Sequential Immunization with Vaccines Based on SARS-CoV-2 Virus-like Particles Induces Broadly Neutralizing Antibodies
Abstract
1. Introduction
2. Methods
2.1. Preparation of SARS-CoV-2 VLPs
2.2. Mouse Immunization
2.3. The Evaluation of Antibody Responses in Sera
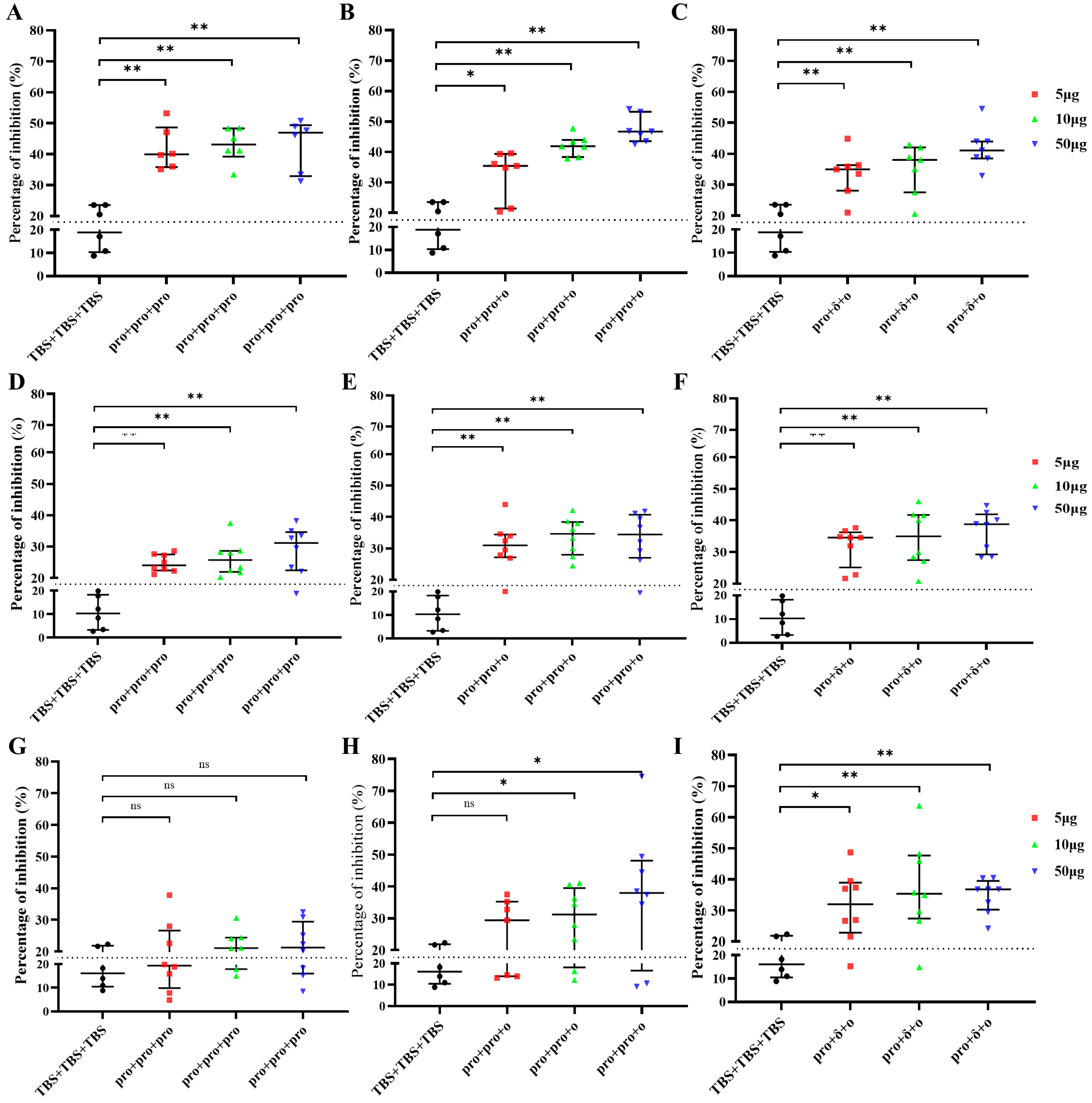
2.4. Surrogate Neutralization Assay
2.5. Immunophenotyping of Mouse Splenocytes
2.6. Cytokine Analysis of Mouse Splenocytes
2.7. Hematoxylin and Eosin (H&E) Staining
2.8. Statistical Analysis
3. Results
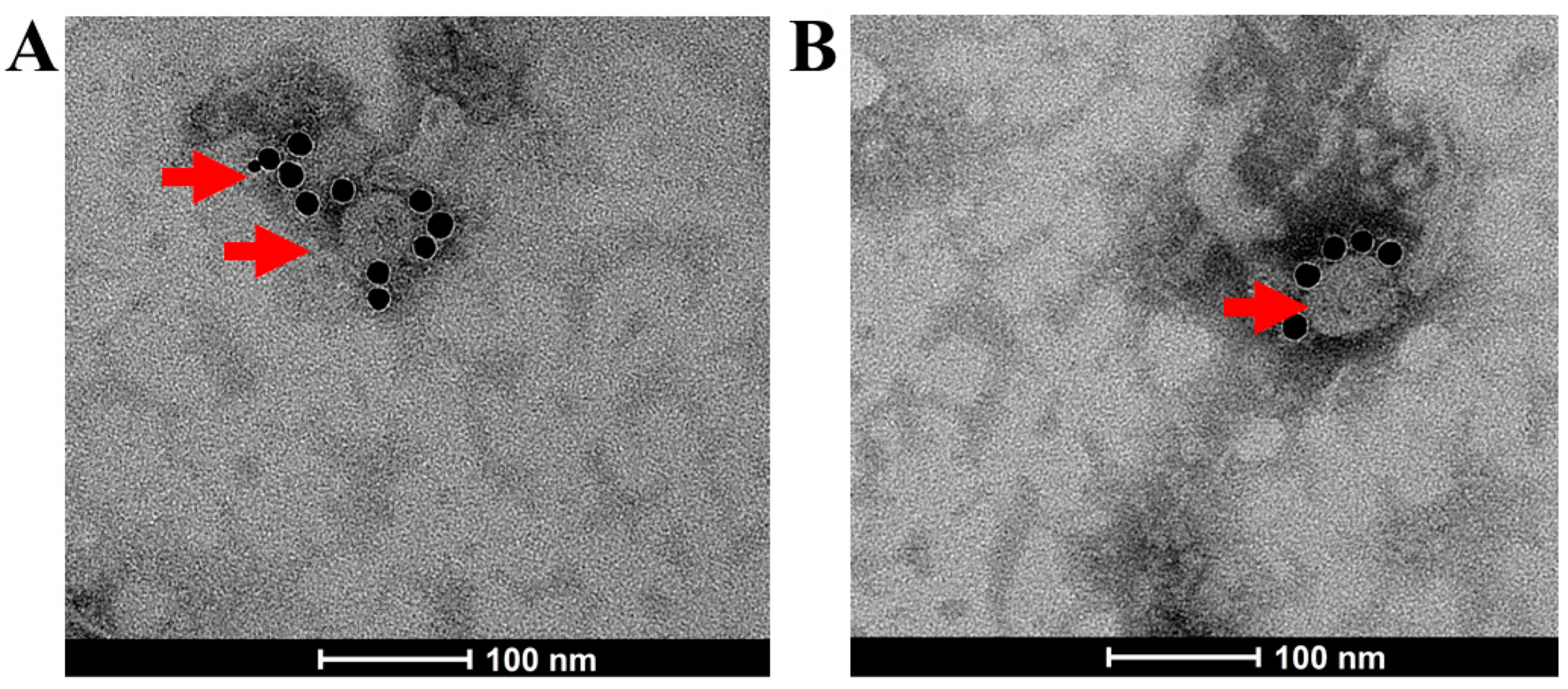
3.1. Generation of SARS-CoV-2 VLPs
3.2. VLPs Induced Antibody Responses
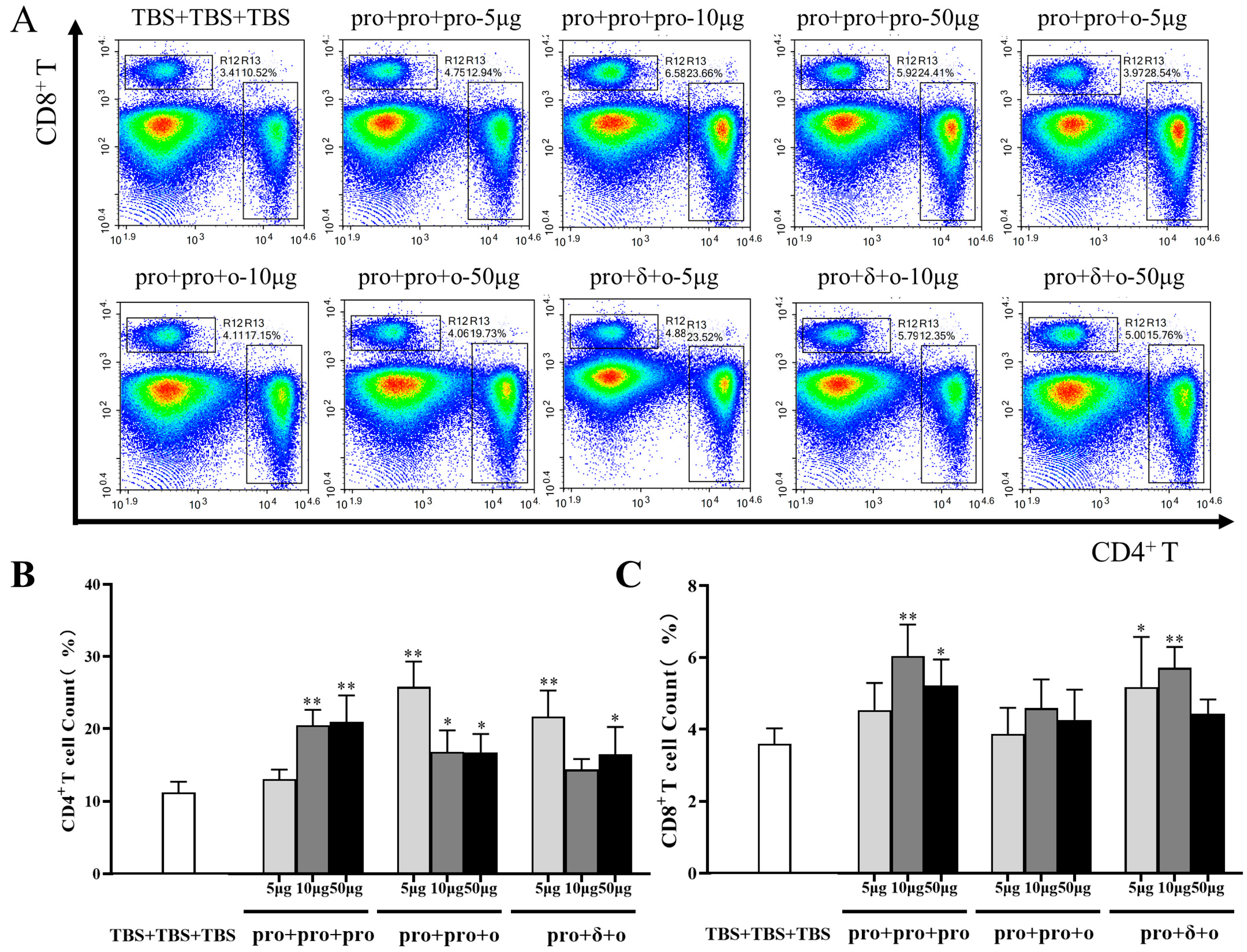
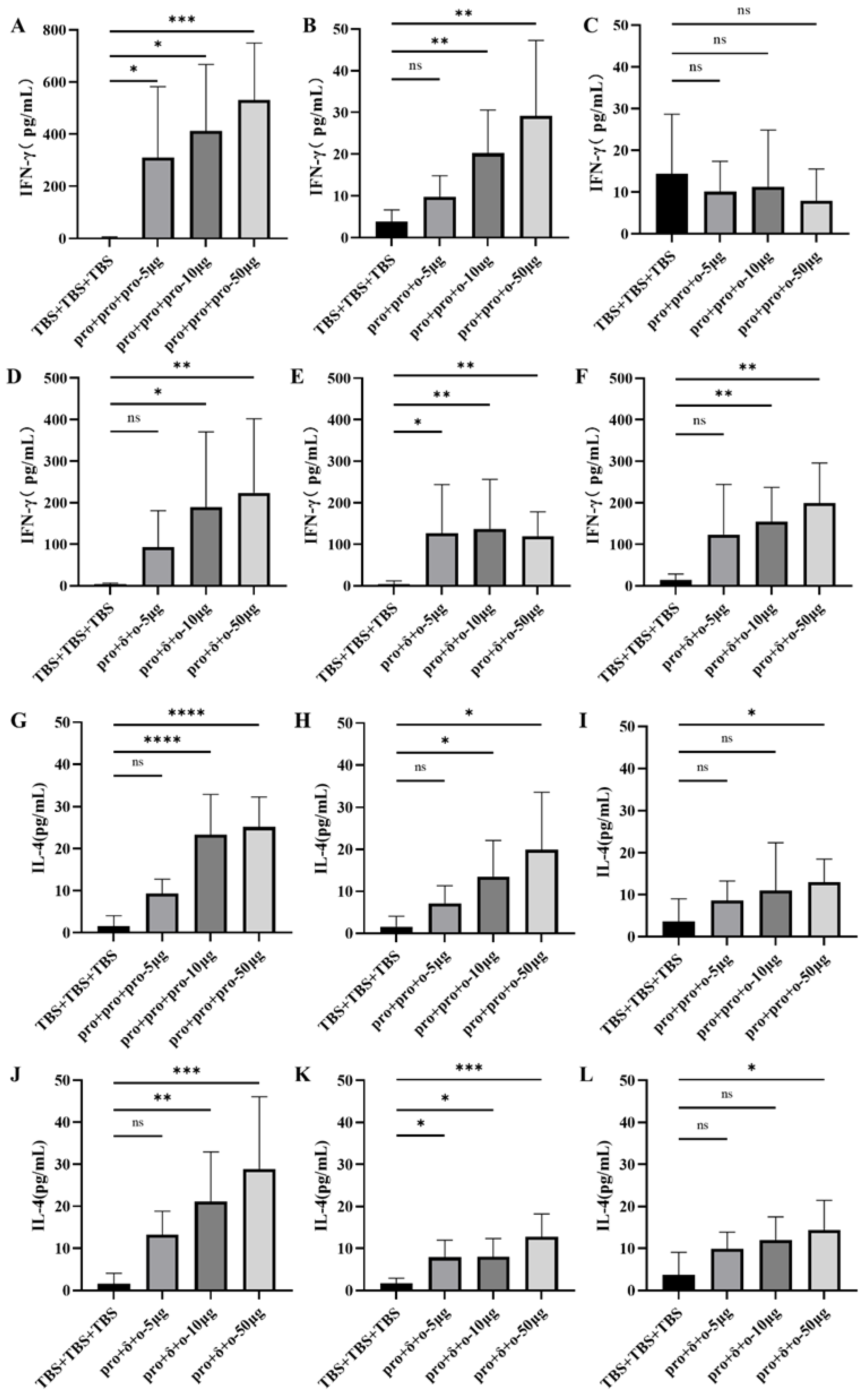
3.3. Cellular Immune Responses Elicited by Different Immunization
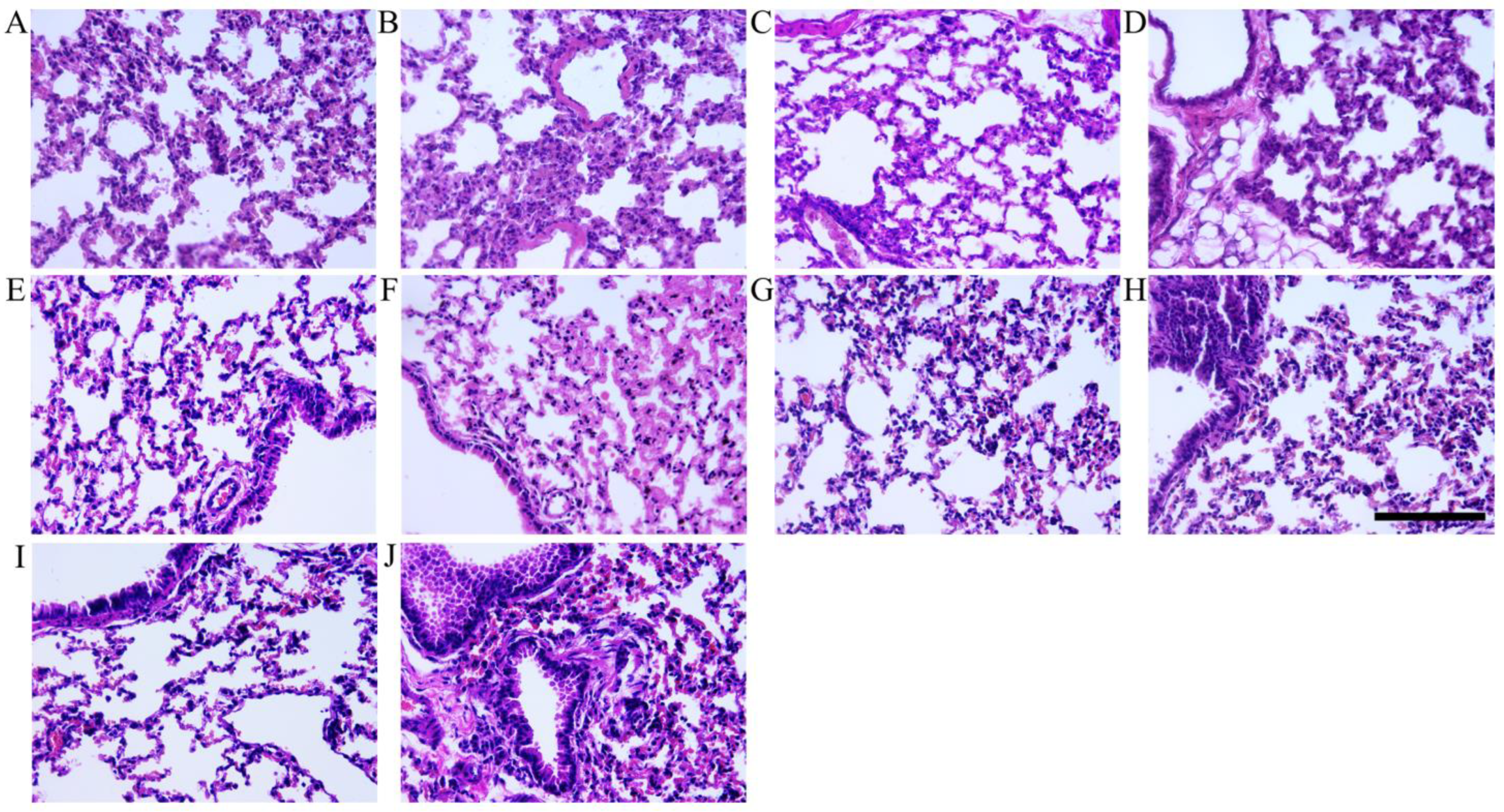
3.4. Histopathologic Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Tian, L.; Pang, Z.; Yang, Q.; Huang, T.; Fan, J.; Song, L.; Tong, Y.; Fan, H. COVID-19 vaccine development: Milestones, lessons and prospects. Sig. Transduct. Target. Ther. 2022, 7, 146. [Google Scholar] [CrossRef]
- WHO. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 30 March 2023).
- Frederiksen, L.S.F.; Zhang, Y.; Foged, C.; Thakur, A. The Long Road Toward COVID-19 Herd Immunity: Vaccine Platform Technologies and Mass Immunization Strategies. Front. Immunol. 2020, 11, 1817. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, R.; Samieefar, N.; Masoumi, N.; Mohseni, S.; Rezaei, N. COVID-19 vaccines mix-and-match: The concept, the efficacy and the doubts. J. Med. Virol. 2022, 94, 1294–1299. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; De Serres, G. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2021, 384, 1576–1577. [Google Scholar] [CrossRef]
- Yilmaz, I.C.; Ipekoglu, E.M.; Bulbul, A.; Turay, N.; Yildirim, M.; Evcili, I.; Yilmaz, N.S.; Guvencli, N.; Aydin, Y.; Gungor, B.; et al. Development and preclinical evaluation of virus-like particle vaccine against COVID-19 infection. Allergy 2022, 77, 258–270. [Google Scholar] [CrossRef]
- Shao, Y.; Wu, Y.; Feng, Y.; Xu, W.; Xiong, F.; Zhang, X. SARS-CoV-2 vaccine research and immunization strategies for improved control of the COVID-19 pandemic. Front. Med. 2022, 16, 185–195. [Google Scholar] [CrossRef]
- Rana, K.; Mohindra, R.; Pinnaka, L. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 385, e7. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M. Understanding Breakthrough Infections Following mRNA SARS-CoV-2 Vaccination. JAMA 2021, 326, 2018–2020. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, J.; Edson, D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Polack, F.P.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.-D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N.; et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.H.G.; de Fátima Goebel de Souza, T.; de Carvalho Araújo, F.M.; de Andrade, L.O.M. Dynamics of antibody response to CoronaVac vaccine. J. Med. Virol. 2022, 94, 2139–2148. [Google Scholar] [CrossRef]
- Chi, W.-Y.; Li, Y.-D.; Huang, H.-C.; Chan, T.E.H.; Chow, S.-Y.; Su, J.-H.; Ferrall, L.; Hung, C.-F.; Wu, T.-C. COVID-19 vaccine update: Vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection. J. Biomed. Sci. 2022, 29, 82. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Pepper, M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2022, 12, 809244. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lanzavecchia, A.; Araki, K.; Ahmed, R. From Vaccines to Memory and Back. Immunity 2010, 33, 451–463. [Google Scholar] [CrossRef]
- He, Q.; Mao, Q.; An, C.; Zhang, J.; Gao, F.; Bian, L.; Li, C.; Liang, Z.; Xu, M.; Wang, J. Heterologous prime-boost: Breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg. Microbes Infect. 2021, 10, 629–637. [Google Scholar] [CrossRef]
- Lv, J.; Wu, H.; Xu, J.; Liu, J. Immunogenicity and safety of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine: A systematic review. Infect. Dis. Poverty 2022, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Chavda, V.P.; Apostolopoulos, V. Is Booster Dose Strategy Sufficient for Omicron Variant of SARS-CoV-2? Vaccines 2022, 10, 367. [Google Scholar] [CrossRef]
- Mohsen, M.O.; Bachmann, M.F. Virus-like particle vaccinology, from bench to bedside. Cell. Mol. Immunol. 2022, 19, 993–1011. [Google Scholar] [CrossRef] [PubMed]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Banoun, H. mRNA: Vaccine or Gene Therapy? The Safety Regulatory Issues. Int. J. Mol. Sci. 2023, 24, 10514. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kamat, I.; Hotez, P.J. Myocarditis With COVID-19 mRNA Vaccines. Circulation 2021, 144, 471–484. [Google Scholar] [CrossRef]
- Mi, Y.; Xie, T.; Zhu, B.; Tan, J.; Li, X.; Luo, Y.; Li, F.; Niu, H.; Han, J.; Lv, W.; et al. Production of SARS-CoV-2 Virus-like Particles in Insect Cells. Vaccines 2021, 9, 554. [Google Scholar] [CrossRef]
- Mi, Y.; Liang, L.; Xu, K.; Li, Q.; Wang, W.; Dang, W.; Deng, J.; Zhi, Y.; Li, X.; Tan, J. Severe acute respiratory syndrome coronavirus 2 virus-like particles induce dendritic cell maturation and modulate T cell immunity. Front. Cell. Infect. Microbiol. 2022, 12, 986350. [Google Scholar] [CrossRef]
- Hao, X.; Yuan, F.; Yao, X. Advances in virus-like particle-based SARS-CoV-2 vaccines. Front. Cell. Infect. Microbiol. 2024, 14, 1406091. [Google Scholar] [CrossRef]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Ciotti, M.; Ciccozzi, M.; Pieri, M.; Bernardini, S. The COVID-19 pandemic: Viral variants and vaccine efficacy. Crit. Rev. Clin. Lab. Sci. 2022, 59, 66–75. [Google Scholar] [CrossRef]
- Singh, D.D.; Parveen, A.; Yadav, D.K. SARS-CoV-2: Emergence of New Variants and Effectiveness of Vaccines. Front. Cell. Infect. Microbiol. 2021, 11, 777212. [Google Scholar] [CrossRef]
- Sapkota, B.; Saud, B.; Shrestha, R.; Al-Fahad, D.; Sah, R.; Shrestha, S.; Rodriguez-Morales, A.J. Heterologous prime-boost strategies for COVID-19 vaccines. J. Travel Med. 2021, 29, taab191. [Google Scholar] [CrossRef] [PubMed]
- Kardani, K.; Bolhassani, A.; Shahbazi, S. Prime-boost vaccine strategy against viral infections: Mechanisms and benefits. Vaccine 2016, 34, 413–423. [Google Scholar] [CrossRef]
- Vierboom, M.P.M.; Chenine, A.L.; Darrah, P.A.; Vervenne, R.A.W.; Boot, C.; Hofman, S.O.; Sombroek, C.C.; Dijkman, K.; Khayum, M.A.; Stammes, M.A.; et al. Evaluation of heterologous prime-boost vaccination strategies using chimpanzee adenovirus and modified vaccinia virus for TB subunit vaccination in rhesus macaques. Npj Vaccines 2020, 5, 39. [Google Scholar] [CrossRef]
- Burm, R.; Maravelia, P.; Ahlen, G.; Ciesek, S.; Perez, N.C.; Pasetto, A.; Urban, S.; Van Houtte, F.; Verhoye, L.; Wedemeyer, H.; et al. Novel prime-boost immune-based therapy inhibiting both hepatitis B and D virus infections. Gut 2023, 72, 1186–1195. [Google Scholar] [CrossRef]
- van Diepen, M.T.; Chapman, R.; Douglass, N.; Galant, S.; Moore, P.L.; Margolin, E.; Ximba, P.; Morris, L.; Rybicki, E.P.; Williamson, A.-L.; et al. Prime-Boost Immunizations with DNA, Modified Vaccinia Virus Ankara, and Protein-Based Vaccines Elicit Robust HIV-1 Tier 2 Neutralizing Antibodies against the CAP256 Superinfecting Virus. J. Virol. 2019, 93, e02155-18. [Google Scholar] [CrossRef]
- Simon, G.; Favresse, J.; Gillot, C.; Closset, M.; Catry, É.; Dogné, J.-M.; Douxfils, J.; Wieërs, G.; Bayart, J.-L. Kinetics and ability of binding antibody and surrogate virus neutralization tests to predict neutralizing antibodies against the SARS-CoV-2 Omicron variant following BNT162b2 booster administration. Clin. Chem. Lab. Med. 2023, 61, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Prusinkiewicz, M.A.; Sediqi, S.; Li, Y.J.; Goldfarb, D.M.; Asamoah-Boaheng, M.; Wall, N.; Lavoie, P.M.; Grunau, B. Effect of vaccine dosing intervals on Omicron surrogate neutralization after three doses of BNT162b2. Heliyon 2023, 9, e17259. [Google Scholar] [CrossRef] [PubMed]
- Lyke, K.E.; Atmar, R.L.; Islas, C.D.; Posavad, C.M.; Szydlo, D.; Chourdhury, R.P.; Deming, M.E.; Eaton, A.; Jackson, L.A.; Branche, A.R.; et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep. Med. 2022, 3, 100679. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Zhang, Y.; Wu, J.; Wan, Y.; Huang, Y.; Song, J.; Fu, Z.; et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 2022, 11, 337–343. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA 2022, 327, 639–651. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.W. Omicron Variant (B.1.1.529): Infectivity, Vaccine Breakthrough, and Antibody Resistance. J. Chem. Inf. Model. 2022, 62, 412–422. [Google Scholar] [CrossRef]
- Ying, B.; Scheaffer, S.M.; Whitener, B.; Liang, C.-Y.; Dmytrenko, O.; Mackin, S.; Wu, K.; Lee, D.; Avena, L.E.; Chong, Z.; et al. Boosting with variant-matched or historical mRNA vaccines protects against Omicron infection in mice. Cell 2022, 185, 1572–1587.e11. [Google Scholar] [CrossRef]
- Nascimento, I.P.; Leite, L.C.C. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111. [Google Scholar] [CrossRef]
- Liu, J.; Xu, K.; Xing, M.; Zhuo, Y.; Guo, J.; Du, M.; Wang, Q.; An, Y.; Li, J.; Gao, P.; et al. Heterologous prime-boost immunizations with chimpanzee adenoviral vectors elicit potent and protective immunity against SARS-CoV-2 infection. Cell Discov. 2021, 7, 123. [Google Scholar] [CrossRef]
- Ding, C.; Ni, S.; Zhang, X.; Xie, J.; Sun, Y.; He, J.; Mei, Q.; Huang, L.; He, H.; Liu, Z.; et al. Evaluation of humoral immune responses induced by different SARS-CoV-2 spike trimers from wild-type and emerging variants with individual, sequential, and combinational delivered strategies. J. Med. Virol. 2022, 94, 5841–5849. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Z.; Yang, J.; Xu, X. Immunogenicity of novel DNA vaccines encoding receptor-binding domain (RBD) dimer-Fc fusing antigens derived from different SARS-CoV-2 variants of concern. J. Med. Virol. 2023, 95, e28563. [Google Scholar] [CrossRef]
- Nowill, A.E.; Caruso, M.; de Campos-Lima, P.O. T-cell immunity to SARS-CoV-2: What if the known best is not the optimal course for the long run? Adapting to evolving targets. Front. Immunol. 2023, 14, 1133225. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, S.; Liu, Y.; Wang, S.; Peng, H.; Hao, Y.; Hong, K.; Li, D.; Shao, Y. Sequential Administration of SARS-CoV-2 Strains-Based Vaccines Effectively Induces Potent Immune Responses against Previously Unexposed Omicron Strain. Pathogens 2023, 12, 655. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Yu, J.; McMahan, K.; Jacob-Dolan, C.; Liu, J.; He, X.; Hope, D.; Anioke, T.; Barrett, J.; Chung, B.; et al. Vaccine protection against the SARS-CoV-2 Omicron variant in macaques. Cell 2022, 185, 1549–1555.e11. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
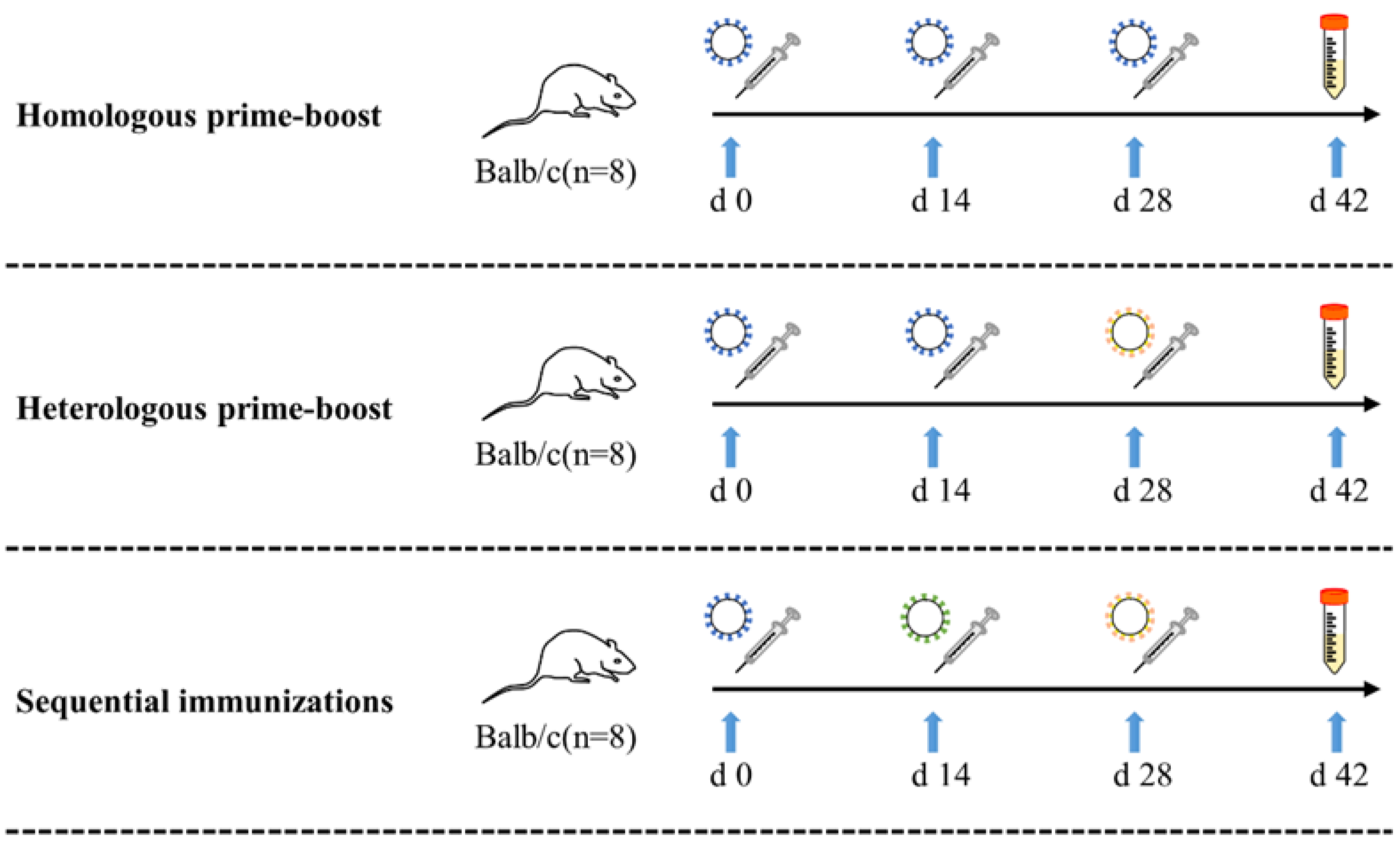

| Vaccination Strategy | Group | μg/Dose | First Dose | Second Dose | Third Dose | n |
|---|---|---|---|---|---|---|
| Control | TBS + TBS + TBS | - | TBS | TBS | TBS | 8 |
| Homologous prime-boost | pro + pro + pro | 5 | pro-VLPs | pro-VLPs | pro-VLPs | 8 |
| 10 | pro-VLPs | pro-VLPs | pro-VLPs | 8 | ||
| 50 | pro-VLPs | pro-VLPs | pro-VLPs | 8 | ||
| Heterologous prime-boost | pro + pro + ο | 5 | pro-VLPs | pro-VLPs | ο-VLPs | 8 |
| 10 | pro-VLPs | pro-VLPs | ο-VLPs | 8 | ||
| 50 | pro-VLPs | pro-VLPs | ο-VLPs | 8 | ||
| Sequential immunizations | pro + δ + ο | 5 | pro-VLPs | δ-VLPs | ο-VLPs | 8 |
| 10 | pro-VLPs | δ-VLPs | ο-VLPs | 8 | ||
| 50 | pro-VLPs | δ-VLPs | ο-VLPs | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mi, Y.; Xu, K.; Wang, W.; Kong, W.; Xu, X.; Rong, X.; Tan, J. Sequential Immunization with Vaccines Based on SARS-CoV-2 Virus-like Particles Induces Broadly Neutralizing Antibodies. Vaccines 2024, 12, 927. https://doi.org/10.3390/vaccines12080927
Mi Y, Xu K, Wang W, Kong W, Xu X, Rong X, Tan J. Sequential Immunization with Vaccines Based on SARS-CoV-2 Virus-like Particles Induces Broadly Neutralizing Antibodies. Vaccines. 2024; 12(8):927. https://doi.org/10.3390/vaccines12080927
Chicago/Turabian StyleMi, Youjun, Kun Xu, Wenting Wang, Weize Kong, Xiaonan Xu, Xifeng Rong, and Jiying Tan. 2024. "Sequential Immunization with Vaccines Based on SARS-CoV-2 Virus-like Particles Induces Broadly Neutralizing Antibodies" Vaccines 12, no. 8: 927. https://doi.org/10.3390/vaccines12080927
APA StyleMi, Y., Xu, K., Wang, W., Kong, W., Xu, X., Rong, X., & Tan, J. (2024). Sequential Immunization with Vaccines Based on SARS-CoV-2 Virus-like Particles Induces Broadly Neutralizing Antibodies. Vaccines, 12(8), 927. https://doi.org/10.3390/vaccines12080927






