Stability Preparedness: The Not-So-Cold Case for Innovations in Vaccine Stability Modelling and Product Release
Abstract
:1. Introduction
- rapid response vaccine manufacturing platforms, formulations, and/or novel delivery systems with improved thermostability;
- methods to rapidly test and predict stability of a new vaccine candidate through leveraging prior knowledge of a manufacturing platform and stability modelling;
- labelling systems to allow flexible update of expiry dates as evidence becomes available.
2. Problem Statement
3. The Need for Thermostable Vaccines
4. The Need for Stability Modelling
4.1. Platform Characterization
- Available platform knowledge
- 1.1.
- Historic stability informationThe extent of long-term, accelerated, and stressed stability data which have been collected for the platform and individual products manufactured, including process intermediates.
- 1.2.
- Known impact of critical factorsPrior knowledge on how modifications of the platform process can impact quality attributes across different products, such as antigen sequence, presence of process- and product-related impurities, differences in concentration, and other variability in physico-chemical properties.Understanding of degradation pathways and impacts due to changes in platform processes or modifications to manufacture a new product on the same platform.Impact of batch-to-batch variability, manufacturing scale, or manufacturing site on the stability of the final product.
- 1.3.
- Maturity of predictive modelThe availability of a stability model which has been used to accurately predict long-term stability based on accelerated stability studies.Understanding of stability study requirements to update an available predictive model for a new product.
- 1.4.
- Product presentationAvailable stability data in different formulations/strengths, as applicable, and when filled in different primary and secondary container combinations. Multidose vials are desirable for outbreak response and require collection of appropriate stability information beforehand.
- Analytical support
- 2.1.
- The quality of the stability knowledge is highly dependent on the suitability of the analytical methods available for product characterization.
- 2.2.
- Determination of the structure–function relationship of the product in its formulation and under various storage conditions requires fit-for-purpose methods.
- 2.3.
- Potency method is key to monitor vaccine efficacy loss.Although platform analytical methods can be adapted for a new product with relative ease, the development of a potency assay is likely on the critical path in a rapid response scenario.
- 2.4.
- Implementation of reference standardsAppropriate reference materials are critical to monitor analytical performance and product quality.
4.2. Predictive Models
- In a study by Clénet, the forced degradation of a multivalent inactivated vaccine used a combined approach of advanced kinetics and statistical analysis to describe the loss of antigenicity [24]. Results showed that six months of data under multiple accelerated conditions were sufficient to accurately predict the product stability out to thirty months. In addition, the kinetic model correctly predicted the loss in antigenicity following experimental temperature excursions.
- In another study by Clénet et. al., a kinetic model for a protein-based vaccine has been validated [22]. Following data collection over six months, the model accurately predicted stability out to 24 months. In the same publication, the validation of an autocatalytic-type kinetic model is presented for an oil-in-water adjuvant formulation.
- Castellanos et. al. describe the use of advanced kinetic modelling for a commercial vaccine [25]. Data from an accelerated stability study performed over 6 months were used to establish the model, which then correctly predicted potency loss of the product for up to 3 years.
- For rapid response vaccine manufacturing, mRNA-based products are considered key. Efforts are ongoing to identify relevant stability-indicating attributes, develop kinetic models, and confirm the validity with experimental data, as described by Kis [23]. To date, degradation of mRNA has been confirmed to demonstrate Arrhenius behaviour [26,27]. In addition, the ability to model mRNA vaccine stability using first-order kinetics has been demonstrated [28].
4.3. Stability Execution
- Stability studiesNote: Subject to available comparability data and/or prior knowledge, it is feasible to pool stability information from multiple lots, material generated across different clinical phases, and lots produced across more than one manufacturing sites to boost the stability data package which is used in subsequent steps.
- 1.1.
- Stability-indicating attribute and assay selection.
- 1.2.
- Samples are incubated using at least three different temperatures.
- 1.3.
- Periodic testing is performed to acquire at least 20 to 30 experimental data points.
- 1.4.
- Replicates should be favoured over additional time points, and the variability of the analytical method as well as knowledge of the variability structure (in terms of between- and within-run variability) are key variables that need to be considered at this step.
- Screening kinetic models to fit experimental dataRun fitting procedures from simple first order to more complex models.
- Identification of the appropriate modelThe model best describing the observed stability data over all temperatures is identified according to statistical parameters (quality of fit, model comparison score).
- Determination of prediction accuracy
- 4.1.
- The selected model is used to predict reaction progress under any time/temperature.
- 4.2.
- Prediction accuracy uncertainties are assessed by statistical methods.
- Verify platform stability
- 5.1.
- Compare the new product model with the available platform stability information.
- 5.2.
- For the new product, confirm a similar trend is observed under accelerated conditions to leverage platform stability information.
- 5.3.
- Claim the shelf life for the new product based on the platform stability data.
- [OPTIONAL] Model refinementWith increased understanding of the stability profile and generation of additional data, the model may be refined to better reflect the observed reaction kinetics.
5. The Need for Agile Labelling Systems
6. Towards Stability Preparedness
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saville, M.; Cramer, J.P.; Downham, M.; Hacker, A.; Lurie, N.; Van der Veken, L.; Whelan, M.; Hatchett, R. Delivering Pandemic Vaccines in 100 Days—What Will It Take? N. Engl. J. Med. 2022, 387, e3. [Google Scholar] [CrossRef] [PubMed]
- CEPI 100 Days Report. Available online: https://static.cepi.net/downloads/2024-02/CEPI-100-Days-Report-Digital-Version_29-11-22.pdf (accessed on 13 June 2024).
- Li, M.; Wang, H.; Tian, L.; Pang, Z.; Yang, Q.; Huang, T.; Fan, J.; Song, L.; Tong, Y.; Fan, H. COVID-19 vaccine development: Milestones, lessons and prospects. Signal Transduct. Target. Ther. 2022, 7, 146–178. [Google Scholar] [CrossRef]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Dumpa, N.; Goel, K.; Guo, Y.; McFall, H.; Pillai, A.R.; Shukla, A.; Repka, M.A.; Murthy, S.N. Stability of Vaccines. AAPS PharmSciTech 2019, 20, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Pambudi, N.A.; Sarifudin, A.; Gandidi, I.M.; Romadhon, R. Vaccine cold chain management and cold storage technology to address the challenges of vaccination programs. Energy Rep. 2022, 8, 955–972. [Google Scholar] [CrossRef]
- Kartoglu, U.; Mistien, J. Tools and approaches to ensure quality of vaccines throughout the cold chain. Expert Rev. Vaccines 2014, 13, 843–854. [Google Scholar] [CrossRef]
- World Health Organisation. Monitoring Vaccine Wastage at Country Level: Guidelines for Programme Managers; Rev 1; WHO: Geneva, Switzerland, 2005.
- Karp, C.L.; Lans, D.; Esparza, J.; Edson, E.B.; Owen, K.E.; Wilson, C.B.; Heaton, P.M.; Levine, O.S.; Rao, R. Evaluating the value proposition for improving vaccine thermostability to increase vaccine impact in low and middle-income countries. Vaccine 2015, 33, 3471–3479. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat. Human Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef]
- Hotez, P.J. SARS-CoV-2 variants offer a second chance to fix vaccine inequities. Nat. Rev. Microbiol. 2023, 21, 127–128. [Google Scholar] [CrossRef]
- Hare, J.; Hesselink, R.; Bongers, A.; Blakeley, P.; Riggall, G. Improving vaccine equity by increasing vaccine thermostability. Sci. Transl. Med. 2024, 16, eadm7471. [Google Scholar] [CrossRef]
- Hotez, P.J. Global Vaccine Access Demands Combating Both Inequity And Hesitancy. Health Aff. 2023, 42, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccine 2021, 9, 1033. [Google Scholar] [CrossRef]
- Mvundura, M.; Frivold, C.; Osborne, A.J.; Soni, P.; Robertson, J.; Kumar, S.; Anena, J.; Gueye, A.; Menozzi-Arnaud, M.; Giersing, B.; et al. Vaccine innovation prioritisation strategy: Findings from three country-stakeholder consultations on vaccine product innovations. Vaccine 2021, 39, 7195–7207. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, Y.; Bai, Y.; Liang, Z.; Mao, Q.; Liu, D.; Wu, X.; Xu, M. Research Advances on the Stability of mRNA Vaccines. Viruses 2023, 15, 668. [Google Scholar] [CrossRef] [PubMed]
- Blenke, E.O.; Örnskov, E.; Schöneich, C.; Nilsson, G.A.; Volkin, D.B.; Mastrobattista, E.; Almarsson, Ö.; Crommelin, D.J. The Storage and In-Use Stability of mRNA Vaccines and Therapeutics: Not A Cold Case. J. Pharm. Sci. 2023, 112, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Campa, C.; Pronce, T.; Paludi, M.; Weusten, J.; Conway, L.; Savery, J.; Richards, C.; Clénet, D. Use of Stability Modeling to Support Accelerated Vaccine Development and Supply. Vaccines 2021, 9, 1114. [Google Scholar] [CrossRef]
- World Health Organisation. Guidelines on Stability Evaluation of Vaccines; WHO/BS/06/2049; WHO: Geneva, Switzerland, 2006.
- Toolbox Guidance on Scientific Elements and Regulatory Tools to Support Quality Data Packages for PRIME and Certain Marketing Authorisation Applications Targeting an Unmet Medical Need; EMA/CHMP/BWP/QWP/IWG/694114/2019; EMA: Amsterdam, The Netherlands, 2022.
- Roduit, B.; Hartmann, M.; Folly, P.; Sarbach, A.; Baltensperger, R. Prediction of thermal stability of materials by modified kinetic and model selection approaches based on limited amount of experimental points. Thermochim. Acta 2014, 579, 31–39. [Google Scholar] [CrossRef]
- Clénet, D.; Imbert, F.; Probeck, P.; Rahman, N.; Ausar, S.F. Advanced kinetic analysis as a tool for formulation development and prediction of vaccine stability. J. Pharm. Sci. 2014, 103, 3055–3064. [Google Scholar] [CrossRef]
- Kis, Z. Stability Modelling of mRNA Vaccine Quality Based on Temperature Monitoring throughout the Distribution Chain. Pharmaceutics 2022, 14, 430. [Google Scholar] [CrossRef]
- Clénet, D. Accurate prediction of vaccine stability under real storage conditions and during temperature excursions. Eur. J. Pharm. Biopharm. 2018, 125, 76–84. [Google Scholar] [CrossRef]
- Castellanos, M.M.; Gressard, H.; Li, X.; Magagnoli, C.; Moriconi, A.; Stranges, D.; Strodiot, L.; Soto, M.T.; Zwierzyna, M.; Campa, C. CMC Strategies and Advanced Technologies for Vaccine Development to Boost Acceleration and Pandemic Preparedness. Vaccines 2023, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Breaker, R.R. Kinetics of RNA Degradation by Specific Base Catalysis of Transesterification Involving the 2′-Hydroxyl Group. J. Am. Chem. Soc. 1999, 121, 5364–5372. [Google Scholar] [CrossRef]
- Fabre, A.-L.; Colotte, M.; Luis, A.; Tuffet, S.; Bonnet, J. An efficient method for long-term room temperature storage of RNA. Eur. J. Human. Genet. 2014, 22, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Assessment Report—COVID-19 Vaccine Moderna—Common Name: COVID-19 mRNA Vaccine (Nucleoside-Modified). Available online: https://www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccine-moderna-epar-public-assessment-report_en.pdf (accessed on 13 June 2024).
- Roduit, B.; Luyet, C.A.; Hartmann, M.; Folly, P.; Sarbach, A.; Dejeaifve, A.; Dobson, R.; Schroeter, N.; Vorlet, O.; Dabros, M.; et al. Continuous Monitoring of Shelf Lives of Materials by Application of Data Loggers with Implemented Kinetic Parameters. Molecules 2019, 24, 2217. [Google Scholar] [CrossRef]
- Campa, C. Stability assessment for vaccines: Recent trends & learnings from accelerated scenarios. Vaccine Insights 2022, 9, 281–291. [Google Scholar]
- Model Packaging Label for Vials of COVID 19 Vaccines. Available online: https://www.who.int/publications/m/item/model-packaging-for-label-for-vials-for-covid-19-vaccines (accessed on 13 June 2024).
- COVID-19 Vaccine Label: User Evaluation. Available online: https://www.path.org/our-impact/resources/covid-19-vaccine-label-user-evaluation/ (accessed on 13 June 2024).
- COVID-19 Vaccine Job Aid: How to Manage COVID-19 Vaccines without VVM at Vaccination Service Points? Available online: https://www.who.int/docs/default-source/coronaviruse/21277_vvm-job-aid_31-august-update_final_for-the-web.pdf (accessed on 13 June 2024).
- COVID-19 Vaccine Job Aid: How to Verify the Expiration Date for Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) Formulation? Available online: https://cdn.who.int/media/docs/default-source/immunization/covid-19/job_aid_pfizer_vcv_expirationdate.pdf (accessed on 13 June 2024).
- Job Aid for COVID-19 Vaccine Administration: Moderna COVID-19 (mRNA-1273) Vaccine, June 2021. Available online: https://www.who.int/europe/publications/i/item/WHO-EURO-2021-2025-41780-57236 (accessed on 13 June 2024).

| Benefit | Description | Impact |
|---|---|---|
| Enhanced Global Access | Vaccines with improved thermostability can be distributed in regions lacking ultracold storage facilities. | Ensures equitable access to vaccines, especially in low-income and remote areas. |
| Reduced Cold Chain Dependency | Stable vaccines reduce the need for ultracold storage and complex logistics. | Simplifies the supply chain and lowers distribution costs. |
| Minimized Vaccine Waste | Thermostable vaccines are less likely to be compromised by temperature excursions. | Reduces the number of doses lost during transportation and storage. |
| Extended Shelf Life | Improved stability extends the usable life of vaccines. | Increases the time available for distribution and administration, reducing waste. |
| Rapid Deployment in Emergencies | Stable vaccines can be rapidly deployed without the need for stringent storage conditions. | Enhances the ability to respond quickly to pandemics and outbreaks. |
| Lower Financial Burden | Reduced need for specialized storage equipment lowers overall costs. | Makes vaccination programmes more affordable and sustainable. |
| Environmental Benefits | Less reliance on energy-intensive cold storage reduces greenhouse gas emissions. | Supports global efforts to mitigate climate change. |
| Increased Public Trust | Reliable vaccines with stable efficacy build confidence in vaccination programmes. | Encourages higher vaccination rates and better public health outcomes. |
| Facilitates Remote and Outreach Programmes | Stable vaccines are easier to transport to and store in remote areas. | Improves vaccination coverage in hard-to-reach populations. |
| Innovation in Vaccine Technology | Drives research and development of new formulations and delivery systems. | Leads to overall advancements in vaccine science and technology. |
| Key Point | Description |
|---|---|
| Environmental Changes | Vaccines face temperature variations, freeze/thaw cycles, light exposure, and contact material changes during manufacturing, distribution, and administration. |
| Degradation Profile Characterization | Requires in-depth knowledge of product properties and external stress factors, often necessitating bespoke assays for monitoring degradation. |
| Critical Path Activity | Establishing stability profiles and expiration dates is critical in vaccine development, typically relying on long-term studies. |
| Regulatory Guidance | Limited specific mathematical approaches in ICH guidelines (Q1A to Q1E, Q5C) and WHO guidelines for evaluating stability data. |
| Real-time Stability Data | Shelf life is based on real-time stability data from at least three batches, but rapid response scenarios limit available time for these studies. |
| Rapid Response Scenarios | In outbreaks, the timeline from recognition to vaccine availability is around three months, limiting stability assessment time. |
| Pooling Stability Information | Initial stability claims may use data from pre-clinical material, engineering runs, and clinical trial lots, though acceptance by health authorities varies. |
| Modelling and Stability Evaluation | ICH Q1E mentions linear and non-linear functions but lacks detailed methodologies; biological products have complex, non-linear degradation pathways. |
| Risk-based Approach | A risk-based approach, leveraging seasonal flu vaccine principles, is needed to claim a shelf life within a 100-day development timeline. |
| Extrapolation of Data | Utilizes data from previous seasonal flu vaccines or similar platform products to quickly establish initial shelf-life claims. |
| Advanced Kinetic Modelling | Combines platform experience with short-term stability studies under multiple accelerated conditions to establish initial shelf-life claims. |
| Review and Update of Shelf Life | Shelf-life claims are reviewed and updated with additional long-term stability data to ensure continued vaccine efficacy and safety. |
| Storage Condition | Example Time Points | ||||
|---|---|---|---|---|---|
| Real storage temperature | T0 | 1 W | 2 W | 4 W | 8 W |
| Accelerated 1 | 0.5 W | 1 W | 2 W | 4 W | 8 W |
| Accelerated 2 | 1 D | 3 D | 6 D | 12 D | |
| Stressed | 1 D | 2 D | 3 D | ||
| # | Topic | Details | Innovation Needs |
|---|---|---|---|
| 1 | Development stage of vaccine manufacturing platforms | Platform development ongoing; focus on mRNA due to highest potential to meet rapid response requirement. | Improvement of unit operations or novel technologies to streamline production and release of vaccine candidates. |
| Lifecycle management; risk of resetting stability knowledge with significant changes to the product. | Enhanced understanding of how process changes or different product attributes may impact stability profile. | ||
| 2 | Thermostable presentation | Increased stability reduces the risk of stability failure, reduces supply chain pressure, and allows for equitable access due to ease of distribution. | Improvement of product formulations or novel technologies for primary and secondary container closure systems. |
| 3 | Stability testing | Pre-approved stability protocols allow rapid generation of additional data to inform expiration dating. However, timelines to notify health authorities and awaiting approval cause delay in applying updated expiration dates to newly manufactured lots. | Identify and agree on a mechanism for health authority notification as a new expiration date is proposed. |
| Appropriate analytical methods are required to observe changes in stability-indicating parameters. Product-specific assay development may be on the critical path for initiation of stability studies. | Platform potency assays or surrogate methods to monitor loss of potency in stability studies and throughout the shelf-life period. | ||
| 4 | Multidose vials | Rapid response/large outbreaks may benefit from multidose vials to increase access/speed of delivery and reduce wastage. | Such storage configurations may require specific characterization studies (e.g., container compatibility, homogeneity, microbial growth). |
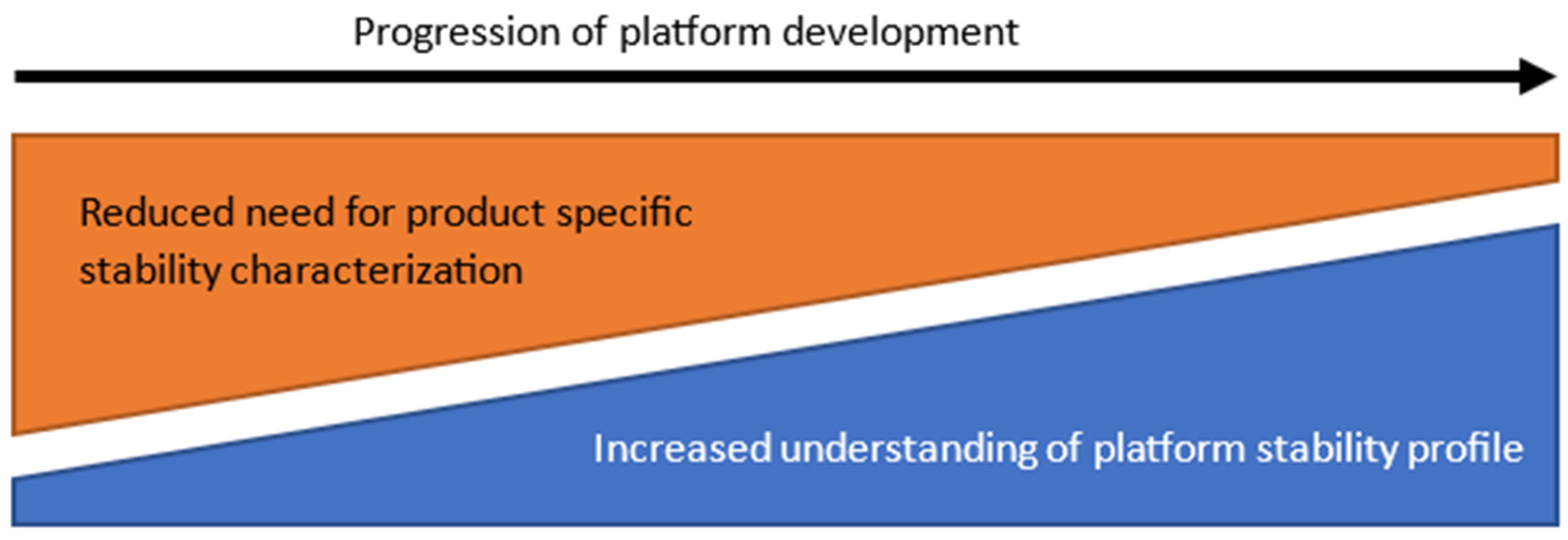
| 5 | Platform stability | Prior platform stability knowledge is used to inform studies of vaccine candidates and provides a baseline for a long-term stability assumption for new vaccine candidates. | Evaluate health authority acceptance and refine conditions which allow the use of platform data. |
| Define similarity conditions for the stability profile of a new vaccine candidate when compared to historic data. | |||
| 6 | Predictive modelling | Apply advanced kinetic models to predict stability of new vaccine candidates based on short-term stability data. | Consider revision of ICH Q1E and Q5C to incorporate best practices for stability modelling. |
| Develop pre-approved protocols for rapid stability assessment of new vaccine candidates. | |||
| 7 | Real-time monitoring | Utilize real-time tracking devices to monitor storage conditions. | Understand infrastructure requirements and potential hurdles. |
| Develop demonstration studies to test the impact the devices can have on cold chain monitoring. | |||
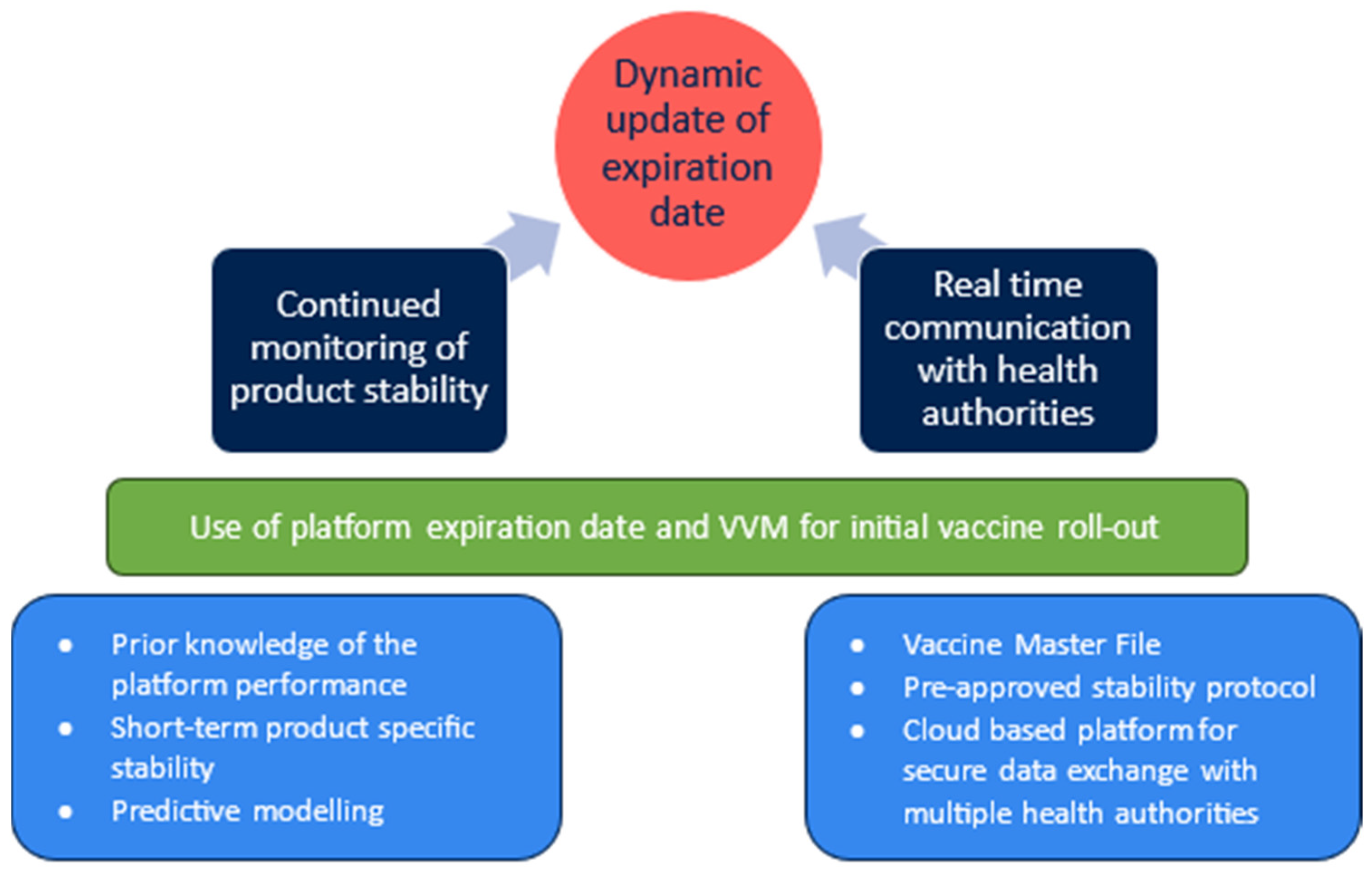
| 8 | Agile label | There are no immediate technical solutions which allow the shipment of vaccine candidates with expiration date and VVM in a rapid response scenario. | With health authorities at national and international level, define a framework for future rapid response supply of vaccine vials through leveraging platform stability and platform VVM. |
| Procedures which require look-up of the latest batch expiry date and the manual update of carton and vial information could be standardized for a rapid response scenario and part of healthcare worker training to increase preparedness. | Work with vaccine delivery partners to understand the user requirements and available infrastructure. | ||
| 9 | Platform expiration date and VVM | Development of thermostable vaccine presentations and investments into platform stability characterization may allow the definition of platform stability and generation of a platform VVM—a minimum stability which is understood to be met by all products manufactured within defined process parameters. | Evaluation of scientific, technical, and regulatory feasibility of platform stability which allows shipment of rapid response vaccine lots using platform expiration date and VVM only. |
| 10 | Pre-approved “Vaccine DMF” | Submission, review, and discussion of clinical trial applications are a bottleneck in deploying vaccines in an outbreak scenario. | Establish “Vaccine Master File” dossiers at a platform level which are harmonized across geographic regions to speed up revision and review of vaccine candidate applications. |
| Segmentation in health authorities across the globe with differing regulations and requirements leads to additional challenges and delays in the product approval process. | Cloud-based platforms for sharing information across multiple health authorities and providing updates in real time as it becomes available in the vaccine development lifecycle can reduce effort in duplicating documentation exchanged with individual health authorities and significantly increase the response time in vaccine deployment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnetzinger, F.; Clénet, D.; Gilbert, P.-A.; Guzzi, A.; Paludi, M.; Weusten, J.; Hesselink, R. Stability Preparedness: The Not-So-Cold Case for Innovations in Vaccine Stability Modelling and Product Release. Vaccines 2024, 12, 1000. https://doi.org/10.3390/vaccines12091000
Schnetzinger F, Clénet D, Gilbert P-A, Guzzi A, Paludi M, Weusten J, Hesselink R. Stability Preparedness: The Not-So-Cold Case for Innovations in Vaccine Stability Modelling and Product Release. Vaccines. 2024; 12(9):1000. https://doi.org/10.3390/vaccines12091000
Chicago/Turabian StyleSchnetzinger, Franz, Didier Clénet, Philippe-Alexandre Gilbert, Antonio Guzzi, Marilena Paludi, Jos Weusten, and Renske Hesselink. 2024. "Stability Preparedness: The Not-So-Cold Case for Innovations in Vaccine Stability Modelling and Product Release" Vaccines 12, no. 9: 1000. https://doi.org/10.3390/vaccines12091000
APA StyleSchnetzinger, F., Clénet, D., Gilbert, P.-A., Guzzi, A., Paludi, M., Weusten, J., & Hesselink, R. (2024). Stability Preparedness: The Not-So-Cold Case for Innovations in Vaccine Stability Modelling and Product Release. Vaccines, 12(9), 1000. https://doi.org/10.3390/vaccines12091000





