Evaluation of Vaccine Strategies among Healthcare Workers during COVID-19 Omicron Outbreak in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
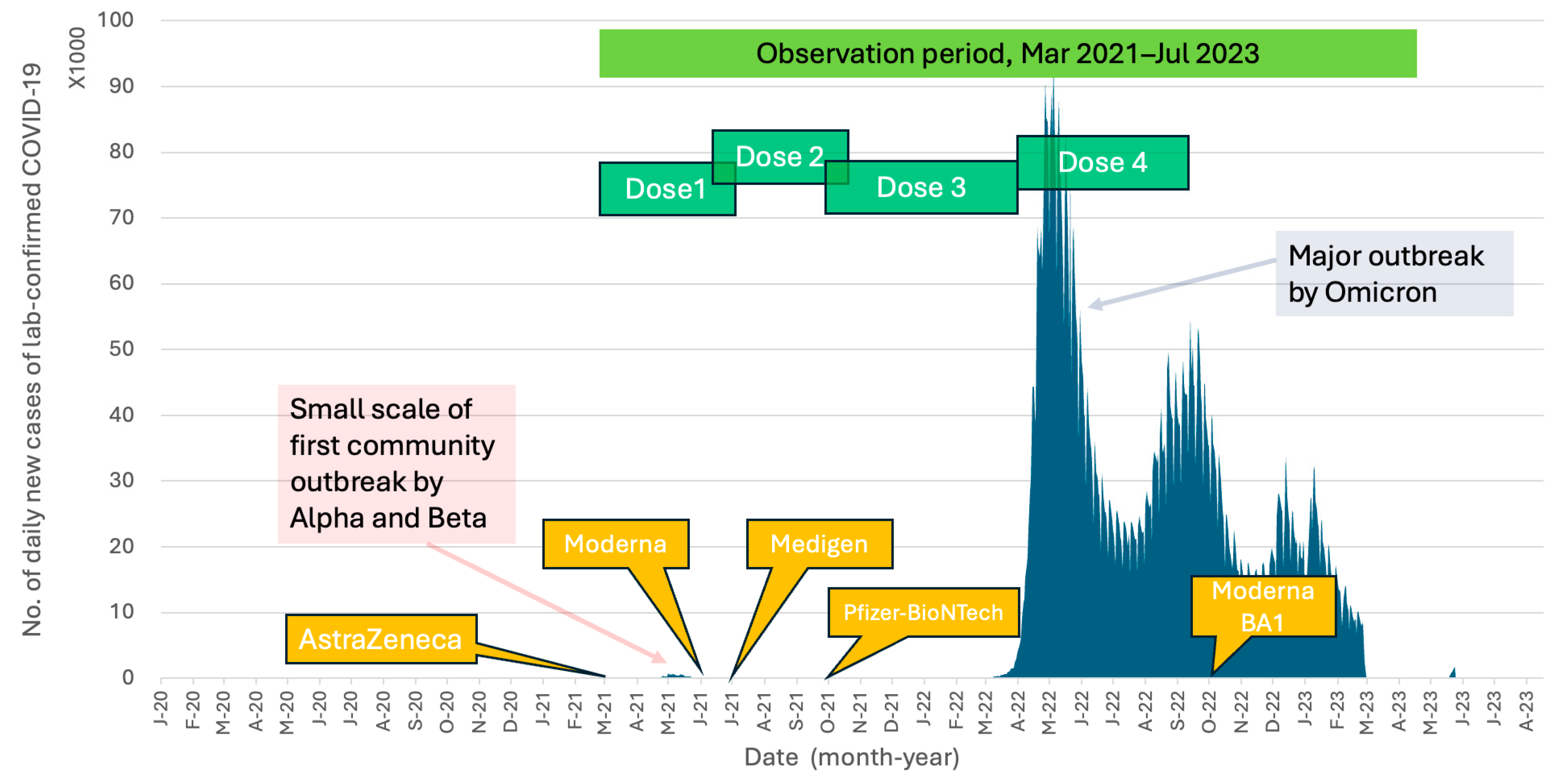
2.2. Study Design
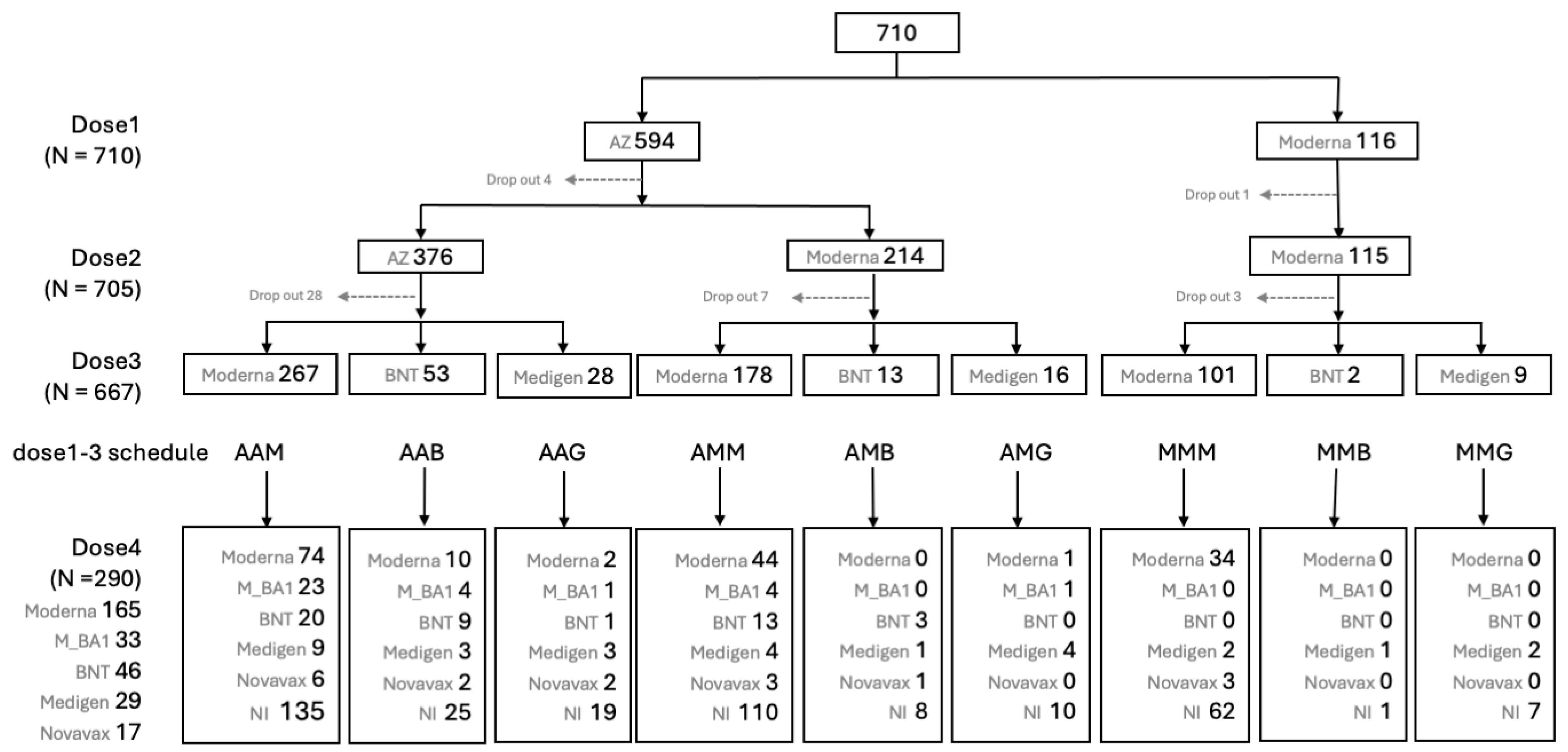
2.3. Participants
2.4. Immunizations, Blood Samplings and Monitoring of Adverse Events
2.5. Measurement of Neutralizing Antibody (nAb) and Anti-N Antibody to SARS-CoV-2
2.6. Statistical Analysis
3. Results
3.1. Reactogenicity of SARS-CoV-2 Vaccines of Different Platforms
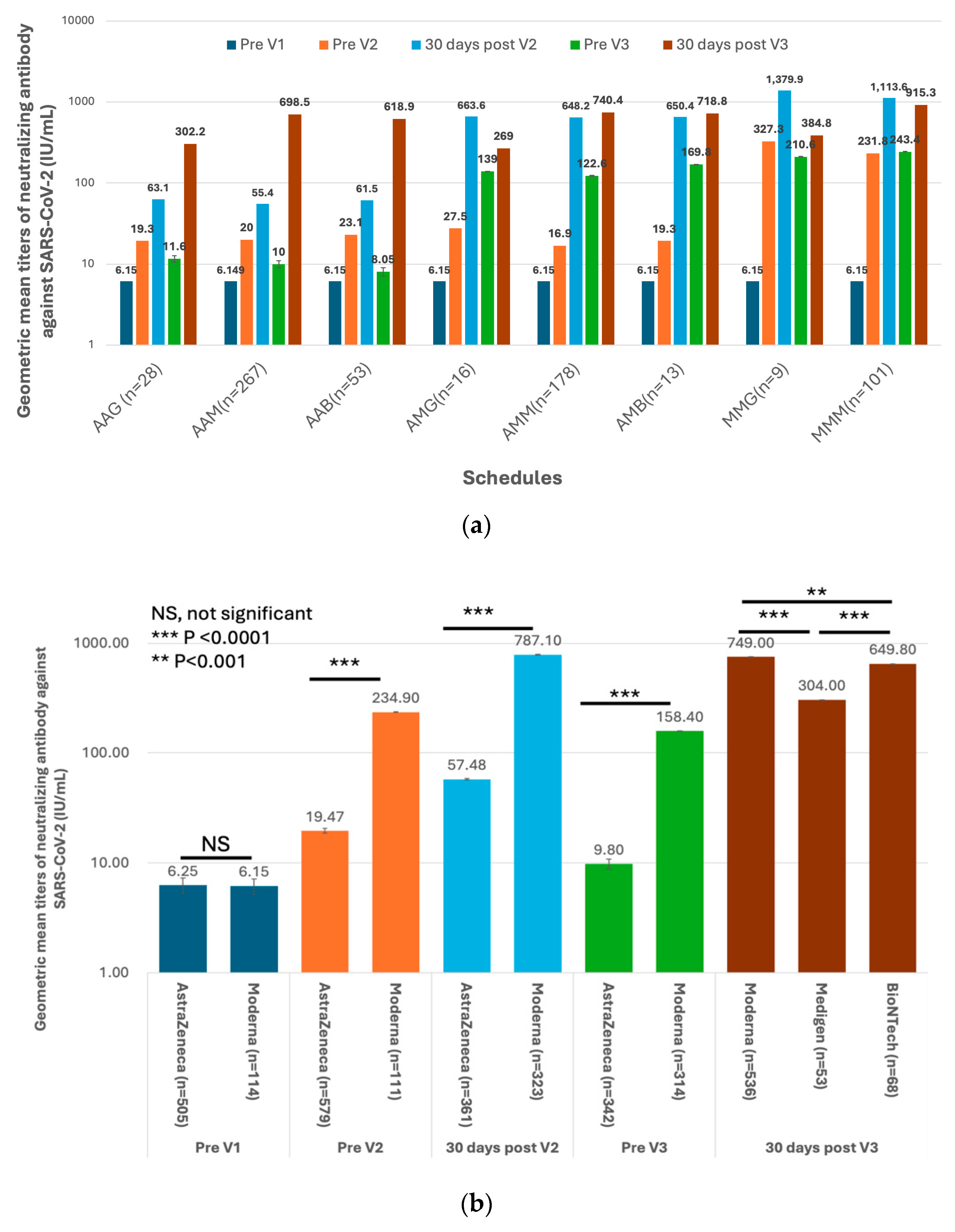
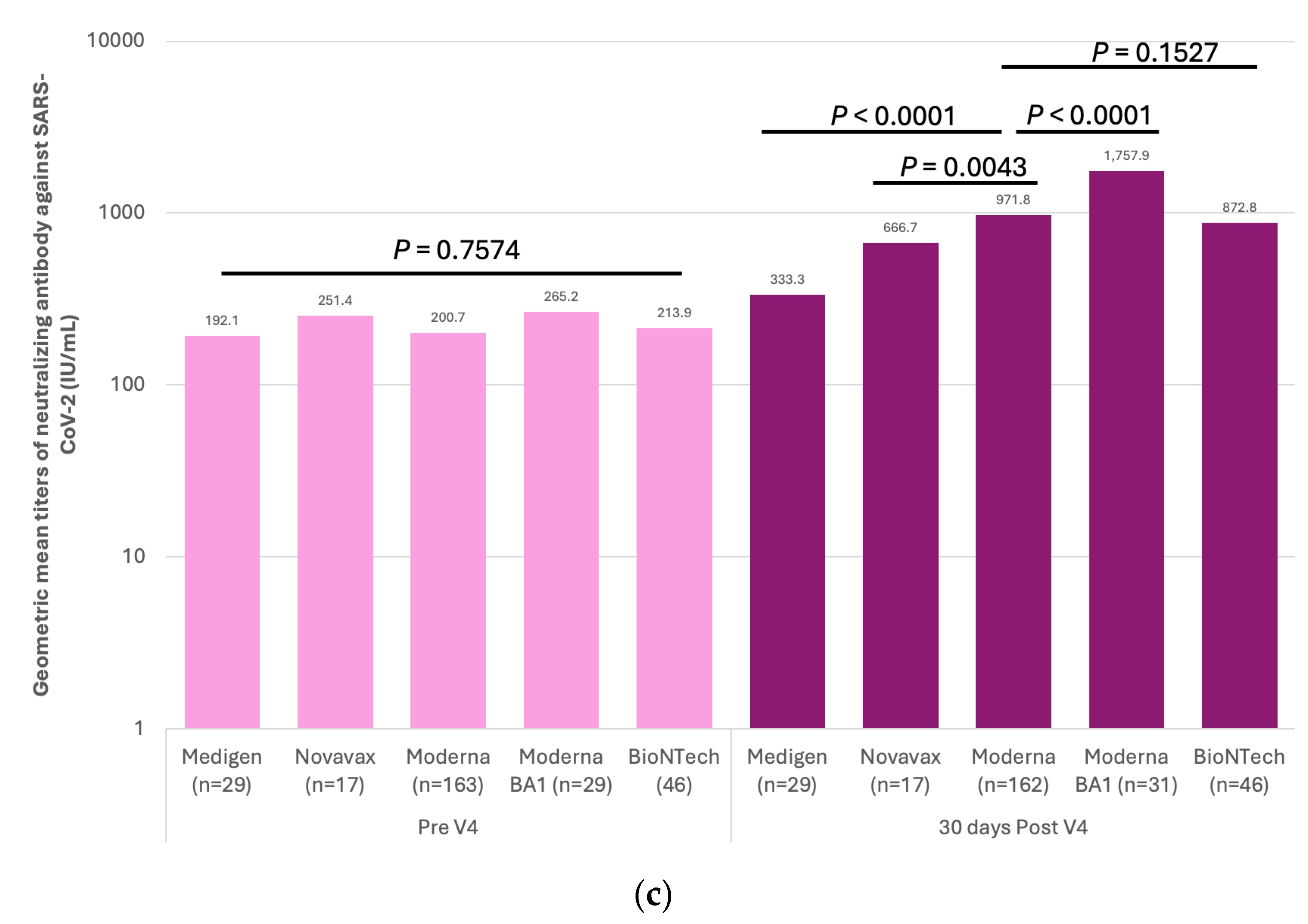
3.2. Immunogenicity of SARS-CoV-2 Vaccines and Dynamics of Vaccine-Induced Neutralizing Antibody (nAb) against SARS-CoV-2
3.3. Factors Associated with the GMTs of Vaccine-Evoked nAb against SARS-CoV2
3.4. Effect of SARS-CoV-2 Vaccine Schedules against COVID-19 during the Omicron Epidemic
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gebru, A.A.; Birhanu, T.; Wendimu, E.; Ayalew, A.F.; Mulat, S.; Abasimel, H.Z.; Kazemi, A.; Tadesse, B.A.; Gebru, B.A.; Deriba, B.S.; et al. Global Burden of COVID-19: Situational Analyis and Review. Hum. Antibodies 2021, 29, 139–148. [Google Scholar] [CrossRef] [PubMed]
- WHO. Solidarity Trial Vaccines. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-trial-of-covid-19-vaccines (accessed on 29 January 2022).
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and Immunogenicity of ChAdOx1 NCoV-19 Vaccine Administered in a Prime-Boost Regimen in Young and Old Adults (COV002): A Single-Blind, Randomised, Controlled, Phase 2/3 Trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-M.; Liu, M.-C.; Chen, Y.-H.; Lee, W.-S.; Hwang, S.-J.; Cheng, S.-H.; Ko, W.-C.; Hwang, K.-P.; Wang, N.-C.; Lee, Y.-L.; et al. Safety and Immunogenicity of CpG 1018 and Aluminium Hydroxide-Adjuvanted SARS-CoV-2 S-2P Protein Vaccine MVC-COV1901: Interim Results of a Large-Scale, Double-Blind, Randomised, Placebo-Controlled Phase 2 Trial in Taiwan. Lancet Respir. Med. 2021, 9, 1396–1406. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by COVID-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef]
- Lyke, K.E.; Atmar, R.L.; Islas, C.D.; Posavad, C.M.; Szydlo, D.; Chourdhury, R.P.; Deming, M.E.; Eaton, A.; Jackson, L.A.; Branche, A.R.; et al. Rapid Decline in Vaccine-Boosted Neutralizing Antibodies against SARS-CoV-2 Omicron Variant. Cell Rep. Med. 2022, 3, 100679. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The Emergence and Epidemic Characteristics of the Highly Mutated SARS-CoV-2 Omicron Variant. J. Méd. Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef]
- Lai, C.-C.; Ko, W.-C.; Chen, C.-J.; Chen, P.-Y.; Huang, Y.-C.; Lee, P.-I.; Hsueh, P.-R. COVID-19 Vaccines and Thrombosis with Thrombocytopenia Syndrome. Expert Rev. Vaccines 2021, 20, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Paterlini, M. COVID-19: Sweden, Norway, and Finland Suspend Use of Moderna Vaccine in Young People “as a Precaution”. BMJ 2021, 375, n2477. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D. Omicron’s Molecular Structure Could Help Explain Its Global Takeover. Nature 2022, 602, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Mannar, D.; Saville, J.W.; Zhu, X.; Srivastava, S.S.; Berezuk, A.M.; Tuttle, K.S.; Marquez, A.C.; Sekirov, I.; Subramaniam, S. SARS-CoV-2 Omicron Variant: Antibody Evasion and Cryo-EM Structure of Spike Protein–ACE2 Complex. Science 2022, 375, 760–764. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.-G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2021, 386, 494–496. [Google Scholar] [CrossRef]
- Chen, C.-J.; Yang, L.-Y.; Chang, W.-Y.; Huang, Y.-C.; Chiu, C.-H.; Shih, S.-R.; Huang, C.-G.; Huang, K.-Y.A. A Randomized Controlled Trial of Heterologous ChAdOx1 NCoV-19 and Recombinant Subunit Vaccine MVC-COV1901 against COVID-19. Nat. Commun. 2022, 13, 5466. [Google Scholar] [CrossRef]
- Wall, E.C.; Wu, M.; Harvey, R.; Kelly, G.; Warchal, S.; Sawyer, C.; Daniels, R.; Hobson, P.; Hatipoglu, E.; Ngai, Y.; et al. Neutralising Antibody Activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 Vaccination. Lancet 2021, 397, 2331–2333. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; Sahly, H.M.E.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and Immunogenicity of Seven COVID-19 Vaccines as a Third Dose (Booster) Following Two Doses of ChAdOx1 NCov-19 or BNT162b2 in the UK (COV-BOOST): A Blinded, Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca Vaccines on COVID-19 Related Symptoms, Hospital Admissions, and Mortality in Older Adults in England: Test Negative Case-Control Study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e15. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]


| Parameter | 30 Days after Second Immunization | 30 Days after Third Immunization | ||||
|---|---|---|---|---|---|---|
| Estimate | Standard Error | p Value | Estimate | Standard Error | p Value | |
| Dose 1–3 Vaccination Schedule $, vs. MMM | ||||||
| AAB | −3.0117 | 0.3166 | <0.0001 | −0.4251 | 0.0822 | <0.0001 |
| AAG | −2.9765 | 0.2779 | <0.0001 | −1.1476 | 0.0981 | <0.0001 |
| AAM | −3.0824 | 0.1861 | <0.0001 | −0.2947 | 0.0656 | <0.0001 |
| AMB | −0.7316 | 0.4072 | 0.0729 | −0.2889 | 0.1417 | 0.0028 |
| AMG | −0.6025 | 0.3893 | 0.1222 | −1.2444 | 0.1348 | <0.0001 |
| AMM | −0.6973 | 0.2803 | 0.0131 | −0.2472 | 0.0977 | <0.0001 |
| MMG | 0.15169 | 0.3835 | 0.6926 | −0.9047 | 0.1333 | <0.0001 |
| Interval since V1 | 0.00404 | 0.0057 | 0.4793 | 0.0012 | 0.0008 | 0.1383 |
| Gender female vs. male | 0.28022 | 0.0947 | 0.0032 | 0.0620 | 0.0331 | 0.0615 |
| Age, in years | −0.0077 | 0.0038 | 0.0452 | −0.0011 | 0.0013 | 0.4196 |
| Parameter | Negative for COVID-19 N= 318 | Positive for COVID-19 N = 299 | Hazard Ratio (HR) | 95% HR Confidence Limits | p |
|---|---|---|---|---|---|
| Female gender (%) | 220 (69.2) | 218 (72.9) | 1.160 | 0.893–1.507 | 0.2674 |
| Age, years, mean ± standard deviation | 41.5 ± 11.3 | 42.7 ± 11.5 | 1.004 | 0.994–1.015 | 0.4464 |
| Dose 4, vs. not immunized | |||||
| Not immunized | 157 (49.4) | 205 (68.6) | … | … | … |
| Medigen | 20 (6.29) | 9 (3.01) | 0.307 | 0.153–0.614 | 0.0008 |
| Moderna | 98 (30.8) | 55 (18.4) | 0.372 | 0.275–0.505 | <0.0001 |
| Moderna_BA1 | 9 (2.83) | 6 (2.01) | 0.191 | 0.077–0.473 | 0.0003 |
| Novavax | 8 (2.52) | 5 (1.67) | 0.447 | 0.182–1.100 | 0.0796 |
| Pfizer-BioNTech | 26 (8.18) | 19 (6.35) | 0.409 | 0.250–0.668 | 0.0004 |
| $ Dose 1–3, vs. MMM | |||||
| MMM | 56 (17.6) | 43 (14.4) | … | … | … |
| AAB | 25 (7.86) | 23 (7.69) | 1.407 | 0.830–2.387 | 0.2051 |
| AAG | 11 (3.46) | 16 (5.35) | 1.386 | 0.772–2.486 | 0.2740 |
| AAM | 117 (36.8) | 117 (39.1) | 1.362 | 0.950–1.953 | 0.0924 |
| AMB | 6 (1.89) | 7 (2.34) | 1.497 | 0.659–3.402 | 0.3350 |
| AMG | 8 (2.52) | 8 (2.68) | 1.408 | 0.649–3.052 | 0.3863 |
| AMM | 88 (27.7) | 80 (26.8) | 1.262 | 0.863–1.845 | 0.2304 |
| MMB | 0 (0) | 2 (0.67) | 2.596 | 0.612–11.016 | 0.1957 |
| MMG | 6 (1.89) | 3 (1.00) | 1.160 | 0.356–3.788 | 0.8052 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-R.; Huang, C.-G.; Chiu, C.-H.; Chen, C.-J. Evaluation of Vaccine Strategies among Healthcare Workers during COVID-19 Omicron Outbreak in Taiwan. Vaccines 2024, 12, 1057. https://doi.org/10.3390/vaccines12091057
Lin M-R, Huang C-G, Chiu C-H, Chen C-J. Evaluation of Vaccine Strategies among Healthcare Workers during COVID-19 Omicron Outbreak in Taiwan. Vaccines. 2024; 12(9):1057. https://doi.org/10.3390/vaccines12091057
Chicago/Turabian StyleLin, Min-Ru, Chung-Guei Huang, Cheng-Hsun Chiu, and Chih-Jung Chen. 2024. "Evaluation of Vaccine Strategies among Healthcare Workers during COVID-19 Omicron Outbreak in Taiwan" Vaccines 12, no. 9: 1057. https://doi.org/10.3390/vaccines12091057






