Effects of Rotavirus NSP4 on the Immune Response and Protection of Rotavirus-Norovirus Recombinant Subunit Vaccines in Different Immune Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antigen Preparation
2.2. Recombinant Protein Expression and Purification
2.3. Western Blot Analysis
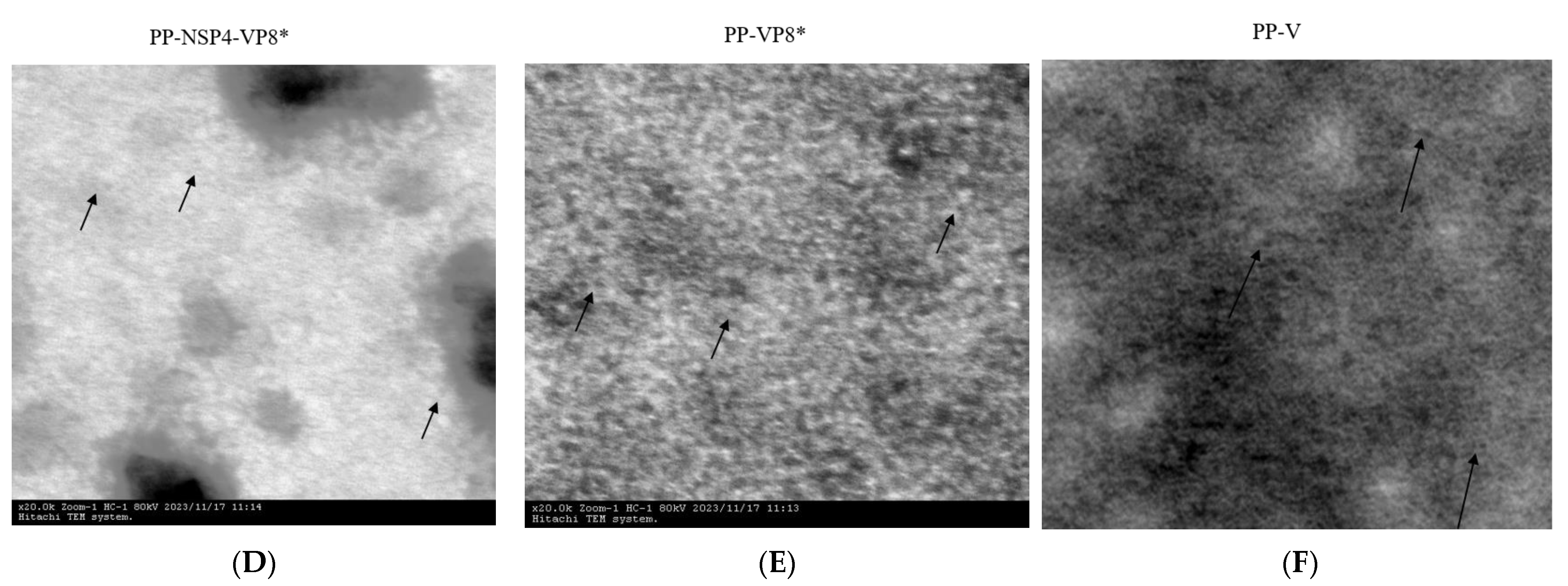
2.4. Transmission Electron Microscopy (TEM)
2.5. Mouse Immunization
2.6. Detection of IgG and IgA Antibody Titers
2.7. Fluorescence-Based Plaque Reduction Neutralization Assays
2.8. HBGA Binding and Blocking Assays
2.9. Suckling Mouse Rotavirus Challenge Model
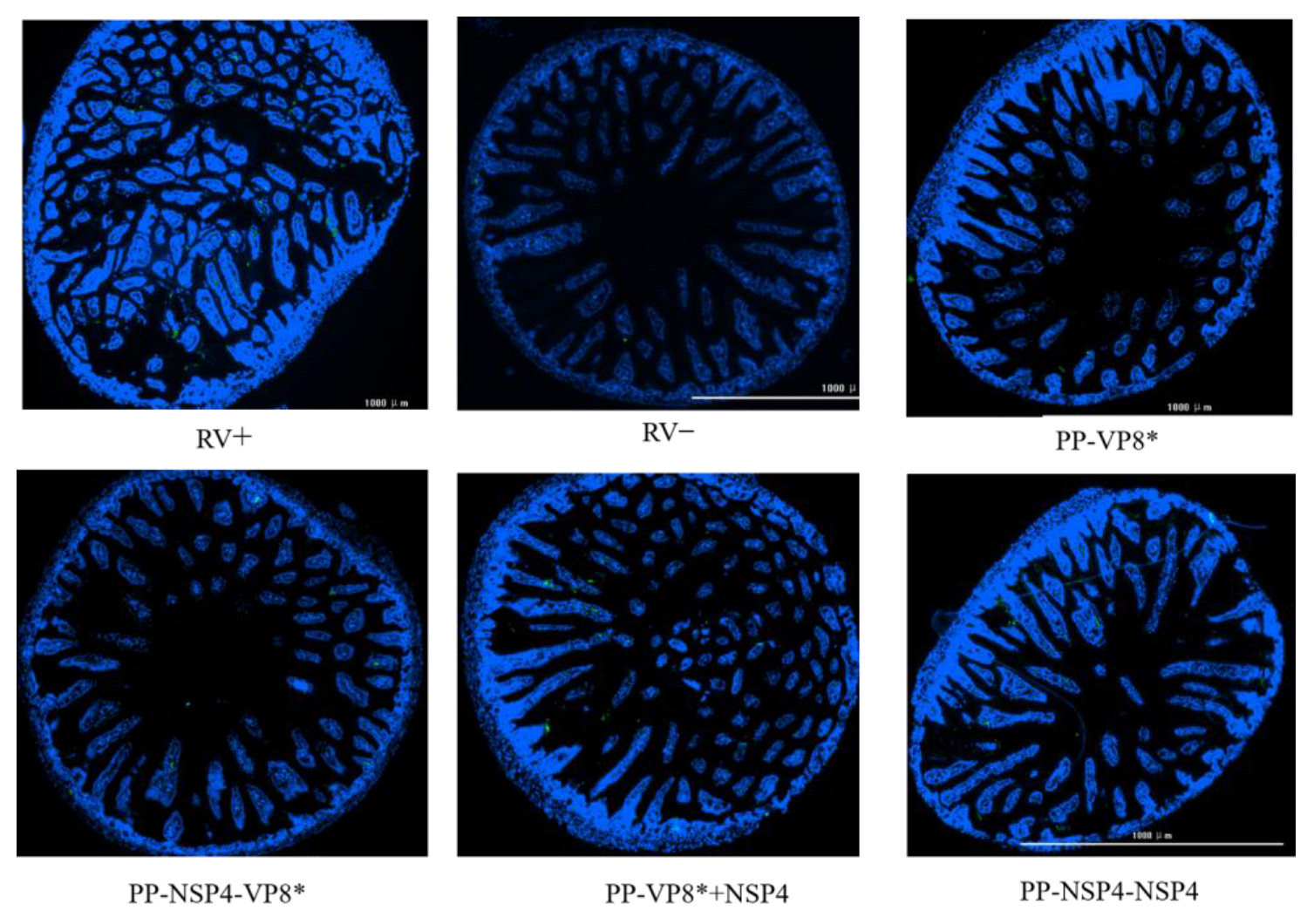
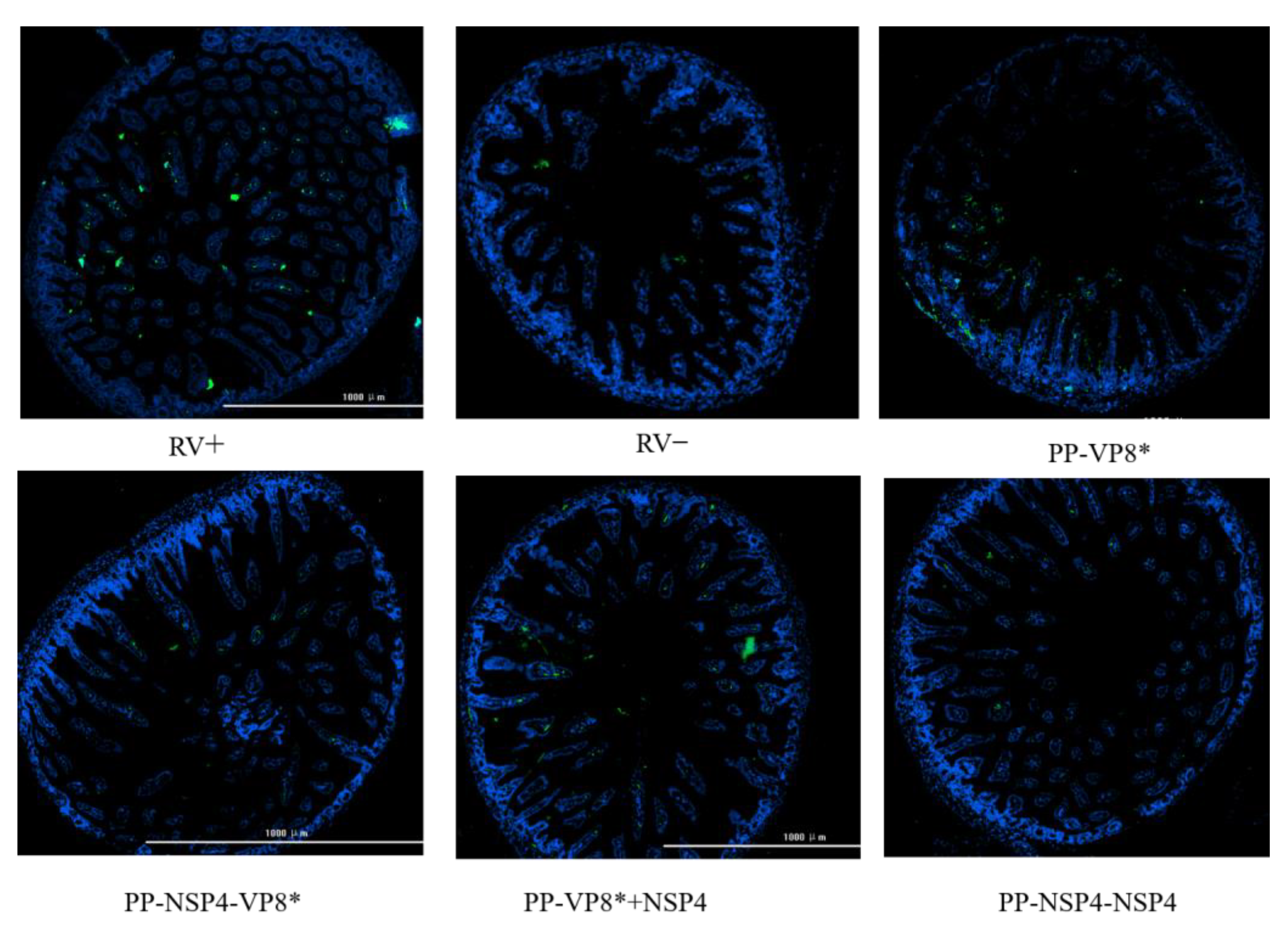
2.10. Immunofluorescence (IF) to Detect the Distribution of Rotavirus in Intestinal Tissue
2.11. Statistical Analysis
3. Results
3.1. Protein Expression and Purification
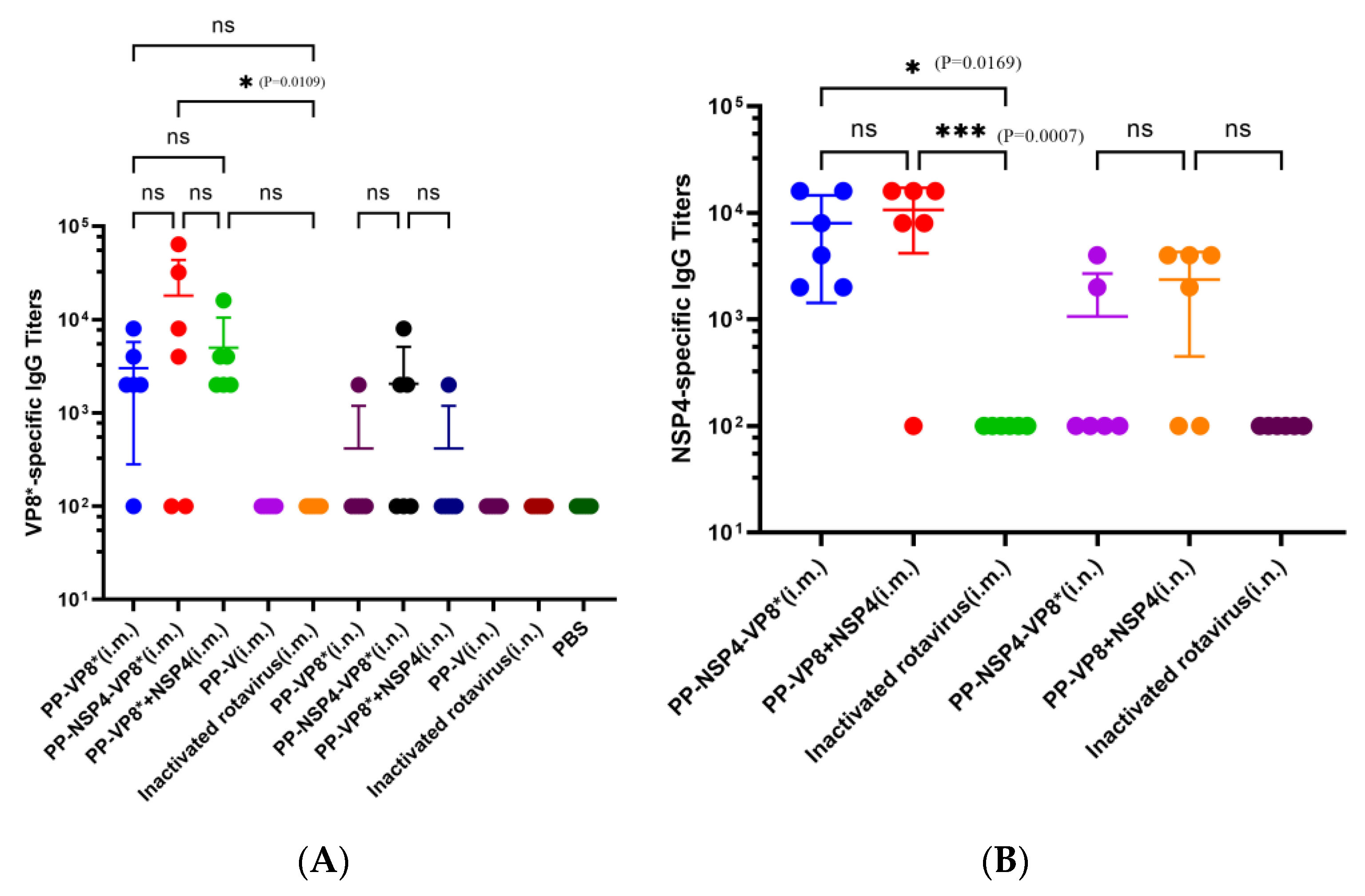
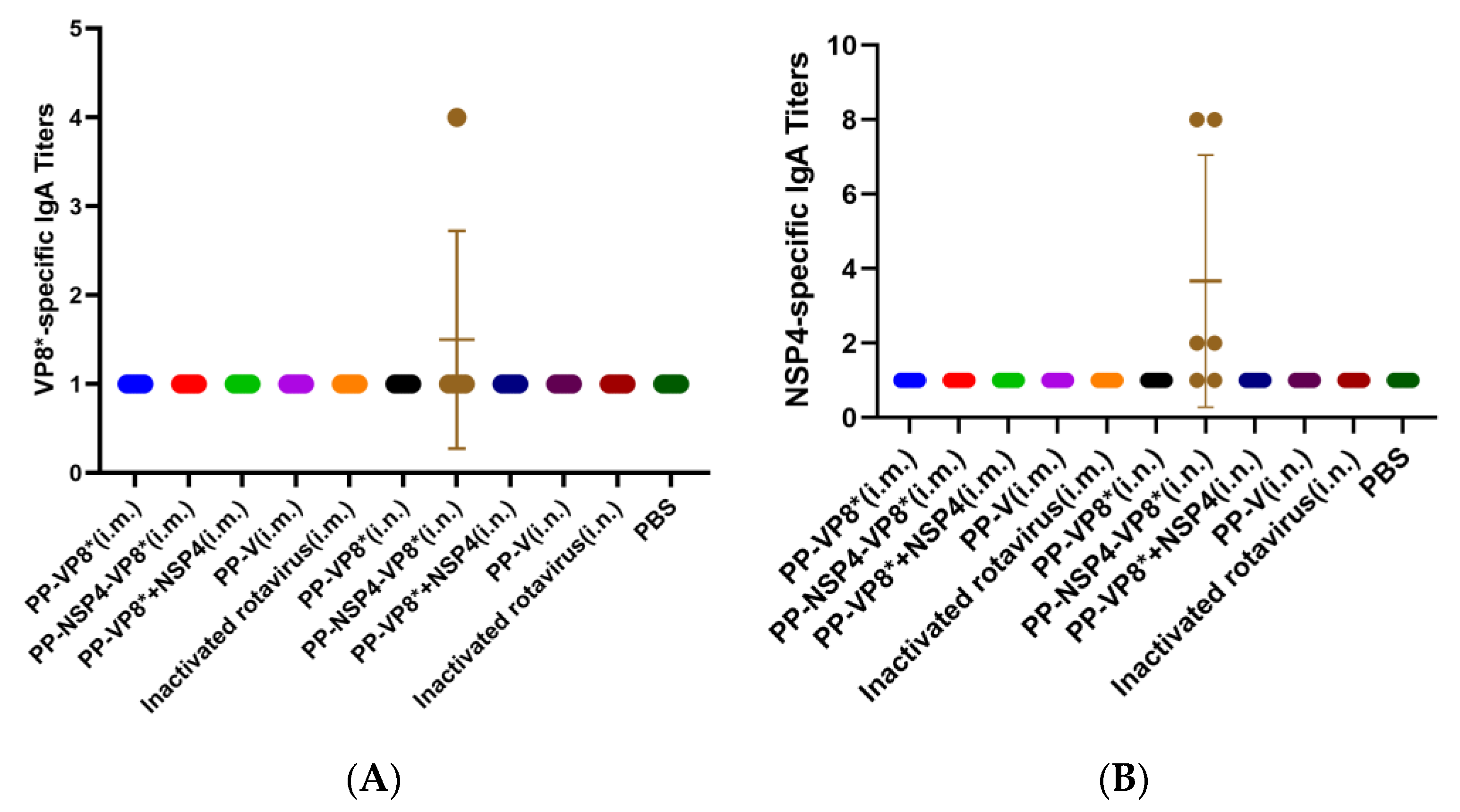
3.2. Results of Specific Antibody Titers from Serum and BALF Samples
3.2.1. Detection of NSP4- and VP8*-Specific Serum IgG Titers
3.2.2. Determination of NSP4- and VP8*-Specific IgA Titers in BALFs
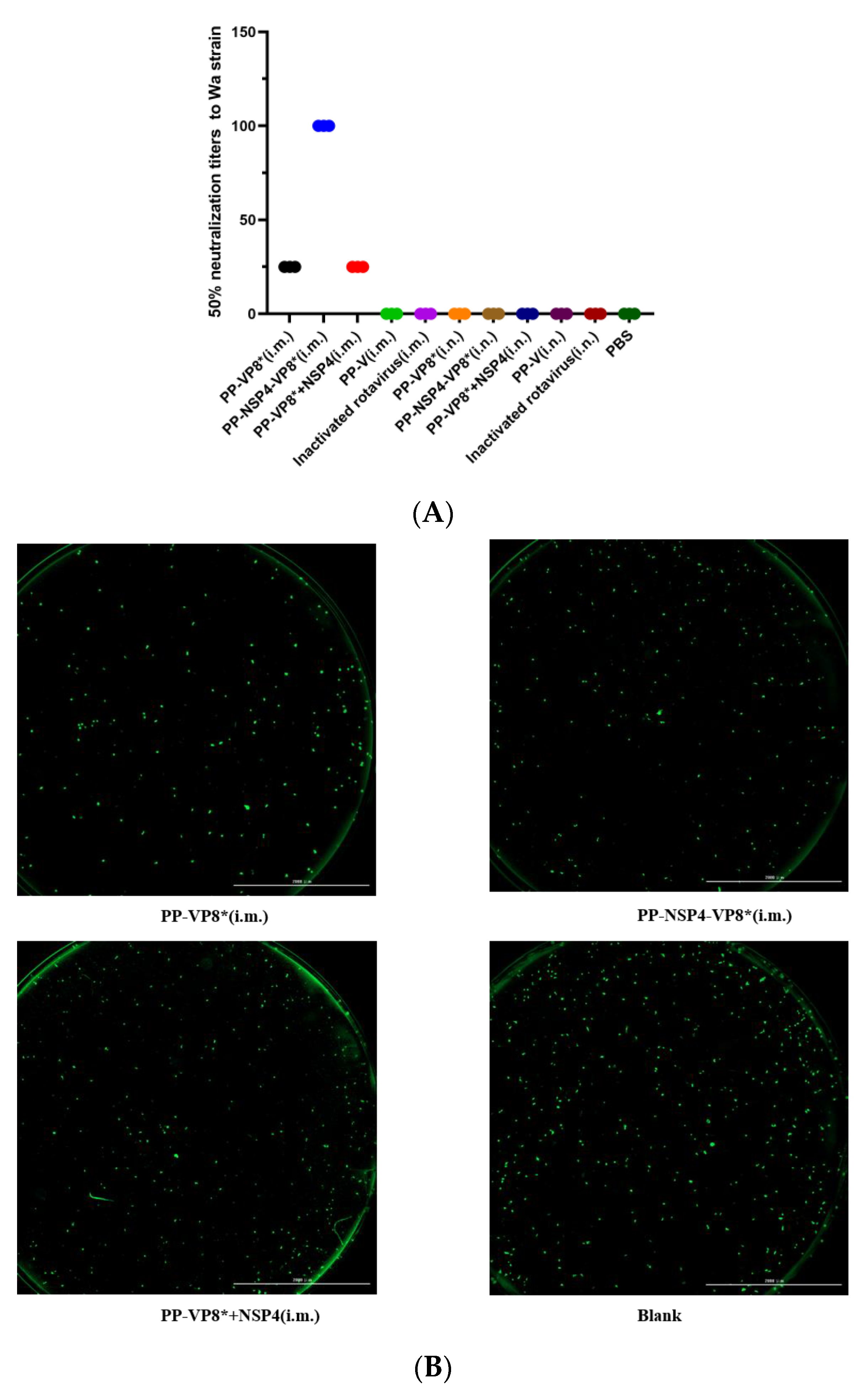
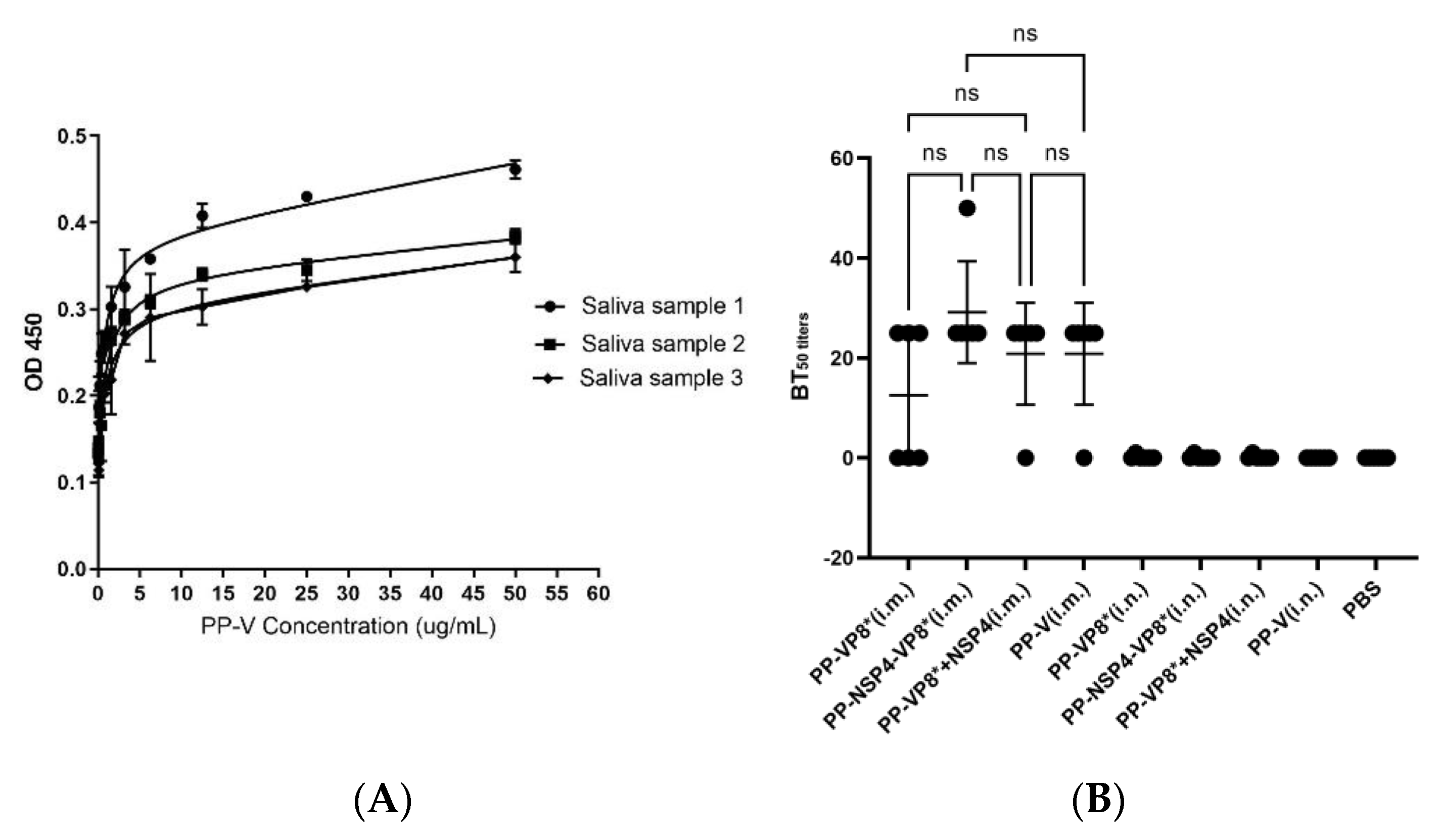
3.3. Results of Fluorescence-Based Plaque Reduction Neutralization Assays
3.4. Results of HBGA Binding and Blocking Assays
3.5. Suckling Mouse Rotavirus Challenge and Immunofluorescence Results of the Small Intestinal Tissue Assay
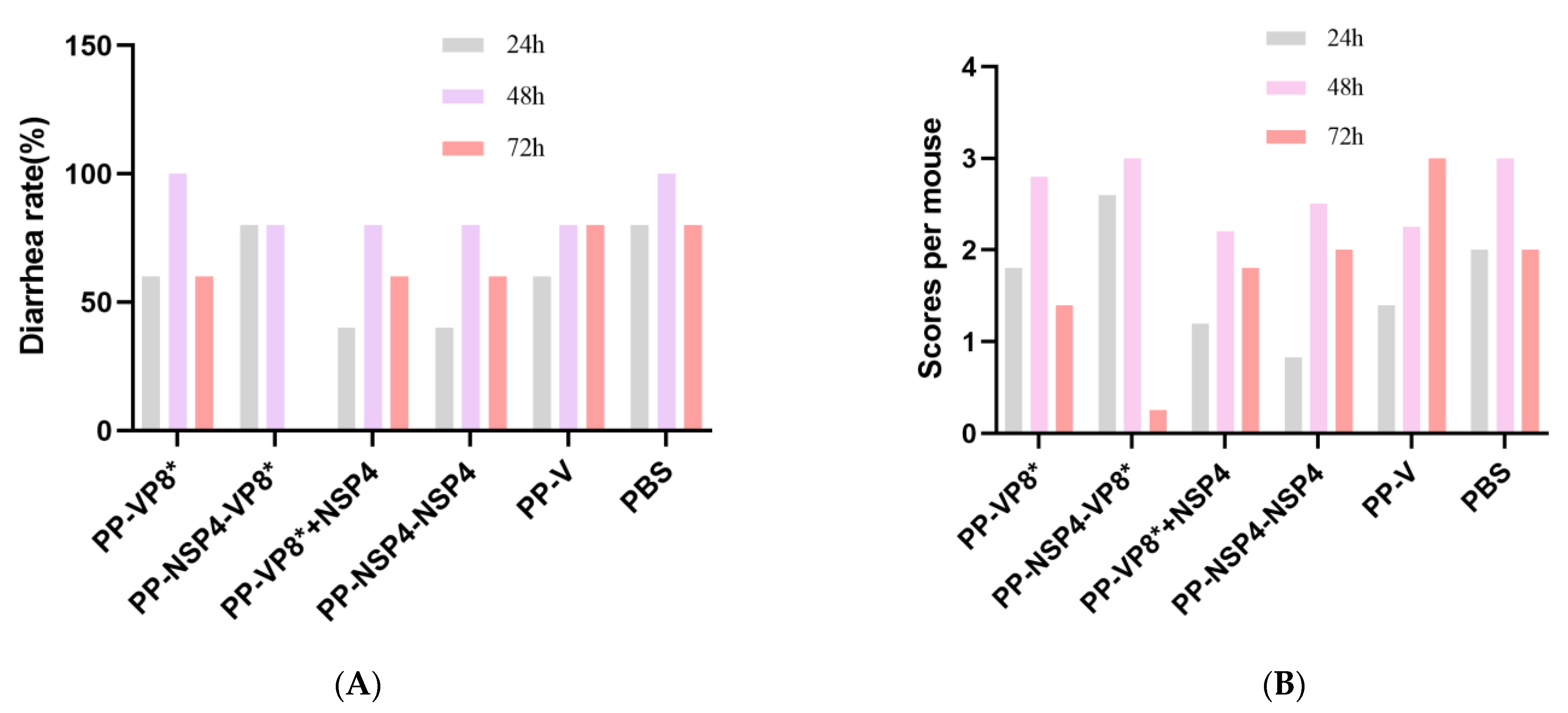
3.5.1. NSP4 Can Protect Suckling Mice against Diarrhea Caused by Rotavirus Wa Strain Challenge
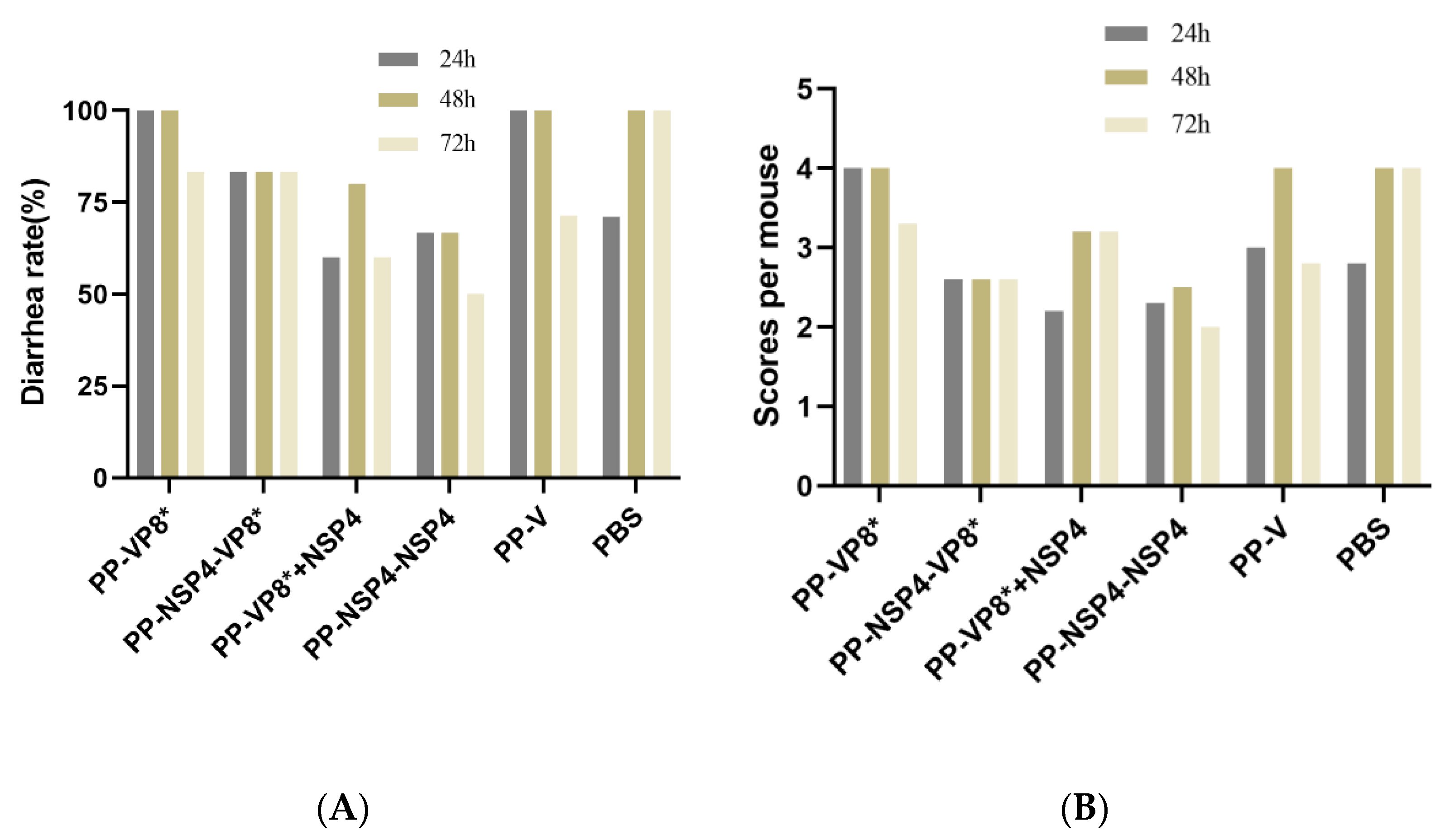
3.5.2. NSP4 Can Protect Suckling Mice against Diarrhea Caused by a Rotavirus SA11 Strain Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desselberger, U. Rotaviruses. Virus Res. 2014, 190, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Matthijnssens, J.; Otto, P.H.; Ciarlet, M.; Desselberger, U.; Van Ranst, M.; Johne, R. VP6-Sequence-Based Cutoff Values as a Criterion for Rotavirus Species Demarcation. Arch. Virol. 2012, 157, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Gómara, M.I.; Wong, C.; Blome, S.; Desselberger, U.; Gray, J. Molecular Characterization of VP6 Genes of Human Rotavirus Isolates: Correlation of Genogroups with Subgroups and Evidence of Independent Segregation. J. Virol. 2002, 76, 6596–6601. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Bostan, N.; Yinda, K.C.; Naseem, S.; Sattar, S. Rotavirus: Genetics, Pathogenesis and Vaccine Advances. Rev. Med. Virol. 2018, 28, e2003. [Google Scholar] [CrossRef]
- Ball, J.M.; Mitchell, D.M.; Gibbons, T.F.; Parr, R.D. Rotavirus NSP4: A Multifunctional Viral Enterotoxin. Viral Immunol. 2005, 18, 27–40. [Google Scholar] [CrossRef]
- Wang, M.X.; Ma, X.; Wei, Y.H.; Peng, R.; Mao, T.Y.; Wang, M.W.; Fan, J.X.; Li, J.S.; Li, D.D. Spread Trend Analysis on Reassortant of Rotavirus G9P[8]-E2 in China. Dis. Surveill. 2021, 36, 780–784. [Google Scholar] [CrossRef]
- Chen, J.; Grow, S.; Iturriza-Gómara, M.; Hausdorff, W.P.; Fix, A.; Kirkwood, C.D. The Challenges and Opportunities of Next-Generation Rotavirus Vaccines: Summary of an Expert Meeting with Vaccine Developers. Viruses 2022, 14, 2565. [Google Scholar] [CrossRef]
- Troeger, C.; Khalil, I.A.; Rao, P.C.; Cao, S.; Blacker, B.F.; Ahmed, T.; Armah, G.; Bines, J.E.; Brewer, T.G.; Colombara, D.V.; et al. Rotavirus Vaccination and the Global Burden of Rotavirus Diarrhea Among Children Younger Than 5 Years. JAMA Pediatr. 2018, 172, 958. [Google Scholar] [CrossRef]
- Anwari, P.; Burnett, E.; Chavers, T.P.; Samsor, A.; Safi, H.; Safi, N.; Clark, A.D.; Parashar, U.D.; Tate, J.E. Post-Marketing Surveillance of Intussusception after Rotarix Administration in Afghanistan, 2018–2022. Vaccine 2024, 42, 2059–2064. [Google Scholar] [CrossRef]
- Buchy, P.; Chen, J.; Zhang, X.H.; Benninghoff, B.; Lee, C.; Bibera, G.L. A Review of Rotavirus Vaccine Use in Asia and the Pacific Regions: Challenges and Future Prospects. Expert Rev. Vaccines 2021, 20, 1499–1514. [Google Scholar] [CrossRef]
- Armah, G.E.; Sow, S.O.; Breiman, R.F.; Dallas, M.J.; Tapia, M.D.; Feikin, D.R.; Binka, F.N.; Steele, A.D.; Laserson, K.F.; Ansah, N.A.; et al. Efficacy of Pentavalent Rotavirus Vaccine against Severe Rotavirus Gastroenteritis in Infants in Developing Countries in Sub-Saharan Africa: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2010, 376, 606–614. [Google Scholar] [CrossRef]
- Harris, V.C.; Haak, B.W.; Handley, S.A.; Jiang, B.; Velasquez, D.E.; Hykes, B.L.; Droit, L.; Berbers, G.A.M.; Kemper, E.M.; van Leeuwen, E.M.M.; et al. Effect of Antibiotic-Mediated Microbiome Modulation on Rotavirus Vaccine Immunogenicity: A Human, Randomized-Control Proof-of-Concept Trial. Cell Host Microbe 2018, 24, 197–207.e4. [Google Scholar] [CrossRef]
- Desselberger, U. Differences of Rotavirus Vaccine Effectiveness by Country: Likely Causes and Contributing Factors. Pathogens 2017, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.; Ali, A.; Fuentes, S.; Korpela, K.; Kazi, M.; Tate, J.; Parashar, U.; Wiersinga, W.J.; Giaquinto, C.; de Weerth, C.; et al. Rotavirus Vaccine Response Correlates with the Infant Gut Microbiota Composition in Pakistan. Gut Microbes 2018, 9, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Parker, E.P.K.; Bronowski, C.; Sindhu, K.N.C.; Babji, S.; Benny, B.; Carmona-Vicente, N.; Chasweka, N.; Chinyama, E.; Cunliffe, N.A.; Dube, Q.; et al. Impact of Maternal Antibodies and Microbiota Development on the Immunogenicity of Oral Rotavirus Vaccine in African, Indian, and European Infants. Nat. Commun. 2021, 12, 7288. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.H.; Armah, G.; Dennis, F.; Wang, L.; Rodgers, R.; Droit, L.; Baldridge, M.T.; Handley, S.A.; Harris, V.C. Enteric Virome Negatively Affects Seroconversion Following Oral Rotavirus Vaccination in a Longitudinally Sampled Cohort of Ghanaian Infants. Cell Host Microbe 2022, 30, 110–123.e5. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M. Parenteral, Non-Live Rotavirus Vaccine: Recent History and Future Perspective. Clin. Exp. Vaccine Res. 2021, 10, 203–210. [Google Scholar] [CrossRef]
- Cao, H.; Wu, J.; Luan, N.; Wang, Y.; Lin, K.; Liu, C. Evaluation of a Bivalent Recombinant Vaccine Candidate Targeting Norovirus and Rotavirus: Antibodies to Rotavirus NSP4 Exert Antidiarrheal Effects without Virus Neutralization. J. Med. Virol. 2022, 94, 3847–3856. [Google Scholar] [CrossRef]
- Hu, L.; Crawford, S.E.; Czako, R.; Cortes-Penfield, N.W.; Smith, D.F.; Le Pendu, J.; Estes, M.K.; Prasad, B.V.V. Cell Attachment Protein VP8* of a Human Rotavirus Specifically Interacts with A-Type Histo-Blood Group Antigen. Nature 2012, 485, 256–259. [Google Scholar] [CrossRef]
- Feng, N.; Hu, L.; Ding, S.; Sanyal, M.; Zhao, B.; Sankaran, B.; Ramani, S.; McNeal, M.; Yasukawa, L.L.; Song, Y.; et al. Human VP8* mAbs Neutralize Rotavirus Selectively in Human Intestinal Epithelial Cells. J. Clin. Investig. 2019, 129, 3839–3851. [Google Scholar] [CrossRef]
- Groome, M.J.; Fairlie, L.; Morrison, J.; Fix, A.; Koen, A.; Masenya, M.; Jose, L.; Madhi, S.A.; Page, N.; McNeal, M.; et al. Safety and Immunogenicity of a Parenteral Trivalent P2-VP8 Subunit Rotavirus Vaccine: A Multisite, Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Infect. Dis. 2020, 20, 851–863. [Google Scholar] [CrossRef]
- Xia, M.; Huang, P.; Jiang, X.; Tan, M. A Nanoparticle-Based Trivalent Vaccine Targeting the Glycan Binding VP8* Domains of Rotaviruses. Viruses 2021, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, C.D.; Palombo, E.A. Genetic Characterization of the Rotavirus Nonstructural Protein, NSP4. Virology 1997, 236, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, L.S.; Tortorici, M.A.; Vasquez-Del Carpio, R.; Patton, J.T. Rotavirus Glycoprotein NSP4 Is a Modulator of Viral Transcription in the Infected Cell. J. Virol. 2005, 79, 15165–15174. [Google Scholar] [CrossRef]
- Tian, P.; Estes, M.K.; Hu, Y.; Ball, J.M.; Zeng, C.Q.; Schilling, W.P. The Rotavirus Nonstructural Glycoprotein NSP4 Mobilizes Ca2+ from the Endoplasmic Reticulum. J. Virol. 1995, 69, 5763–5772. [Google Scholar] [CrossRef]
- Pham, T.; Perry, J.L.; Dosey, T.L.; Delcour, A.H.; Hyser, J.M. The Rotavirus NSP4 Viroporin Domain Is a Calcium-Conducting Ion Channel. Sci. Rep. 2017, 7, 43487. [Google Scholar] [CrossRef]
- Chang-Graham, A.L.; Perry, J.L.; Engevik, M.A.; Engevik, K.A.; Scribano, F.J.; Gebert, J.T.; Danhof, H.A.; Nelson, J.C.; Kellen, J.S.; Strtak, A.C.; et al. Rotavirus Induces Intercellular Calcium Waves through ADP Signaling. Science 2020, 370, eabc3621. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, D.; Sastri, N.P.; Marathahalli, J.R.; Indi, S.S.; Pamidimukkala, K.; Suguna, K.; Rao, C.D. The Flexible C Terminus of the Rotavirus Non-Structural Protein NSP4 Is an Important Determinant of Its Biological Properties. J. Gen. Virol. 2008, 89, 1485–1496. [Google Scholar] [CrossRef]
- Lorrot, M.; Vasseur, M. How Do the Rotavirus NSP4 and Bacterial Enterotoxins Lead Differently to Diarrhea? Virol. J. 2007, 4, 31. [Google Scholar] [CrossRef]
- Hou, Z.; Huang, Y.; Huan, Y.; Pang, W.; Meng, M.; Wang, P.; Yang, M.; Jiang, L.; Cao, X.; Wu, K.K. Anti-NSP4 Antibody Can Block Rotavirus-Induced Diarrhea in Mice. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 376–385. [Google Scholar] [CrossRef]
- Deepa, R.; Durga Rao, C.; Suguna, K. Structure of the Extended Diarrhea-Inducing Domain of Rotavirus Enterotoxigenic Protein NSP4. Arch.Virol. 2007, 152, 847–859. [Google Scholar] [CrossRef]
- Xie, L.; Yan, M.; Wang, X.; Ye, J.; Mi, K.; Yan, S.; Niu, X.; Li, H.; Sun, M. Immunogenicity and Efficacy in Mice of an Adenovirus-Based Bicistronic Rotavirus Vaccine Expressing NSP4 and VP7. Virus Res. 2015, 210, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, O.V.; Ajami, N.J.; Cheng, E.; Ciarlet, M.; Guerrero, R.A.; Zeng, C.Q.-Y.; Crawford, S.E.; Estes, M.K. Rotavirus Enterotoxin NSP4 Has Mucosal Adjuvant Properties. Vaccine 2010, 28, 3106–3111. [Google Scholar] [CrossRef]
- Ge, Y.; Mansell, A.; Ussher, J.E.; Brooks, A.E.S.; Manning, K.; Wang, C.J.H.; Taylor, J.A. Rotavirus NSP4 Triggers Secretion of Proinflammatory Cytokines from Macrophages via Toll-Like Receptor 2. J. Virol. 2013, 87, 11160–11167. [Google Scholar] [CrossRef]
- Liu, C.; Huang, P.; Zhao, D.; Xia, M.; Zhong, W.; Jiang, X.; Tan, M. Effects of Rotavirus NSP4 Protein on the Immune Response and Protection of the SR69A-VP8* Nanoparticle Rotavirus Vaccine. Vaccine 2021, 39, 263–271. [Google Scholar] [CrossRef]
- Pan, J.; Wang, Q.; Qi, M.; Chen, J.; Wu, X.; Zhang, X.; Li, W.; Zhang, X.-E.; Cui, Z. An Intranasal Multivalent Epitope-Based Nanoparticle Vaccine Confers Broad Protection against Divergent Influenza Viruses. ACS Nano 2023, 17, 13474–13487. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, C.; Cox, T.M.; Li, C.; Son, Y.M.; Cheon, I.S.; Wu, Y.; Behl, S.; Taylor, J.J.; Chakaraborty, R.; et al. Respiratory Mucosal Immunity against SARS-CoV-2 after mRNA Vaccination. Sci. Immunol. 2022, 7, eadd4853. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhuang, C.; Chu, K.; Zhang, L.; Zhao, H.; Huang, S.; Su, Y.; Lin, H.; Yang, C.; Jiang, H.; et al. Safety and Immunogenicity of a Live-Attenuated Influenza Virus Vector-Based Intranasal SARS-CoV-2 Vaccine in Adults: Randomised, Double-Blind, Placebo-Controlled, Phase 1 and 2 Trials. Lancet Respir. Med. 2022, 10, 749–760. [Google Scholar] [CrossRef]
- Tan, M.; Huang, P.; Xia, M.; Fang, P.-A.; Zhong, W.; McNeal, M.; Wei, C.; Jiang, W.; Jiang, X. Norovirus P Particle, a Novel Platform for Vaccine Development and Antibody Production. J. Virol. 2011, 85, 753–764. [Google Scholar] [CrossRef]
- Tan, M.; Jiang, X. Norovirus P Particle: A Subviral Nanoparticle for Vaccine Development against Norovirus, Rotavirus and Influenza Virus. Nanomedicine 2012, 7, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Mao, J.; Lei, S.; Twitchell, E.; Shiraz, A.; Jiang, X.; Tan, M.; Yuan, A.L. Parenterally Administered P24-VP8* Nanoparticle Vaccine Conferred Strong Protection against Rotavirus Diarrhea and Virus Shedding in Gnotobiotic Pigs. Vaccines 2019, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Boshuizen, J.A.; Reimerink, J.H.J.; Korteland-van Male, A.M.; van Ham, V.J.J.; Koopmans, M.P.G.; Büller, H.A.; Dekker, J.; Einerhand, A.W.C. Changes in Small Intestinal Homeostasis, Morphology, and Gene Expression during Rotavirus Infection of InfantMice. J. Virol. 2003, 77, 13005–13016. [Google Scholar] [CrossRef] [PubMed]
- Chacko, A.R.; Jeyakanthan, J.; Ueno, G.; Sekar, K.; Rao, C.D.; Dodson, E.J.; Suguna, K.; Read, R.J. A New Pentameric Structure of Rotavirus NSP4 Revealed by Molecular Replacement. Acta Crystallogr. Sect. D Biol. Crystallogr. 2012, 68, 57–61. [Google Scholar] [CrossRef]
- Kumar, S.; Ramappa, R.; Pamidimukkala, K.; Rao, C.D.; Suguna, K. New Tetrameric Forms of the Rotavirus NSP4 with Antiparallel Helices. Arch. Virol. 2018, 163, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Czerkinsky, C. Mucosal Immunity and Vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef]
- Kim, S.-H.; Jang, Y.-S. The Development of Mucosal Vaccines for Both Mucosal and Systemic Immune Induction and the Roles Played by Adjuvants. Clin. Exp. Vaccine Res. 2017, 6, 15. [Google Scholar] [CrossRef]
- Iosef, C.; Chang, K.-O.; Azevedo, M.S.P.; Saif, L.J. Systemic and Intestinal Antibody Responses to NSP4 Enterotoxin of Wa Human Rotavirus in a Gnotobiotic Pig Model of Human Rotavirus Disease. J. Med. Virol. 2002, 68, 119–128. [Google Scholar] [CrossRef]
- Afchangi, A.; Arashkia, A.; Shahosseini, Z.; Jalilvand, S.; Marashi, S.M.; Roohvand, F.; Mohajel, N.; Shoja, Z. Immunization of Mice by Rotavirus NSP4-VP6 Fusion Protein Elicited Stronger Responses Compared to VP6 Alone. Viral Immunol. 2018, 31, 233–241. [Google Scholar] [CrossRef]
- Tan, M.; Jiang, X. The p Domain of Norovirus Capsid Protein Forms a Subviral Particle That Binds to Histo-Blood Group Antigen Receptors. J. Virol. 2005, 79, 14017–14030. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Wu, J.; Cao, H.; Luan, N.; Lin, K.; Zhang, H.; Gao, D.; Lei, Z.; Li, H.; Liu, C. Effects of Rotavirus NSP4 on the Immune Response and Protection of Rotavirus-Norovirus Recombinant Subunit Vaccines in Different Immune Pathways. Vaccines 2024, 12, 1025. https://doi.org/10.3390/vaccines12091025
Hu J, Wu J, Cao H, Luan N, Lin K, Zhang H, Gao D, Lei Z, Li H, Liu C. Effects of Rotavirus NSP4 on the Immune Response and Protection of Rotavirus-Norovirus Recombinant Subunit Vaccines in Different Immune Pathways. Vaccines. 2024; 12(9):1025. https://doi.org/10.3390/vaccines12091025
Chicago/Turabian StyleHu, Jingping, Jinyuan Wu, Han Cao, Ning Luan, Kangyang Lin, Haihao Zhang, Dandan Gao, Zhentao Lei, Hongjun Li, and Cunbao Liu. 2024. "Effects of Rotavirus NSP4 on the Immune Response and Protection of Rotavirus-Norovirus Recombinant Subunit Vaccines in Different Immune Pathways" Vaccines 12, no. 9: 1025. https://doi.org/10.3390/vaccines12091025





