Safety and Immunogenicity of a Carbohydrate Fatty Acid Monosulphate Ester Adjuvant Combined with a Low-Dose Quadrivalent Split-Virion Inactivated Influenza Vaccine: A Randomised, Observer-Blind, Active-Controlled, First-in-Human, Phase 1 Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Study Vaccine
2.3. Study Procedures
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Study Population Demographics
3.2. Safety and Reactogenicity
3.3. Humoral Immune Response
3.4. Cell-Mediated Immune Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Influenza (Seasonal) Factsheet. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 19 December 2023).
- Carregaro, R.L.; Roscani, A.N.C.P.; Raimundo, A.C.S.; Ferreira, L.; Vanni, T.; da Graça Salomão, M.; Probst, L.F.; Viscondi, J.Y.K. Immunogenicity and safety of inactivated quadrivalent influenza vaccine compared with the trivalent vaccine for influenza infection: An overview of systematic reviews. BMC Infect. Dis. 2023, 23, 563. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Factsheet about Seasonal Influenza. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet (accessed on 14 May 2020).
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Erbelding, E.J.; Post, D.J.; Stemmy, E.J.; Roberts, P.C.; Augustine, A.D.; Ferguson, S.; Paules, C.I.; Graham, B.S.; Fauci, A.S. A Universal Influenza Vaccine: The Strategic Plan for the National Institute of Allergy and Infectious Diseases. J. Infect. Dis. 2018, 218, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Comber, L.; Eamon, O.M.; Jordan, K.; Hawkshaw, S.; Marshall, L.; O’Neill, M.; Teljeur, C.; Ryan, M.; Carnahan, A.; Pérez Martín, J.J.; et al. Systematic review of the efficacy, effectiveness and safety of high-dose seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals ≥18 years of age. Rev. Med. Virol. 2023, 33, e2330. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Recommended Composition of Influenza Virus Vaccines for Use in the 2022–2023 Northern Hemisphere Influenza Season. Available online: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2022-2023-northern-hemisphere-influenza-season (accessed on 18 July 2024).
- Moore, K.A.; Ostrowsky, J.T.; Kraigsley, A.M.; Mehr, A.J.; Bresee, J.S.; Friede, M.H.; Gellin, B.G.; Golding, J.P.; Hart, P.J.; Moen, A.; et al. A Research and Development (R&D) roadmap for influenza vaccines: Looking toward the future. Vaccine 2021, 39, 6573–6584. [Google Scholar] [CrossRef]
- Sparrow, E.; Wood, J.G.; Chadwick, C.; Newall, A.T.; Torvaldsen, S.; Moen, A.; Torelli, G. Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine 2021, 39, 512–520. [Google Scholar] [CrossRef]
- Hilgers, L.A.T.; Platenburg, P.; Bajramovic, J.; Veth, J.; Sauerwein, R.; Roeffen, W.; Pohl, M.; van Amerongen, G.; Stittelaar, K.J.; van den Bosch, J.F. Carbohydrate fatty acid monosulphate esters are safe and effective adjuvants for humoral responses. Vaccine 2017, 35, 3249–3255. [Google Scholar] [CrossRef]
- Platenburg, P.; Deschamps, F.; Jung, J.; Leonard, C.; Rusconi, S.; Mohan Kumar, S.B.; Sulaiman, S.M.; de Waal, L.; Hilgers, L.A.T. Carbohydrate fatty acid monosulphate ester is a potent adjuvant for low-dose seasonal influenza vaccines. Vaccine 2023, 41, 6980–6990. [Google Scholar] [CrossRef]
- US Food and Drug Administration; Center for Biologics Evaluation and Research. Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials; US Food and Drug Administration: Silver Spring, MD, USA, 2007. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical (accessed on 18 July 2024).
- Leroux-Roels, I.; Borkowski, A.; Vanwolleghem, T.; Dramé, M.; Clement, F.; Hons, E.; Devaster, J.M.; Leroux-Roels, G. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: A randomised controlled trial. Lancet 2007, 370, 580–589. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Freeman, E.E.; Saff, R.R.; Robinson, L.B.; Wolfson, A.R.; Foreman, R.K.; Hashimoto, D.; Banerji, A.; Li, L.; Anvari, S.; et al. Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2. N. Engl. J. Med. 2021, 384, 1273–1277. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Portuondo, D.; Carlos, I.Z.; Pérez, O. An approach to local immunotoxicity induced by adjuvanted vaccines. Int. Immunopharmacol. 2013, 17, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Martínez, D.T.; Carlos, I.Z. Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed. Pharmacother. 2018, 105, 616–624. [Google Scholar] [CrossRef]
- Silcock, R.; Crawford, N.W.; Perrett, K.P. Subcutaneous nodules: An important adverse event following immunization. Expert. Rev. Vaccines 2019, 18, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Engler, R.J.; Nelson, M.R.; Klote, M.M.; VanRaden, M.J.; Huang, C.Y.; Cox, N.J.; Klimov, A.; Keitel, W.A.; Nichol, K.L.; Carr, W.W.; et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004–2005): Age, dose, and sex effects on immune responses. Arch. Intern. Med. 2008, 168, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Versage, E.; van Twuijver, E.; Jansen, W.; Theeuwes, A.; Sawlwin, D.; Hohenboken, M. Analyses of Safety Profile and Homologous Antibody Responses to a Mammalian Cell-Based, MF59-Adjuvanted, A/H5N1, Pandemic Influenza Vaccine across Four Phase II/III Clinical Trials in Healthy Children, Adults, and Older Adults. Vaccines 2021, 9, 1468. [Google Scholar] [CrossRef]
- Reisinger, K.S.; Holmes, S.J.; Pedotti, P.; Arora, A.K.; Lattanzi, M. A dose-ranging study of MF59®-adjuvanted and non-adjuvanted A/H1N1 pandemic influenza vaccine in young to middle-aged and older adult populations to assess safety, immunogenicity, and antibody persistence one year after vaccination. Hum. Vaccin. Immunother. 2014, 10, 2395–2407. [Google Scholar] [CrossRef]
- Vanni, T.; Thomé, B.C.; Sparrow, E.; Friede, M.; Fox, C.B.; Beckmann, A.M.; Huynh, C.; Mondini, G.; Silveira, D.H.; Viscondi, J.Y.K.; et al. Dose-sparing effect of two adjuvant formulations with a pandemic influenza A/H7N9 vaccine: A randomized, double-blind, placebo-controlled, phase 1 clinical trial. PLoS ONE 2022, 17, e0274943. [Google Scholar] [CrossRef]
- Gorse, G.J.; Grimes, S.; Buck, H.; Mulla, H.; White, P.; Hill, H.; May, J.; Frey, S.E.; Blackburn, P. A phase 1 dose-sparing, randomized clinical trial of seasonal trivalent inactivated influenza vaccine combined with MAS-1, a novel water-in-oil adjuvant/delivery system. Vaccine 2022, 40, 1271–1281. [Google Scholar] [CrossRef]
- Korenkov, D.; Isakova-Sivak, I.; Rudenko, L. Basics of CD8 T-cell immune responses after influenza infection and vaccination with inactivated or live attenuated influenza vaccine. Expert. Rev. Vaccines 2018, 17, 977–987. [Google Scholar] [CrossRef]
- Thomas, S.; Pak, J.; Doss-Gollin, S.; Ryff, K.; Beijnen, E.; Pedersen, G.K.; Christensen, D.; Levy, O.; van Haren, S.D. Human In vitro Modeling Identifies Adjuvant Combinations that Unlock Antigen Cross-presentation and Promote T-helper 1 Development in Newborns, Adults and Elders. J. Mol. Biol. 2024, 436, 168446. [Google Scholar] [CrossRef] [PubMed]
- van Haren, S.D.; Dowling, D.J.; Foppen, W.; Christensen, D.; Andersen, P.; Reed, S.G.; Hershberg, R.M.; Baden, L.R.; Levy, O. Age-Specific Adjuvant Synergy: Dual TLR7/8 and Mincle Activation of Human Newborn Dendritic Cells Enables Th1 Polarization. J. Immunol. 2016, 197, 4413–4424. [Google Scholar] [CrossRef]
- Della Cioppa, G.; Nicolay, U.; Lindert, K.; Leroux-Roels, G.; Clement, F.; Castellino, F.; Galli, G.; Groth, N.; Del Giudice, G. Superior immunogenicity of seasonal influenza vaccines containing full dose of MF59® adjuvant: Results from a dose-finding clinical trial in older adults. Hum. Vaccin. Immunother. 2012, 8, 216–227. [Google Scholar] [CrossRef]
- Cowling, B.J.; Perera, R.; Valkenburg, S.A.; Leung, N.H.L.; Iuliano, A.D.; Tam, Y.H.; Wong, J.H.F.; Fang, V.J.; Li, A.P.Y.; So, H.C.; et al. Comparative Immunogenicity of Several Enhanced Influenza Vaccine Options for Older Adults: A Randomized, Controlled Trial. Clin. Infect. Dis. 2020, 71, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Haralambieva, I.H.; Quach, H.Q.; Ovsyannikova, I.G.; Goergen, K.M.; Grill, D.E.; Poland, G.A.; Kennedy, R.B. T Cell Transcriptional Signatures of Influenza A/H3N2 Antibody Response to High Dose Influenza and Adjuvanted Influenza Vaccine in Older Adults. Viruses 2022, 14, 2763. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Kuang, Y.; Liang, J.; Jones, M.; Swain, S.L. Influenza Vaccine-Induced CD4 Effectors Require Antigen Recognition at an Effector Checkpoint to Generate CD4 Lung Memory and Antibody Production. J. Immunol. 2020, 205, 2077–2090. [Google Scholar] [CrossRef]
- Levie, K.; Leroux-Roels, I.; Hoppenbrouwers, K.; Kervyn, A.D.; Vandermeulen, C.; Forgus, S.; Leroux-Roels, G.; Pichon, S.; Kusters, I. An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J. Infect. Dis. 2008, 198, 642–649. [Google Scholar] [CrossRef]
- Frey, S.E.; Shakib, S.; Chanthavanich, P.; Richmond, P.; Smith, T.; Tantawichien, T.; Kittel, C.; Jaehnig, P.; Mojares, Z.; Verma, B.; et al. Safety and Immunogenicity of MF59-Adjuvanted Cell Culture–Derived A/H5N1 Subunit Influenza Virus Vaccine: Dose-Finding Clinical Trials in Adults and the Elderly. Open Forum Infect. Dis. 2019, 6, ofz107. [Google Scholar] [CrossRef]
- Weinberger, B.; Herndler-Brandstetter, D.; Schwanninger, A.; Weiskopf, D.; Grubeck-Loebenstein, B. Biology of immune responses to vaccines in elderly persons. Clin. Infect. Dis. 2008, 46, 1078–1084. [Google Scholar] [CrossRef]
- Nichol, K.L.; Nordin, J.D.; Nelson, D.B.; Mullooly, J.P.; Hak, E. Effectiveness of influenza vaccine in the community-dwelling elderly. N. Engl. J. Med. 2007, 357, 1373–1381. [Google Scholar] [CrossRef]
- Kang, K.S.; Lee, N.; Shin, M.S.; Kim, S.D.; Yu, Y.; Mohanty, S.; Belshe, R.B.; Montgomery, R.R.; Shaw, A.C.; Kang, I. An altered relationship of influenza vaccine-specific IgG responses with T cell immunity occurs with aging in humans. Clin. Immunol. 2013, 147, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Begue, S.; Waerlop, G.; Salaun, B.; Janssens, M.; Bellamy, D.; Cox, R.J.; Davies, R.; Gianchecchi, E.; Medaglini, D.; Montomoli, E.; et al. Harmonization and qualification of intracellular cytokine staining to measure influenza-specific CD4+ T cell immunity within the FLUCOP consortium. Front. Immunol. 2022, 13, 982887. [Google Scholar] [CrossRef]




| Parameter | Category | VaxigripTetra (15 µg) (N = 20) | VaxigripTetra (3 µg) + 0.5 mg CMS (N = 20) | VaxigripTetra (3 µg) + 2 mg CMS (N = 20) | Total (N = 60) |
|---|---|---|---|---|---|
| Age (years (SD)) | 39.8 (9.8) | 38.9 (10.0) | 34.1 (10.0) | 37.6 (10.1) | |
| Sex (n, %) | Female | 12 (60.0%) | 14 (70.0%) | 16 (80.0%) | 42 (70.0%) |
| Male | 8 (40.0%) | 6 (30.0%) | 4 (20.0%) | 18 (30.0%) | |
| Age and sex distribution (n,%) | 18–25 y—Male | 2 (10.0%) | 0 (0.0%) | 0 (0.0%) | 2 (3.3%) |
| 18–25 y—Female | 1 (5.0%) | 3 (15.0%) | 6 (30.0%) | 10 (16.7%) | |
| 26–35 y—Male | 1 (5.0%) | 1 (5.0%) | 1 (5.0%) | 3 (5.0%) | |
| 26–35 y—Female | 1 (5.0%) | 4 (20.0%) | 4 (20.0%) | 9 (15.0%) | |
| 36–50 y—Male | 5 (25.0%) | 5 (25.0%) | 3 (15.0%) | 13 (21.7%) | |
| 36–50 y—Female | 10 (50.0%) | 7 (35.0%) | 6 (30.0%) | 23 (38.3%) | |
| Race (n, %) | White | 20 (100.0%) | 19 (95.0%) | 18 (90.0%) | 57 (95.0%) |
| Black or African American | 0 (0.0%) | 1 (5.0%) | 0 (0.0%) | 1 (1.7%) | |
| Asian | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 1 (1.7%) | |
| Other | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 1 (1.7%) | |
| Weight (kg (SD)) | 74.34 (13.4) | 73.96 (12.78) | 68.49 (8.68) | 72.26 (11.91) | |
| BMI (kg/m2 (SD)) | 24.33 (3.43) | 24.33 (3.07) | 23.84 (2.69) | 24.17 (3.03) |
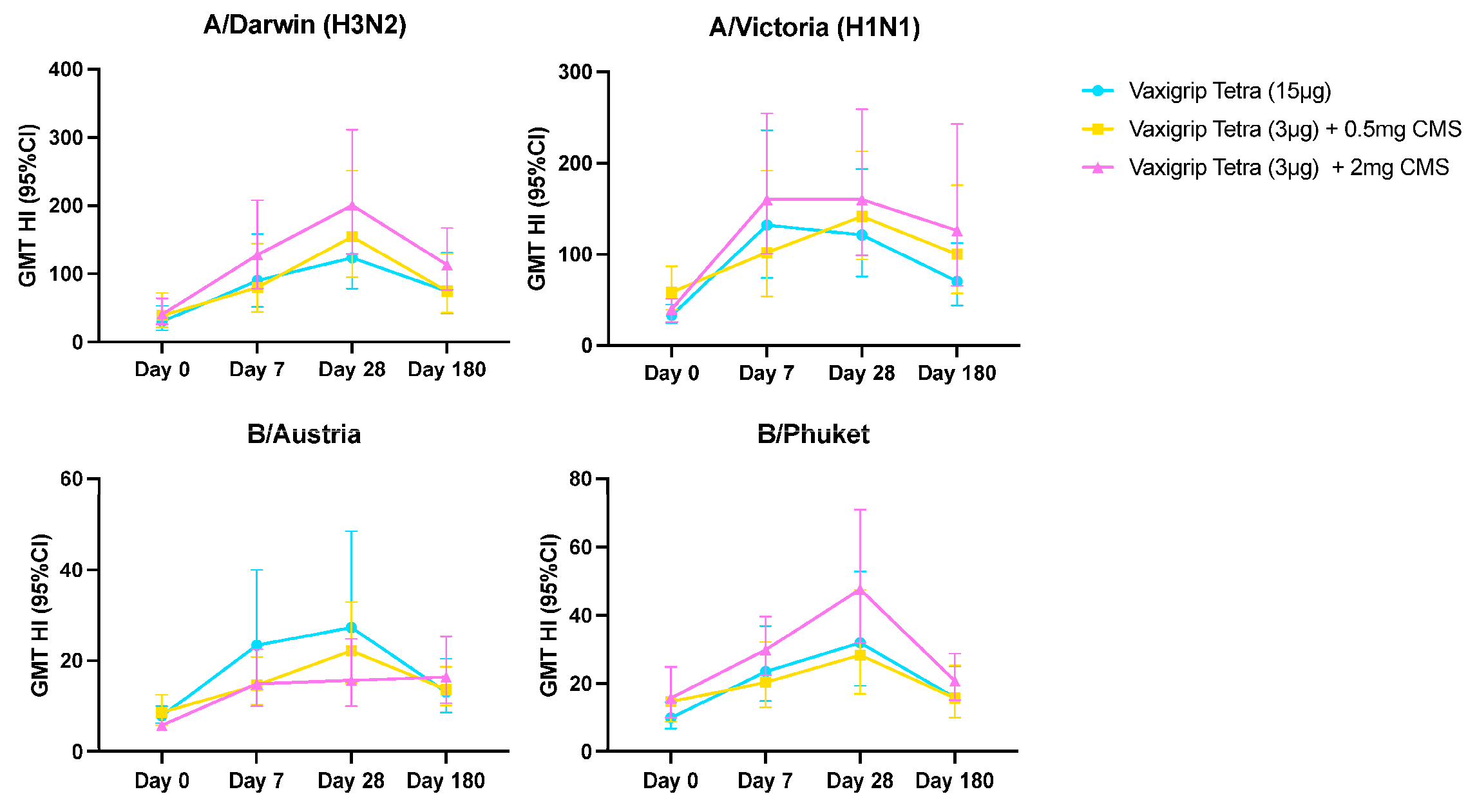
| VaxigripTetra (15 µg) | VaxigripTetra (3 µg) + 0.5 mg CMS | VaxigripTetra (3 µg) + 2 mg CMS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/Darwin (H3N2) | A/Victoria (H1N1) | B/Austria | B/Phuket | A/Darwin (H3N2) | A/Victoria (H1N1) | B/Austria | B/Phuket | A/Darwin (H3N2) | A/Victoria (H1N1) | B/Austria | B/Phuket | ||
| Day 0 | GMT (95% CI) | 30.3 (17.3–53.1) | 33.1 (24.4–44.8) | 7.8 (6.2–9.9) | 9.8 (6.7–14.4) | 39.3 (21.6–71.7) | 58.6 (39.5–86.8) | 8.6 (5.8–12.5) | 14.6 (8.7–24.7) | 40.7 (25.8–64.3) | 40 (25.9–61.7) | 5.7 (4.9–6.7) | 15.7 (9.9–24.8) |
| Day 7 | GMT (95% CI) | 90.3 (51.5–158.5) | 132.2 (74.0–236.2) | 23.4 (13.7–40.0) | 23.4 (14.8–36.9) | 80.0 (44.3–144.4) | 102.0 (54.2–191.8) | 14.6 (10.3–20.8) | 20.3 (12.9–32.1) | 127.7 (78.6–207.7) | 160.0 (100.5–254.7) | 14.9 (9.9–22.5) | 29.8 (22.4–39.6) |
| GMR (95% CI) | 3.0 (1.9–4.6) | 4.0 (2.2–7.4) | 3.0 (1.9–4.7) | 2.4 (1.5–3.7) | 2.0 (1.5–2.8) | 1.7 (1.1–2.7) | 1.7 (1.2–2.4) | 1.4 (1.0–1.9) | 3.1 (2.1–4.6) | 4.0 (2.7–6.0) | 2.6 (1.8–3.7) | 1.9 (1.2–3.0) | |
| Day 28 | GMT (95% CI) | 123.4 (78.5–193.9) | 121.3 (75.8–193.9) | 27.3 (15.4–48.5) | 31.9 (19.3–52.9) | 154.5 (95.2–251.0) | 141.7 (94.2–213.2) | 22.2 (15.0–32.9) | 28.3 (16.9–47.4) | 200.4 (128.9–311.6) | 160.0 (98.8–259.2) | 15.7 (9.9–24.8) | 47.6 (31.9–70.9) |
| GMR (95% CI) | 4.1 (2.4–7.0) | 3.7 (2.1–6.5) | 3.5 (2.1–5.8) | 3.2 (1.9–5.7) | 3.9 (2.5–6.1) | 2.4 (1.6–3.6) | 2.6 (1.8–3.7) | 1.9 (1.3–2.9) | 4.9 (3.3–7.4) | 4.0 (2.6–6.2) | 2.7 (1.8–4.0) | 3.0 (1.9–4.9) | |
| SPR (n, %) | 19 (95.0%) | 20 (100.0%) | 8 (40.0%) | 11 (55.0%) | 19 (95.0%) | 19 (95.0%) | 7 (35.0%) | 11 (55.0%) | 20 (100.0%) | 20 (100.0%) | 3 (15.0%) | 15 (75.0%) | |
| SCR (n, %) | 9 (45.0%) | 9 (45.0%) | 8 (40.0%) | 8 (40.0%) | 9 (45.0%) | 7 (35.0%) | 6 (30.0%) | 5 (25.0%) | 14 (70.0%) | 8 (40.0%) | 6 (30.0%) | 9 (45.0%) | |
| Day 180 | GMT (95% CI) | 74.4 (42.2–131.1) | 70.4 (44.0–112.6) | 13.1 (8.5–20.4) | 15.8 (10.0–25.0) | 74.6 (43.1–129.2) | 100.2 (57.1–175.9) | 13.7 (10.1–18.6) | 15.7 (9.8–25.2) | 113.1 (76.6–167.1) | 126.2 (65.5–243.4) | 16.4 (10.6–25.3) | 20.7 (15.0–28.7) |
| GMR (95% CI) | 2.2 (1.4–3.6) | 2.0 (1.2–3.3) | 1.6 (1.1–2.4) | 1.6 (0.9–2.7) | 1.9 (1.2–2.9) | 1.7 (1.1–2.8) | 1.6 (1.1–2.4) | 1.1 (0.8–1.5) | 3.0 (2.0–4.5) | 3.1 (1.8–5.3) | 2.8 (1.9–4.2) | 1.3 (0.8–2.0) | |
| SPR (n, %) | 15 (75.0%) | 16 (80.0%) | 2 (10.0%) | 2 (10.0%) | 14 (70.0%) | 18 (90.0%) | 3 (15.0%) | 4 (20.0%) | 18 (90.0%) | 16 (80.0%) | 4 (20.0%) | 5 (25.0%) | |
| SCR (n, %) | 4 (20.0%) | 5 (25.0%) | 3 (15.0%) | 6 (30.0%) | 5 (25.0%) | 5 (25.0%) | 4 (20.0%) | 2 (10.0%) | 8 (40.0%) | 6 (30.0%) | 7 (35.0%) | 4 (20.0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Onofrio, V.; Porrez, S.; Jacobs, B.; Alhatemi, A.; De Boever, F.; Waerlop, G.; Michels, E.; Vanni, F.; Manenti, A.; Leroux-Roels, G.; et al. Safety and Immunogenicity of a Carbohydrate Fatty Acid Monosulphate Ester Adjuvant Combined with a Low-Dose Quadrivalent Split-Virion Inactivated Influenza Vaccine: A Randomised, Observer-Blind, Active-Controlled, First-in-Human, Phase 1 Study. Vaccines 2024, 12, 1036. https://doi.org/10.3390/vaccines12091036
D’Onofrio V, Porrez S, Jacobs B, Alhatemi A, De Boever F, Waerlop G, Michels E, Vanni F, Manenti A, Leroux-Roels G, et al. Safety and Immunogenicity of a Carbohydrate Fatty Acid Monosulphate Ester Adjuvant Combined with a Low-Dose Quadrivalent Split-Virion Inactivated Influenza Vaccine: A Randomised, Observer-Blind, Active-Controlled, First-in-Human, Phase 1 Study. Vaccines. 2024; 12(9):1036. https://doi.org/10.3390/vaccines12091036
Chicago/Turabian StyleD’Onofrio, Valentino, Sharon Porrez, Bart Jacobs, Azhar Alhatemi, Fien De Boever, Gwenn Waerlop, Els Michels, Francesca Vanni, Alessandro Manenti, Geert Leroux-Roels, and et al. 2024. "Safety and Immunogenicity of a Carbohydrate Fatty Acid Monosulphate Ester Adjuvant Combined with a Low-Dose Quadrivalent Split-Virion Inactivated Influenza Vaccine: A Randomised, Observer-Blind, Active-Controlled, First-in-Human, Phase 1 Study" Vaccines 12, no. 9: 1036. https://doi.org/10.3390/vaccines12091036







