Immunogenicity and Safety of SARS-CoV-2 Protein Subunit Recombinant Vaccine (IndoVac®) as a Heterologous Booster Dose against COVID-19 in Indonesian Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Vaccines
2.3. Sample Size and Study Analysis
2.4. Immunogenicity Measurements
2.5. Safety Measurements
2.6. Demographics and Baseline Characteristics
3. Results
3.1. Immunogenicity
3.1.1. Primary Criteria
3.1.2. Secondary Criteria
Neutralizing Antibody
IgG Antibody
3.1.3. Comparison with Previous Study
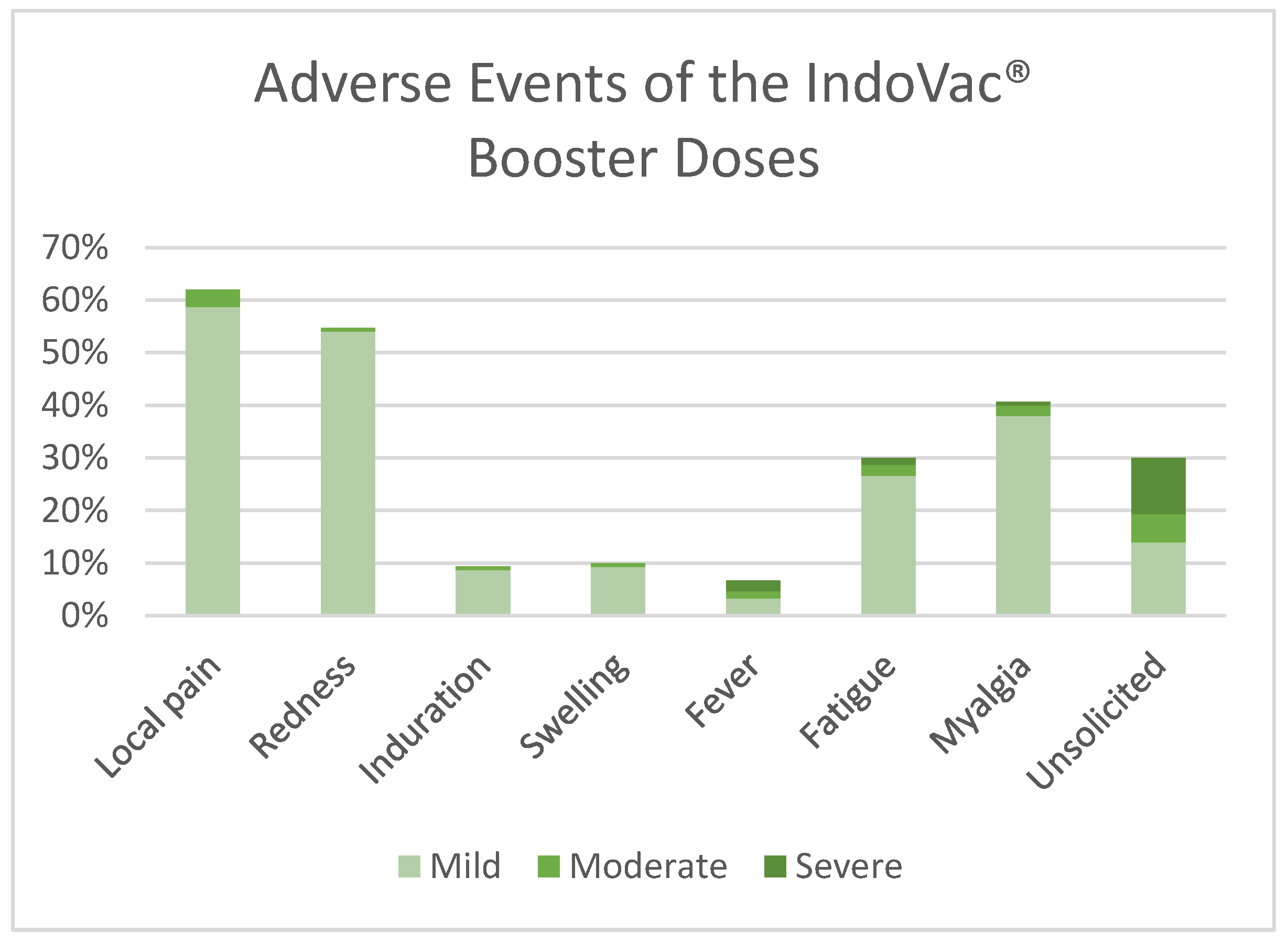
3.2. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, P.; Kang, L.Y.; Liu, J.; Liu, M. Immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents aged 2–18 years: An updated systematic review and meta-analysis. World J. Pediatr. 2023, 19, 1041–1054. [Google Scholar] [CrossRef]
- Du, Y.; Chen, L.; Shi, Y. Safety, immunogenicity, and efficacy of COVID-19 vaccines in adolescents, children, and infants: A systematic review and meta-analysis. Front. Public Health 2022, 10, 829176. [Google Scholar] [CrossRef]
- COVID-19 Cases | WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 8 May 2024).
- Pudjiadi, A.H.; Putri, N.D.; Sjakti, H.A.; Yanuarso, P.B.; Gunardi, H.; Roeslani, R.D.; Pasaribu, A.D.; Nurmalia, L.D.; Sambo, C.M.; Ugrasena, I.D.G.; et al. Pediatric COVID-19: Report from Indonesian pediatric society data registry. Front. Pediatr. 2021, 9, 716898. [Google Scholar] [CrossRef]
- Cavalcante Pinto Júnior, V.; Moura, L.F.W.G.; Cavalcante, R.C.; Lima, J.R.C.; Bezerra, A.S.; de Sousa Dantas, D.R.; Amaral, C.M.L.; Lima, D.F.; Júnior, A.B.V.; Florindo Guedes, M.I. Prevalence of COVID-19 in children, adolescents and adults in remote education situations in the city of Fortaleza, Brazil. Int. J. Infect. Dis. 2021, 108, 20–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Naja, M.; Wedderburn, L.; Ciurtin, C. COVID-19 infection in children and adolescents. Br. J. Hosp. Med. 2020, 81, 1–10. [Google Scholar] [CrossRef]
- Cooper, D.M.; Afghani, B.; Byington, C.L.; Cunningham, C.K.; Golub, S.; Lu, K.D.; Radom-Aizik, S.; Ross, L.F.; Singh, J.; Smoyer, W.E.; et al. SARS-CoV-2 vaccine testing and trials in the pediatric population: Biologic, ethical, research, and implementation challenges. Pediatr. Res. 2021, 90, 966–970. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Shi, D.S. Hospitalizations of children aged 5–11 years with laboratory-confirmed COVID-19—COVID-NET, 14 states, March 2020–February 2022. MMWR Morb Mortal Wkly Rep. 2022, 71, 574–581. [Google Scholar] [CrossRef]
- Ward, J.L.; Harwood, R.; Kenny, S.; Cruz, J.; Clark, M.; Davis, P.J.; Draper, E.S.; Hargreaves, D.; Ladhani, S.N.; Gent, N.; et al. Pediatric hospitalizations and ICU admissions due to COVID-19 and pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 in England. JAMA Pediatr. 2023, 177, 947–955. [Google Scholar] [CrossRef]
- Loades, M.E.; Chatburn, E.; Higson-Sweeney, N.; Reynolds, S.; Shafran, R.; Brigden, A.; Linney, C.; McManus, M.N.; Borwick, C.; Crawley, E. Rapid systematic review: The impact of social isolation and loneliness on the mental health of children and adolescents in the context of COVID-19. J. Am. Acad. Child. Adolesc. Psychiatry 2020, 59, 1218–1239.e3. [Google Scholar] [CrossRef]
- Bera, L.; Souchon, M.; Ladsous, A.; Colin, V.; Lopez-Castroman, J. Emotional and behavioral impact of the COVID-19 epidemic in adolescents. Curr. Psychiatry Rep. 2022, 24, 37–46. [Google Scholar] [CrossRef]
- Panchal, U.; de Pablo, G.S.; Franco, M.; Moreno, C.; Parellada, M.; Arango, C.; Fusar-Poli, P. The impact of COVID-19 lockdown on child and adolescent mental health: Systematic review. Eur. Child. Adolesc. Psychiatry 2023, 32, 1151–1177. [Google Scholar] [CrossRef]
- Bates, L.C.; Zieff, G.; Stanford, K.; Moore, J.B.; Kerr, Z.Y.; Hanson, E.D.; Barone Gibbs, B.; Kline, C.E.; Stoner, L. COVID-19 impact on behaviors across the 24-hour day in children and adolescents: Physical activity, sedentary behavior, and sleep. Children 2020, 7, 138. [Google Scholar] [CrossRef]
- Yang, S.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; Wu, C.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; He, P.; Chen, Z.; Yang, H.; Li, F.; Zhang, S.; Wang, D.; Wang, G.; Yang, S.; et al. Safety and immunogenicity of a protein subunit COVID-19 vaccine (ZF2001) in healthy children and adolescents aged 3-17 years in China: A randomised, double-blind, placebo-controlled, phase 1 trial and an open-label, non-randomised, non-inferiority, phase 2 trial. Lancet Child. Adolesc. Health 2023, 7, 269–279. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Dhama, K. SARS-CoV-2 Vaccines, Vaccine Development Technologies, and Significant Efforts in Vaccine Development during the Pandemic: The Lessons Learned Might Help to Fight against the Next Pandemic. Vaccines 2023, 11, 682. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- WHO Target Product Profiles for COVID-19 Vaccines. Available online: https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines (accessed on 28 April 2024).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Reasons for Adults to Be Vaccinated. CDC. Available online: https://www.cdc.gov/vaccines-adults/reasons/?CDC_AAref_Val=https://www.cdc.gov/vaccines/adults/reasons-to-vaccinate.html (accessed on 28 April 2024).
- Why Childhood Vaccines Matter—Mayo Clinic Health System. Available online: https://www.mayoclinichealthsystem.org/hometown-health/speaking-of-health/the-facts-about-vaccinations (accessed on 28 April 2024).
- WHO. COVID-19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/agency/who/ (accessed on 28 April 2024).
- WHO Issues Emergency Use Listing for Eighth COVID-19 Vaccine. Available online: https://www.who.int/news/item/03-11-2021-who-issues-emergency-use-listing-for-eighth-covid-19-vaccine (accessed on 28 April 2024).
- IndoVac Can Be Used by the Elderly for the Second Booster of the COVID-19 Vaccine. Available online: https://www.biofarma.co.id/en/latest-news/detail/indovac-can-be-used-by-the-elderly-for-the-second-booster-of-the-covid19-vaccine- (accessed on 28 April 2024).
- Indonesia Approves First Homegrown COVID Vaccine for Emergency Use—Media | Reuters. Available online: https://www.reuters.com/world/asia-pacific/indonesia-approves-first-home-grown-covid-vaccine-emergency-use-media-2022-09-28/ (accessed on 28 April 2024).
- Rusmil, K.; Fadlyana, E.; Girsang, R.T.; Adrizain, R.; Rahmadi, A.R.; Suryadinata, H.; Putra, M.G.D.; Fulendry, F.P.; Nashsyah, D.T.; Utami, R.K.; et al. Immunogenicity and safety of SARS-CoV-2 protein subunit recombinant vaccine (IndoVac®) as a booster dose against COVID-19 in Indonesian adults. Vaccines 2024, 12, 540. [Google Scholar] [CrossRef]
- Kim, S.; Liu, Y.; Ziarnik, M.; Seo, S.; Cao, Y.; Zhang, X.F.; Im, W. Binding of human ACE2 and RBD of omicron enhanced by unique interaction patterns among SARS-CoV-2 variants of concern. J. Comput. Chem. 2023, 44, 594–601. [Google Scholar] [CrossRef]
- Ha, E.K.; Kim, J.H.; Han, M.Y. Long COVID in children and adolescents: Prevalence, clinical manifestations, and management strategies. Clin. Exp. Pediatr. 2023, 66, 465–474. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Zahedipour, F.; Mirzaei, H.; Kazemi Oskuee, R. Potential SARS-CoV-2 vaccines: Concept, progress, and challenges. Int. Immunopharmacol. 2021, 97, 107622. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Adhikari, R.; Chen, W.-H.; Chen, Y.-L.; Gillespie, P.; Islam, N.Y.; Keegan, B.; Kundu, R.T.; Lee, J.; Liu, Z.; et al. From concept to delivery: A yeast-expressed recombinant protein-based COVID-19 vaccine technology suitable for global access. Expert. Rev. Vaccines 2023, 22, 495–500. [Google Scholar] [CrossRef]
- Pollet, J.; Chen, W.H.; Strych, U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv. Rev. 2021, 170, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Sample Size for Before-After Study (Paired T-Test). Sample Size Calculators. Available online: https://sample-size.net/sample-size-study-paired-t-test/ (accessed on 15 May 2024).
- Gondokesumo, M.E.; Purnamayanti, A.; Hanum, P.S.; Santosa, W.N.; Wardhana, A.P.; Avanti, C. Anti-SARS-CoV-2 receptor binding domain antibodies after the second dose of Sinovac and AstraZeneca vaccination. Clin. Exp. Vaccine Res. 2023, 12, 224–231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ISO 9001; Internationally Recognized Standard for Quality Management Systems (QMS). ISO: Geneva, Switzerland, 2015.
- ISO 15189; Internationally Recognized Standard for Medical Laboratories. ISO: Geneva, Switzerland, 2022.
- Company Profile. Available online: https://www.prodia.co.id/en/profil-perusahaan (accessed on 16 May 2024).
- ISO 45001; Internationally Recognized Standard for Occupational Health and Safety Management Systems. ISO: Geneva, Switzerland, 2018.
- Certification. Available online: https://www.biofarma.co.id/en/certification (accessed on 16 May 2024).
- Bullen, M.; Heriot, G.S.; Jamrozik, E. Herd immunity, vaccination and moral obligation. J. Med. Ethics. 2023, 49, 636–641. [Google Scholar] [CrossRef]
- Velavan, T.P.; Pollard, A.J.; Kremsner, P.G. Herd immunity and vaccination of children for COVID-19. Int. J. Infect. Dis. 2020, 98, 14–15. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Nguyen, K.; Mansfield, K.; Allen, J.D.; Corlin, L. Child and adolescent COVID-19 vaccination status and reasons for non-vaccination by parental vaccination status. Public Health 2022, 209, 82–89. [Google Scholar] [CrossRef]
- Vaccines for Teens 11 to 12 Years | CDC. Available online: https://www.cdc.gov/vaccines/parents/by-age/years-11-12.html (accessed on 28 May 2024).
- Bubar, K.M.; Reinholt, K.; Kissler, S.M.; Lipsitch, M.; Cobey, S.; Grad, Y.H.; Larremore, D.B. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 2021, 371, 916–921. [Google Scholar] [CrossRef]
- Munzert, S.; Ramirez-Ruiz, S.; Çalı, B.; Stoetzer, L.F.; Gohdes, A.; Lowe, W. Prioritization preferences for COVID-19 vaccination are consistent across five countries. Humanit. Soc. Sci. Commun. 2022, 9, 439. [Google Scholar] [CrossRef]
- Santi, T.; Hegar, B.; Munasir, Z.; Prayitno, A.; Werdhani, R.A.; Bandar, I.N.S.; Jo, J.; Uswa, R.; Widia, R.; Vandenplas, Y. Factors associated with parental intention to vaccinate their preschool children against COVID-19: A cross-sectional survey in urban area of Jakarta, Indonesia. Clin. Exp. Vaccine Res. 2023, 12, 240–248. [Google Scholar] [CrossRef]
- Khemiri, H.; Ayouni, K.; Triki, H.; Haddad-Boubaker, S. SARS-CoV-2 infection in pediatric population before and during the Delta (B.1.617.2) and omicron (B.1.1.529) variants era. Virol. J. 2022, 19, 144. [Google Scholar] [CrossRef] [PubMed]
- Advice for the Public. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public?adgroupsurvey={adgroupsurvey}&gad_source=1&gclid=Cj0KCQjwsPCyBhD4ARIsAPaaRf2PBRH9WbwkVr2Fy7cqZqEo21KP2IbAcWSf5av6V6emoL_GL1R5XE8aAhzYEALw_wcB (accessed on 3 June 2024).
- What Parents Need to Know about Long COVID in Children. UNICEF Parenting. Available online: https://www.unicef.org/parenting/health/long-COVID-children (accessed on 22 May 2024).
- Ladhani, S.N. COVID-19 vaccination for children aged 5–11 years. Lancet 2022, 400, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, A.; Nency, Y.M.; Maddeppungeng, M.; Sekartini, R.; Sari, R.M.; Surachman, F.; Yani, F.F.; Anggrainy, F.; Hafiz, A.; Machmud, R.; et al. Immunogenicity and safety of SARS-CoV-2 recombinant protein subunit vaccine (IndoVac) adjuvanted with alum and CpG 1018 in Indonesian adults: A phase 3, randomized, active-controlled, multicenter trial. Vaccine 2024, 42, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Harimurti, K.; Jasmin, H.; Sekartini, R.; Aini, M.; Yuniar, I.; Indawati, W.; Wirahmadi, A. Evaluation of safety and anti-RBD IgG SARS-CoV-2 after Indovac Administration in Depok. ejki 2023, 11, 118–125. [Google Scholar] [CrossRef]
- Huang, T.; Hu, Q.; Zhou, X.; Yang, H.; Xia, W.; Cao, F.; Deng, M.; Teng, X.; Ding, F.; Zhong, Z.; et al. Immunogenicity and safety of a recombinant COVID-19 vaccine (ZF2001) as heterologous booster after priming with inactivated vaccine in healthy children and adolescents aged 3–17 years: An open-labeled, single-arm clinical trial. BMC Infect. Dis. 2024, 24, 413. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, H.; Zhang, Q.; Zhang, Y.; Lin, K.; Fu, Z.; Song, J.; Zhao, Y.; Fan, M.; Wang, H.; et al. Recombinant protein subunit vaccine booster following two-dose inactivated vaccines dramatically enhanced anti-RBD responses and neutralizing titers against SARS-CoV-2 and variants of concern. Cell Res. 2022, 32, 103–106. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, Y.; Chen, B.; Liang, Z.; Hu, X.; Xing, B.; Yang, J.; Zheng, Q.; Hua, Q.; Yan, C.; et al. Safety and immunogenicity of heterologous recombinant protein subunit vaccine (ZF2001) booster against COVID-19 at 3–9-month intervals following two-dose inactivated vaccine (CoronaVac). Front. Immunol. 2022, 13, 1017590. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, J.; Wang, X.; Zhang, Z.; Wang, B.; Zhang, J.; Jiang, H.; Guo, G.; Wang, Y.; Wan, J.; et al. Safety and Immunogenicity of Homologous Recombinant Adenovirus Type 5-Vectored COVID-19 Vaccine Booster Dose in Healthy Adults Aged 18–60 Years: A Single-Center, Open-Label Trial. Infect. Dis. Ther. 2023, 12, 2757–2769. [Google Scholar] [CrossRef]
- Park, H.J.; Gonsalves, G.S.; Tan, S.T.; Kelly, J.D.; Rutherford, G.W.; Wachter, R.M.; Schechter, R.; Paltiel, A.D.; Lo, N.C. Comparing frequency of booster vaccination to prevent severe COVID-19 by risk group in the United States. Nat. Commun. 2024, 15, 1883. [Google Scholar] [CrossRef]
- Puthanakit, T.; Chantasrisawad, N.; Yoohat, K.; Nantanee, R.; Sophonphan, J.; Meepuksom, T.; Sodsai, P.; Phanthanawiboon, S.; Jantarabenjakul, W.; Hirankarn, N.; et al. Immunogenicity of a fractional dose of mRNA BNT162b2 COVID-19 vaccine for primary series and booster vaccination among healthy adolescents. Vaccines 2022, 10, 1646. [Google Scholar] [CrossRef]
- Xin, Q.; Wu, Q.; Chen, X.; Han, B.; Chu, K.; Song, Y.; Jin, H.; Chen, P.; Lu, W.; Yang, T.; et al. Six-month follow-up of a booster dose of CoronaVac in two single-centre phase 2 clinical trials. Nat. Commun. 2022, 13, 3100. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe. 2022, 3, e52–e61. [Google Scholar] [CrossRef] [PubMed]
- Áñez, G.; Dunkle, L.M.; Gay, C.L.; Kotloff, K.L.; Adelglass, J.M.; Essink, B.; Campbell, J.D.; Cloney-Clark, S.; Zhu, M.; Plested, J.S.; et al. Safety, immunogenicity, and efficacy of the NVX-CoV2373 COVID-19 vaccine in adolescents: A randomized clinical trial. JAMA Netw. Open 2023, 6, e239135. [Google Scholar] [CrossRef] [PubMed]
- Tukhvatulin, A.I.; Dolzhikova, I.V.; Dzharullaeva, A.S.; Grousova, D.M.; Kovyrshina, A.V.; Zubkova, O.V.; Gintsburg, A.L. Safety and immunogenicity of rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine against SARS-CoV-2 in healthy adolescents: An open-label, non-randomized, multicenter, phase 1/2, dose-escalation study. Front. Immunol. 2023, 14, 1228461. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Gan, J.; Luo, Z.; Li, S.; Wang, Z.; Wu, J.; Zhang, H.; Xian, J.; Cheng, R.; Tang, X.; et al. Safety, immunogenicity and protective effectiveness of heterologous boost with a recombinant COVID-19 vaccine (Sf9 cells) in adult recipients of inactivated vaccines. Signal Transduct. Target. Ther. 2024, 9, 41. [Google Scholar] [CrossRef]
- Gunale, B.; Kapse, D.; Kar, S.; Bavdekar, A.; Kohli, S.; Lalwani, S.; Meshram, S.; Raut, A.; Kulkarni, P.; Samuel, C.; et al. Safety and Immunogenicity of SARS-CoV-2 Recombinant Spike Protein Vaccine in Children and Adolescents in India: A Phase 2–3 Randomized Clinical Trial. JAMA Pediatr. 2023, 177, 911–920. [Google Scholar] [CrossRef]
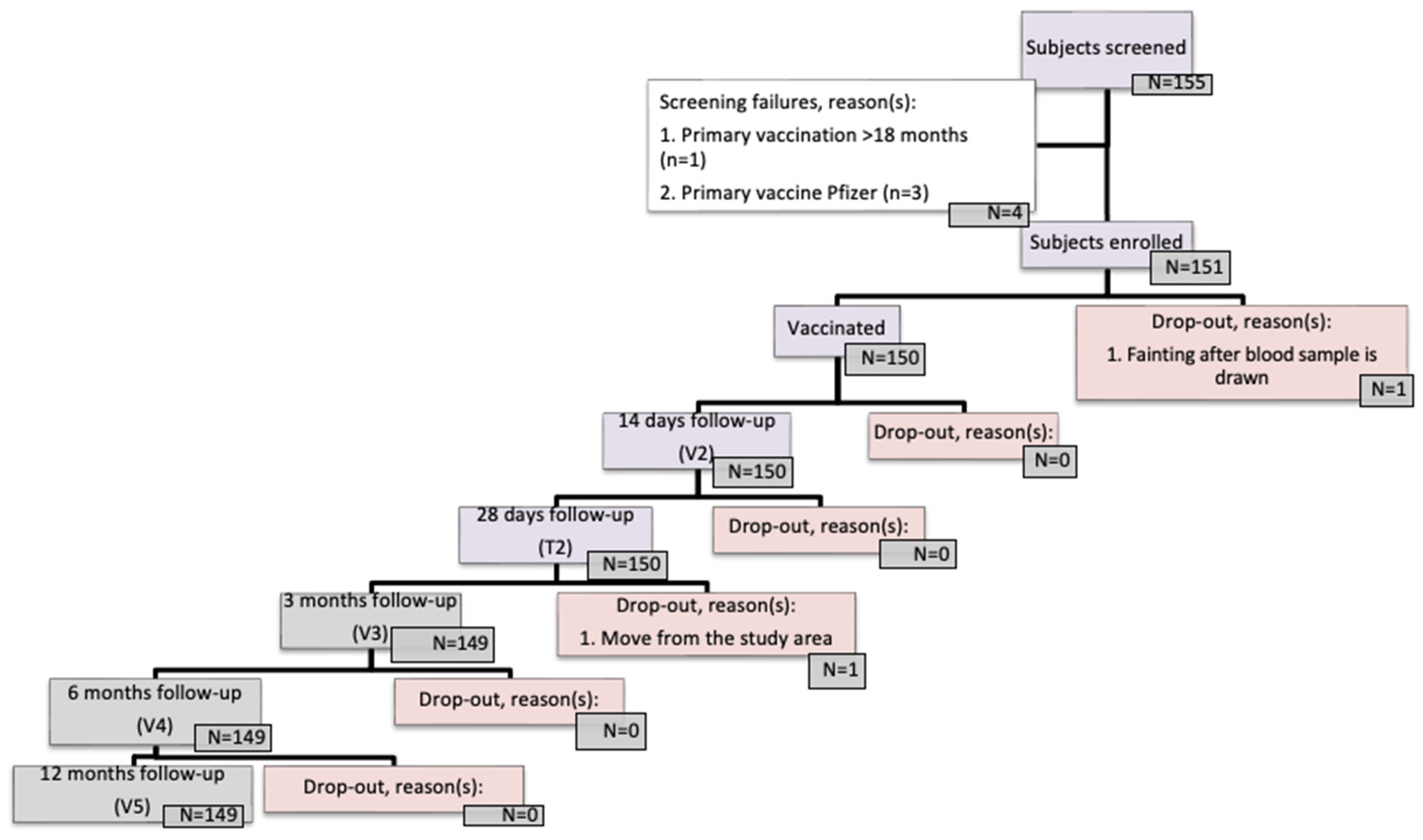


| Parameter | Total (N = 150) | |
|---|---|---|
| Mean age [years] (SD) | 14.7 | (1.8) |
| Mean height [cm] (SD) | 155.6 | (9.2) |
| Mean weight [kg] (SD) | 50.3 | (12.3) |
| BMI (kg/m2) | 20.7 | (4.4) |
| Sex, n (%) | ||
| Male | 65 | (43.3%) |
| Female | 85 | (56.7%) |
| Education, n (%) | ||
| Primary school | 37 | (24.7%) |
| Junior high school | 50 | (33.3%) |
| Senior high school | 63 | (42.0%) |
| Timepoint | Parameter | Booster Dose in Adults | Booster Dose in Adolescents |
|---|---|---|---|
| Before Vaccination | Seropositive Rate | ||
| n | 113 | 139 | |
| (%) | 76.87 | 92.67 | |
| (95% CI) | 69.207–83.419 | 87.257–96.282 | |
| GMT (IU/mL) | 147.52 | 303.26 | |
| (95% CI) | 117.84–184.665 | 240.835–381.859 | |
| Median | 130.15 | 260.42 | |
| 14 Days After Booster Dose | Seropositive Rate | ||
| n | 145 | 150 | |
| (%) | 98.64 | 100 | |
| (95% CI) | 95.171–99.834 | 97.57–100 | |
| GMT (IU/mL) | 1266.69 | 2661.21 | |
| (95% CI) | 1014.731–1581.21 | 2275.086–3112.873 | |
| Median | 1041.57 | 2946 | |
| 3 Months After Booster Dose | Seropositive Rate | ||
| n | 144 | 149 | |
| (%) | 99.31 | 100 | |
| (95% CI) | 96.217–99.982 | 97.554–100 | |
| GMT (IU/mL) | 717.36 | 2021.09 | |
| (95% CI) | 594.713–865.309 | 1735.892–2353.143 | |
| Median | 736.5 | 2083.14 | |
| 6 Months After Booster Dose | Seropositive Rate | ||
| n | 140 | 149 | |
| (%) | 99.29 | 100 | |
| (95% CI) | 96.111–99.982 | 97.554–100 | |
| GMT (IU/mL) | 758.54 | 1172.74 | |
| (95% CI) | 637.309–902.827 | 1009.258–1362.695 | |
| Median | 736.5 | 1041.57 | |
| Timepoint | Parameter | Booster Dose in Adults | Booster Dose in Adolescents |
|---|---|---|---|
| Before Vaccination | Seropositive Rate | ||
| n | 145 | 150 | |
| (%) | 98.64 | 100 | |
| (95% CI) | 95.171–99.834 | 97.57–100 | |
| IgG (BAU/mL) | |||
| GMT | 266.2 | 277.75 | |
| (95% CI) | 218.103–324.904 | 235.62–327.4 | |
| Median | 274.8 | 259.06 | |
| 14 Days After Booster Dose | Seropositive Rate | ||
| n | 147 | 150 | |
| (%) | 100 | 100 | |
| (95% CI) | 97.521–100.00 | 97.57–100 | |
| IgG (BAU/mL) | |||
| GMT | 2817.08 | 3479.22 | |
| (95% CI) | 2460.41–3225.458 | 3182.65–3803.417 | |
| Median | 3941.41 | 4225.86 | |
| 3 Months After Booster Dose | Seropositive Rate | ||
| n | 145 | 149 | |
| (%) | 100 | 100 | |
| (95% CI) | 97.488–100 | 97.554–100 | |
| IgG (BAU/mL) | |||
| GMT | 1804.82 | 2631.52 | |
| (95% CI) | 1590.084–2048.555 | 2378.491–2911.46 | |
| Median | 2017.64 | 2677.17 | |
| 6 Months After Booster Dose | Seropositive Rate | ||
| n | 141 | 149 | |
| (%) | 100 | 100 | |
| (95% CI) | 97.417–100 | 97.554–100 | |
| IgG (BAU/mL) | |||
| GMT | 1090.63 | 1686.36 | |
| (95% CI) | 954.267–1246.474 | 1504.862–1889.743 | |
| Median | 1052.9 | 1751.73 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadlyana, E.; Rusmil, K.; Dwi Putra, M.G.; Fulendry, F.P.; Somantri, N.K.; Putri, A.D.; Sari, R.M.; Puspita, M.; Dewi, G.P. Immunogenicity and Safety of SARS-CoV-2 Protein Subunit Recombinant Vaccine (IndoVac®) as a Heterologous Booster Dose against COVID-19 in Indonesian Adolescents. Vaccines 2024, 12, 938. https://doi.org/10.3390/vaccines12080938
Fadlyana E, Rusmil K, Dwi Putra MG, Fulendry FP, Somantri NK, Putri AD, Sari RM, Puspita M, Dewi GP. Immunogenicity and Safety of SARS-CoV-2 Protein Subunit Recombinant Vaccine (IndoVac®) as a Heterologous Booster Dose against COVID-19 in Indonesian Adolescents. Vaccines. 2024; 12(8):938. https://doi.org/10.3390/vaccines12080938
Chicago/Turabian StyleFadlyana, Eddy, Kusnandi Rusmil, Muhammad Gilang Dwi Putra, Frizka Primadewi Fulendry, Nitta Kurniati Somantri, Alvira Dwilestarie Putri, Rini Mulia Sari, Mita Puspita, and Gianita Puspita Dewi. 2024. "Immunogenicity and Safety of SARS-CoV-2 Protein Subunit Recombinant Vaccine (IndoVac®) as a Heterologous Booster Dose against COVID-19 in Indonesian Adolescents" Vaccines 12, no. 8: 938. https://doi.org/10.3390/vaccines12080938






