (×103 per μL) | 124; 322.56 ± 93.70 | 23; 387.13 ± 124.91 | 58; 322.02 ± 85.07 | 43; 288.74 ± 65.31 | 0.0002 |
| ESR (mm/h) (normal range 0–20 mm/h) | 171; 7.08 ± 6.55 | 65; 8.05 ± 7.23 | 76; 6.99 ± 6.80 | 30; 5.20 ± 3.23 | 0.14 |
| hsCRP (mg/dL) (normal range 0.04–1.12 mg/dL) | 353; 0.19 ± 0.62 | 107; 0.27 ± 0.81 | 160; 0.15 ± 0.49 | 86; 0.19 ± 0.56 | 0.31 |
| LDH (U/L) (normal range 120–330 U/L) | 312; 201.21 ± 57.47 | 95; 242.97 ± 67.13 | 142; 198.73 ± 35.52 | 75; 153.00 ± 33.90 | <0.0001 |
| Ferritin (ng/mL) (normal range 10–300 ng/mL) | 297; 52.80 ± 43.14 | 89; 43.74 ± 48.43 | 132; 51.81 ± 29.01 | 76; 65.15 ± 53.55 | 0.01 |
| D-Dimer (ng/mL) (normal range ≤ 500 ng/mL) | 347; 312.24 ± 185.50 | 105; 383.49 ± 251.73 | 157; 270.18 ± 101.43 | 85; 301.90 ± 185.49 | <0.0001 |
| AST (U/L) (normal range 15–45 U/L) | 322; 24.12 ± 10.65 | 98; 30.09 ± 11.60 | 146; 23.50 ± 7.89 | 78; 17.77 ± 9.95 | <0.0001 |
| ALT (U/L) (normal range 5–45 U/L) | 323; 15.92 ± 19.48 | 99; 15.16 ± 18.65 | 146; 15.49 ± 15.61 | 78; 17.71 ± 26.09 | 0.65 |
| CPK (U/L) (normal range 5–200 U/L) | 311; 117.62 ± 98.16 | 94; 128.71 ± 88.75 | 141; 117.75 ± 85.27 | 76; 103.66 ± 127.04 | 0.26 |
| Total IgE (IU/mL) (normal range < 100 IU/mL) | 269; 315.46 ± 562.40 | 79; 291.32 ± 478.39 | 117; 321.96 ± 658.14 | 73; 331.18 ± 480.21 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-L.; Chen, P.-C.; Tsai, Y.-F.; Wei, C.-H.; Wu, L.S.-H.; Hsieh, K.-S.; Hsieh, M.-H.; Lai, H.-C.; Lin, C.-H.; Lin, H.-C.; et al. Clinical Features and Vaccination Effects among Children with Post-Acute Sequelae of COVID-19 in Taiwan. Vaccines 2024, 12, 910. https://doi.org/10.3390/vaccines12080910
Hsu Y-L, Chen P-C, Tsai Y-F, Wei C-H, Wu LS-H, Hsieh K-S, Hsieh M-H, Lai H-C, Lin C-H, Lin H-C, et al. Clinical Features and Vaccination Effects among Children with Post-Acute Sequelae of COVID-19 in Taiwan. Vaccines. 2024; 12(8):910. https://doi.org/10.3390/vaccines12080910
Chicago/Turabian StyleHsu, Yu-Lung, Pei-Chi Chen, Yi-Fen Tsai, Chi-Hung Wei, Lawrence Shi-Hsin Wu, Kai-Sheng Hsieh, Miao-Hsi Hsieh, Huan-Cheng Lai, Chien-Heng Lin, Hsiao-Chuan Lin, and et al. 2024. "Clinical Features and Vaccination Effects among Children with Post-Acute Sequelae of COVID-19 in Taiwan" Vaccines 12, no. 8: 910. https://doi.org/10.3390/vaccines12080910
APA StyleHsu, Y.-L., Chen, P.-C., Tsai, Y.-F., Wei, C.-H., Wu, L. S.-H., Hsieh, K.-S., Hsieh, M.-H., Lai, H.-C., Lin, C.-H., Lin, H.-C., Chen, C.-H., Chen, A.-C., Lin, H.-C., Chou, I.-C., Soong, W.-J., Hwang, K.-P., Lu, H. H.-S., Pawankar, R., Tsai, H.-J., & Wang, J.-Y. (2024). Clinical Features and Vaccination Effects among Children with Post-Acute Sequelae of COVID-19 in Taiwan. Vaccines, 12(8), 910. https://doi.org/10.3390/vaccines12080910







