Investigation of the Hepatitis-B Vaccine’s Immune Response in a Non-Alcoholic Fatty Liver Disease Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diets and Diet-Induced NAFLD Model
2.3. Vaccination Protocol
2.4. Blood and Tissue Samples
2.5. Analysis of Mononuclear Cells
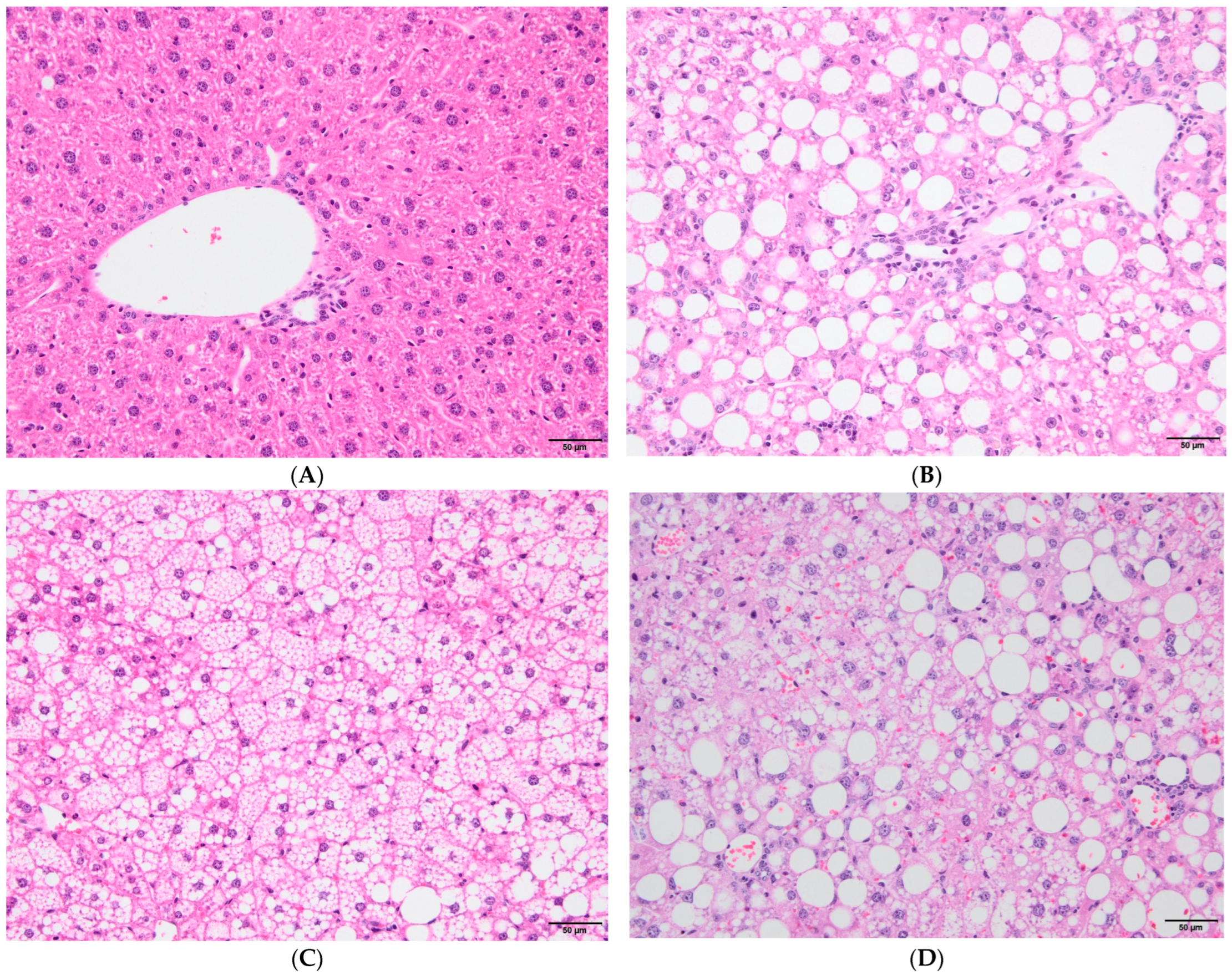
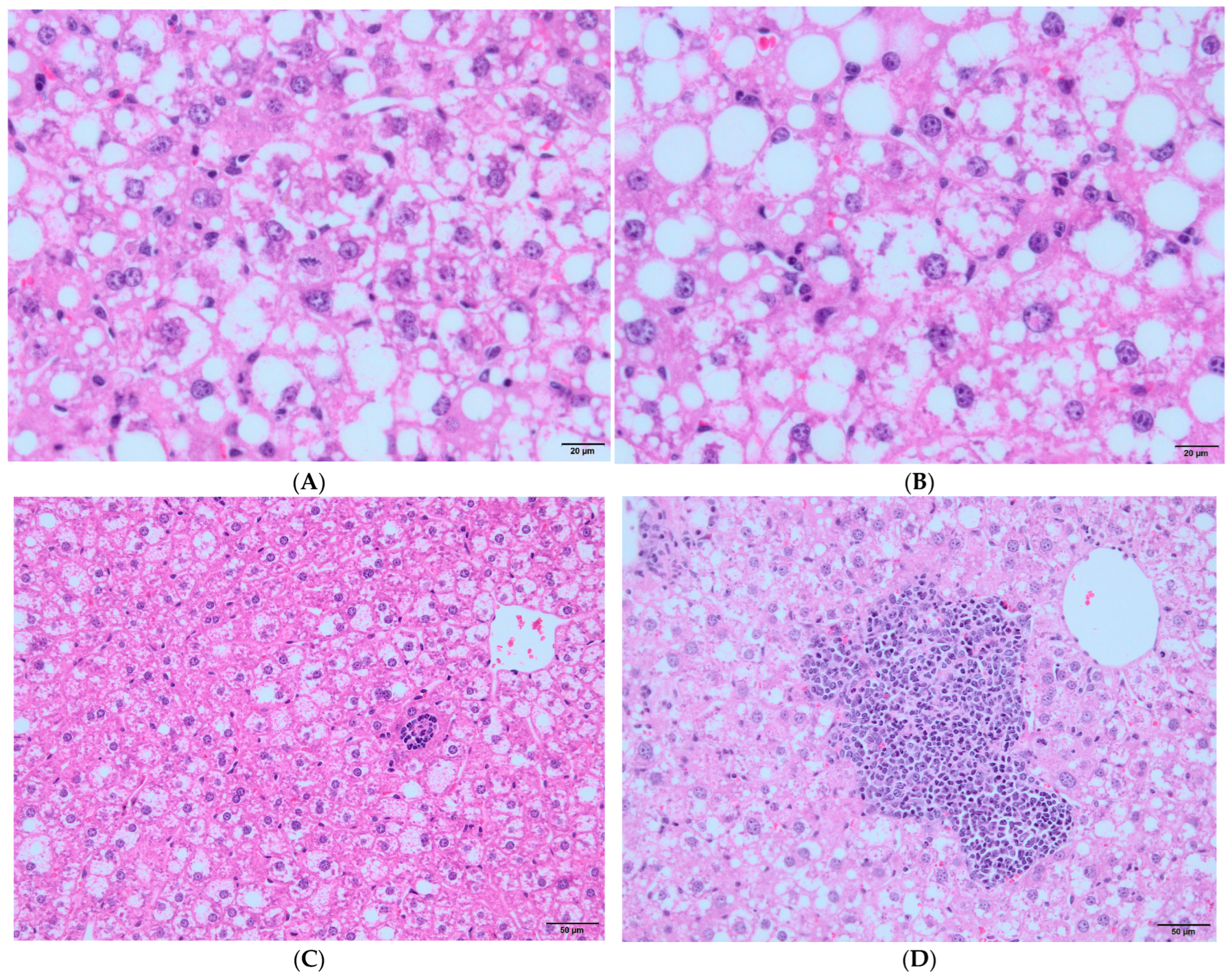
2.6. Histopatologic Evaluation
2.7. Anti-HB Titers
2.8. Flow-Cytometric Counting of Blood Helper T and Regulatory T Cells
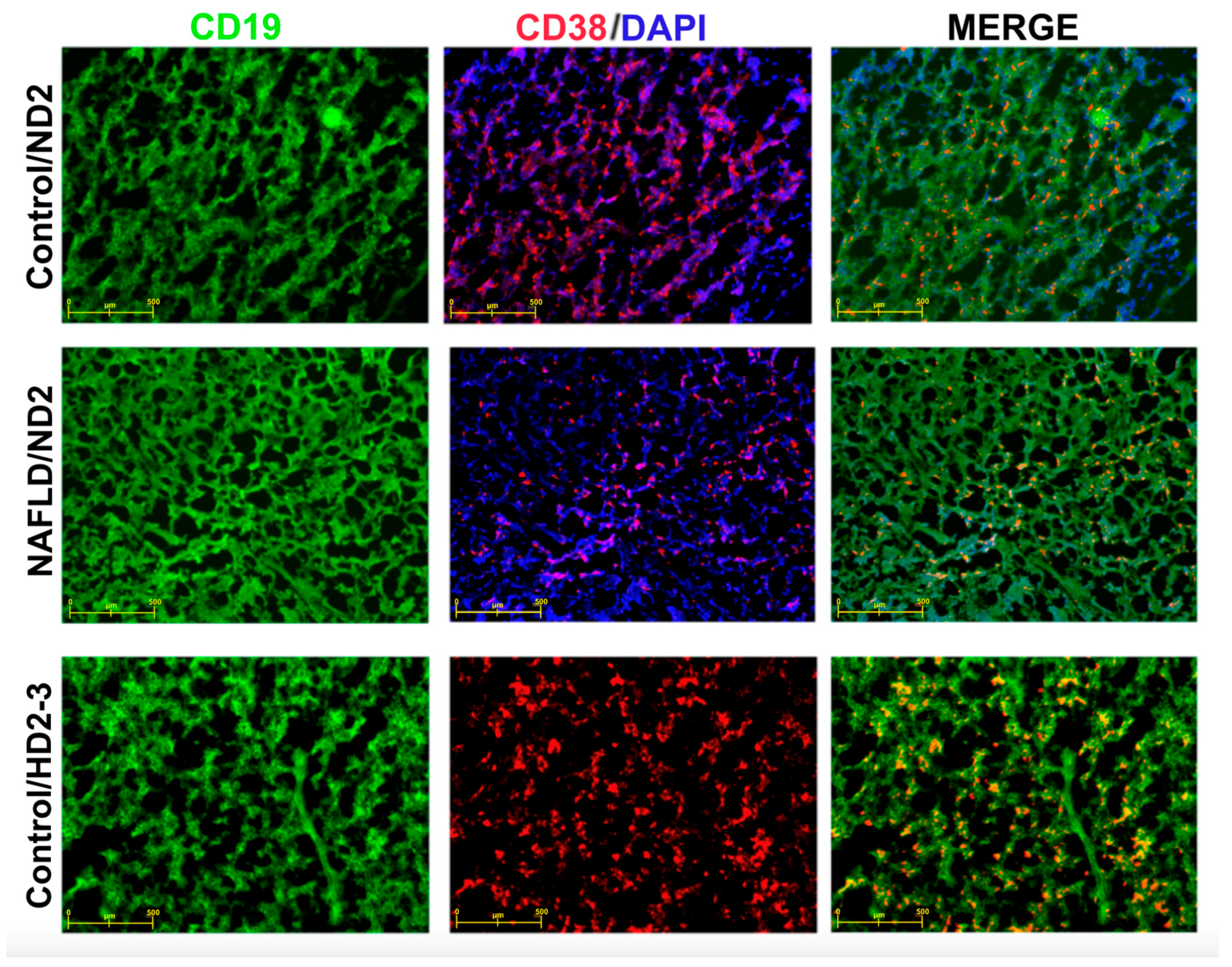
2.9. Immunofluorescent Staining of Mouse Thymus and Spleen Sections
2.10. Statistical Analysis
3. Results
3.1. Nutritional/Growth Parameters of Experimental Groups

3.2. Immunological Parameters in Experimental Groups According to HBV Vaccination Program
3.3. Hepatitis B Vaccine Responses in Experimental Groups
3.4. Correlation between Hepatitis B Vaccine Responses and Immunological Parameter Levels in the Control and NAFLD Groups
| Control Groups | NAFLD Groups | |||
|---|---|---|---|---|
| rs | p | rs | p | |
| Treg | −0.18 | 0.499 | −0.02 | 0.931 |
| Tfh | −0.21 | 0.422 | −0.47 | 0.030 |
| Treg/Tfh | −0.21 | 0.444 | 0.67 | 0.002 |
| CD27+ | −0.32 | 0.202 | −0.59 | 0.005 |
| CD38+ | 0.07 | 0.769 | 0.64 | 0.002 |
| CD27+CD38+ | 0.10 | 0.698 | −0.04 | 0.849 |
3.5. Immunophenotyping in Experimental Groups
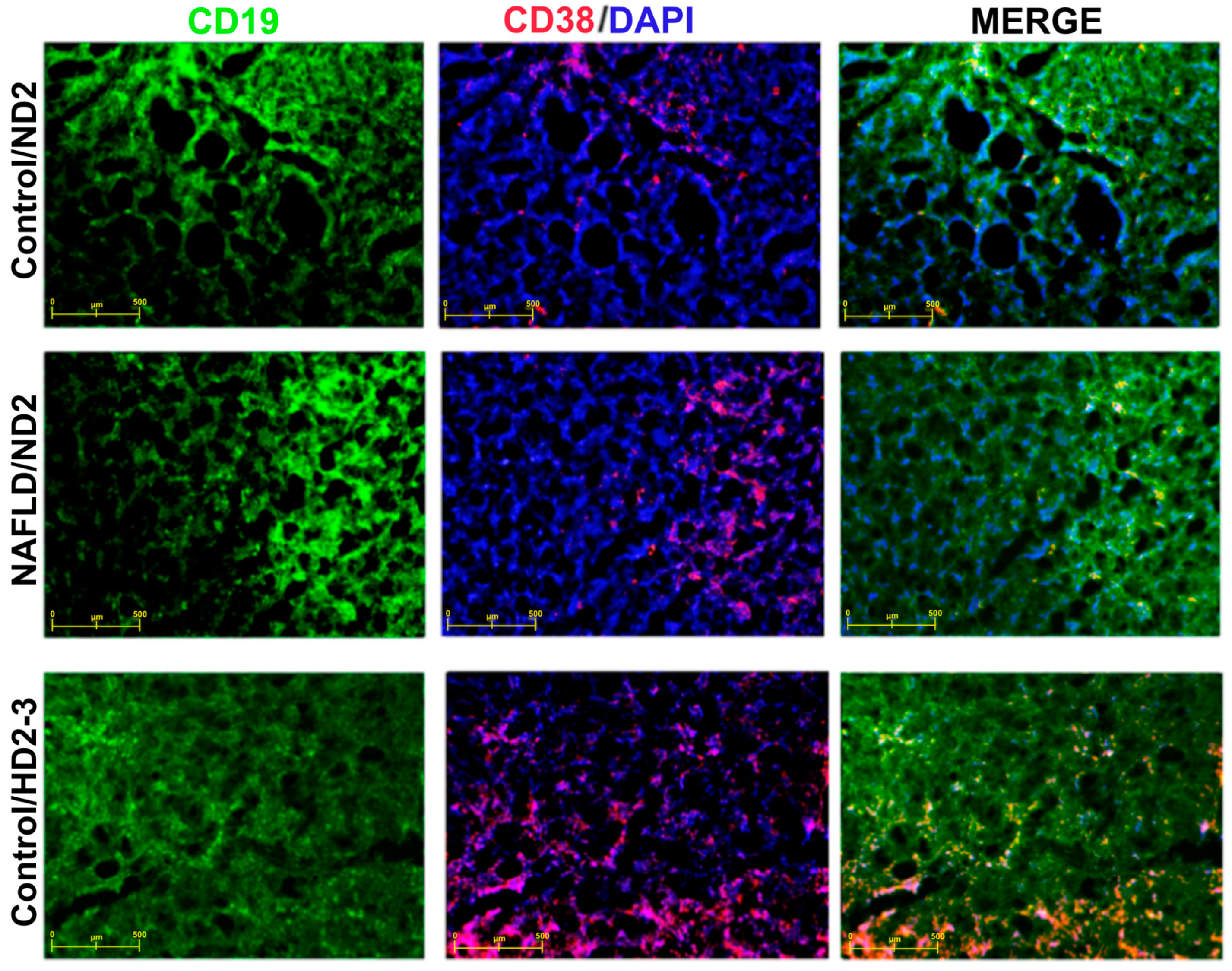
4. Discussion
Strengths and Difficulties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tourkochristou, E.; Assimakopoulos, S.F.; Thomopoulos, K.; Marangos, M.; Triantos, C. NAFLD and HBV interplay—Related mechanisms underlying liver disease progression. Front. Immunol. 2022, 13, 965548. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- The Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Zheng, Q.; Zou, B.; Wu, Y.; Yeo, Y.; Wu, H.; Stave, C.D.; Cheung, R.C.; Nguyen, M.H. Systematic review with meta-analysis: Prevalence of hepatic steatosis, fibrosis and associated factors in chronic hepatitis B. Aliment. Pharmacol. Ther. 2021, 54, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Luci, C.; Vieira, E.; Perchet, T.; Gual, P.; Golub, R. Natural Killer Cells and Type 1 Innate Lymphoid Cells Are New Actors in Non-alcoholic Fatty Liver Disease. Front. Immunol. 2019, 10, 1192. [Google Scholar] [CrossRef]
- Karlsson, E.A.; Beck, M.A. The burden of obesity on infectious disease. Exp. Biol. Med. 2010, 235, 1412–1424. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; Kay, T.; Scholz, M.H.; Diggs, B.; Jobe, B.A.; Lewinsohn, D.M.; Bakke, A.C. Alterations in T-cell subset frequency in peripheral blood in obesity. Obes. Surg. 2005, 15, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, D.; Cawood, T.J.; O’Farrelly, C.; Lynch, L. Natural killer cells in obesity: Impaired function and increased susceptibility to the effects of cigarette smoke. PLoS ONE 2010, 5, e8660. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Isoda, F.; Ishihara, Y.; Kimura, M.; Yamakawa, T. T lymphopaenia in relation to body mass index and TNF-alpha in human obesity: Adequate weight reduction can be corrective. Clin. Endocrinol. 2001, 54, 347–354. [Google Scholar] [CrossRef]
- Averhoff, F.; Mahoney, F.; Coleman, P.; Schatz, G.; Hurwitz, E.; Margolis, H. Immunogenicity of hepatitis B Vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am. J. Prev. Med. 1998, 15, 1–8. [Google Scholar] [CrossRef]
- Tolosa Martínez, N.; Tenías Burillo, J.M.; Pérez Bermúdez, B.; Sanchis Álvarez, J.B. Factores asociados a una respuesta inadecuada a la vacunación contra la hepatitis B en personal sanitario. Rev. Española Salud Pública 1998, 72, 509–515. [Google Scholar] [CrossRef]
- Weber, D.J.; Rutala, W.A.; Samsa, G.P.; Santimaw, J.E.; Lemon, S.M. Obesity as a Predictor of Poor Antibody Response to Hepatitis B Plasma Vaccine. JAMA 1985, 254, 3187–3189. [Google Scholar] [CrossRef] [PubMed]
- Middleman, A.B.; Anding, R.; Tung, C. Effect of needle length when immunizing obese adolescents with hepatitis B vaccine. Pediatrics 2010, 125, e508–e512. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.N.; Zuckerman, A.J.; Symington, I.; Du, W.; Williams, A.; Dickson, B.; Young, M.D. Evaluation of a new hepatitis B triple-antigen vaccine in inadequate responders to current vaccines. Hepatology 2001, 34, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tajes, S.; Pocurull, A.; Lens, S.; Mariño, Z.; Olivas, I.; Soy, G.; Alonso, A.; Vilella, A.; Forns, X. Efficacy of an accelerated double-dose hepatitis B vaccine regimen in patients with cirrhosis. J. Viral Hepat. 2021, 28, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Davis, R.P.; Ma, M.M.; Tam, E.; Cooper, C.L.; Ramji, A.; Kelly, E.M.; Jayakumar, S.; Swain, M.G.; Jenne, C.N.; et al. Reduced immune responses to hepatitis B primary vaccination in obese individuals with nonalcoholic fatty liver disease (NAFLD). NPJ Vaccines 2021, 6, 9. [Google Scholar] [CrossRef]
- World Health Organization. Global Hepatitis Report 2017. Available online: https://iris.who.int/bitstream/handle/10665/255016/9789241565455-eng.pdf (accessed on 14 May 2024).
- Yin, M.; Xiong, Y.; Liang, D.; Tang, H.; Hong, Q.; Liu, G.; Zeng, J.; Lian, T.; Huang, J.; Ni, J. Circulating Tfh cell and subsets distribution are associated with low-responsiveness to hepatitis B vaccination. Mol. Med. 2021, 27, 32. [Google Scholar] [CrossRef]
- WHO. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis. 2016. Available online: http://apps.who.int/iris/handle/10665/246177 (accessed on 8 November 2021).
- Asgharpour, A.; Cazanave, S.C.; Pacana, T.; Seneshaw, M.; Vincent, R.; Banini, B.A.; Kumar, D.P.; Daita, K.; Min, H.K.; Mirshahi, F.; et al. A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. J. Hepatol. 2016, 65, 579–588. [Google Scholar] [CrossRef]
- Liu, X.J.; Duan, N.N.; Liu, C.; Niu, C.; Liu, X.P.; Wu, J. Characterization of a murine nonalcoholic steatohepatitis model induced by high fat high calorie diet plus fructose and glucose in drinking water. Lab. Invest. 2018, 98, 1184–1199. [Google Scholar] [CrossRef]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qi, F.; Yang, Y.; Yuan, Q.; Zou, J.; Guo, K.; Yao, Z. Neonatal hepatitis B vaccination impaired the behavior and neurogenesis of mice transiently in early adulthood. Psychoneuroendocrinology 2016, 73, 166–176. [Google Scholar] [CrossRef]
- Duseja, A.; Singh, S.P.; De, A.; Madan, K.; Rao, P.N.; Shukla, A.; Choudhuri, G.; Saigal, S.; Shalimar; Arora, A.; et al. Indian National Association for Study of the Liver (INASL) Guidance Paper on Nomenclature, Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease (NAFLD). J. Clin. Exp. Hepatol. 2023, 13, 273–302. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Bandaru, P.; Rajkumar, H.; Nappanveettil, G. Altered or impaired immune response upon vaccination in WNIN/Ob rats. Vaccine 2011, 29, 3038–3042. [Google Scholar] [CrossRef] [PubMed]
- Estévez, Z.C.; Betancourt, A.A.; Muzio González, V.; Baile, N.F.; Silva, C.V.; Bernal, F.H.; Arias, E.P.; Delhanty Fernández, A.; Olazábal, N.M.; del Río Martín, A.; et al. Immunogenicity and safety assessment of the Cuban recombinant hepatitis B vaccine in healthy adults. Biologicals 2007, 35, 115–122. [Google Scholar] [CrossRef]
- Janssen, J.M.; Jackson, S.; Heyward, W.L.; Janssen, R.S. Immunogenicity of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (HBsAg-1018) compared with a licensed hepatitis B vaccine in subpopulations of healthy adults 18–70 years of age. Vaccine 2015, 33, 3614–3618. [Google Scholar] [CrossRef]
- Dinelli, M.I.; Moraes-Pinto, M.I. Seroconvertion to hepatitis B vaccine after weight reduction in obese non-responder. Rev. Inst. Med. Trop Sao Paulo 2008, 50, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Shim, S.H.; Lee, E.Y.; Cho, W.; Park, S.; Jeon, H.J.; Ahn, S.Y.; Kim, H.; Nam, J.H. Obesity-induced chronic inflammation is associated with the reduced efficacy of influenza vaccine. Hum. Vaccin. Immunother. 2014, 10, 1181–1186. [Google Scholar] [CrossRef]
- Paich, H.A.; Sheridan, P.A.; Handy, J.; Karlsson, E.A.; Schultz-Cherry, S.; Hudgens, M.G.; Noah, T.L.; Weir, S.S.; Beck, M.A. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity 2013, 21, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, A.E.; Taylor, K.R.; Dutt, S.; Han, P.P.; Fujioka, K.; Jameson, J.M. Obesity impairs γδ T cell homeostasis and antiviral function in humans. PLoS ONE 2015, 10, e0120918. [Google Scholar] [CrossRef]
- Kosaraju, R.; Guesdon, W.; Crouch, M.J.; Teague, H.L.; Sullivan, E.M.; Karlsson, E.A.; Schultz-Cherry, S.; Gowdy, K.; Bridges, L.C.; Reese, L.R.; et al. B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection. J. Immunol. 2017, 198, 4738–4752. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.E.; Adler, B.J.; Green, D.E.; Rubin, C.T. Bone structure and B-cell populations, crippled by obesity, are partially rescued by brief daily exposure to low-magnitude mechanical signals. Faseb J. 2012, 26, 4855–4863. [Google Scholar] [CrossRef]
- Liu, R.; Nikolajczyk, B.S. Tissue Immune Cells Fuel Obesity-Associated Inflammation in Adipose Tissue and Beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Akbar, S.M.; Yoshida, O.; Chen, S.; Hiasa, Y.; Matsuura, B.; Abe, M.; Onji, M. Impaired dendritic cell functions disrupt antigen-specific adaptive immune responses in mice with nonalcoholic fatty liver disease. J. Gastroenterol. 2010, 45, 859–867. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Wu, L.; Xie, W.; Shao, Y.; Jiang, J.; Zhao, Z.; Yan, M.; Chen, Z.; Cui, D. The imbalance of Th17/Treg cells is involved in the progression of nonalcoholic fatty liver disease in mice. BMC Immunol. 2017, 18, 33. [Google Scholar] [CrossRef]
- Ma, X.; Hua, J.; Mohamood, A.R.; Hamad, A.R.; Ravi, R.; Li, Z. A high-fat diet and regulatory T cells influence susceptibility to endotoxin-induced liver injury. Hepatology 2007, 46, 1519–1529. [Google Scholar] [CrossRef]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F.; et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef]
- Lu, L.; Wang, J.; Yang, Q.; Xie, X.; Huang, Y. The role of CD38 in HIV infection. AIDS Res. Ther. 2021, 18, 11. [Google Scholar] [CrossRef]
- Lund, J.M.; Hsing, L.; Pham, T.T.; Rudensky, A.Y. Coordination of early protective immunity to viral infection by regulatory T cells. Science 2008, 320, 1220–1224. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef] [PubMed]
- de Wolf, A.; van Aalst, S.; Ludwig, I.S.; Bodinham, C.L.; Lewis, D.J.; van der Zee, R.; van Eden, W.; Broere, F. Regulatory T cell frequencies and phenotypes following anti-viral vaccination. PLoS ONE 2017, 12, e0179942. [Google Scholar] [CrossRef]
- van der Veeken, J.; Gonzalez, A.J.; Cho, H.; Arvey, A.; Hemmers, S.; Leslie, C.S.; Rudensky, A.Y. Memory of Inflammation in Regulatory T Cells. Cell 2016, 166, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Chekol Abebe, E.; Asmamaw Dejenie, T.; Mengie Ayele, T.; Dagnew Baye, N.; Agegnehu Teshome, A.; Tilahun Muche, Z. The Role of Regulatory B Cells in Health and Diseases: A Systemic Review. J. Inflamm. Res. 2021, 14, 75–84. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Nahid-Samiei, M.; Abadi, M.S.S.; Arjmand, M.H.; Ferns, G.A.; Bashash, D.; Rahimian, G.; Bagheri, N. New insights into regulatory B cells biology in viral, bacterial, and parasitic infections. Infect. Genet. Evol. 2021, 89, 104753. [Google Scholar] [CrossRef]
- Körber, N.; Pohl, L.; Weinberger, B.; Grubeck-Loebenstein, B.; Wawer, A.; Knolle, P.A.; Roggendorf, H.; Protzer, U.; Bauer, T. Hepatitis B Vaccine Non-Responders Show Higher Frequencies of CD24(high)CD38(high) Regulatory B Cells and Lower Levels of IL-10 Expression Compared to Responders. Front. Immunol. 2021, 12, 713351. [Google Scholar] [CrossRef]
- Jansen, K.; Cevhertas, L.; Ma, S.; Satitsuksanoa, P.; Akdis, M.; van de Veen, W. Regulatory B cells, A to Z. Allergy 2021, 76, 2699–2715. [Google Scholar] [CrossRef]
- Wąsik, M.; Nazimek, K.; Bryniarski, K. Regulatory B cell phenotype and mechanism of action: The impact of stimulating conditions. Microbiol. Immunol. 2018, 62, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Mauri, C. Regulatory B cells: Origin, phenotype, and function. Immunity 2015, 42, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Bolther, M.; Andersen, K.L.D.; Tolstrup, M.; Visvanathan, K.; Woolley, I.; Skinner, N.; Millen, R.; Warner, N.; Østergaard, L.; Jensen-Fangel, S. Levels of regulatory B cells do not predict serological responses to hepatitis B vaccine. Hum. Vaccin. Immunother. 2018, 14, 1483–1488. [Google Scholar] [CrossRef]
- de Souza, A.B.F.; Kozima, E.T.; Castro, T.F.; de Matos, N.A.; Oliveira, M.; de Souza, D.M.S.; Talvani, A.; de Menezes, R.C.A.; Cangussú, S.D.; Bezerra, F.S. Chronic Oral Administration of Aluminum Hydroxide Stimulates Systemic Inflammation and Redox Imbalance in BALB/c Mice. Biomed. Res. Int. 2023, 2023, 4499407. [Google Scholar] [CrossRef] [PubMed]
- Hamza, H.; Cao, J.; Li, X.; Li, C.; Zhu, M.; Zhao, S. Hepatitis B vaccine induces apoptotic death in Hepa1-6 cells. Apoptosis 2012, 17, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Guo, Y.; Lei, W.; Wang, J.; Hu, X.; Yang, J.; He, Q. Metal Ion Imbalance-Related Oxidative Stress Is Involved in the Mechanisms of Liver Injury in a Rat Model of Chronic Aluminum Exposure. Biol. Trace Elem. Res. 2016, 173, 126–131. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
| Pre-Vaccination, PV (10th Week) | ND | HD2 | HD3 | Sign, p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet Types | n | Median (25th–75th Percentile) | n | Median (25th–75th Percentile) | n | Median (25th–75th Percentile) | n | Median (25th–75th Percentile) | PV vs. ND & | PV vs. HD2 & | PV vs. HD3 & | ND-HD2-HD3 $ | |
| Treg | Control | 18 | 10.07 (7.41–11.95) | 5 | 11.70 (7.30–19.30) | 6 | 9.58 (5.13–16.20) | 5 | 12.30 (8.38–18.90) | 0.446 | 0.923 | 0.150 | 0.673 |
| NAFLD | 21 | 7.04 (4.84–11.95) | 7 | 9.56 (7.76–12.20) | 7 | 7.31 (6.78–13.90) | 7 | 13.90 (10.50–16.90) | 0.155 | 0.321 | 0.012 | 0.138 | |
| p # | 0.321 | 0.755 | 1.000 | 1.000 | |||||||||
| Tfh | Control | 17 | 2.81 (1.13–3.54) | 5 | 3.48 (2.59–4.94) | 6 | 5.67 (4.28–6.46) | 6 | 3.55 (2.16–5.22) | 0.319 | 0.010 | 0.201 | 0.146 |
| NAFLD | 21 | 3.84 (2.81–4.28) | 7 | 2.74 (1.47–4.60) | 7 | 3.55 (2.01–9.68) | 7 | 2.48 (0.00–6.06) | 0.376 | 0.979 | 0.499 | 0.598 | |
| p # | 0.064 | 0.432 | 0.366 | 0.628 | |||||||||
| Treg/Tfh | Control | 17 | 3.87 (1.36–12.17) | 5 | 3.21 (1.93–6.48) | 6 | 1.81 (1.38–2.29) | 6 | 3.31 (2.13–6.53) | 0.880 | 0.135 | 0.940 | 0.217 |
| NAFLD | 21 | 1.95 (1.11–5.16) | 7 | 3.97 (1.53–3.55) | 7 | 3.33 (1.53–3.55) | 7 | 3.21 (1.98–3.92) | 0.189 | 0.756 | 0.642 | 0.426 | |
| p # | 0.199 | 0.755 | 0.295 | 0.730 | |||||||||
| CD27+ | Control | 18 | 14.55 (6.98–60.40) | 6 | 9.99 (5.04–20.20) | 6 | 11.48 (3.19–20.88) | 6 | 14.15 (2.90–27.33) | 0.343 | 0.343 | 0.454 | 0.999 |
| NAFLD | 21 | 6.50 (1.95–18.75) | 7 | 4.17 (2.16–7.14) | 7 | 6.68 (3.23–9.09) | 7 | 7.14 (0.75–15.00) | 0.499 | 0.466 | 0.533 | 0.816 | |
| p # | 0.037 | 0.073 | 0.366 | 0.295 | |||||||||
| CD38+ | Control | 18 | 47.25 (9.69–78.30) | 6 | 69.20 (48.00–78.40) | 6 | 70.80 (56.28–79.90) | 6 | 55.95 (35.03–69.18) | 0.537 | 0.251 | 0.923 | 0.258 |
| NAFLD | 21 | 78.10 (67.70–83.75) | 7 | 85.40 (65.20–92.10) | 7 | 73.90 (64.50–84.70) | 7 | 72.30 (20.00–84.40) | 0.155 | 0.876 | 0.717 | 0.305 | |
| p # | 0.024 | 0.138 | 0.534 | 0.234 | |||||||||
| CD27+CD38+ | Control | 18 | 15.15 (9.95–18.98) | 6 | 11.10 (8.96–14.13) | 6 | 9.20 (6.58–11.55) | 6 | 19.90 (7.07–26.03) | 0.177 | 0.033 | 0.673 | 0.244 |
| NAFLD | 21 | 11.10 (10.15–13.90) | 7 | 8.96 (3.78–14.20) | 7 | 5.65 (2.78–12.90) | 7 | 9.77 (0.00–13.10) | 0.155 | 0.048 | 0.249 | 0.791 | |
| p # | 0.112 | 0.366 | 0.534 | 0.181 | |||||||||
| Vaccination Program | n | Control Groups | n | NAFLD Groups | p # |
|---|---|---|---|---|---|
| ND | 6 | 48.1 (18.2–62.3) | 7 | 26.5 (24.5–66.9) | 0.503 |
| HD2 | 6 | 22.5 (7.8–49.4) | 7 | 39.7 (21.2–44.7) | 0.833 |
| HD3 | 6 | 26.5 (17.8–40.4) | 7 | 26.3 (19.5–74.5) | 0.295 |
| p $ | 0.421 | 0.911 |
| Model Groups (A) | Vaccination Protocol (B) | Sign, p | ||||||
|---|---|---|---|---|---|---|---|---|
| Control Group | NAFLD Group | ND | HD2 | HD3 | A | B | AXB | |
| n | 18 | 21 | 13 | 13 | 13 | |||
| Anti-HB titers, IU/mL | 37.0 [28.7–45.4] | 32.5 [23.5–41.4] | 41.4 [30.8–52.0] | 29.2 [18.6–39.8] | 33.6 [23.0–44.2] | 0.462 | 0.274 | 0.555 |
| Treg | 12.3 [9.9–14.7] | 11.8 [9.7–13.9] | 12.3 [9.5–15.1] | 10.4 [7.7–13.1] | 13.6 [10.8–16.4] | 0.756 | 0.267 | 0.903 |
| Tfh | 4.37 [3.29–5.44] | 3.60 [0.63–4.56] | 3.34 [2.05–4.64] | 5.14 [3.91–6.80] | 3.46 [2.23–4.69] | 0.297 | 0.078 | 0.983 |
| Treg/ Tfh | 3.34 [2.07–4.62] | 3.77 [2.53–5.01] | 4.69 [3.21–6.18] | 2.39 [0.98–3.81] | 3.58 [1.88–5.29] | 0.637 | 0.089 | 0.528 |
| CD27+ | 13.6 [10.0–17.2] | 6.2 [2.9–9.6] | 9.2 [5.0–13.5] | 9.2 [5.0–13.5] | 11.3 [7.1–15.6] | 0.003 | 0.735 | 0.967 |
| CD38+ | 59.9 [49.6–70.2] | 70.5 [61.0–80.0] | 70.6 [58.5–82.7] | 69.2 [57.1–81.4] | 55.8 [43.7–67.9] | 0.138 | 0.173 | 0.528 |
| CD27+ CD38+ | 12.3 [9.6–15.1] | 8.1 [5.6–10.7] | 10.1 [6.9–13.3] | 7.9 [4.6–11.1] | 12.7 [9.5–15.9] | 0.027 | 0.113 | 0.280 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kütük, T.; Onbaşilar, İ.; Oskay-Halaçli, S.; Babaoğlu, B.; Ayhan, S.; Yalçin, S.S. Investigation of the Hepatitis-B Vaccine’s Immune Response in a Non-Alcoholic Fatty Liver Disease Mouse Model. Vaccines 2024, 12, 934. https://doi.org/10.3390/vaccines12080934
Kütük T, Onbaşilar İ, Oskay-Halaçli S, Babaoğlu B, Ayhan S, Yalçin SS. Investigation of the Hepatitis-B Vaccine’s Immune Response in a Non-Alcoholic Fatty Liver Disease Mouse Model. Vaccines. 2024; 12(8):934. https://doi.org/10.3390/vaccines12080934
Chicago/Turabian StyleKütük, Tuğba, İlyas Onbaşilar, Sevil Oskay-Halaçli, Berrin Babaoğlu, Selda Ayhan, and Sıddika Songül Yalçin. 2024. "Investigation of the Hepatitis-B Vaccine’s Immune Response in a Non-Alcoholic Fatty Liver Disease Mouse Model" Vaccines 12, no. 8: 934. https://doi.org/10.3390/vaccines12080934






