A Recombinant Mosaic HAs Influenza Vaccine Elicits Broad-Spectrum Immune Response and Protection of Influenza a Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics Statement
2.3. Mosaic HA Sequences Design
2.4. Gene Cloning, Protein Expression, and Purification
2.5. Cells and Influenza Viruses
2.6. Immunization and Challenges of Mice
2.7. Hemagglutination Inhibition (HAI) Assay
2.8. Microneutralization (MN) Assay
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Enzyme-Linked Immunospot (ELISpot) Assay
2.11. Flow Cytometry and Intracellular Cytokine Staining
2.12. Histological Examination of the Mice’s Lung
2.13. Statistical Analysis
3. Results
3.1. Mosaic HA Sequences Construction and Structural Prediction
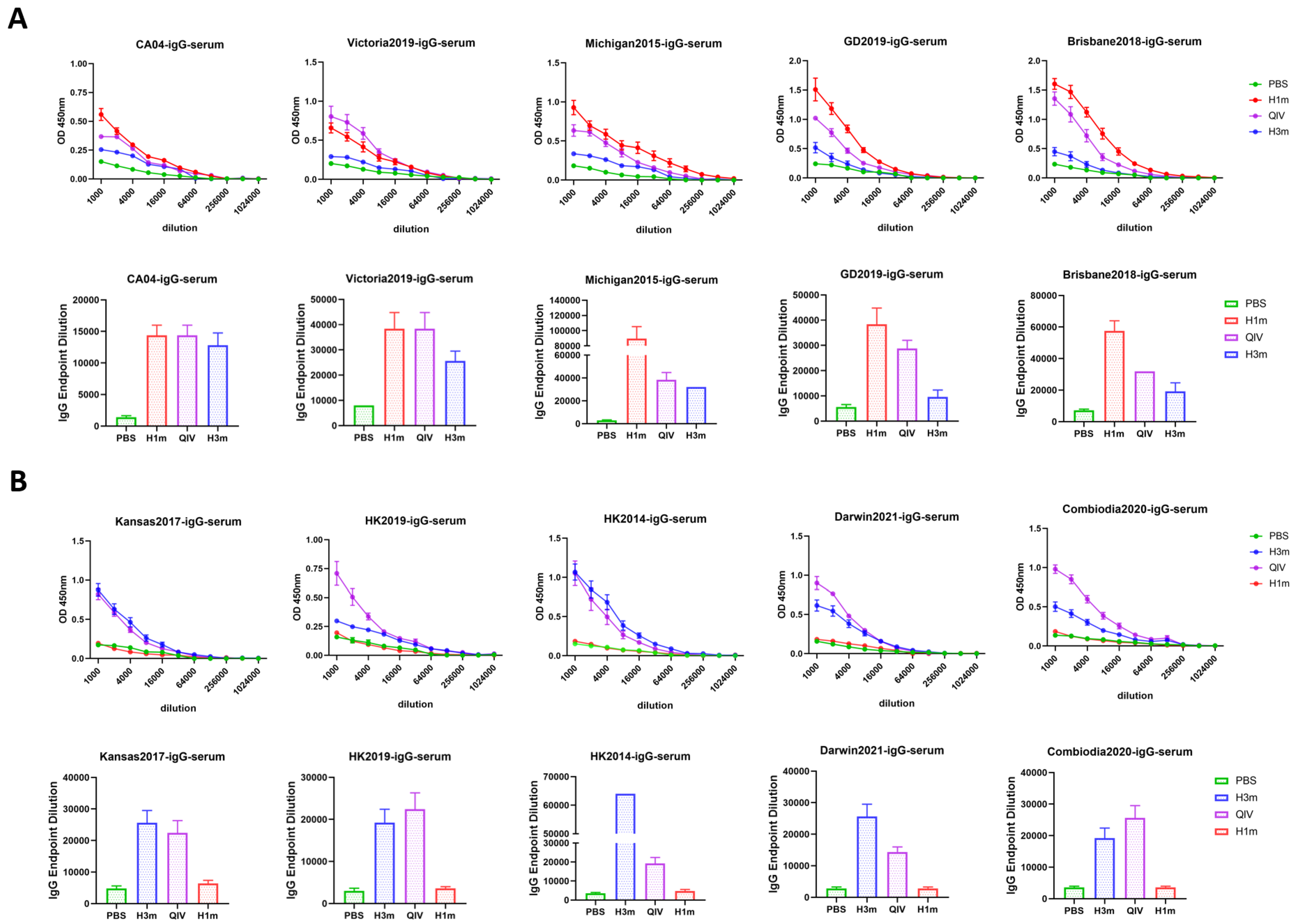
3.2. Recombinant Mosaic HAs Enhanced HAI Breadth and Humoral Immune Responses Compared with That Induced by QIV Vaccines in Mice
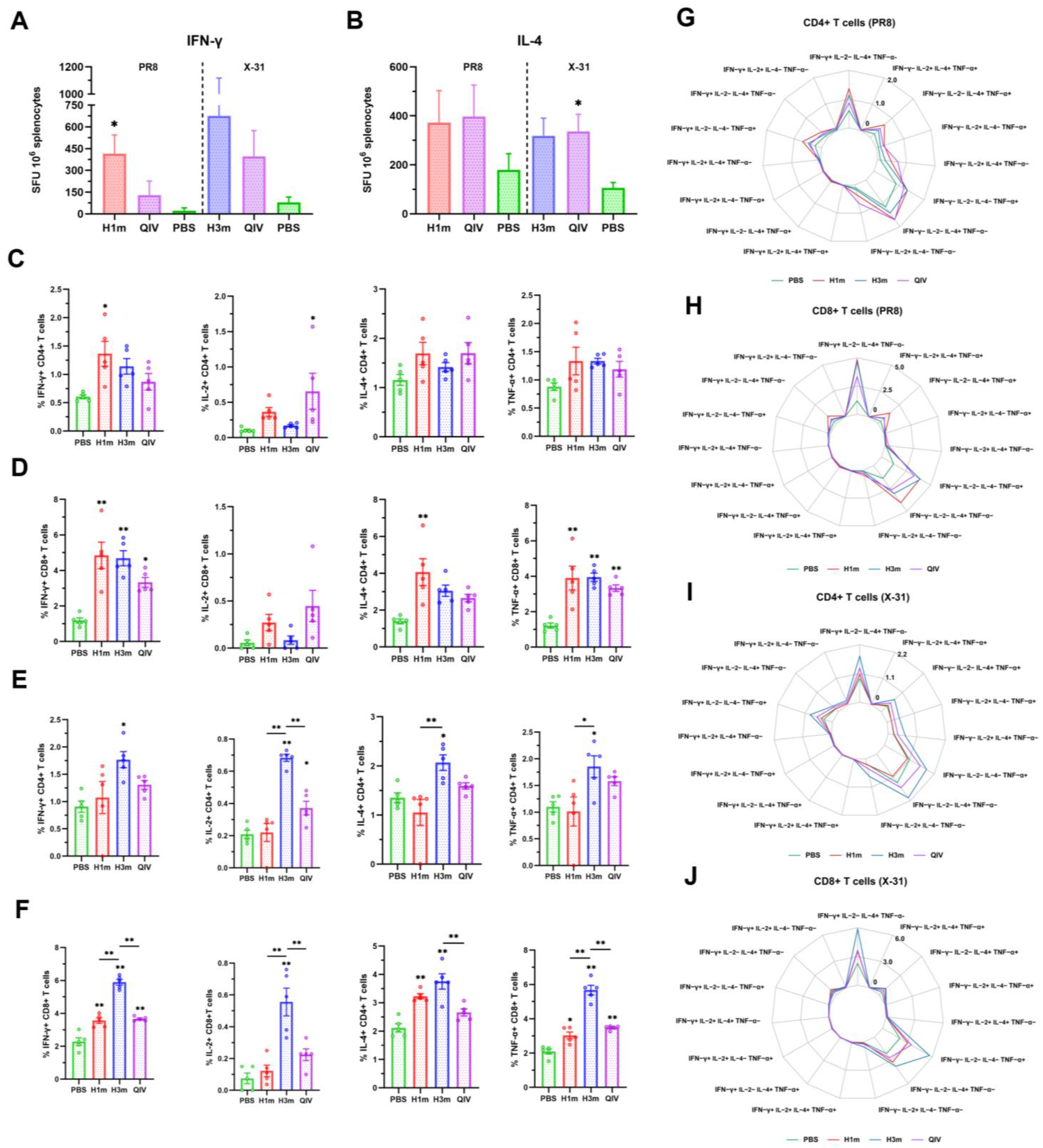
3.3. Recombinant Mosaic HA Proteins Elicited More T Cell Immune Responses Compared with That Induced by QIV Vaccines in Mice
3.4. Recombinant Mosaic HA Proteins Immunized Mice Were Protected from Lethal Virus Challenges
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Chen, J.; Shen, C.; Wang, G.; Lu, Z.; Zeng, D.; Gao, Y.; Chen, H.; Xia, N.; Chen, Y. Vaccination with Deglycosylated Modified Hemagglutinin Broadly Protects against Influenza Virus Infection in Mice and Ferrets. Vaccines 2022, 10, 1304. [Google Scholar] [CrossRef]
- Strohmeier, S.; Amanat, F.; Zhu, X.; McMahon, M.; Deming, M.E.; Pasetti, M.F.; Neuzil, K.M.; Wilson, I.A.; Krammer, F. A Novel Recombinant Influenza Virus Neuraminidase Vaccine Candidate Stabilized by a Measles Virus Phosphoprotein Tetramerization Domain Provides Robust Protection from Virus Challenge in the Mouse Model. mBio 2021, 12, e0224121. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.P.; Gruber, M.F. An Overview of the Regulation of Influenza Vaccines in the United States. Influenza Other Respir. Viruses 2016, 10, 354–360. [Google Scholar] [CrossRef]
- Krammer, F. The Human Antibody Response to Influenza A Virus Infection and Vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- Kotey, E.N.; Ampofo, W.K.; Daines, R.; Sadeyen, J.-R.; Iqbal, M.; Quaye, O. Immune Response in Mice Immunized with Chimeric H1 Antigens. Vaccines 2021, 9, 1182. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Hong, K.-J.; Kim, H.; Nam, J.-H. Influenza Vaccines: Past, Present, and Future. Rev. Med. Virol. 2022, 32, e2243. [Google Scholar] [CrossRef]
- Bouvier, N.M. The Future of Influenza Vaccines: A Historical and Clinical Perspective. Vaccines 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.; Gresset-Bourgeois, V.; Macabeo, B.; Woods, A.; Samson, S.I. A Trivalent, Inactivated Influenza Vaccine (Vaxigrip®): Summary of Almost 50 Years of Experience and More than 1.8 Billion Doses Distributed in over 120 Countries. Expert Rev. Vaccines 2017, 16, 545–564. [Google Scholar] [CrossRef]
- Tao, Y.-Y.; Li, J.-X.; Hu, Y.-M.; Hu, Y.-S.; Zeng, G.; Zhu, F.-C. Quadrivalent Influenza Vaccine (Sinovac Biotech) for Seasonal Influenza Prophylaxis. Expert Rev. Vaccines 2021, 20, 1–11. [Google Scholar] [CrossRef]
- Statler, V.A.; Albano, F.R.; Airey, J.; Sawlwin, D.C.; Graves Jones, A.; Matassa, V.; Heijnen, E.; Edelman, J.; Marshall, G.S. Immunogenicity and Safety of a Quadrivalent Inactivated Influenza Vaccine in Children 6-59 months of Age: A Phase 3, Randomized, Noninferiority Study. Vaccine 2019, 37, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.J.; Izikson, R.; Post, P.; Dunkle, L. Safety, Efficacy, and Immunogenicity of Flublok in the Prevention of Seasonal Influenza in Adults. Ther. Adv. Vaccines 2015, 3, 97–108. [Google Scholar] [CrossRef]
- Kelso, J.M. The Adverse Reactions to Vaccines Practice Parameter 10 Years On-What Have We Learned? Ann. Allergy Asthma Immunol. 2022, 129, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Dunkle, L.M.; Izikson, R. Recombinant Hemagglutinin Influenza Vaccine Provides Broader Spectrum Protection. Expert Rev. Vaccines 2016, 15, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Orsi, A.; Pariani, E.; Lai, P.L.; Guarona, G.; Pellegrinelli, L.; Ebranati, E.; Icardi, G.; Panatto, D. In-Depth Phylogenetic Analysis of the Hemagglutinin Gene of Influenza A(H3N2) Viruses Circulating during the 2016–2017 Season Revealed Egg-Adaptive Mutations of Vaccine Strains. Expert Rev. Vaccines 2020, 19, 115–122. [Google Scholar] [CrossRef]
- Richards, K.A.; Moritzky, S.; Shannon, I.; Fitzgerald, T.; Yang, H.; Branche, A.; Topham, D.J.; Treanor, J.J.; Nayak, J.; Sant, A.J. Recombinant HA-Based Vaccine Outperforms Split and Subunit Vaccines in Elicitation of Influenza-Specific CD4 T Cells and CD4 T Cell-Dependent Antibody Responses in Humans. npj Vaccines 2020, 5, 77. [Google Scholar] [CrossRef]
- Manini, I.; Domnich, A.; Amicizia, D.; Rossi, S.; Pozzi, T.; Gasparini, R.; Panatto, D.; Montomoli, E. Flucelvax (Optaflu) for Seasonal Influenza. Expert Rev. Vaccines 2015, 14, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.J.; Hollister, J.R. FluBlok, a next Generation Influenza Vaccine Manufactured in Insect Cells. Biol. J. Int. Assoc. Biol. Stand. 2009, 37, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.J.; Patriarca, P.A.; Treanor, J. FluBlok, a Recombinant Hemagglutinin Influenza Vaccine. Influenza Other Respir. Viruses 2008, 2, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Margine, I.; Palese, P.; Krammer, F. Expression of Functional Recombinant Hemagglutinin and Neuraminidase Proteins from the Novel H7N9 Influenza Virus Using the Baculovirus Expression System. JoVE 2013, 81, 51112. [Google Scholar] [CrossRef]
- Magadán, J.G.; Khurana, S.; Das, S.R.; Frank, G.M.; Stevens, J.; Golding, H.; Bennink, J.R.; Yewdell, J.W. Influenza A Virus Hemagglutinin Trimerization Completes Monomer Folding and Antigenicity. J. Virol. 2013, 87, 9742–9753. [Google Scholar] [CrossRef]
- Impagliazzo, A.; Milder, F.; Kuipers, H.; Wagner, M.V.; Zhu, X.; Hoffman, R.M.B.; van Meersbergen, R.; Huizingh, J.; Wanningen, P.; Verspuij, J.; et al. A Stable Trimeric Influenza Hemagglutinin Stem as a Broadly Protective Immunogen. Science 2015, 349, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Huang, M.-H.; Tsou, P.-C.; Huang, L.-M.; Chong, P.; Wu, S.-C. Recombinant Trimeric HA Protein Immunogenicity of H5N1 Avian Influenza Viruses and Their Combined Use with Inactivated or Adenovirus Vaccines. PLoS ONE 2011, 6, e20052. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, T.; Wang, L.; Li, M.; Sun, C.; Shu, Y. Strategies Targeting Hemagglutinin Cocktail as a Potential Universal Influenza Vaccine. Front. Microbiol. 2022, 13, 1014122. [Google Scholar] [CrossRef] [PubMed]
- Corder, B.N.; Bullard, B.L.; DeBeauchamp, J.L.; Ilyushina, N.A.; Webby, R.J.; Weaver, E.A. Influenza H1 Mosaic Hemagglutinin Vaccine Induces Broad Immunity and Protection in Mice. Vaccines 2019, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Kamlangdee, A.; Kingstad-Bakke, B.; Osorio, J.E. Mosaic H5 Hemagglutinin Provides Broad Humoral and Cellular Immune Responses against Influenza Viruses. J. Virol. 2016, 90, 6771–6783. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, T.; Wang, L.; Yang, Z.; Luo, C.; Li, M.; Luo, H.; Sun, C.; Yan, H.; Shu, Y. A Mosaic Influenza Virus-like Particles Vaccine Provides Broad Humoral and Cellular Immune Responses against Influenza A Viruses. npj Vaccines 2023, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, K.; Lanfermeijer, J.; van Dijken, H.; Muramatsu, H.; Vilas Boas de Melo, C.; Lenz, S.; Peters, F.; Beattie, M.B.; Lin, P.J.C.; Ferreira, J.A.; et al. A Universal Influenza mRNA Vaccine Candidate Boosts T Cell Responses and Reduces Zoonotic Influenza Virus Disease in Ferrets. Sci. Adv. 2022, 8, eadc9937. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Gan, P.; Hu, X.; Mai, Z.; Ji, C.; Yi, H.; Li, M.; Li, S.; Ji, Y.; Huang, J.; et al. Protective Effect of Bivalent H1N1 and H3N2 VLP Vaccines against Eurasian Avian-like H1N1 and Recent Human-like H3N2 Influenza Viruses in a Mouse Model. Vet. Microbiol. 2022, 266, 109370. [Google Scholar] [CrossRef] [PubMed]
- Bullard, B.L.; DeBeauchamp, J.; Pekarek, M.J.; Petro-Turnquist, E.; Vogel, P.; Webby, R.J.; Weaver, E.A. An Epitope-Optimized Human H3N2 Influenza Vaccine Induces Broadly Protective Immunity in Mice and Ferrets. npj Vaccines 2022, 7, 65. [Google Scholar] [CrossRef]
- Wang, L.; Yang, L.; Zhang, F.; Liu, X.; Xie, Q.; Liu, Q.; Yuan, L.; Zhao, T.; Xie, S.; Xu, Q.; et al. A Microneedle-Based Delivery System for Broad-Protection Seasonal Influenza A DNA Nanovaccines. Cell Rep. Phys. Sci. 2023, 4, 101430. [Google Scholar] [CrossRef]
- Granados, A.; Peci, A.; McGeer, A.; Gubbay, J.B. Influenza and Rhinovirus Viral Load and Disease Severity in Upper Respiratory Tract Infections. J. Clin. Virol. 2017, 86, 14–19. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Kondor, R.J.G.; Chung, J.R.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; et al. Effect of Antigenic Drift on Influenza Vaccine Effectiveness in the United States-2019-2020. Clin. Infect. Dis. 2021, 73, e4244–e4250. [Google Scholar] [CrossRef] [PubMed]
- Boni, M.F. Vaccination and Antigenic Drift in Influenza. Vaccine 2008, 26 (Suppl. S3), C8–C14. [Google Scholar] [CrossRef]
- Hendin, H.E.; Lavoie, P.-O.; Gravett, J.M.; Pillet, S.; Saxena, P.; Landry, N.; D’Aoust, M.-A.; Ward, B.J. Elimination of Receptor Binding by Influenza Hemagglutinin Improves Vaccine-Induced Immunity. npj Vaccines 2022, 7, 42. [Google Scholar] [CrossRef]
- Ward, B.J.; Pillet, S.; Charland, N.; Trepanier, S.; Couillard, J.; Landry, N. The Establishment of Surrogates and Correlates of Protection: Useful Tools for the Licensure of Effective Influenza Vaccines? Hum. Vaccines Immunother. 2018, 14, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M.; Krammer, F.; McMahon, M. The Human Antibody Response to the Influenza Virus Neuraminidase Following Infection or Vaccination. Vaccines 2021, 9, 846. [Google Scholar] [CrossRef]
- Black, S.; Nicolay, U.; Vesikari, T.; Knuf, M.; Del Giudice, G.; Della Cioppa, G.; Tsai, T.; Clemens, R.; Rappuoli, R. Hemagglutination Inhibition Antibody Titers as a Correlate of Protection for Inactivated Influenza Vaccines in Children. Pediatr. Infect. Dis. J. 2011, 30, 1081–1085. [Google Scholar] [CrossRef]
- Benoit, A.; Beran, J.; Devaster, J.-M.; Esen, M.; Launay, O.; Leroux-Roels, G.; McElhaney, J.E.; Oostvogels, L.; van Essen, G.A.; Gaglani, M.; et al. Hemagglutination Inhibition Antibody Titers as a Correlate of Protection Against Seasonal A/H3N2 Influenza Disease. Open Forum Infect. Dis. 2015, 2, ofv067. [Google Scholar] [CrossRef]
- Ravina; Manjeet; Mohan, H.; Narang, J.; Pundir, S.; Pundir, C.S. A Changing Trend in Diagnostic Methods of Influenza A (H3N2) Virus in Human: A Review. 3 Biotech 2021, 11, 87. [Google Scholar] [CrossRef]
- Lee, H.; Shim, E.H.; You, S. Immunodominance Hierarchy of Influenza Subtype-Specific Neutralizing Antibody Response as a Hurdle to Effectiveness of Polyvalent Vaccine. Hum. Vaccin. Immunother. 2018, 14, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.B.; Kirchenbaum, G.A.; Clutter, E.F.; Sautto, G.A.; Ross, T.M. Preexisting Subtype Immunodominance Shapes Memory B Cell Recall Response to Influenza Vaccination. JCI Insight 2020, 5, 132155. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, C.P.; Singh, P.; Russell, M.L.; Bowdish, D.M.E.; Brewer, A.; Cyr, L.; Ward, B.J.; Loeb, M. Microneutralization Assay Titres Correlate with Protection against Seasonal Influenza H1N1 and H3N2 in Children. PLoS ONE 2015, 10, e0131531. [Google Scholar] [CrossRef] [PubMed]
- Sant, A.J.; Chaves, F.A.; Krafcik, F.R.; Lazarski, C.A.; Menges, P.; Richards, K.; Weaver, J.M. Immunodominance in CD4 T-Cell Responses: Implications for Immune Responses to Influenza Virus and for Vaccine Design. Expert Rev. Vaccines 2007, 6, 357–368. [Google Scholar] [CrossRef]
- Schmidt, A.; Lapuente, D. T Cell Immunity against Influenza: The Long Way from Animal Models Towards a Real-Life Universal Flu Vaccine. Viruses 2021, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Auladell, M.; Jia, X.; Hensen, L.; Chua, B.; Fox, A.; Nguyen, T.H.O.; Doherty, P.C.; Kedzierska, K. Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses. Front. Immunol. 2019, 10, 1400. [Google Scholar] [CrossRef]
- Jansen, J.M.; Gerlach, T.; Elbahesh, H.; Rimmelzwaan, G.F.; Saletti, G. Influenza Virus-Specific CD4+ and CD8+ T Cell-Mediated Immunity Induced by Infection and Vaccination. J. Clin. Virol. 2019, 119, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-Z.J.; Thomas, P.G. New Fronts Emerge in the Influenza Cytokine Storm. Semin. Immunopathol. 2017, 39, 541–550. [Google Scholar] [CrossRef]
- Snapper, C.M.; Mond, J.J. Towards a Comprehensive View of Immunoglobulin Class Switching. Immunol. Today 1993, 14, 15–17. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Li, Y.; Gu, W.; Halimu, G.; Li, Y.; Zhang, Z.; Zhou, L.; Liao, H.; Yao, S.; et al. A Recombinant Protein Containing Influenza Viral Conserved Epitopes and Superantigen Induces Broad-Spectrum Protection. eLife 2021, 10, e71725. [Google Scholar] [CrossRef] [PubMed]
- Cargnelutti, D.E.; Sánchez, M.V.; Mattion, N.M.; Scodeller, E.A. Development of a Universal CTL-Based Vaccine for Influenza. Bioengineered 2013, 4, 374–378. [Google Scholar] [CrossRef]
- Spitaels, J.; Roose, K.; Saelens, X. Influenza and Memory T Cells: How to Awake the Force. Vaccines 2016, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, A.F.; Rimmelzwaan, G.F.; de Vries, R.D. Virus-Specific T Cells as Correlate of (Cross-)Protective Immunity against Influenza. Vaccine 2015, 33, 500–506. [Google Scholar] [CrossRef]
- Janssens, Y.; Joye, J.; Waerlop, G.; Clement, F.; Leroux-Roels, G.; Leroux-Roels, I. The Role of Cell-Mediated Immunity against Influenza and Its Implications for Vaccine Evaluation. Front. Immunol. 2022, 13, 959379. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.L.; Agrewala, J.N.; Brown, D.M.; Jelley-Gibbs, D.M.; Golech, S.; Huston, G.; Jones, S.C.; Kamperschroer, C.; Lee, W.-H.; McKinstry, K.K.; et al. CD4+ T-Cell Memory: Generation and Multi-Faceted Roles for CD4+ T Cells in Protective Immunity to Influenza. Immunol. Rev. 2006, 211, 8–22. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Luo, C.; Yang, Z.; Zhao, T.; Yuan, L.; Xie, Q.; Liao, Q.; Liao, X.; Wang, L.; Yuan, J.; et al. A Recombinant Mosaic HAs Influenza Vaccine Elicits Broad-Spectrum Immune Response and Protection of Influenza a Viruses. Vaccines 2024, 12, 1008. https://doi.org/10.3390/vaccines12091008
Liu X, Luo C, Yang Z, Zhao T, Yuan L, Xie Q, Liao Q, Liao X, Wang L, Yuan J, et al. A Recombinant Mosaic HAs Influenza Vaccine Elicits Broad-Spectrum Immune Response and Protection of Influenza a Viruses. Vaccines. 2024; 12(9):1008. https://doi.org/10.3390/vaccines12091008
Chicago/Turabian StyleLiu, Xuejie, Chuming Luo, Zhuolin Yang, Tianyi Zhao, Lifang Yuan, Qian Xie, Qijun Liao, Xinzhong Liao, Liangliang Wang, Jianhui Yuan, and et al. 2024. "A Recombinant Mosaic HAs Influenza Vaccine Elicits Broad-Spectrum Immune Response and Protection of Influenza a Viruses" Vaccines 12, no. 9: 1008. https://doi.org/10.3390/vaccines12091008







