Cost-Effectiveness Analysis of Routine Childhood Immunization with 20-Valent versus 15-Valent Pneumococcal Conjugate Vaccines in Germany
Abstract
:1. Introduction
2. Methods
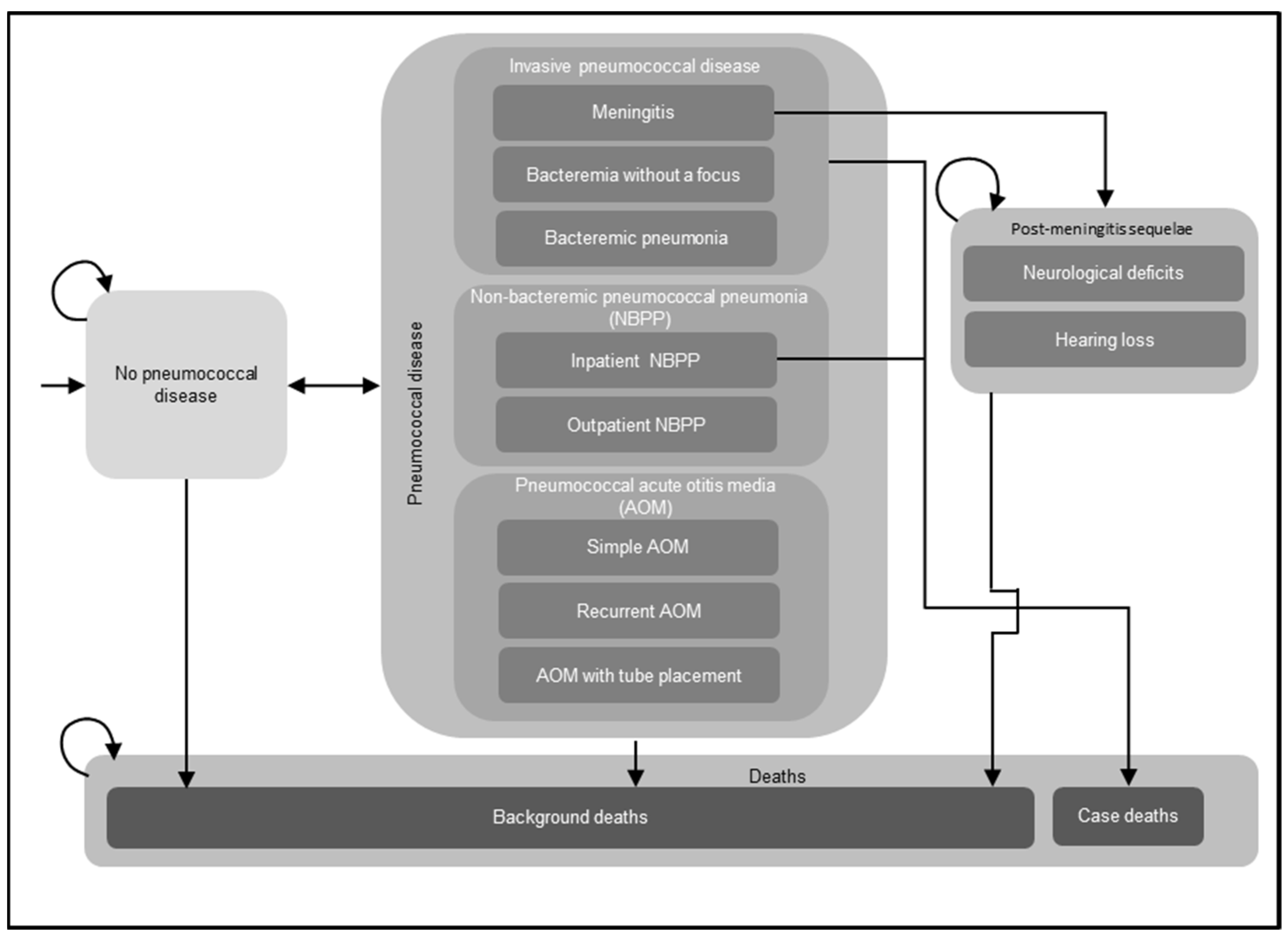
2.1. Model Overview
2.2. Model Inputs
2.2.1. Population Inputs
2.2.2. Epidemiological Inputs
2.2.3. Vaccine Coverage Rates
2.2.4. Vaccine Effectiveness
Direct Effects Estimation
Direct-Effects Inputs
Estimations of PPS VE and PTD VE for V114 and PCV20
Vaccine Effectiveness Onset and Waning
2.2.5. Indirect Effects
2.2.6. Utility Inputs
2.2.7. Cost Inputs
Vaccine Acquisition and Administration Costs
Costs Associated with Pneumococcal Disease
Costs Associated with PMS
2.3. Scenario and Sensitivity Analyses
3. Results
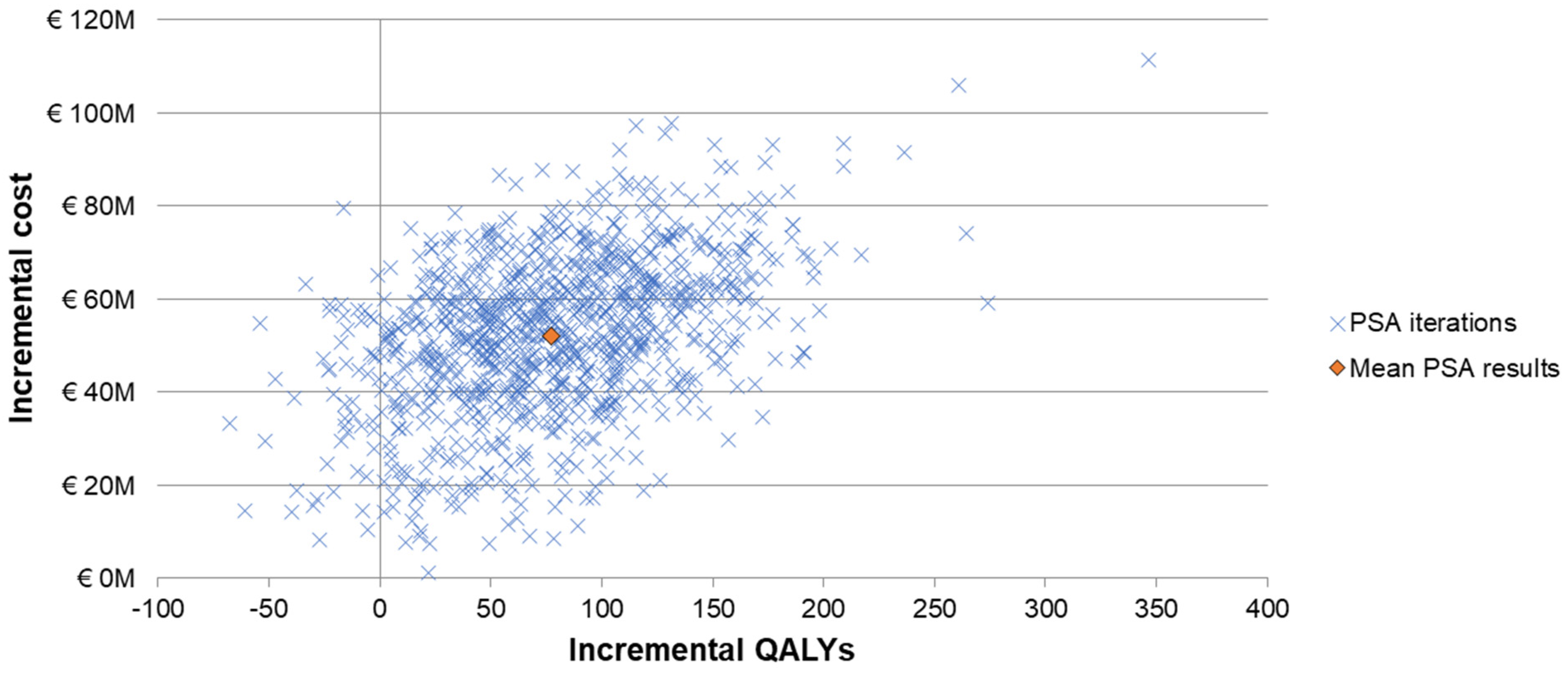
3.1. Base Case
3.2. Scenario and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldman, C.; Anderson, R. Recent advances in the epidemiology and prevention of Streptococcus pneumoniae infections. F1000Reserch 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Lower Respiratory Infections and Antimicrobial Resistance Collaborators. Global, regional, and national incidence and mortality burden of non-COVID-19 lower respiratory infections and aetiologies, 1990–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect. Dis. 2024, 9, 974–1002. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Factsheet about Pneumococcal Disease. Available online: https://www.ecdc.europa.eu/en/pneumococcal-disease/facts (accessed on 21 May 2024).
- Schiess, N.; Groce, N.E.; Dua, T. The Impact and Burden of Neurological Sequelae Following Bacterial Meningitis: A Narrative Review. Microorganisms 2021, 9, 900. [Google Scholar] [CrossRef]
- Yildirim, I.; Shea, K.M.; Pelton, S.I. Pneumococcal Disease in the Era of Pneumococcal Conjugate Vaccine. Infect. Dis. Clin. 2015, 29, 679–697. [Google Scholar] [CrossRef]
- Gierke, R.W.; Patricia, A.; Kobayashi, M. Pneumococcal Disease. Available online: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html (accessed on 6 September 2024).
- van der Linden, M.; Falkenhorst, G.; Perniciaro, S.; Imöhl, M. Effects of Infant Pneumococcal Conjugate Vaccination on Serotype Distribution in Invasive Pneumococcal Disease among Children and Adults in Germany. PLoS ONE 2015, 10, e0131494. [Google Scholar] [CrossRef]
- Noharet-Koenig, R.; Lasota, K.; Faivre, P.; Langevin, E. Evolution of Pneumococcal Vaccine Recommendations and Criteria for Decision Making in 5 Western European Countries and the United States. MDM Policy Pract. 2023, 8, 23814683231174432. [Google Scholar] [CrossRef]
- Whitney, C.G.; Goldblatt, D.; O’Brien, K.L. Dosing schedules for pneumococcal conjugate vaccine: Considerations for policy makers. Pediatr. Infect. Dis. J. 2014, 33 (Suppl. S2), S172–S181. [Google Scholar] [CrossRef]
- Weinberger, R.; von Kries, R.; van der Linden, M.; Rieck, T.; Siedler, A.; Falkenhorst, G. Invasive pneumococcal disease in children under 16 years of age: Incomplete rebound in incidence after the maximum effect of PCV13 in 2012/13 in Germany. Vaccine 2018, 36, 572–577. [Google Scholar] [CrossRef]
- European Medicines Agency. Label for Pneumococcal 15-Valent Conjugate Vaccine. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaxneuvance (accessed on 7 April 2024).
- Benfield, T.; Rämet, M.; Valentini, P.; Seppä, I.; Dagan, R.; Richmond, P.; Mercer, S.; Churchill, C.; Lupinacci, R.; McFetridge, R.; et al. Safety, tolerability, and immunogenicity of V114 pneumococcal vaccine compared with PCV13 in a 2+1 regimen in healthy infants: A phase III study (PNEU-PED-EU-2). Vaccine 2023, 41, 2456–2465. [Google Scholar] [CrossRef]
- Martinon-Torres, F.; Wysocki, J.; Szenborn, L.; Carmona-Martinez, A.; Poder, A.; Dagan, R.; Richmond, P.; Gilbert, C.; Trudel, M.C.; Flores, S.; et al. A Phase III, multicenter, randomized, double-blind, active comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of V114 compared with PCV13 in healthy infants (PNEU-PED-EU-1). Vaccine 2023, 41, 3387–3398. [Google Scholar] [CrossRef] [PubMed]
- Robert-Koch-Institut. STIKO: Stellungnahme zum Einsatz von Pneumokokken-Konjugatimpfstoffen im Säuglings-, Kindes- und Jugendalter. Available online: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2023/Ausgaben/20_23.pdf?__blob=publicationFile (accessed on 20 May 2023).
- European Medicines Agency. Label for Pneumococcal 20-Valent Conjugate Vaccine. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/prevenar-20-previously-apexxnar (accessed on 7 April 2024).
- European Medicines Agency Committee for Medicinal Products for Human Use. Assessment Report: Prevenar 20. Available online: https://www.ema.europa.eu/en/documents/variation-report/prevenar-20-previously-apexxnar-h-c-005451-ii-0012-epar-assessment-report-variation_en.pdf (accessed on 31 May 2024).
- Huang, M.; Hu, T.; Weaver, J.; Owusu-Edusei, K.; Elbasha, E. Cost-Effectiveness Analysis of Routine Use of 15-Valent Pneumococcal Conjugate Vaccine in the US Pediatric Population. Vaccines 2023, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Claes, C.; Reinert, R.R.; von der Schulenburg, J.M. Cost effectiveness analysis of heptavalent pneumococcal conjugate vaccine in Germany considering herd immunity effects. Eur. J. Health Econ. 2009, 10, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, A.; von der Schulenburg, J.G. Modeling the cost-effectiveness of infant vaccination with pneumococcal conjugate vaccines in Germany. Eur. J. Health Econ. 2017, 18, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Ta, A.; Kühne, F.; Laurenz, M.; von Eiff, C.; Warren, S.; Perdrizet, J. Cost-effectiveness of PCV20 to Prevent Pneumococcal Disease in the Pediatric Population: A German Societal Perspective Analysis. Infect. Dis. Ther. 2024, 13, 1333–1358. [Google Scholar] [CrossRef]
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). General Methods, version 7.0; Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG): Köln, Germany, 2023. [Google Scholar]
- Destatis Statistisches Bundesamt. Population: Births: Population Movement. Available online: https://www.destatis.de/EN/Themes/Society-Environment/Population/Births/Tables/birth-deaths.html (accessed on 31 May 2024).
- Destatis Statistisches Bundesamt. Population: Deaths, Life Expectancy. Available online: https://www.destatis.de/EN/Themes/Society-Environment/Population/Deaths-Life-Expectancy/_node.html (accessed on 31 May 2024).
- Deb, A.; Podmore, B.; Barnett, R.; Beier, D.; Galetzka, W.; Qizilbash, N.; Haeckl, D.; Mihm, S.; Johnson, K.D.; Weiss, T. Clinical and economic burden of pneumococcal disease among individuals aged 16 years and older in Germany. Epidemiol. Infect. 2022, 150, e204. [Google Scholar] [CrossRef]
- Weaver, J.; Hu, T.; Podmore, B.; Barnett, R.; Obermüller, D.; Galetzka, W.; Qizilbash, N.; Haeckl, D.; Weiss, T.; Mohanty, S.; et al. Incidence of pneumococcal disease in children in Germany, 2014–2019: A retrospective cohort study. 2024; Forthcoming. [Google Scholar]
- Ieven, M.; Coenen, S.; Loens, K.; Lammens, C.; Coenjaerts, F.; Vanderstraeten, A.; Henriques-Normark, B.; Crook, D.; Huygen, K.; Butler, C.C.; et al. Aetiology of lower respiratory tract infection in adults in primary care: A prospective study in 11 European countries. Clin. Microbiol. Infect. 2018, 24, 1158–1163. [Google Scholar] [CrossRef]
- Hu, T.; Podmore, B.; Barnett, R.; Beier, D.; Galetzka, W.; Qizilbash, N.; Haeckl, D.; Weaver, J.; Boellinger, T.; Mihm, S.; et al. Incidence of acute otitis media in children < 16 years old in Germany during 2014–2019. BMC Pediatr. 2022, 22, 204. [Google Scholar] [CrossRef]
- Imöhl, M.; Perniciaro, S.; Busse, A.; van der Linden, M. Bacterial Spectrum of Spontaneously Ruptured Otitis Media in a 7-Year, Longitudinal, Multicenter, Epidemiological Cross-Sectional Study in Germany. Front. Med. 2021, 8, 675225. [Google Scholar] [CrossRef]
- von Kries, R.; Siedler, A.; Schmitt, H.J.; Reinert, R.R. Proportion of invasive pneumococcal infections in German children preventable by pneumococcal conjugate vaccines. Clin. Infect. Dis. 2000, 31, 482–487. [Google Scholar] [CrossRef]
- van der Linden, M.; Itzek, A. Effects of the National Immunization Program for Pneumococcal Conjugate Vaccination (PCV7, PCV10, PCV13, PCV15) and Pneumococcal Polysaccharide Vaccination (PCV20, PPV23) on IPD in Children and Adults in Germany, Reporting period: 1 July 1992–30 June 2023, Merck & Co., Inc.: Rahway, NJ, USA, Unpublished report, Microsoft Word file.
- Laurenz, M.; von Eiff, C.; Borchert, K.; Jacob, C.; Seidel, K.; Schley, K. Vaccination rates and adherence in pneumococcal conjugate vaccination in mature born infants before and after vaccination schedule change—A claims database analysis. Vaccine 2021, 39, 3287–3295. [Google Scholar] [CrossRef] [PubMed]
- Savulescu, C.; Krizova, P.; Valentiner-Branth, P.; Ladhani, S.; Rinta-Kokko, H.; Levy, C.; Mereckiene, J.; Knol, M.; Winje, B.A.; Ciruela, P.; et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine 2022, 40, 3963–3974. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.G.; Pilishvili, T.; Farley, M.M.; Schaffner, W.; Craig, A.S.; Lynfield, R.; Nyquist, A.C.; Gershman, K.A.; Vazquez, M.; Bennett, N.M.; et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: A matched case-control study. Lancet 2006, 368, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Holtzman, C.; Harrison, L.H.; Zansky, S.M.; Rosen, J.B.; Reingold, A.; Scherzinger, K.; et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: A matched case-control study. Lancet Respir. Med. 2016, 4, 399–406. [Google Scholar] [CrossRef]
- Lewnard, J.A.; Givon-Lavi, N.; Dagan, R. Effectiveness of Pneumococcal Conjugate Vaccines Against Community-acquired Alveolar Pneumonia Attributable to Vaccine-serotype Streptococcus pneumoniae among Children. Clin. Infect. Dis. 2021, 73, e1423–e1433. [Google Scholar] [CrossRef]
- Eskola, J.; Kilpi, T.; Palmu, A.; Jokinen, J.; Haapakoski, J.; Herva, E.; Takala, A.; Käyhty, H.; Karma, P.; Kohberger, R.; et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 2001, 344, 403–409. [Google Scholar] [CrossRef]
- Pichichero, M.; Kaur, R.; Scott, D.A.; Gruber, W.C.; Trammel, J.; Almudevar, A.; Center, K.J. Effectiveness of 13-valent pneumococcal conjugate vaccination for protection against acute otitis media caused by Streptococcus pneumoniae in healthy young children: A prospective observational study. Lancet Child. Adolesc. Health 2018, 2, 561–568. [Google Scholar] [CrossRef]
- Black, S.; Shinefield, H.; Fireman, B.; Lewis, E.; Ray, P.; Hansen, J.R.; Elvin, L.; Ensor, K.M.; Hackell, J.; Siber, G.; et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr. Infect. Dis. J. 2000, 19, 187–195. [Google Scholar] [CrossRef]
- Kaur, R.; Morris, M.; Pichichero, M.E. Epidemiology of Acute Otitis Media in the Postpneumococcal Conjugate Vaccine Era. Pediatrics 2017, 140, e20170181. [Google Scholar] [CrossRef]
- Joloba, M.L.; Windau, A.; Bajaksouzian, S.; Appelbaum, P.C.; Hausdorff, W.P.; Jacobs, M.R. Pneumococcal conjugate vaccine serotypes of Streptococcus pneumoniae isolates and the antimicrobial susceptibility of such isolates in children with otitis media. Clin. Infect. Dis. 2001, 33, 1489–1494. [Google Scholar] [CrossRef]
- Yeh, S.H.; Gurtman, A.; Hurley, D.C.; Block, S.L.; Schwartz, R.H.; Patterson, S.; Jansen, K.U.; Love, J.; Gruber, W.C.; Emini, E.A.; et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics 2010, 126, e493–e505. [Google Scholar] [CrossRef] [PubMed]
- Lupinacci, R.; Rupp, R.; Wittawatmongkol, O.; Jones, J.; Quinones, J.; Ulukol, B.; Dagan, R.; Richmond, P.; Stek, J.E.; Romero, L.; et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine 2023, 41, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.J.; Waight, P.A.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: A postlicensure indirect cohort study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Treskova, M.; Scholz, S.M.; Kuhlmann, A. Cost Effectiveness of Elderly Pneumococcal Vaccination in Presence of Higher-Valent Pneumococcal Conjugate Childhood Vaccination: Systematic Literature Review with Focus on Methods and Assumptions. Pharmacoeconomics 2019, 37, 1093–1127. [Google Scholar] [CrossRef] [PubMed]
- Pletz, M.W.; Ewig, S.; Rohde, G.; Schuette, H.; Rupp, J.; Welte, T.; Suttorp, N.; Forstner, C. Impact of pneumococcal vaccination in children on serotype distribution in adult community-acquired pneumonia using the serotype-specific multiplex urinary antigen detection assay. Vaccine 2016, 34, 2342–2348. [Google Scholar] [CrossRef]
- Rubin, J.L.; McGarry, L.J.; Strutton, D.R.; Klugman, K.P.; Pelton, S.I.; Gilmore, K.E.; Weinstein, M.C. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine 2010, 28, 7634–7643. [Google Scholar] [CrossRef]
- Mangen, M.J.; Rozenbaum, M.H.; Huijts, S.M.; van Werkhoven, C.H.; Postma, D.F.; Atwood, M.; van Deursen, A.M.; van der Ende, A.; Grobbee, D.E.; Sanders, E.A.; et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur. Respir. J. 2015, 46, 1407–1416. [Google Scholar] [CrossRef]
- Szende, A.; Janssen, B.; Cabases, J. Self-Reported Population Health: An International Perspective Based on EQ-5D; Springer Nature: Dordrecht, The Nederland, 2014. [Google Scholar]
- Destatis Statistisches Bundesamt. Price: Consumer Price Index. Available online: https://www.destatis.de/EN/Themes/Economy/Prices/Consumer-Price-Index/_node.html#266062 (accessed on 31 May 2024).
- Vaccine Price: BAPO® LAUER-Taxe. Pricing Database. Available online: www.lauer-taxe-online.de (accessed on 22 March 2024).
- Hu, T.; Podmore, B.; Barnett, R.; Beier, D.; Galetzka, W.; Qizilbash, N.; Heckl, D.; Boellinger, T.; Weaver, J. Healthcare resource utilization and cost of pneumococcal disease in children in Germany, 2014–2019: A retrospective cohort study. Pneumonia 2023, 15, 7. [Google Scholar] [CrossRef]
- Li, X.; Warren, S.; Rozenbaum, M.H.; Perdrizet, J. Reanalysis of the Clinical and Economic Burden of Pneumococcal Disease Due to Serotypes Contained in Current and Investigational Pneumococcal Conjugate Vaccines in Children < 5 Age: A Societal Perspective. Infect. Dis. Ther. 2023, 12, 997–1006. [Google Scholar] [CrossRef]
- Destatis Statistisches Bundesamt. Earnings and Earnings Differences: Average Gross Earnings in April 2023. Available online: https://www.destatis.de/EN/Themes/Labour/Earnings/Earnings-Earnings-Differences/current-economic-activity.html (accessed on 31 May 2024).
- Destatis Statistisches Bundesamt. Employment: Labor Force Participation. Available online: https://www.destatis.de/EN/Themes/Labour/Labour-Market/Employment/Tables/et-etq-2021.html (accessed on 31 May 2024).
- Talbird, S.E.; Taylor, T.N.; Caporale, J.; Ismaila, A.S.; Gomez, J. Residual economic burden of Streptococcus pneumoniae- and nontypeable Haemophilus influenzae-associated disease following vaccination with PCV-7: A multicountry analysis. Vaccine 2010, 28 (Suppl. S6), G14–G22. [Google Scholar] [CrossRef]
- Ryman, J.; Sachs, J.R.; Banniettis, N.; Weiss, T.; Ahsman, M.; Yee, K.L.; Weaver, J. Potential serotype-specific effectiveness against IPD of pneumococcal conjugate vaccines V114 and PCV20 in children given a 2+1 dosing regimen. Expert. Rev. Vaccines 2024, 23, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ryman, J.; Sachs, J.R.; Yee, K.L.; Banniettis, N.; Weaver, J.; Weiss, T. Predicted serotype-specific effectiveness of pneumococcal conjugate vaccines V114 and PCV20 against invasive pneumococcal disease in children. Expert Rev. Vaccines 2024, 23, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, J.; Shao, X.; Feng, S.; Xu, X.; Zheng, B.; Liu, C.; Dai, Z.; Jiang, Q.; Gessner, B.D.; et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against community acquired pneumonia among children in China, an observational cohort study. Vaccine 2021, 39, 4620–4627. [Google Scholar] [CrossRef]
- Stoecker, C.; Hampton, L.M.; Link-Gelles, R.; Messonnier, M.L.; Zhou, F.; Moore, M.R. Cost-effectiveness of using 2 vs 3 primary doses of 13-valent pneumococcal conjugate vaccine. Pediatrics 2013, 132, e324–e332. [Google Scholar] [CrossRef] [PubMed]
- King, L. Pediatric Outpatient ARI Visits and Antibiotic Use Attributable to Serotypes in Higher Valency PCVs. In Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (CDC ACIP) Meeting Presentation Slides. 22–24 February 2023. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-22/Pneumococcal-03-King-508.pdf (accessed on 13 July 2024).
- Tang, Z.; Matanock, A.; Jeon, S.; Leidner, A.J. A review of health-related quality of life associated with pneumococcal disease: Pooled estimates by age and type of disease. J. Public Health 2022, 44, e234–e240. [Google Scholar] [CrossRef]
- Iino, H.; Hashiguchi, M.; Hori, S. Estimating the range of incremental cost-effectiveness thresholds for healthcare based on willingness to pay and GDP per capita: A systematic review. PLoS ONE 2022, 17, e0266934. [Google Scholar] [CrossRef]
- Stoecker, C. Economic Assessment of Routine PCV20 for Children. In Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (CDC ACIP) Pneumococcal Vaccines Meeting Presentations. 22 June 2023. Available online: https://stacks.cdc.gov/view/cdc/130005 (accessed on 7 April 2024).
- Centers for Disease Control and Prevention. Summary of Three Economic Analyses of the Use of 20 Valent Pneumococcal Conjugate Vaccine (PCV20) in Children in the United States. In Advisory Committee on Immunization Practices (ACIP) Meeting Pneumococcal vaccines. 22 June 2023. Available online: https://stacks.cdc.gov/view/cdc/130006/cdc_130006_DS1.pdf (accessed on 13 July 2024).
- Rozenbaum, M.H.; Huang, L.; Perdrizet, J.; Cane, A.; Arguedas, A.; Hayford, K.; Tort, M.J.; Chapman, R.; Dillon-Murphy, D.; Snow, V.; et al. Cost-effectiveness of 20-valent pneumococcal conjugate vaccine in US infants. Vaccine 2024, 42, 573–582. [Google Scholar] [CrossRef]
- Black, S.B.; Shinefield, H.R.; Ling, S.; Hansen, J.; Fireman, B.; Spring, D.; Noyes, J.; Lewis, E.; Ray, P.; Lee, J.; et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 2002, 21, 810–815. [Google Scholar] [CrossRef]
- Hansen, J.; Black, S.; Shinefield, H.; Cherian, T.; Benson, J.; Fireman, B.; Lewis, E.; Ray, P.; Lee, J. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: Updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr. Infect. Dis. J. 2006, 25, 779–781. [Google Scholar] [CrossRef]


| Parameters | Input Value | ||||||
|---|---|---|---|---|---|---|---|
| Age Group (Years) | |||||||
| 0–1 | 2–4 | 5–15 | 16–49 | 50–59 | 60–69 | 70–100 | |
| Pneumococcal disease annual incidence rates (cases per 100,000) | |||||||
| IPD a | 11.04 | 3.69 | 1.76 | 1.69 | 8.76 | 14.47 | 24.77 |
| IPD—meningitis (%) | 27.08 | 20.00 | 65.91 | 19.82 | 14.07 | 10.09 | 3.98 |
| IPD—bacteremia without a focus (%) | 60.42 | 52.00 | 15.91 | 27.93 | 34.67 | 31.65 | 29.65 |
| IPD—bacteremic pneumonia (%) | 12.50 | 28.00 | 18.18 | 52.25 | 51.26 | 58.26 | 66.37 |
| NBPP b | |||||||
| Inpatient NBPP | 109.14 | 62.67 | 13.53 | 10.25 | 33.63 | 84.13 | 290.05 |
| Outpatient NBPP | 221.02 | 296.16 | 82.15 | 36.92 | 60.78 | 69.74 | 102.99 |
| Pneumococcal AOM c | |||||||
| Simple AOM | 950.27 | 1115.92 | 272.88 | NA | NA | NA | NA |
| Recurrent AOM | 200.80 | 252.67 | 26.36 | NA | NA | NA | NA |
| AOM with TT placement | 181.04 | 414.28 | 165.18 | NA | NA | NA | NA |
| Case fatality rates | |||||||
| Meningitis d | 0.0980 | 0.0980 | 0.0980 | 0.0874 | 0.1316 | 0.1250 | 0.2229 |
| Bacteremia without a focus/bacteremic pneumonia d | 0.0200 | 0.0200 | 0.0200 | 0.0874 | 0.1316 | 0.1250 | 0.2229 |
| Inpatient NBPP e | 0.0022 | 0.0013 | 0.0038 | 0.0442 | 0.1095 | 0.1410 | 0.2036 |
| PCV20 Non-V114 Serotypes (%) | Age Group (Years) | ||||||
|---|---|---|---|---|---|---|---|
| 0–1 | 2–4 | 5–15 | 16–49 | 50–59 | 60–69 | 70–100 | |
| IPD a | 14.9 | 4.5 | 10.6 | 8.1 | 6.6 | 7.6 | 7.6 |
| NBPP b | 14.9 | 4.5 | 10.6 | 8.1 | 6.6 | 7.6 | 7.6 |
| Pneumococcal AOM c | 9.8 | 9.8 | 9.8 | NA | NA | NA | NA |
| Disease | Vaccine Product | Serotype-Specific VE | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPS | Serotype | PCV13 | V114-non-PCV13 | PCV20-non-V114 | |||||||||||||||||
| 1 | 3 | 4 | 5 | 6A | 6B | 7F | 9V | 14 | 18C | 19A | 19F | 23F | 22F | 33F | 8 | 10A | 11A | 12F | 15B | ||
| IPD a | V114 | 85.1 | 64.5 | 93.0 | 79.0 | 92.7 | 92.7 | 91.4 | 97.3 | 96.8 | 93.0 | 83.2 | 84.4 | 93.0 | 84.2 | 84.2 | |||||
| PCV20 b | 65.3 | 40.0 | 69.8 | 87.0 | 86.0 | 94.0 | 97.0 | 75.0 | 94.0 | 97.0 | 86.0 | 87.0 | 73.5 | 86.0 | 86.0 | 86.0 | 86.0 | 86.0 | 43.0 | 86.0 | |
| NBPP c | V114 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | |||||
| PCV20 b | 57.8 | 38.5 | 57.8 | 77.0 | 77.0 | 77.0 | 77.0 | 57.8 | 77.0 | 77.0 | 77.0 | 77.0 | 57.8 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 38.5 | 77.0 | |
| Pneumococcal AOM (simple or recurrent) d | V114 | 86.0 | 15.0 | 57.0 | 86.0 | 100.0 | 57.0 | 86.0 | 57.0 | 57.0 | 57.0 | 91.0 | 57.0 | 57.0 | 57.0 | 57.0 | |||||
| PCV20 b | 64.5 | 7.5 | 42.8 | 86.0 | 100.0 | 57.0 | 86.0 | 42.8 | 57.0 | 57.0 | 91.0 | 57.0 | 42.8 | 57.0 | 57.0 | 57.0 | 57.0 | 57.0 | 28.5 | 57.0 | |
| Pneumococcal AOM (with TT placement) e | V114 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | |||||
| PCV20 b | 51.6 | 34.4 | 51.6 | 68.8 | 68.8 | 68.8 | 68.8 | 51.6 | 68.8 | 68.8 | 68.8 | 68.8 | 51.6 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 34.4 | 68.8 | |
| PTD | Serotype | PCV13 | V114-non-PCV13 | PCV20-non-V114 | |||||||||||||||||
| 1 | 3 | 4 | 5 | 6A | 6B | 7F | 9V | 14 | 18C | 19A | 19F | 23F | 22F | 33F | 8 | 10A | 11A | 12F | 15B | ||
| IPD a,f | V114 | 87.0 | 80.0 | 96.1 | 87.0 | 95.9 | 95.9 | 97.0 | 100.0 | 97.6 | 97.0 | 92.0 | 92.3 | 98.0 | 86.0 | 86.0 | |||||
| PCV20 | 87.0 | 80.0 | 96.1 | 87.0 | 95.9 | 95.9 | 97.0 | 100.0 | 97.6 | 97.0 | 92.0 | 92.3 | 98.0 | 86.0 | 86.0 | 86.0 | 86.0 | 86.0 | 86.0 | 86.0 | |
| NBPP c | V114 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | |||||
| PCV20 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | 77.0 | |
| Pneumococcal AOM(Simple or recurrent AOM) d | V114 | 86.0 | 15.0 | 57.0 | 86.0 | 100.0 | 57.0 | 86.0 | 57.0 | 57.0 | 57.0 | 91.0 | 57.0 | 57.0 | 57.0 | 57.0 | |||||
| PCV20 | 86.0 | 15.0 | 57.0 | 86.0 | 100.0 | 57.0 | 86.0 | 57.0 | 57.0 | 57.0 | 91.0 | 57.0 | 57.0 | 57.0 | 57.0 | 57.0 | 57.0 | 57.0 | 57.0 | 57.0 | |
| Pneumococcal AOM(with TT placement) e | V114 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | |||||
| PCV20 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | 68.8 | |
| Parameters | Input Value | ||||||
|---|---|---|---|---|---|---|---|
| Age Group (Years) | |||||||
| 0–24 | 25–34 | 35–45 | 45–54 | 55–64 | 65–74 | 75–100 | |
| Health state utility values | |||||||
| Baseline (general population without pneumococcal disease or PMS) a | 0.972 | 0.973 | 0.966 | 0.945 | 0.922 | 0.891 | 0.839 |
| PMS, neurological deficits b | 0.68 | 0.68 | 0.68 | 0.68 | 0.68 | 0.68 | 0.68 |
| PMS, hearing loss b | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 |
| 0–15 | 16–100 | ||||||
| QALY decrements related to each pneumococcal disease episode c | |||||||
| Meningitis | 0.023 | 0.071 | |||||
| Bacteremia without a focus | 0.008 | 0.071 | |||||
| Bacteremic pneumonia | 0.008 | 0.071 | |||||
| Inpatient NBPP | 0.006 | 0.071 | |||||
| Outpatient NBPP | 0.004 | 0.005 | |||||
| Simple pneumococcal AOM | 0.005 | NA | |||||
| Recurrent pneumococcal AOM | 0.005 | NA | |||||
| AOM with TT placement | 0.005 | NA | |||||
| Parameters | Input Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine Costs (in 2023 EUR) | ||||||||||
| Vaccine acquisition costs (per dose) a | ||||||||||
| V114 | 59.80 | |||||||||
| PCV20 | 66.95 | |||||||||
| Vaccine administration costs (per dose) b | ||||||||||
| V114 | 8.00 | |||||||||
| PCV20 | 8.00 | |||||||||
| Age group (years) | ||||||||||
| 0–15 | 16–100 | |||||||||
| Costs per pneumococcal disease episode (in 2023 EUR) | ||||||||||
| Direct medical costs c | ||||||||||
| Meningitis | 6464 | 8584 | ||||||||
| Bacteremia without focus | 6464 | 8584 | ||||||||
| Bacteremic pneumonia | 6464 | 8584 | ||||||||
| Inpatient NBPP | 2841 | 6614 | ||||||||
| Outpatient NBPP | 71 | 58 | ||||||||
| Simple pneumococcal AOM | 61 | NA | ||||||||
| Recurrent pneumococcal AOM | 69 | NA | ||||||||
| AOM with TT placement | 443 | NA | ||||||||
| Age group (years) | ||||||||||
| 0–4 | 5–15 | 15–20 | 20–25 | 25–30 | 30–40 | 40–55 | 55–60 | 60–65 | 65–100 | |
| Indirect costs d | ||||||||||
| Meningitis | 1875 | 1875 | 527 | 1316 | 1543 | 1585 | 1628 | 1558 | 1226 | 169 |
| Bacteremia without a focus | 919 | 919 | 258 | 645 | 756 | 777 | 798 | 764 | 601 | 83 |
| Bacteremic pneumonia | 919 | 919 | 258 | 645 | 756 | 777 | 798 | 764 | 601 | 83 |
| Inpatient NBPP | 1221 | 1221 | 343 | 857 | 1005 | 1033 | 1061 | 1015 | 799 | 110 |
| Outpatient NBPP | 473 | 473 | 133 | 332 | 389 | 400 | 411 | 393 | 309 | 43 |
| Simple pneumococcal AOM | 473 | 473 | NA | NA | NA | NA | NA | NA | NA | NA |
| Recurrent pneumococcal AOM | 473 | 473 | NA | NA | NA | NA | NA | NA | NA | NA |
| AOM with TT placement | 473 | 473 | NA | NA | NA | NA | NA | NA | NA | NA |
| Annual direct costs of PMS(in 2023 EUR) | ||||||||||
| Direct medical and non-medical costs of PMS e | ||||||||||
| PMS, neurological deficits | 1694 | 1694 | 1694 | 1694 | 1694 | 1694 | 1694 | 1694 | 1694 | 1694 |
| PMS, hearing loss | 2974 | 2974 | 2974 | 2974 | 2974 | 2974 | 2974 | 2974 | 2974 | 2974 |
| Average annual earnings f | NA | NA | 14,577 | 36,417 | 42,694 | 43,861 | 45,046 | 43,109 | 33,927 | 4669 |
| Outcomes | PCV20 (3 + 1 Schedule) | V114 (2 + 1 Schedule) | Incremental Outcomes (PCV20 versus V114) |
|---|---|---|---|
| Cost outcomes (in 2023 EUR, discounted) | |||
| Vaccine acquisition costs | 160,757,092 | 111,144,118 | 49,612,973 |
| Vaccine administration costs | 19,209,212 | 14,868,778 | 4,340,433 |
| IPD treatment costs | 6,984,679 | 7,232,406 | −247,727 |
| NBPP treatment costs | 41,650,604 | 42,032,280 | −381,676 |
| Pneumococcal AOM treatment costs | 9,059,576 | 9,283,841 | −224,266 |
| PMS treatment costs | 1,370,853 | 1,496,730 | −125,876 |
| Indirect costs | 55,157,026 | 57,861,839 | −2,704,813 |
| Costs of premature death | 131,785,723 | 133,696,348 | −1,910,625 |
| Total Costs | 425,974,765 | 377,616,341 | 48,358,424 |
| Clinical outcomes (undiscounted) | |||
| IPD cases | 4659 | 4745 | −86 |
| NBPP cases | 82,521 | 83,194 | −672 |
| Pneumococcal AOM cases | 75,888 | 78,107 | −2219 |
| PMS cases | 65 | 68 | −3 |
| IPD deaths | 790 | 800 | −10 |
| NBPP deaths | 7064 | 7065 | 0 |
| QALYs and life years (discounted) | |||
| Total QALYs | 21,556,792 | 21,556,696 | 96 |
| Total life years | 22,448,853 | 22,448,779 | 75 |
| ICERs | |||
| Cost per QALY gained | 503,620 | ||
| Cost per life year gained | 648,546 | ||
| Scenario | Assumptions or Inputs in the Base Case | Assumptions or Inputs in the Scenario Analysis | Incremental Costs (in 2023 EUR) | Incremental QALYs Gained | ICER for PCV20 vs. V114 (Cost per QALY Gained, in 2023 EUR) |
|---|---|---|---|---|---|
| 1. Time horizon | Lifetime | 10 years | 52,041,409 | 19 | 2,672,457 |
| 2. Model perspective | Societal perspective (including vaccine acquisition and administration costs, direct medical costs, direct non-medical costs, and indirect costs) | Healthcare sector perspective (only including vaccine acquisition and administration costs and direct medical costs) | 52,973,862 | 96 | 551,687 |
| 3. Target population | A single birth cohort | Entire German population | 1,278,538,860 | 6986 | 183,006 |
| 4. Target population and time horizon | Target population: a single birth cohort Time horizon: lifetime | Target population: entire German population Time horizon: 10 years | 429,468,264 | 528 | 813,056 |
| 5. IPD serotype distribution | Use of most recent German data (2022–2023) from van der Linden et al., 2024 [31] | Use of data from prior to the COVID-19 pandemic (2018–2019) from van der Linden et al., 2024 [31] | 41,828,610 | 199 | 210,403 |
| 6. Proportion of pneumonia and AOM cases attributable to S. pneumoniae | Inputs from studies conducted in Germany | Inputs based on recent US data presented at the CDC ACIP meetings by King et al. [61] a | 46,926,936 | 110 | 424,805 |
| 7. PCV20 VE reduction | Assuming reductions in PPS VE for the six serotypes that did not meet the statistical non-inferiority criterion in the PCV20 (3 + 1) clinical trial (serotypes 1, 3, 4, 9V, 12F, and 23F) in all pneumococcal disease categories | No VE reductions assumed b | 46,978,220 | 114 | 412,159 |
| 8. IPD and NBPP VE | VE against IPD: VE of V114 was derived from an observational study of PCV13 (2 + 1); VE of PCV20 was derived from PCV7 (3 + 1) and PCV13 (3 + 1) case–control studies VE against NBPP: For both V114 and PCV20, VE was obtained from a surveillance study conducted in southern Israel by Lewnard et al., 2021 [36] | VE against IPD: For both V114 and PCV20, VE was obtained from modeling predictions for V114 (2 + 1) and PCV20 (3 + 1) by Ryman et al., 2024 [57,58] VE against NBPP: For both V114 and PCV20, VE was obtained from an observational cohort study conducted in China by Zhang et al., 2021 [59] | 49,749,027 | 82 | 606,835 |
| 9. Waning of vaccine effects | Full VE for first five years after vaccination followed by a linear reduction to 0% over the next five years | Full VE for the first 10 years after vaccination and no VE thereafter | 48,293,570 | 97 | 499,005 |
| 10. Indirect effects | Herd protection effects and serotype replacement from a study conducted in Germany by van der Linden et al., 2015 [8] c | Herd protection effects and no serotype replacement based on a CEA study conducted in the US by Stoecker et al., 2013 [60] c | 46,729,650 | 158 | 295,248 |
| 11. Re-emergence of IPD with PCV20 (3 + 1) | No re-emergence of IPD caused by serotypes included in PCV13 | Re-emergence of IPD caused by serotypes shared between PCV13 and PCV20 for which PCV20 was assumed to have reduced VE (serotypes 1, 4, 9V, and 23F) d | 78,477,554 | −765 | PCV20 is dominated by V114 |
| 12. Utility inputs | Inputs for QALY decrements for pneumococcal disease were sourced from Rubin et al., 2010 [47] and Mangen et al., 2015 [48] | Inputs for QALY decrements for pneumococcal disease were sourced from a pooled analysis performed by Tang et al., 2022 [62] | 48,358,424 | 93 | 518,169 |
| 13. Vaccine coverage rate | Different vaccine coverage rates for 3 + 1 and 2 + 1 schedules were applied, based on a German study conducted by Laurenz et al., 2021 [32] | Vaccine coverage rates for PCV20 (3 + 1) were assumed to be the same as those for V114 (2 + 1) | 55,762,365 | 112 | 500,069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Weaver, J.P.; Elbasha, E.; Weiss, T.; Banniettis, N.; Feemster, K.; White, M.; Kelly, M.S. Cost-Effectiveness Analysis of Routine Childhood Immunization with 20-Valent versus 15-Valent Pneumococcal Conjugate Vaccines in Germany. Vaccines 2024, 12, 1045. https://doi.org/10.3390/vaccines12091045
Huang M, Weaver JP, Elbasha E, Weiss T, Banniettis N, Feemster K, White M, Kelly MS. Cost-Effectiveness Analysis of Routine Childhood Immunization with 20-Valent versus 15-Valent Pneumococcal Conjugate Vaccines in Germany. Vaccines. 2024; 12(9):1045. https://doi.org/10.3390/vaccines12091045
Chicago/Turabian StyleHuang, Min, Jessica P. Weaver, Elamin Elbasha, Thomas Weiss, Natalie Banniettis, Kristen Feemster, Meghan White, and Matthew S. Kelly. 2024. "Cost-Effectiveness Analysis of Routine Childhood Immunization with 20-Valent versus 15-Valent Pneumococcal Conjugate Vaccines in Germany" Vaccines 12, no. 9: 1045. https://doi.org/10.3390/vaccines12091045
APA StyleHuang, M., Weaver, J. P., Elbasha, E., Weiss, T., Banniettis, N., Feemster, K., White, M., & Kelly, M. S. (2024). Cost-Effectiveness Analysis of Routine Childhood Immunization with 20-Valent versus 15-Valent Pneumococcal Conjugate Vaccines in Germany. Vaccines, 12(9), 1045. https://doi.org/10.3390/vaccines12091045






