Adjuvant Use of the Invariant-Natural-Killer-T-Cell Agonist α-Galactosylceramide Leads to Vaccine-Associated Enhanced Respiratory Disease in Influenza-Vaccinated Pigs
Abstract
1. Introduction
2. Material and Methods
2.1. Pigs
2.2. Viruses
2.3. Virus Titration
2.4. Experimental Design
2.5. Tissue Processing for Single-Cell Isolation
2.6. Flow Cytometry and Antibodies
2.7. Cytokine Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Next-Generation Sequencing
2.9. Epitope Mapping of Nonsynonymous Mutations
2.10. IFN-γ ELISpot Assay
2.11. Antibody Detection and Quantification
2.12. Anti-HA2 ELISA
2.13. Pathology and Histopathology
2.14. Statistical Analysis
3. Results
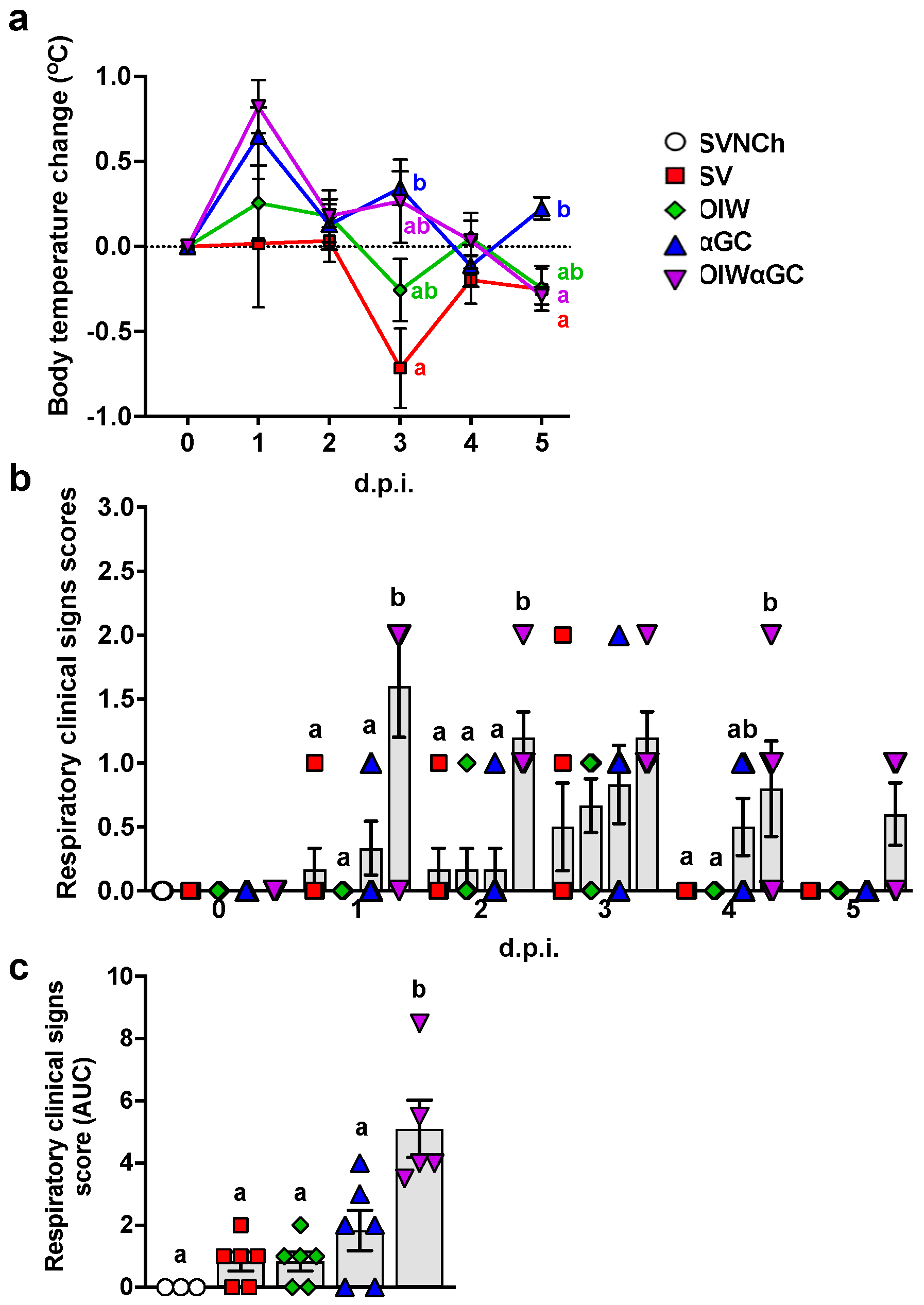
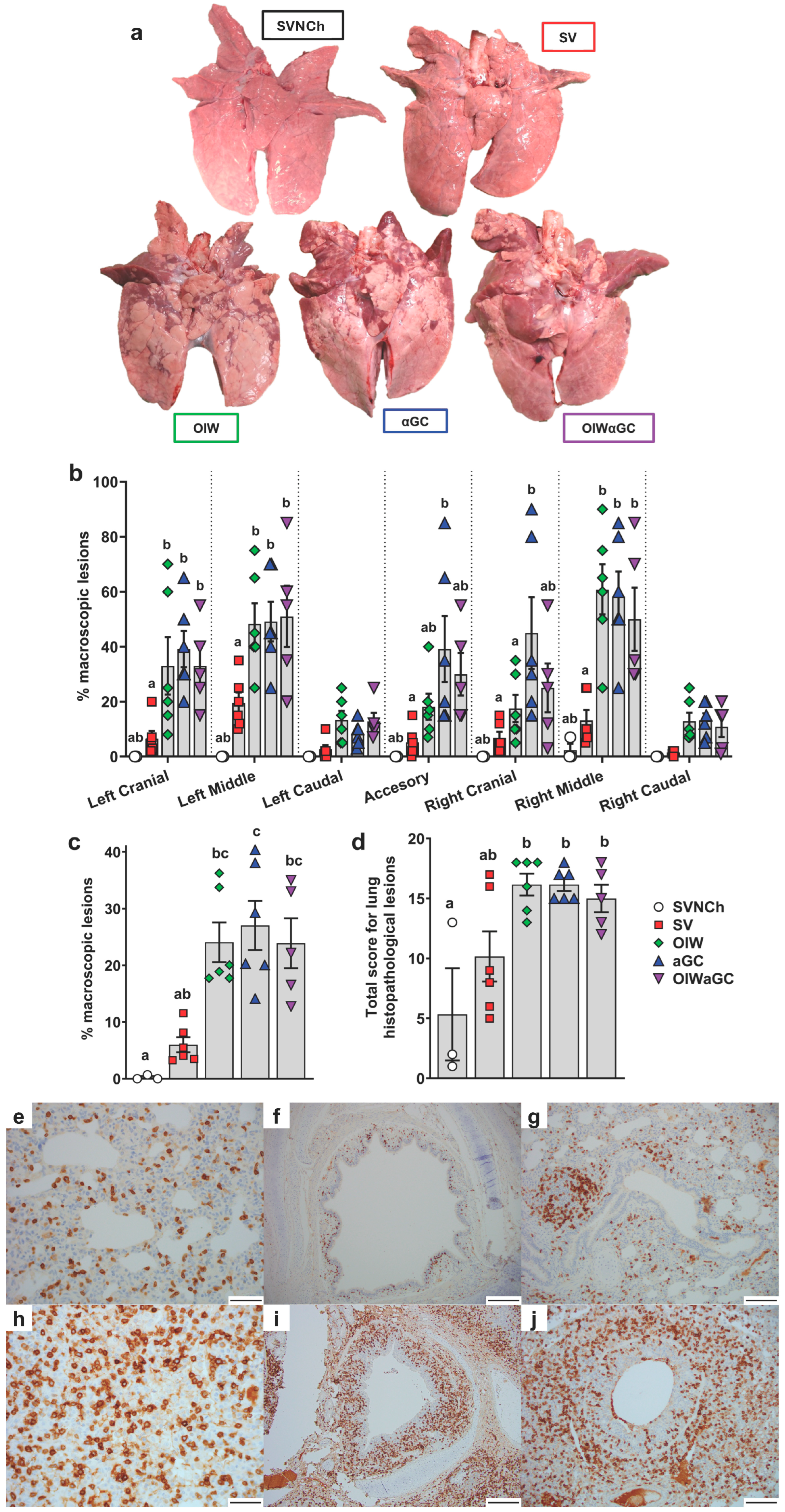
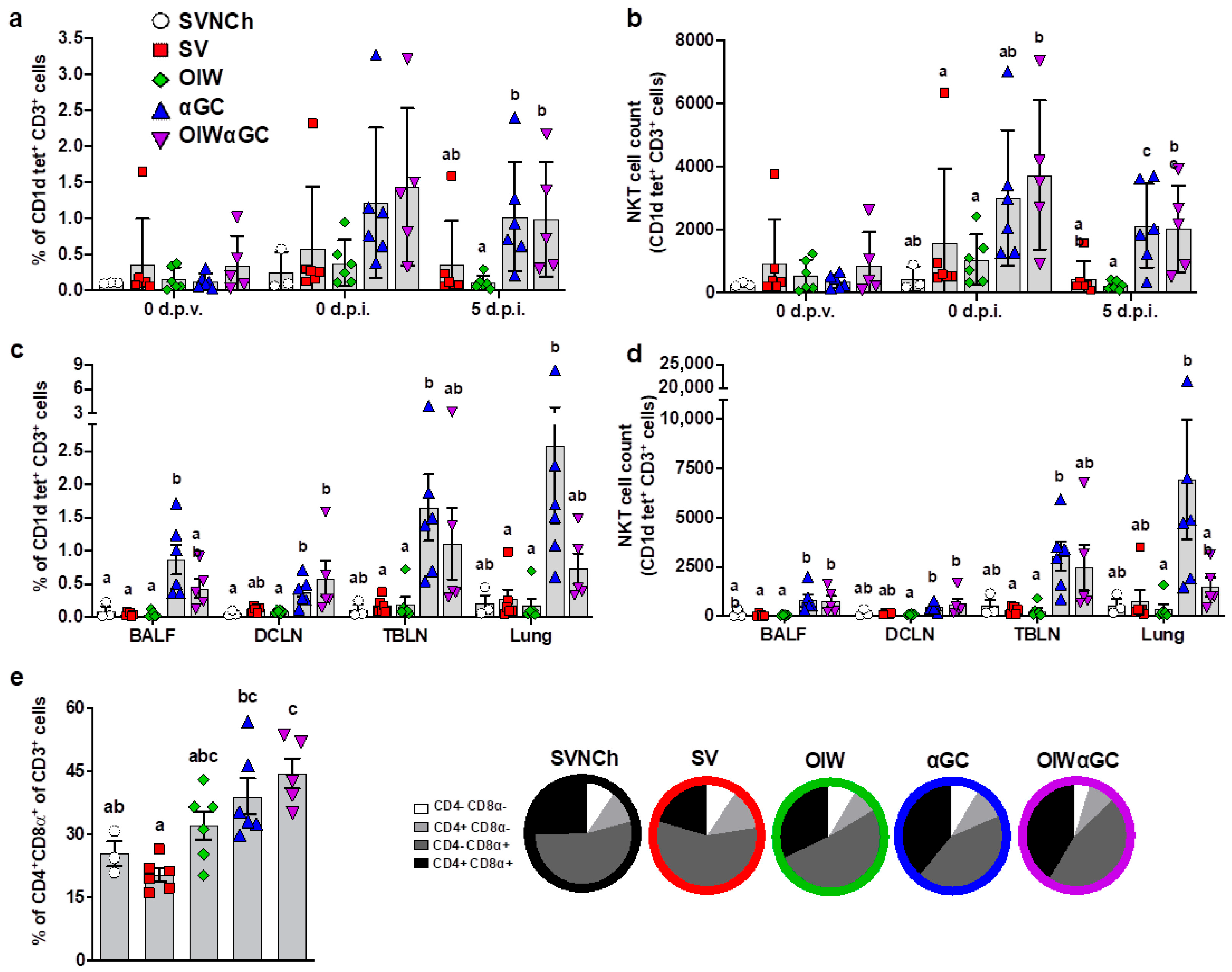
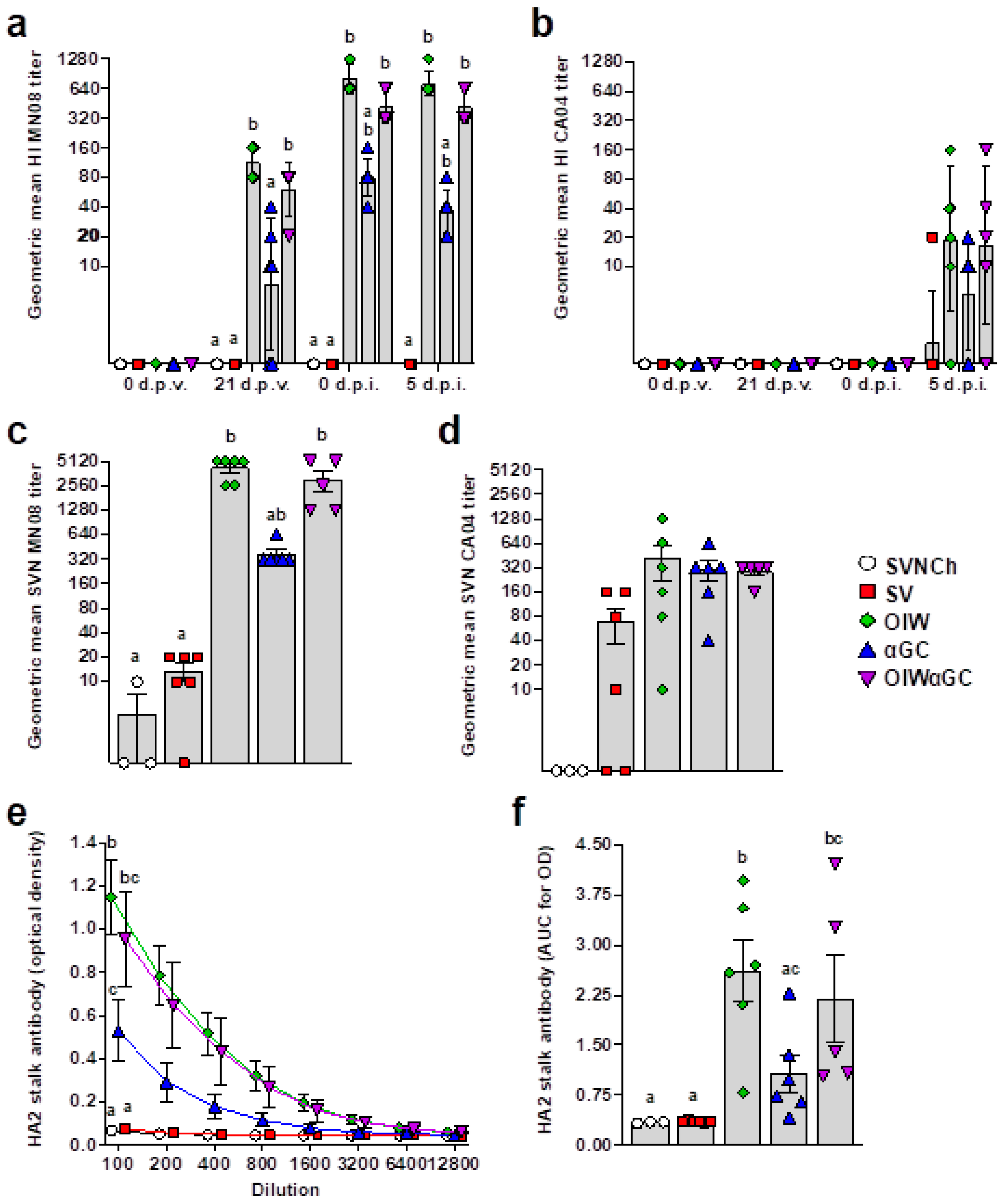
3.1. iNKT-Cell Activation with Heterologous IAV Challenge Induces VAERD
3.2. Analysis of Immune Cells and Cytokines
3.3. Virus Shedding and Replication
3.4. Virus- and HA2 Stalk-Specific Antibody Responses
3.5. iNKT-Cell Activation Elicits High Concentrations of IFN-γ-Secreting Cells
3.6. Vaccine-Induced Antibodies Correlate with Increased Genetic Diversity of IAV Genomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, A.; Foo, S.S.; Bruzzone, R.; Vu Dinh, L.; King, N.J.; Mahalingam, S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015, 268, 340–364. [Google Scholar] [CrossRef] [PubMed]
- Takada, A.; Kawaoka, Y. Antibody-dependent enhancement of viral infection: Molecular mechanisms and in vivo implications. Rev. Med. Virol. 2003, 13, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Smatti, M.K.; Al Thani, A.A.; Yassine, H.M. Viral-Induced Enhanced Disease Illness. Front. Microbiol. 2018, 9, 2991. [Google Scholar] [CrossRef] [PubMed]
- Kobinger, G.P.; Meunier, I.; Patel, A.; Pillet, S.; Gren, J.; Stebner, S.; Leung, A.; Neufeld, J.L.; Kobasa, D.; von Messling, V. Assessment of the efficacy of commercially available and candidate vaccines against a pandemic H1N1 2009 virus. J. Infect. Dis. 2010, 201, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.L.; Lager, K.M.; Janke, B.H.; Gramer, M.R.; Richt, J.A. Failure of protection and enhanced pneumonia with a US H1N2 swine influenza virus in pigs vaccinated with an inactivated classical swine H1N1 vaccine. Vet. Microbiol. 2008, 126, 310–323. [Google Scholar] [CrossRef]
- Gauger, P.C.; Vincent, A.L.; Loving, C.L.; Henningson, J.N.; Lager, K.M.; Janke, B.H.; Kehrli, M.E.; Roth, J.A. Kinetics of lung lesion development and pro-inflammatory cytokine response in pigs with vaccine-associated enhanced respiratory disease induced by challenge with pandemic (2009) A/H1N1 influenza virus. Vet. Pathol. 2012, 49, 900–912. [Google Scholar] [CrossRef]
- Gauger, P.C.; Vincent, A.L.; Loving, C.L.; Lager, K.M.; Janke, B.H.; Kehrli, M.E.; Roth, J.A. Enhanced pneumonia and disease in pigs vaccinated with an inactivated human-like (δ-cluster) H1N2 vaccine and challenged with pandemic 2009 H1N1 influenza virus. Vaccine 2011, 29, 2712–2719. [Google Scholar] [CrossRef]
- Kimble, J.B.; Wymore Brand, M.; Kaplan, B.S.; Gauger, P.; Coyle, E.M.; Chilcote, K.; Khurana, S.; Vincent, A.L. Vaccine-Associated Enhanced Respiratory Disease following Influenza Virus Infection in Ferrets Recapitulates the Model in Pigs. J. Virol. 2022, 96, e0172521. [Google Scholar] [CrossRef]
- Ochiai, H.; Kurokawa, M.; Hayashi, K.; Niwayama, S. Antibody-mediated growth of influenza A NWS virus in macrophagelike cell line P388D1. J. Virol. 1988, 62, 20–26. [Google Scholar] [CrossRef]
- Ochiai, H.; Kurokawa, M.; Kuroki, Y.; Niwayama, S. Infection enhancement of influenza A H1 subtype viruses in macrophage-like P388D1 cells by cross-reactive antibodies. J. Med. Virol. 1990, 30, 258–265. [Google Scholar] [CrossRef]
- Monsalvo, A.C.; Batalle, J.P.; Lopez, M.F.; Krause, J.C.; Klemenc, J.; Hernandez, J.Z.; Maskin, B.; Bugna, J.; Rubinstein, C.; Aguilar, L.; et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat. Med. 2011, 17, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; De Serres, G.; Crowcroft, N.S.; Janjua, N.Z.; Boulianne, N.; Hottes, T.S.; Rosella, L.C.; Dickinson, J.A.; Gilca, R.; Sethi, P.; et al. Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: Four observational studies from Canada. PLoS Med. 2010, 7, e1000258. [Google Scholar] [CrossRef] [PubMed]
- Janjua, N.Z.; Skowronski, D.M.; Hottes, T.S.; Osei, W.; Adams, E.; Petric, M.; Sabaiduc, S.; Chan, T.; Mak, A.; Lem, M.; et al. Seasonal influenza vaccine and increased risk of pandemic A/H1N1-related illness: First detection of the association in British Columbia, Canada. Clin. Infect. Dis. 2010, 51, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Loving, C.L.; Manischewitz, J.; King, L.R.; Gauger, P.C.; Henningson, J.; Vincent, A.L.; Golding, H. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci. Transl. Med. 2013, 5, 200ra114. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.K.; Rajão, D.S.; Sandbulte, M.R.; Lopes, S.; Lewis, N.S.; Loving, C.L.; Gauger, P.C.; Vincent, A.L. The type of adjuvant in whole inactivated influenza a virus vaccines impacts vaccine-associated enhanced respiratory disease. Vaccine 2018, 36, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Rajão, D.S.; Loving, C.L.; Gauger, P.C.; Kitikoon, P.; Vincent, A.L. Influenza A virus hemagglutinin protein subunit vaccine elicits vaccine-associated enhanced respiratory disease in pigs. Vaccine 2014, 32, 5170–5176. [Google Scholar] [CrossRef]
- Gauger, P.C.; Loving, C.L.; Lager, K.M.; Janke, B.H.; Kehrli, M.E., Jr.; Roth, J.A.; Vincent, A.L. Vaccine-associated enhanced respiratory disease does not interfere with the adaptive immune response following challenge with pandemic A/H1N1 2009. Viral Immunol. 2013, 26, 314–321. [Google Scholar] [CrossRef]
- Rajão, D.S.; Chen, H.; Perez, D.R.; Sandbulte, M.R.; Gauger, P.C.; Loving, C.L.; Shanks, G.D.; Vincent, A. Vaccine-associated enhanced respiratory disease is influenced by haemagglutinin and neuraminidase in whole inactivated influenza virus vaccines. J. Gen. Virol. 2016, 97, 1489–1499. [Google Scholar] [CrossRef]
- Souza, C.K.; Rajão, D.S.; Loving, C.L.; Gauger, P.C.; Pérez, D.R.; Vincent, A.L. Age at Vaccination and Timing of Infection Do Not Alter Vaccine-Associated Enhanced Respiratory Disease in Influenza A Virus-Infected Pigs. Clin. Vaccine Immunol. 2016, 23, 470–482. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Joyce, S.; Okoye, G.D.; Driver, J.P. Die Kämpfe únd schláchten-the struggles and battles of innate-like effector T lymphocytes with microbes. Front. Immunol. 2023, 14, 1117825. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Takeda, K.; Yagita, H.; Van Kaer, L.; Saiki, I.; Okumura, K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J. Immunol. 2001, 166, 6012–6018. [Google Scholar] [CrossRef]
- Van Kaer, L.; Parekh, V.V.; Wu, L. Invariant natural killer T cells: Bridging innate and adaptive immunity. Cell Tissue Res. 2011, 343, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Bendelac, A.; Savage, P.B.; Teyton, L. The biology of NKT cells. Annu Rev. Immunol. 2007, 25, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Emoto, M.; Emoto, Y.; Yoshizawa, I.; Kita, E.; Shimizu, T.; Hurwitz, R.; Brinkmann, V.; Kaufmann, S.H. Alpha-GalCer ameliorates listeriosis by accelerating infiltration of Gr-1+ cells into the liver. Eur. J. Immunol. 2010, 40, 1328–1341. [Google Scholar] [CrossRef]
- Sidobre, S.; Naidenko, O.V.; Sim, B.C.; Gascoigne, N.R.; Garcia, K.C.; Kronenberg, M. The V alpha 14 NKT cell TCR exhibits high-affinity binding to a glycolipid/CD1d complex. J. Immunol. 2002, 169, 1340–1348. [Google Scholar] [CrossRef]
- Speir, M.; Hermans, I.F.; Weinkove, R. Engaging Natural Killer T Cells as ’Universal Helpers’ for Vaccination. Drugs 2017, 77, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cerundolo, V.; Silk, J.D.; Masri, S.H.; Salio, M. Harnessing invariant NKT cells in vaccination strategies. Nat. Rev. Immunol. 2009, 9, 28–38. [Google Scholar] [CrossRef]
- Burn, O.K.; Pankhurst, T.E.; Painter, G.F.; Connor, L.M.; Hermans, I.F. Harnessing NKT cells for vaccination. Oxf. Open Immunol. 2021, 2, iqab013. [Google Scholar] [CrossRef]
- Fujii, S.-i.; Yamasaki, S.; Sato, Y.; Shimizu, K. Vaccine Designs Utilizing Invariant NKT-Licensed Antigen-Presenting Cells Provide NKT or T Cell Help for B Cell Responses. Front. Immunol. 2018, 9, 01267. [Google Scholar] [CrossRef]
- Barral, P.; Eckl-Dorna, J.; Harwood, N.E.; De Santo, C.; Salio, M.; Illarionov, P.; Besra, G.S.; Cerundolo, V.; Batista, F.D. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 8345–8350. [Google Scholar] [CrossRef]
- Chang, P.P.; Barral, P.; Fitch, J.; Pratama, A.; Ma, C.S.; Kallies, A.; Hogan, J.J.; Cerundolo, V.; Tangye, S.G.; Bittman, R.; et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 2011, 13, 35–43. [Google Scholar] [CrossRef]
- Driver, J.P.; de Carvalho Madrid, D.M.; Gu, W.; Artiaga, B.L.; Richt, J.A. Modulation of Immune Responses to Influenza A Virus Vaccines by Natural Killer T Cells. Front. Immunol. 2020, 11, 2172. [Google Scholar] [CrossRef]
- Sponseller, B.A.; Strait, E.; Jergens, A.; Trujillo, J.; Harmon, K.; Koster, L.; Jenkins-Moore, M.; Killian, M.; Swenson, S.; Bender, H.; et al. Influenza A pandemic (H1N1) 2009 virus infection in domestic cat. Emerg. Infect. Dis. 2010, 16, 534–537. [Google Scholar] [CrossRef]
- WHO. WHO Manual on Animal Influenza Diagnosis and Surveillance; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Solórzano, A.; Webby, R.J.; Lager, K.M.; Janke, B.H.; García-Sastre, A.; Richt, J.A. Mutations in the NS1 protein of swine influenza virus impair anti-interferon activity and confer attenuation in pigs. J. Virol. 2005, 79, 7535–7543. [Google Scholar] [CrossRef]
- Artiaga, B.L.; Morozov, I.; Ransburgh, R.; Kwon, T.; Balaraman, V.; Indran, S.V.; De Carvalho Madrid, D.M.; Gu, W.; Henningson, J.; Ma, W.; et al. Evaluating α-galactosylceramide as an adjuvant for live attenuated influenza vaccines in pigs. Anim. Dis. 2022, 2, 19. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method for estimating fifty percent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Artiaga, B.L.; Whitener, R.L.; Staples, C.R.; Driver, J.P. Adjuvant effects of therapeutic glycolipids administered to a cohort of NKT cell-diverse pigs. Vet. Immunol. Immunopathol. 2014, 162, 1–13. [Google Scholar] [CrossRef]
- Artiaga, B.L.; Yang, G.; Hackmann, T.J.; Liu, Q.; Richt, J.A.; Salek-Ardakani, S.; Castleman, W.L.; Lednicky, J.A.; Driver, J.P. α-Galactosylceramide protects swine against influenza infection when administered as a vaccine adjuvant. Sci. Rep. 2016, 6, 23593. [Google Scholar] [CrossRef]
- Zhou, B.; Wentworth, D.E. Influenza A virus molecular virology techniques. Methods Mol. Biol. 2012, 865, 175–192. [Google Scholar] [CrossRef]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef]
- Kitikoon, P.; Gauger, P.C.; Vincent, A.L. Hemagglutinin inhibition assay with swine sera. Methods Mol. Biol. 2014, 1161, 295–301. [Google Scholar] [CrossRef]
- Sunwoo, S.Y.; Schotsaert, M.; Morozov, I.; Davis, A.S.; Li, Y.; Lee, J.; McDowell, C.; Meade, P.; Nachbagauer, R.; García-Sastre, A.; et al. A Universal Influenza Virus Vaccine Candidate Tested in a Pig Vaccination-Infection Model in the Presence of Maternal Antibodies. Vaccines 2018, 6, 64. [Google Scholar] [CrossRef]
- Halbur, P.G.; Paul, P.S.; Frey, M.L.; Landgraf, J.; Eernisse, K.; Meng, X.J.; Lum, M.A.; Andrews, J.J.; Rathje, J.A. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet. Pathol. 1995, 32, 648–660. [Google Scholar] [CrossRef]
- Pescovitz, M.D.; Hsu, S.M.; Katz, S.I.; Lunney, J.K.; Shimada, S.; Sachs, D.H. Characterization of a porcine CD1-specific mAb that distinguishes CD4/CD8 double-positive thymic from peripheral T lymphocytes. Tissue Antigens 1990, 35, 151–156. [Google Scholar] [CrossRef]
- Yang, H.; Parkhouse, R.M. Phenotypic classification of porcine lymphocyte subpopulations in blood and lymphoid tissues. Immunology 1996, 89, 76–83. [Google Scholar] [CrossRef]
- Zuckermann, F.A.; Gaskins, H.R. Distribution of porcine CD4/CD8 double-positive T lymphocytes in mucosa-associated lymphoid tissues. Immunology 1996, 87, 493–499. [Google Scholar] [CrossRef]
- Iyoda, T.; Yamasaki, S.; Ueda, S.; Shimizu, K.; Fujii, S.I. Natural Killer T and Natural Killer Cell-Based Immunotherapy Strategies Targeting Cancer. Biomolecules 2023, 13, 348. [Google Scholar] [CrossRef]
- Nelson, A.; Lukacs, J.D.; Johnston, B. The Current Landscape of NKT Cell Immunotherapy and the Hills Ahead. Cancers 2021, 13, 5174. [Google Scholar] [CrossRef]
- Fernandez, C.S.; Jegaskanda, S.; Godfrey, D.I.; Kent, S.J. In-vivo stimulation of macaque natural killer T cells with α-galactosylceramide. Clin. Exp. Immunol. 2013, 173, 480–492. [Google Scholar] [CrossRef]
- Schäfer, A.; Hühr, J.; Schwaiger, T.; Dorhoi, A.; Mettenleiter, T.C.; Blome, S.; Schröder, C.; Blohm, U. Porcine Invariant Natural Killer T Cells: Functional Profiling and Dynamics in Steady State and Viral Infections. Front Immunol. 2019, 10, 1380. [Google Scholar] [CrossRef]
- Thierry, A.; Robin, A.; Giraud, S.; Minouflet, S.; Barra, A.; Bridoux, F.; Hauet, T.; Touchard, G.; Herbelin, A.; Gombert, J.M. Identification of invariant natural killer T cells in porcine peripheral blood. Vet. Immunol. Immunopathol. 2012, 149, 272–279. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Manickam, C.; Khatri, M.; Rauf, A.; Li, X.; Tsuji, M.; Rajashekara, G.; Dwivedi, V. Functional invariant NKT cells in pig lungs regulate the airway hyperreactivity: A potential animal model. J. Clin. Immunol. 2011, 31, 228–239. [Google Scholar] [CrossRef]
- Madrid, D.M.C.; Gu, W.; Artiaga, B.L.; Yang, G.; Loeb, J.; Hawkins, I.K.; Castleman, W.L.; Lednicky, J.A.; Richt, J.A.; Driver, J.P. Comparison of oseltamivir and α-galactosylceramide for reducing disease and transmission in pigs infected with 2009 H1N1 pandemic influenza virus. Front. Vet. Sci. 2022, 9, 999507. [Google Scholar] [CrossRef]
- Gu, W.; Madrid, D.M.D.; Yang, G.; Artiaga, B.L.; Loeb, J.C.; Castleman, W.L.; Richt, J.A.; Lednicky, J.A.; Driver, J.P. Unaltered influenza disease outcomes in swine prophylactically treated with α-galactosylceramide. Dev. Comp. Immunol. 2021, 114, 103843. [Google Scholar] [CrossRef]
- Artiaga, B.L.; Yang, G.; Hutchinson, T.E.; Loeb, J.C.; Richt, J.A.; Lednicky, J.A.; Salek-Ardakani, S.; Driver, J.P. Rapid control of pandemic H1N1 influenza by targeting NKT-cells. Sci. Rep. 2016, 6, 37999. [Google Scholar] [CrossRef]
- Cheon, I.S.; Son, Y.M.; Sun, J. Tissue-resident memory T cells and lung immunopathology. Immunol. Rev. 2023, 316, 63–83. [Google Scholar] [CrossRef]
- Xue, K.S.; Moncla, L.H.; Bedford, T.; Bloom, J.D. Within-Host Evolution of Human Influenza Virus. Trends Microbiol. 2018, 26, 781–793. [Google Scholar] [CrossRef]


| Group | Experimental Group | Vaccine | αGC (μg/kg) | Emulsigen-D d | Challenge Virus e | N |
|---|---|---|---|---|---|---|
| 1 | SVNCh | Vehicle a | Vehicle c | 0 | None | 3 |
| 2 | SV | Vehicle | Vehicle | 0 | H1N1 CA04 | 6 |
| 3 | OIW | MN08 b | Vehicle | 20% | H1N1 CA04 | 6 |
| 4 | αGC | MN08 | 100 | 0 | H1N1 CA04 | 6 |
| 5 | OIWαGC | MN08 | 100 | 20% | H1N1 CA04 | 5 f |
| Group | Experimental Group | Pig ID | Total Nonsynonymous Mutations | Number of Epitopes with Mutations a | |||
|---|---|---|---|---|---|---|---|
| 2 | SV | 7785 | 6 | 9 HA | |||
| 7791 | 10 | 25 HA | |||||
| 7800 | 4 | 7 HA | |||||
| 7804 | 2 | 4 HA | |||||
| 7809 | 7 | 12 HA | |||||
| 7813 | 2 | - | |||||
| Average | 5.2 | ||||||
| 3 | OIW | 7786 | 32 | 30 HA | 2 NA | 1 M1 | 2 NP |
| 7793 | 5 | 8 HA | |||||
| 7801 | 10 | 18 HA | 2 NA | ||||
| 7805 | 19 | 17 HA | 1 PB2 | 1 NEP/NS1 | |||
| 7810 | 26 | 50 HA | 5 NA | ||||
| 7814 | 11 | 14 HA | |||||
| Average | 17.2 | ||||||
| 4 | αGC | 7787 | 21 | 33 HA | 2 NA | 1 PB1 | |
| 7795 | 2 | 4 HA | |||||
| 7802 | 18 | 15 HA | 3 NA | 3 NP | |||
| 7806 | 4 | 13 HA | |||||
| 7811 | 12 | 14 HA | |||||
| 7815 | 3 | - | |||||
| Average | 10 | ||||||
| 5 | OIWαGC | 7789 | 7 | 23 HA | 1 M1 | ||
| 7796 | 1 | - | |||||
| 7803 | 10 | 33 HA | 1 M1 | ||||
| 7808 | 23 | 26 HA | 2 M1 | 1 PB2 | |||
| 7812 | 11 | 25 HA | 3 NA | ||||
| 7816 | 4 | 13 HA | |||||
| Average | 9.3 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artiaga, B.L.; Madden, D.; Kwon, T.; McDowell, C.; Keating, C.; Balaraman, V.; de Carvahlo Madrid, D.M.; Touchard, L.; Henningson, J.; Meade, P.; et al. Adjuvant Use of the Invariant-Natural-Killer-T-Cell Agonist α-Galactosylceramide Leads to Vaccine-Associated Enhanced Respiratory Disease in Influenza-Vaccinated Pigs. Vaccines 2024, 12, 1068. https://doi.org/10.3390/vaccines12091068
Artiaga BL, Madden D, Kwon T, McDowell C, Keating C, Balaraman V, de Carvahlo Madrid DM, Touchard L, Henningson J, Meade P, et al. Adjuvant Use of the Invariant-Natural-Killer-T-Cell Agonist α-Galactosylceramide Leads to Vaccine-Associated Enhanced Respiratory Disease in Influenza-Vaccinated Pigs. Vaccines. 2024; 12(9):1068. https://doi.org/10.3390/vaccines12091068
Chicago/Turabian StyleArtiaga, Bianca L., Daniel Madden, Taeyong Kwon, Chester McDowell, Cassidy Keating, Velmurugan Balaraman, Darling Melany de Carvahlo Madrid, Laurie Touchard, Jamie Henningson, Philip Meade, and et al. 2024. "Adjuvant Use of the Invariant-Natural-Killer-T-Cell Agonist α-Galactosylceramide Leads to Vaccine-Associated Enhanced Respiratory Disease in Influenza-Vaccinated Pigs" Vaccines 12, no. 9: 1068. https://doi.org/10.3390/vaccines12091068
APA StyleArtiaga, B. L., Madden, D., Kwon, T., McDowell, C., Keating, C., Balaraman, V., de Carvahlo Madrid, D. M., Touchard, L., Henningson, J., Meade, P., Krammer, F., Morozov, I., Richt, J. A., & Driver, J. P. (2024). Adjuvant Use of the Invariant-Natural-Killer-T-Cell Agonist α-Galactosylceramide Leads to Vaccine-Associated Enhanced Respiratory Disease in Influenza-Vaccinated Pigs. Vaccines, 12(9), 1068. https://doi.org/10.3390/vaccines12091068









