Pseudorabies Virus Glycoproteins E and B Application in Vaccine and Diagnosis Kit Development
Abstract
:1. Introduction
1.1. PRV gE
1.2. PRV gB
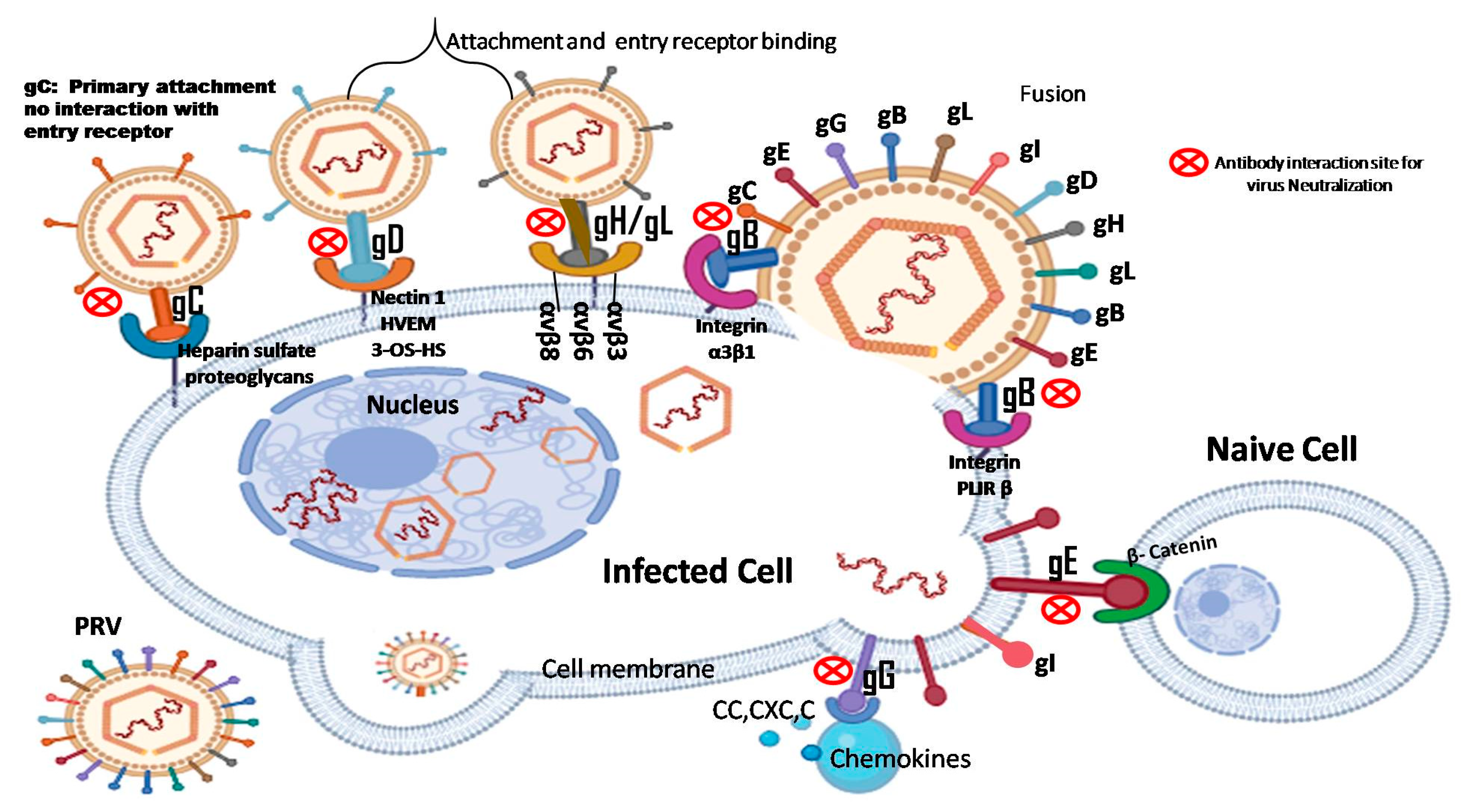
2. Application of PRV Glycoprotein in Vaccine Development
2.1. gE as a Vaccine Target
2.2. Live Attenuated Vaccines
Deletion of gE Gene and Other PRV Proteins from Live Attenuated Vaccines
2.3. Inactivated PRV Vaccine
2.4. PRV gE Subunit Vaccines
2.5. PRV gE DNA Vaccine
2.6. gB as a Vaccine Target
3. Development of Immune Response Vaccine against PRV gB
3.1. PRV gB Subunit Vaccines
3.2. PRV gB DNA Vaccine
3.3. Viral Vector Vaccines
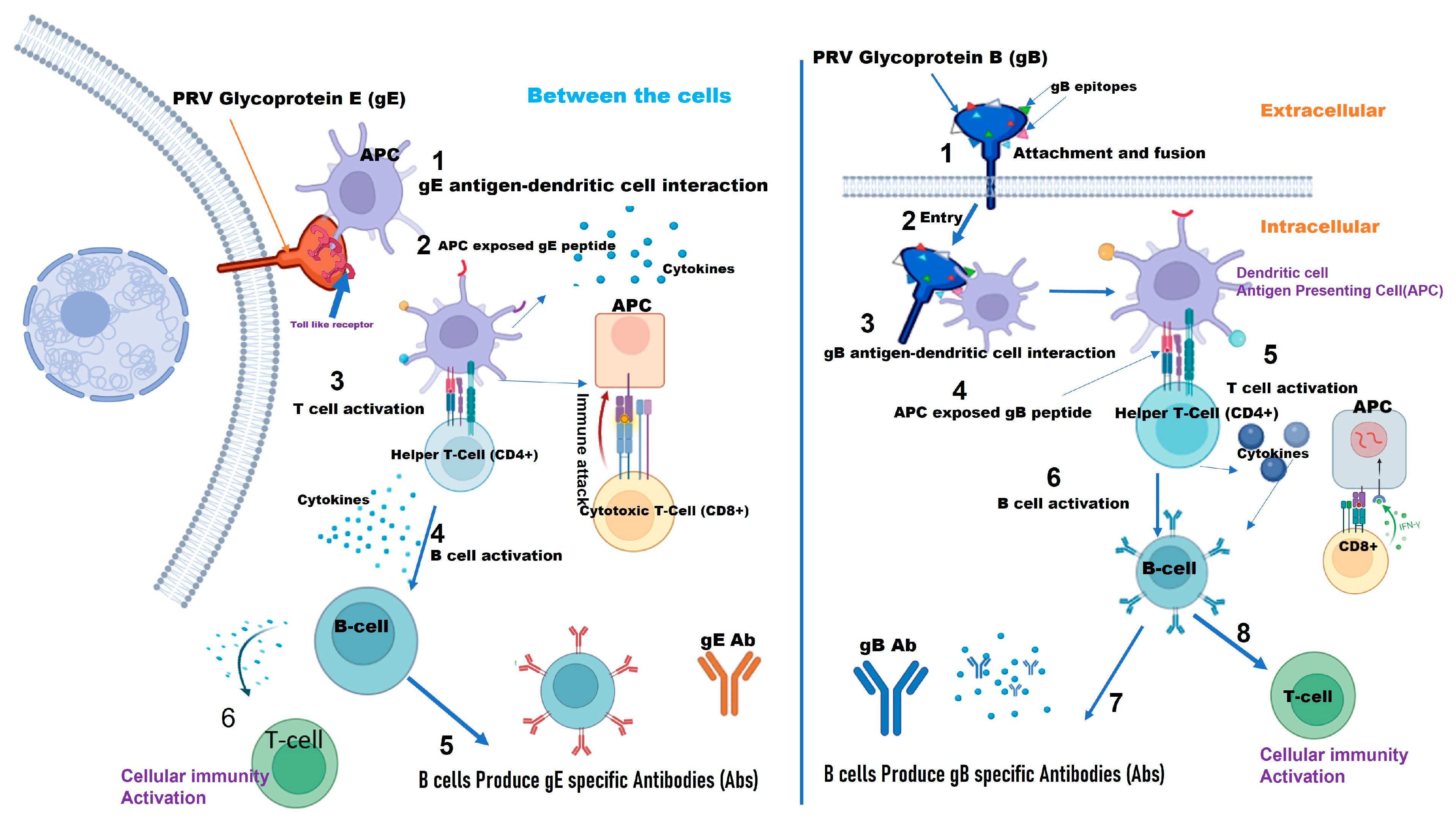
4. Comparative Analysis of Vaccine Effectiveness, Safety, and Field Application
| Vaccine Approaches | Characteristics | Protective Efficacy | Effective on | Vaccine Status |
|---|---|---|---|---|
| gE Live attenuated vaccine [50,57,123] | Attenuated strain with gE gene deleted | High | Classical and variant strains | Approved |
| gE Inactivated PRV vaccine [85,90,124,125] | Inactivated strain with gE gene deleted | Moderate | Classical strains | In development |
| gE subunit vaccine [61,88,91,126] | Recombinant gE protein Generally safe | Moderate | Classical strains | Approved |
| gE DNA vaccine [76,89] | DNA encoding gE antigen | Low | Classical strains | In development |
| gB subunit vaccine [127,128,129] | Recombinant gB protein Generally safe | High | Classical and variant strains | Approved |
| gB DNA vaccine [62,129] | DNA encoding gB antigen | Low | Classical strains | In development |
| gB live attenuated vaccine [59,130] | Attenuated strain with gB gene deleted | High | Classical and variant strains | Approved |
| Recombined PRV gB vaccine [99,131] | Recombinant virus expressing the gB antigen, Expressing both native and foreign antigens | High | Classical and variant strains | Approved |
5. PRV Glycoproteins in Diagnosis Kit Development
5.1. ELISA Kit Development
5.2. Neutralization-Assay
5.3. Antibody Test Kit
5.4. PCR Detection Kit
5.5. Rapid Test Kit
| Diagnostic Kit | Characteristics | Performance |
|---|---|---|
| PRV gE Antibody Test Kit [150,164] | Detect PRV gE antibody in serum Solid-phase microchip platform | High Sensitivity (98.1%) and specificity (98.8%), Suitable for the laboratory and field test, 10–30 min test duration, Low cost |
| PRV gE Real-time PCR Kit [163,165,166] | Detect PRV gE gene sequence in swine serum, plasma, tissue, sperm, and environmental samples. Used for DIVA strategy | Very high sensitivity (100%) and specificity (100%). Suitable for laboratory test 1–4 h test duration, Moderate to high cost |
| PRV gB Antibody Test Kit (BioChek) [126] | Semi-quantitative ELISA Detect PRV gB antibody Confirms herd disease. | High Sensitivity (98.1%) and specificity (98.1%) Suitable for field and laboratory test, 10–30 min test duration, Low cost |
| PRV gB ELISA [143,144] | Detect PRV gB antibody in serum, Provides quantitative measurement of antibodies | High Sensitivity (>80.9%) and specificity (>96.4%) Suitable for laboratory test 2–4 h test duration, Moderate to high cost |
| New immuno chromatographic strip [167] | Rapidly detect PRV gB antibody, Easily applicable with no special skill | High Specificity (95.4%) and sensitivity (98.1%) Suitable for field test 5–10 min test duration, Low cost |
| PRV gB real time PCR [126,163,166,168] | Detect PRV gB gene in tissue, blood, and vaccine samples. Aids disease surveillance and control. | Very high sensitivity (100%) and specificity (100%), Suitable for laboratory test, 1–4 h test duration, Moderate to high cost |
| PRV gE-based Latex Agglutination Kit (gE-LAT) [169,170] | Detect PRV gE antibodies Provides rapid results Reliable and easy to use | High sensitivity (95.76%) and high specificity (96.77%) Suitable for field test, 30–90 min test duration, Moderate cost |
| PRV gB Agar gel immuno diffusion (AGID) test kit [147,171] | Detect PRV gB antibodies in serum. Simple, cost-effective, and reliable | High sensitivity (95%) and specificity (96.6%) Suitable for laboratory test 24 to 48 h test duration, Moderate cost |
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGID | Agar Gel Immunodiffusion |
| Bac-gB | Baculovirus-expressed Glycoprotein B |
| BHV | Bovine Herpesvirus |
| CMV | Cytomegalovirus |
| CTD | Cytoplasm Domain |
| DIVA | Differentiating Infected from Vaccinated Animals |
| EGRT-PCR | Enhanced Green Fluorescent Protein-Tagged Real-Time PCR |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ERK | Extracellular Signal-Regulated Kinase |
| ETD | Extracellular Domain |
| gB | Glycoprotein B |
| gE | Glycoprotein E |
| gE-LAT | Glycoprotein E Latex Agglutination Test |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HSV | Herpes Simplex Virus |
| HVEM | Herpesvirus Entry Mediator |
| IFA | Immunofluorescence Assay |
| IgG | Immunoglobin G |
| INF-γ | Interferon-gamma |
| mAb | Monoclonal Antibody |
| MHC | Major Histocompatibility Complex |
| NK | Natural killer |
| PCR | Polymerase Chain Reaction |
| PFU | Plaque-Forming Unit |
| PILR-β | Paired Immunoglobulin-like Type 2 Receptor Beta |
| PRV | Pseudorabies Virus |
| SN | Serum Neutralization |
| SuHV-1 | Suid Herpes virus-1 |
| TCR | T Cell Receptor |
| TK | thymidine kinase |
| TMD | Transmembrane Domain |
| UL | Unique long |
| US | Unique short |
| VN | Virus Neutralization |
References
- Wang, Y.; Wang, T.; Yan, H.; Yang, F.; Guo, L.; Yang, Q.; Hu, X.; Tan, F.; Xiao, Y.; Li, X.; et al. Research and Development of a Novel Subunit Vaccine for the Currently Circulating Pseudorabies Virus Variant in China. Front. Agric. Sci. Eng. 2015, 2, 216–222. [Google Scholar] [CrossRef]
- Klupp, B.G.; Hengartner, C.J.; Mettenleiter, T.C.; Enquist, L.W. Complete, Annotated Sequence of the Pseudorabies Virus Genome. J. Virol. 2004, 78, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Leedom Larson, K.R. Pseudorabies Virus; Swine Health Information Center; Center for Food Security and Public Health; Iowa State University: Ames, IA, USA, 2016. [Google Scholar]
- Mettenleiter, T.C. Pseudorabies Virus. In Encyclopedia of Virology; Academic Press: Cambridge, MA, USA, 2008; pp. 341–351. [Google Scholar] [CrossRef]
- Lin, M.; Jia, R.; Wang, M.; Gao, X.; Zhu, D.; Chen, S.; Liu, M.; Yin, Z.; Wang, Y.; Chen, X.; et al. Molecular Characterization of Duck Enteritis Virus CHv Strain UL49.5 Protein and Colocalization with Glycoprotein M. J. Vet. Sci. 2014, 15, 389–398. [Google Scholar] [CrossRef]
- Sun, K.F.; Cheng, A.C.; Wang, M.S. Bioinformatic Analysis and Characteristics of Glycoprotein C Encoded by the Newly Identified UL44 Gene of Duck Plague Virus. Genet. Mol. Res. 2014, 13, 4505–4515. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liu, T.; Wang, M.; Cheng, A.; Jia, R. Duck Plague Virus Glycoprotein J Is Functional but Slightly Impaired in Viral Replication and Cell-to-Cell Spread. Sci. Rep. 2018, 8, 4069. [Google Scholar] [CrossRef]
- Zhao, C.; He, T.; Xu, Y.; Wang, M.; Cheng, A. Molecular Characterization and Antiapoptotic Function Analysis of the Duck Plague Virus Us5 Gene. Sci. Rep. 2019, 9, 4851. [Google Scholar] [CrossRef]
- Ning, Y.; Huang, Y.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Tian, B.; Ou, X.; Huang, J.; Mao, S.; et al. Alphaherpesvirus Glycoprotein E: A Review of Its Interactions with Other Proteins of the Virus and Its Application in Vaccinology. Front. Microbiol. 2022, 13, 970545. [Google Scholar] [CrossRef]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular Biology of Pseudorabies Virus: Impact on Neurovirology and Veterinary Medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef]
- Li, X.; Yang, F.; Hu, X.; Tan, F.; Qi, J.; Peng, R.; Wang, M.; Chai, Y.; Hao, L.; Deng, J.; et al. Two Classes of Protective Antibodies against Pseudorabies Virus Variant Glycoprotein B: Implications for Vaccine Design. PLoS Pathog. 2017, 13, e1006777. [Google Scholar] [CrossRef]
- Mettenleiter, T.C. Intriguing Interplay between Viral Proteins during Herpesvirus Assembly or: The Herpesvirus Assembly Puzzle. Vet. Microbiol. 2006, 113, 163–169. [Google Scholar] [CrossRef]
- Avitabile, E.; Forghieri, C.; Campadelli-Fiume, G. Complexes between Herpes Simplex Virus Glycoproteins GD, GB, and GH Detected in Cells by Complementation of Split Enhanced Green Fluorescent Protein. J. Virol. 2007, 81, 11532–11537. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.P.; Geraghty, R.J. Herpes Simplex Virus Type 1 Mediates Fusion through a Hemifusion Intermediate by Sequential Activity of Glycoproteins D, H, L, and B. Proc. Natl. Acad. Sci. USA 2007, 104, 2903–2908. [Google Scholar] [CrossRef]
- Backovic, M.; DuBois, R.M.; Cockburn, J.J.; Sharff, A.J.; Vaney, M.C.; Granzow, H.; Klupp, B.G.; Bricogne, G.; Mettenleiter, T.C.; Rey, F.A. Structure of a Core Fragment of Glycoprotein H from Pseudorabies Virus in Complex with Antibody. Proc. Natl. Acad. Sci. USA 2010, 107, 22635–22640. [Google Scholar] [CrossRef] [PubMed]
- Klupp, B.; Altenschmidt, J.; Granzow, H.; Fuchs, W.; Mettenleiter, T.C. Glycoproteins Required for Entry Are Not Necessary for Egress of Pseudorabies Virus. J. Virol. 2008, 82, 6299–6309. [Google Scholar] [CrossRef] [PubMed]
- Heldwein, E.E.; Krummenacher, C. Entry of Herpesviruses into Mammalian Cells. Cell. Mol. Life Sci. 2008, 65, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Qi, H.; Wang, B.; Li, M.; Qu, L.; Li, S.; Luo, Y.; Li, L.F.; Zheng, G.L.; Qiu, H.J.; et al. The Mutations on the Envelope Glycoprotein D Contribute to the Enhanced Neurotropism of the Pseudorabies Virus Variant. J. Biol. Chem. 2023, 299, 105347. [Google Scholar] [CrossRef]
- Zheng, H.H.; Fu, P.F.; Chen, H.Y.; Wang, Z.Y. Pseudorabies Virus: From Pathogenesis to Prevention Strategies. Viruses 2022, 14, 1638. [Google Scholar] [CrossRef]
- El Kasmi, I.; Lippé, R. Herpes Simplex Virus 1 GN Partners with GM To Modulate the Viral Fusion Machinery. J. Virol. 2015, 89, 2313–2323. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Cheng, A.; Jia, R.; Yang, Q.; Wu, Y.; Zhu, D.; Zhao, X.; Chen, S.; Liu, M.; et al. The Roles of Envelope Glycoprotein M in the Life Cycle of Some Alphaherpesviruses. Front. Microbiol. 2021, 12, 631523. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Dong, S.; Wang, C.; Wang, Y.; Zhang, H. Transcriptomic Analysis Reveals Impact of GE/GI/TK Deletions on Host Response to PRV Infection. Virol. J. 2023, 20, 303. [Google Scholar] [CrossRef]
- Mayr, T.; Claes, L. Pseudorabies (Aujeszky’s Disease) and Its Eradication; Mayr, T., Claes, L., Eds.; Nova Science Publishers: New York, NY, USA, 2010; ISBN 9781607416555. [Google Scholar]
- Zhang, T.; Liu, Y.; Chen, Y.; Wang, A.; Feng, H.; Wei, Q.; Zhou, E.; Zhang, G. A Single Dose Glycoprotein D-Based Subunit Vaccine against Pseudorabies Virus Infection. Vaccine 2020, 38, 6153–6161. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Zhang, L.; Zheng, J.; Niu, G.; Yang, L.; Zhang, X.; Ren, L. Mutation and Interaction Analysis of the Glycoprotein D and L and Thymidine Kinase of Pseudorabies Virus. Int. J. Mol. Sci. 2022, 23, 11597. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, L.; Sharada, R.; Ratnamma, D.; Isloor, S.; Chandranaik, B.M. Bovine Alphaherpesvirus-1 (BoHV-1) Infection in Cattle: An Overview of Epidemiology, Role of Envelope Proteins in Disease and Control. Indian J. Anim. Res. 2024, 1, 1–12. [Google Scholar] [CrossRef]
- Ch’ng, T.H.; Enquist, L.W. Efficient Axonal Localization of Alphaherpesvirus Structural Proteins in Cultured Sympathetic Neurons Requires Viral Glycoprotein E. J. Virol. 2005, 79, 8835–8846. [Google Scholar] [CrossRef] [PubMed]
- Delva, J.L.; Nauwynck, H.J.; Mettenleiter, T.C.; Favoreel, H.W. The Attenuated Pseudorabies Virus Vaccine Strain Bartha K61: A Brief Review on the Knowledge Gathered during 60 Years of Research. Pathogens 2020, 9, 897. [Google Scholar] [CrossRef]
- Jambunathan, N.; Clark, C.M.; Musarrat, F.; Chouljenko, V.N.; Rudd, J.; Kousoulas, K.G. Two Sides to Every Story: Herpes Simplex Type-1 Viral Glycoproteins Gb, Gd, Gh/Gl, Gk, and Cellular Receptors Function as Key Players in Membrane Fusion. Viruses 2021, 13, 1849. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Tong, W.; Zheng, H.; Li, L.W.; Li, G.X.; Gao, F.; Wang, T.; Liang, C.; Ye, C.; Wu, J.Q.; et al. Variations in Glycoprotein B Contribute to Immunogenic Difference between PRV Variant JS-2012 and Bartha-K61. Vet. Microbiol. 2017, 208, 97–105. [Google Scholar] [CrossRef]
- Pötzsch, S.; Spindler, N.; Wiegers, A.K.; Fisch, T.; Rücker, P.; Sticht, H.; Grieb, N.; Baroti, T.; Weisel, F.; Stamminger, T.; et al. B Cell Repertoire Analysis Identifies New Antigenic Domains on Glycoprotein b of Human Cytomegalovirus Which Are Target of Neutralizing Antibodies. PLoS Pathog. 2011, 7, 1002172. [Google Scholar] [CrossRef]
- Bo, Z.; Li, X. A Review of Pseudorabies Virus Variants: Genomics, Vaccination, Transmission, and Zoonotic Potential. Viruses 2022, 14, 1003. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Z.; Tao, Q.; Xu, L.; Gu, S.; Huang, Y.; Liu, Z.; Zhang, Y.; Wen, J.; Lai, S.; et al. Construction of Recombinant Pseudorabies Virus Expressing PCV2 Cap, PCV3 Cap, and IL-4: Investigation of Their Biological Characteristics and Immunogenicity. Front. Immunol. 2024, 15, 1339387. [Google Scholar] [CrossRef]
- Zaripov, M.M.; Morenkov, O.S.; Shmatchenko, V.V.; Smirnov, S.V.; Funtikov, V.A.; Fodor, I. Immunological and Functional Characteristics of Epitopes and Regions of GB Glycoprotein of Aujeszky’s Disease Virus. Vopr. Virusol. 2001, 46, 41–45. [Google Scholar] [PubMed]
- Dong, J.; Gu, Z.; Jin, L.; Lv, L.; Wang, J.; Sun, T.; Bai, J.; Sun, H.; Wang, X.; Jiang, P. Polymorphisms Affecting the GE and GI Proteins Partly Contribute to the Virulence of a Newly-Emergent Highly Virulent Chinese Pseudorabies Virus. Virology 2018, 519, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Creative Diagnostics. Gene Structure and Function of Porcine Pseudorabies Virus; Creative Diagnostics: New York, NY, USA, 2022. [Google Scholar]
- Polcicova, K.; Goldsmith, K.; Rainish, B.L.; Wisner, T.W.; Johnson, D.C. The Extracellular Domain of Herpes Simplex Virus GE Is Indispensable for Efficient Cell-to-Cell Spread: Evidence for GE/GI Receptors. J. Virol. 2005, 79, 11990–12001. [Google Scholar] [CrossRef] [PubMed]
- Diwaker, D.; Murray, J.W.; Barnes, J.; Wolkoff, A.W.; Wilson, D.W. Deletion of the Pseudorabies Virus GE/GI-US9p Complex Disrupts Kinesin KIF1A and KIF5C Recruitment during Egress, and Alters the Properties of Microtubule-Dependent Transport in Vitro. PLoS Pathog. 2020, 16, 1008597. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhuang, D.; Li, H.; Zhao, M.; Zhu, E.; Xie, B.; Chen, J.; Zhao, M. Recombinant Pseudorabies Virus with GI/GE Deletion Generated by Overlapping Polymerase Chain Reaction and Homologous Recombination Technology Induces Protection against the PRV Variant PRV-GD2013. BMC Vet. Res. 2021, 17, 164. [Google Scholar] [CrossRef]
- Nixdorf, R.; Klupp, B.G.; Mettenleiter, T.C. Role of the Cytoplasmic Tails of Pseudorabies Virus Glycoproteins B, E and M Intracellular Localization and Virion Incorporation. J. Gen. Virol. 2001, 82, 215–226. [Google Scholar] [CrossRef]
- Johnson, D.C.; Webb, M.; Wisner, T.W.; Brunetti, C. Herpes Simplex Virus GE/GI Sorts Nascent Virions to Epithelial Cell Junctions, Promoting Virus Spread. J. Virol. 2001, 75, 821–833. [Google Scholar] [CrossRef]
- Jung, K.; Hu, H.; Eyerly, B.; Lu, Z.; Chepngeno, J.; Saif, L.J. Pathogenicity of 2 porcine deltacoronavirus strains in gnotobiotic pigs. Emerg. Infect. Dis. 2015, 21, 650–654. [Google Scholar] [CrossRef]
- Klingbeil, K.; Lange, E.; Teifke, J.P.; Mettenleiter, T.C.; Fuchs, W. Immunization of Pigs with an Attenuated Pseudorabies Virus Recombinant Expressing the Haemagglutinin of Pandemic Swine Origin H1N1 Influenza A Virus. J. Gen. Virol. 2014, 95, 948–959. [Google Scholar] [CrossRef]
- Noronha, L.E.; Harman, R.M.; Wagner, B.; Antczak, D.F. Generation and Characterization of Monoclonal Antibodies to Equine NKp46. Vet. Immunol. Immunopathol. 2012, 147, 60–68. [Google Scholar] [CrossRef]
- Karasneh, G.A.; Shukla, D. Herpes Simplex Virus Infects Most Cell Types in Vitro: Clues to Its Success. Virol. J. 2011, 8, 481. [Google Scholar] [CrossRef] [PubMed]
- Agelidis, A.M.; Shukla, D. Cell Entry Mechanisms of HSV: What We Have Learned in Recent Years. Future Virol. 2015, 10, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- De Pelsmaeker, S.; Dierick, E.; Klupp, B.; Mettenleiter, T.C.; Cantoni, C.; Vitale, M.; Favoreel, H.W. Expression of the Pseudorabies Virus GB Glycoprotein Triggers NK Cell Cytotoxicity and Increases Binding of the Activating NK Cell Receptor PILRβ. J. Virol. 2019, 93, e02107-18. [Google Scholar] [CrossRef] [PubMed]
- Wisner, T.; Brunetti, C.; Dingwell, K.; Johnson, D.C. The Extracellular Domain of Herpes Simplex Virus GE Is Sufficient for Accumulation at Cell Junctions but Not for Cell-to-Cell Spread. J. Virol. 2000, 74, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Viejo-Borbolla, A.; Muñoz, A.; Tabarés, E.; Alcamí, A. Glycoprotein G from Pseudorabies Virus Binds to Chemokines with High Affinity and Inhibits Their Function. J. Gen. Virol. 2010, 91, 23–31. [Google Scholar] [CrossRef]
- Lin, J.; Li, Z.; Feng, Z.; Fang, Z.; Chen, J.; Chen, W.; Liang, W.; Chen, Q. Pseudorabies Virus (PRV) Strain with Defects in GE, GC, and TK Genes Protects Piglets against an Emerging PrV Variant. J. Vet. Med. Sci. 2020, 82, 846–855. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Wu, X.M.; Che, Y.L.; Chen, R.J.; Hou, B.; Wang, C.Y.; Wang, L.B.; Zhou, L.J. The Immune Efficacy of Inactivated Pseudorabies Vaccine Prepared from FJ-2012ΔgE/GI Strain. Microorganisms 2022, 10, 1880. [Google Scholar] [CrossRef]
- Wang, L.; Su, S. Forum Bat-Origin Coronaviruses Expand Their Host Range to Pigs. Trends Microbiol. 2018, 26, 466–470. [Google Scholar] [CrossRef]
- Wang, G.; Cao, J.; Gui, M.; Huang, P.; Zhang, L.; Qi, R.; Chen, R.; Lin, L.; Han, Q.; Lin, Y.; et al. The Potential of Swine Pseudorabies Virus Attenuated Vaccine for Oncolytic Therapy against Malignant Tumors. J. Exp. Clin. Cancer Res. 2023, 42, 284. [Google Scholar] [CrossRef]
- Yan, S.; Huang, B.; Bai, X.; Zhou, Y.; Guo, L.; Wang, T.; Shan, Y.; Wang, Y.; Tan, F.; Tian, K. Construction and Immunogenicity of a Recombinant Pseudorabies Virus Variant With TK/GI/GE/11k/28k Deletion. Front. Vet. Sci. 2022, 8, 797611. [Google Scholar] [CrossRef]
- Freuling, C.M.; Müller, T.F.; Mettenleiter, T.C. Vaccines against Pseudorabies Virus (PrV). Vet. Microbiol. 2017, 206, 3–9. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, H.J.; Ko, H.L.; Lee, J.E.; Lee, H.J.; Kim, H.; Nam, J.H. Evaluation of Glycoprotein E Subunit and Live Attenuated Varicella-Zoster Virus Vaccines Formulated with a Single-Strand RNA-Based Adjuvant. Immun. Inflamm. Dis. 2020, 8, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Guo, W.; Xu, Z. Fluctuant Rule of Colostral Antibodies and the Date of Initial Immunization for the Piglet from Sows Inoculated with Pseudorabies Virus Gene-Deleted Vaccine SA215. Chin. J. Vet. Sci. 2004, 24, 320–322. [Google Scholar]
- Sperimentale, I.Z. A Study of the Ability of a TK-Negative and GI/GE-Negative Pseudorabies Virus (PRV) Mutant Inoculated by Different Routes to Protect Pigs Against PRV Infection. J. Vet. Med. Sci. 2000, 47, 753–762. [Google Scholar]
- Gu, Z.; Dong, J.; Wang, J.; Hou, C.; Sun, H.; Yang, W.; Bai, J.; Jiang, P. A Novel Inactivated GE/GI Deleted Pseudorabies Virus (PRV) Vaccine Completely Protects Pigs from an Emerged Variant PRV Challenge. Virus Res. 2015, 195, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhou, Q.; Song, W.; Sun, E.; Zhang, M.; He, Q.; Chen, H.; Wu, B.; Liu, Z. Novel Pseudorabies Virus Variant with Defects in TK, GE and GI Protects Growing Pigs against Lethal Challenge. Vaccine 2015, 33, 5733–5740. [Google Scholar] [CrossRef]
- Tong, W.; Li, G.; Liang, C.; Liu, F.; Tian, Q.; Cao, Y.; Li, L.; Zheng, X.; Zheng, H.; Tong, G. A Live, Attenuated Pseudorabies Virus Strain JS-2012 Deleted for GE/GI Protects against Both Classical and Emerging Strains. Antivir. Res. 2016, 130, 110–117. [Google Scholar] [CrossRef]
- Mettenleiter, T.C. Aujeszky’s Disease (Pseudorabies) Virus: The Virus and Molecular Pathogenesis—State of the Art, June 1999. Vet. Res. 2000, 31, 99–115. [Google Scholar] [CrossRef]
- Zhou, P.; Fan, H.; Lan, T.; Yang, X.L.; Shi, W.F.; Zhang, W.; Zhu, Y.; Zhang, Y.W.; Xie, Q.M.; Mani, S.; et al. Fatal Swine Acute Diarrhoea Syndrome Caused by an HKU2-Related Coronavirus of Bat Origin. Nature 2018, 556, 255–259. [Google Scholar] [CrossRef]
- Nie, Z.; Zhu, S.; Wu, L.; Sun, R.; Shu, J.; He, Y.; Feng, H. Progress on Innate Immune Evasion and Live Attenuated Vaccine of Pseudorabies Virus. Front. Microbiol. 2023, 14, 1138016. [Google Scholar] [CrossRef]
- Wu, Q.; Zhao, X.; Bai, Y.; Sun, B.; Xie, Q.; Ma, J. The First Identification and Complete Genome of Senecavirus A Affecting Pig with Idiopathic Vesicular Disease in China. Transbound. Emerg. Dis. 2016, 64, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Kim, H.; Choi, S.; Hyun, B. An Oral Aujeszky’s Disease Vaccine (YS-400) Induces Neutralizing Antibody in Pigs. Clin. Exp. Vaccine Res. 2016, 5, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Mettenleiter, T.C. Aujeszky’s Disease and the Development of the Marker/DIVA Vaccination Concept. Pathogens 2020, 9, 563. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, Y.; Zhang, Y.; Li, P.; Liu, X.; Zhang, X.; Li, J.; Zhang, T.; Li, X.; Xiao, D.; et al. Comprehensive Evaluation of the Safety and Immunogenicity of a Gene-Deleted Variant Pseudorabies Virus Attenuated Vaccine. Vet. Res. 2022, 53, 73. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wu, L.; Wang, M.; Cheng, A.; Yang, Q.; Wu, Y.; Jia, R.; Zhu, D.; Zhao, X.; Chen, S.; et al. Alpha-Herpesvirus Thymidine Kinase Genes Mediate Viral Virulence and Are Potential Therapeutic Targets. Front. Microbiol. 2019, 10, 941. [Google Scholar] [CrossRef]
- Ferrari, M.; Mettenleiter, T.C.; Romanelli, M.G.; Cabassi, E.; Corradi, A.; Mas, N.D.; Silini, R. A Comparative Study of Pseudorabies Virus (PRV) Strains with Defects in Thymidine Kinase and Glycoprotein Genes. J. Comp. Pathol. 2000, 123, 152–163. [Google Scholar] [CrossRef]
- Xu, L.; Wei, J.; Zhao, J.; Xu, S.; Lee, F.; Nie, M. The Immunity Protection of Central Nervous System Induced by Pseudorabies Virus DelgI/GE/TK in Mice. Front. Microbiol. 2022, 13, 862907. [Google Scholar] [CrossRef]
- Li, J.; Fang, K.; Rong, Z.; Li, X.; Ren, X.; Ma, H.; Chen, H.; Li, X.; Qian, P. Comparison of GE/GI- and TK/GE/GI-Gene-Deleted Pseudorabies Virus Vaccines Mediated by CRISPR/Cas9 and Cre/Lox Systems Jianglong. Viruses 2020, 12, 369. [Google Scholar] [CrossRef]
- Sun, L.; Tang, Y.; Yan, K.; Zhang, H. Construction of a Quadruple Gene—Deleted Vaccine Confers Complete Protective Immunity against Emerging PRV Variant Challenge in Piglets. Virol. J. 2022, 19, 19. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, Z.; Liu, X.; Li, P.; Yang, F.; Zhao, J.; Fan, Y.; Sun, X.; Zhu, L. A Live GI/GE-Deleted Pseudorabies Virus (PRV) Protects Weaned Piglets against Lethal Variant PRV Challenge. Virus Genes. 2017, 53, 565–572. [Google Scholar] [CrossRef]
- Petrini, S.; Righi, C.; Iscaro, C.; Viola, G.; Gobbi, P.; Scoccia, E.; Rossi, E.; Pellegrini, C.; De Mia, G.M. Evaluation of Passive Immunity Induced by Immunisation Using Two Inactivated Ge-Deleted Marker Vaccines against Infectious Bovine Rhinotracheitis (IBR) in Calves. Vaccines 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Romera, S.A.; Puntel, M.; Quattrocchi, V.; Zajac, P.D.M.; Zamorano, P.; Blanco Viera, J.; Carrillo, C.; Chowdhury, S.; Borca, M.V.; Sadir, A.M. Protection Induced by a Glycoprotein E-Deleted Bovine Herpesvirus Type 1 Marker Strain Used Either as an Inactivated or Live Attenuated Vaccine in Cattle. BMC Vet. Res. 2014, 10, 8. [Google Scholar] [CrossRef]
- Wang, C.H.; Yuan, J.; Qin, H.Y.; Luo, Y.; Cong, X.; Li, Y.; Chen, J.; Li, S.; Sun, Y.; Qiu, H.J. A Novel GE-Deleted Pseudorabies Virus (PRV) Provides Rapid and Complete Protection from Lethal Challenge with the PRV Variant Emerging in Bartha-K61-Vaccinated Swine Population in China. Vaccine 2014, 32, 3379–3385. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, Z.; Ge, A.; Guo, R.; Qiao, Y.; Xu, M.; Wang, Z.; Liu, Y.; Zheng, Y.; Fan, H.; et al. Safety and Immunogenicity of an Attenuated Chinese Pseudorabies Variant by Dual Deletion of TK&gE Genes. BMC Vet. Res. 2018, 14, 287. [Google Scholar] [CrossRef]
- Wang, J.; Guo, R.; Qiao, Y.; Xu, M.; Wang, Z.; Liu, Y.; Gu, Y.; Liu, C.; Hou, J. An Inactivated GE-Deleted Pseudorabies Vaccine Provides Complete Clinical Protection and Reduces Virus Shedding against Challenge by a Chinese Pseudorabies Variant. BMC Vet. Res. 2016, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Lei, J.; Xia, S.; Wang, Y.; Li, Y.; Li, S.; Luo, Y.; Sun, Y.; Qiu, H. Pathogenicity and Immunogenicity of a GE/GI/TK Gene-Deleted Pseudorabies Virus Variant in Susceptible Animals. Vet. Microbiol. 2016, 182, 170–177. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, Z.; Lv, L.; Xiao, Y.; Qu, Y.; Ma, H.; Niu, Y.; Wang, G.; Liu, S. Outbreak of Variant Pseudorabies Virus in Bartha-K61—Vaccinated Piglets in Central Shandong Province, China. J. Vet. Diagn. Investig. 2015, 27, 600–605. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, L.; Jia, X.; Wang, T.; Wang, J.; Sun, Z.; Wang, L.; Li, X.; Tan, F.; Tian, K. Construction of a Triple Gene-Deleted Chinese Pseudorabies Virus Variant and Its Efficacy Study as a Vaccine Candidate on Suckling Piglets. Vaccine 2015, 33, 2432–2437. [Google Scholar] [CrossRef]
- Dong, J.; Bai, J.; Sun, T.; Gu, Z.; Wang, J.; Sun, H.; Jiang, P. Comparative Pathogenicity and Immunogenicity of Triple and Double Gene-Deletion Pseudorabies Virus Vaccine Candidates. Res. Vet. Sci. 2017, 115, 17–23. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, K.; Zhang, H.; Zhang, H.; Yu, Y.; Yin, D.; Shan, H.; Qin, Z. Efficacy of a GB + GD-Based Subunit Vaccine and the Adjuvant Granulocyte-Macrophage Colony Stimulating Factor for Pseudorabies Virus in Rabbits. Front. Microbiol. 2022, 13, 965997. [Google Scholar] [CrossRef]
- Jin, Y.L.; Yin, D.; Xing, G.; Huang, Y.M.; Fan, C.M.; Fan, C.F.; Qiu, X.H.; Dong, W.R.; Yan, Y.; Gu, J.Y.; et al. The Inactivated GE/TK Gene-Deleted Vaccine Against Pseudorabies Virus Type II Confers Effective Protection in Mice and Pigs. Front. Microbiol. 2022, 13, 943707. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, L.; Fu, Z.F. Effective Cross-Protection of a Lyophilized Live GE/GI/TK-Deleted Pseudorabies Virus (PRV) Vaccine against Classical and Variant PRV Challenges. Vet. Microbiol. 2022, 267, 109387. [Google Scholar] [CrossRef] [PubMed]
- Merck U.S. PRV/Marker Gold Pseudorabies Vaccine. Available online: https://www.drugs.com/vet/prv-marker-gold.html (accessed on 2 September 2024).
- Brun, A.; Albina, E.; Barret, T.; Chapman, D.A.G.; Czub, M.; Dixon, L.K.; Keil, G.M.; Klonjkowski, B.; Le Potier, M.F.; Libeau, G.; et al. Antigen Delivery Systems for Veterinary Vaccine Development. Viral-Vector Based Delivery Systems. Vaccine 2008, 26, 6508–6528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Abid, M.; Cao, S.; Zhu, S. Recombinant Pseudorabies Virus Usage in Vaccine Development against Swine Infectious Disease. Viruses 2023, 15, 370. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xiao, Y.; Yang, Q.; Wang, Y.; Sun, Z.; Zhang, C.; Yan, S.; Wang, J.; Guo, L.; Yan, H.; et al. Construction of a GE-Deleted Pseudorabies Virus and Its Efficacy to the New-Emerging Variant PRV Challenge in the Form of Killed Vaccine. Biomed. Res. Int. 2015, 2015, 684945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Zhang, R.H.; Liu, G.; Li, G.M.; Wang, F.X.; Wen, Y.J.; Shan, H. Evaluation of Immunogenicity of Gene-Deleted and Subunit Vaccines Constructed against the Emerging Pseudorabies Virus Variants. Virol. J. 2023, 20, 98. [Google Scholar] [CrossRef]
- Mettenleiter, T.C.; Ehlers, B.; Müller, T.; Yoon, K.J. Vaccines against Pseudorabies Virus and Their Impact on Marker Vaccine Strategies. Vet. Microbiol. 2012, 165, 42–50. [Google Scholar]
- Ma, W.; Lager, K.M.; Vincent, A.L.; Janke, B.H.; Richt, J.A. The Role of Viral Glycoproteins in the Pathogenesis and Immunity of Swine Pseudorabies Virus Infections. Vet. Res. 2008, 39, 137. [Google Scholar]
- Cochran, M.D. Attenuated, Genetically-Engineered Pseudorabies Virus S-PRV-155. U.S. Patent US5240703A, 1995. [Google Scholar]
- Kit, S.; Sheppard, M.; Ichimura, H.; Kit, M. Second-Generation Pseudorabies Virus Vaccine with Deletions in Thymidine Kinase and Glycoprotein Genes. Am. J. Vet. Res. 1987, 48, 780–793. [Google Scholar]
- Du, L.; Pang, F.; Yu, Z.; Xu, X.; Fan, B.; Huang, K.; He, K.; Li, B. Assessment of the Efficacy of Two Novel DNA Vaccine Formulations against Highly Pathogenic Porcine Reproductive and Respiratory Syndrome Virus. Sci. Rep. 2017, 7, srep41886. [Google Scholar] [CrossRef]
- Zaripov, M.M.; Morenkov, O.S.; Fodor, N.; Brown, A.; Schmatchenko, V.V.; Fodor, I. Distribution of B-Cell Epitopes on the Pseudorabies Virus Glycoprotein B. J. Gen. Virol. 1999, 80, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Gut-Winiarska, M.; Jacobs, L.; Kerstens, H.; Bienkowska-Szewczyk, K. A Highly Specific and Sensitive Sandwich Blocking ELISA Based on Baculovirus Expressed Pseudorabies Virus Glycoprotein B. J. Virol. Methods 2000, 88, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, G.; Wan, C.; Li, Y.; Peng, L.; Fang, R.; Peng, Y.; Ye, C. A Comparison of Pseudorabies Virus Latency to Other A-Herpesvirinae Subfamily Members. Viruses 2022, 14, 1386. [Google Scholar] [CrossRef] [PubMed]
- Treat, B.R.; Bidula, S.M.; Ramachandran, S.; St Leger, A.J.; Hendricks, R.L.; Kinchington, P.R. Influence of an Immunodominant Herpes Simplex Virus Type 1 CD8+T Cell Epitope on the Target Hierarchy and Function of Subdominant CD8+T Cells. PLoS Pathog. 2017, 13, e1006732. [Google Scholar] [CrossRef] [PubMed]
- Takada, A.; Okazaki, K.; Kida, H. Protective Effects of Intranasal Vaccination with Plasmid Encoding Pseudorabies Virus Glycoprotein B in Mice. J. Vet. Res. 1999, 47, 25–33. [Google Scholar]
- Hua, T.; Chang, C.; Zhang, X.; Huang, Y.; Wang, H.; Zhang, D.; Tang, B. Protective Efficacy of Intranasal Inactivated Pseudorabies Vaccine Is Improved by Combination Adjuvant in Mice. Front. Microbiol. 2022, 13, 976220. [Google Scholar] [CrossRef]
- Liu, Y.; Heim, K.P.; Che, Y.; Chi, X.; Qiu, X.; Han, S.; Dormitzer, P.R.; Yang, X. Prefusion Structure of Human Cytomegalovirus Glycoprotein B and Structural Basis for Membrane Fusion. Sci. Adv. 2021, 7, abf3178. [Google Scholar] [CrossRef]
- Xiao, S.; Chen, H.; Fang, L.; Liu, C.; Zhang, H.; Jiang, Y.; Hong, W. Comparison of Immune Responses and Protective Efficacy of Suicidal DNA Vaccine and Conventional DNA Vaccine Encoding Glycoprotein C of Pseudorabies Virus in Mice. Vaccine 2004, 22, 345–351. [Google Scholar] [CrossRef]
- van Rooij, E.; Haagmans, B.; Glansbeek, H.; de Visser, Y.; Boersma, W.; Bianchi, A. DNA Vaccine Coding for Glycoprotein B of PRV Induces Cytotoxic T Cell Responses in Pigs against PRV. Vet. Res. 2000, 31, 132–133. [Google Scholar] [CrossRef]
- Vanrooij, E.M.A.; Haagmans, B.L.; Glansbeek, H.L.; De Visser, Y.E.; De Bruin, M.G.M.; Boersma, W.; Bianchi, A.T.J. A DNA Vaccine Coding for Glycoprotein B of Pseudorabies Virus Induces Cell-Mediated Immunity in Pigs and Reduces Virus Excretion Early after Infection. Vet. Immunol. Immunopathol. 2000, 74, 121–136. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Publ. Gr. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Xiao, S.; Zhou, R.; Fang, L.; He, Q.; Wu, B.; Zhou, F.; Chen, H. Protection Induced by Intramuscular Immunization with DNA Vaccines of Pseudorabies in Mice, Rabbits and Piglets. Vaccine 2002, 20, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.A.; Aleyas, A.G.; George, J.A.; Park, S.O.; Han, Y.W.; Lee, J.H.; Kang, H.Y.; Kang, S.H.; Cho, J.G.; Eo, S.K. Modulation of Immune Responses Induced by DNA Vaccine Expressing Glycoprotein B of Pseudorabies Virus via Coadministration of IFN-γ-Associated Cytokines. J. Interf. Cytokine Res. 2006, 26, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.A.; Han, Y.W.; Aleyas, A.G.; George, J.A.; Kim, S.J.; Kim, H.K.; Song, H.J.; Cho, J.G.; Eo, S.K. Protective Immunity Induced by Systemic and Mucosal Delivery of DNA Vaccine Expressing Glycoprotein B of Pseudorabies Virus. J. Microbiol. Biotechnol. 2008, 18, 591–599. [Google Scholar] [PubMed]
- Grabowska, A.K.; Lipińska, A.D.; Rohde, J.; Szewczyk, B.; Bienkowska-Szewczyk, K.; Rziha, H.J. New Baculovirus Recombinants Expressing Pseudorabies Virus (PRV) Glycoproteins Protect Mice against Lethal Challenge Infection. Vaccine 2009, 27, 3584–3591. [Google Scholar] [CrossRef]
- Bai, B.; Lu, X.; Meng, J.; Hu, Q.; Mao, P.; Lu, B.; Chen, Z.; Yuan, Z.; Wang, H. Vaccination of Mice with Recombinant Baculovirus Expressing Spike or Nucleocapsid Protein of SARS-like Coronavirus Generates Humoral and Cellular Immune Responses. Mol. Immunol. 2008, 45, 868–875. [Google Scholar] [CrossRef]
- Takashima, Y.; Nagane, N.; Hushur, O.; Matsumoto, Y.; Otsuka, H. Bovine Herpesvirus-1 (BHV-1) Recombinant Expressing Pseudorabies Virus (PrV) Glycoproteins B and C Induces Type 1 Immune Response in BALB/c Mice. J. Vet. Med. Sci. 2002, 64, 589–596. [Google Scholar] [CrossRef]
- Aoki, H.; Sakoda, Y.; Jukuroki, K. Induction of Antibodies in Mice by a Recombinant Baculovirus Expressing Pseudorabies Virus Glycoprotein B in Mammalian Cells. Vet. Microbiol. 1999, 68, 197–207. [Google Scholar] [CrossRef]
- Otsuka, H.; Xuan, X. Construction of Bovine Herpesvirus-1 (BHV-1) Recombinants Which Express Pseudorabies Virus (PRV) Glycoproteins GB, GC, GD, and GE H. Arch. Virol. 1996, 141, 57–71. [Google Scholar] [CrossRef]
- Riviere, M.; Tartaglia, J.; Perkus, M.E.; Norton, E.K.; Bongermino, C.M.; Lacoste, F.; Duret, C.; Desmettre, P.; Paoletti, E. Protection of Mice and Swine from Pseudorabies Virus Conferred by Vaccinia Virus-Based Recombinants. J. Virol. 1992, 66, 3424–3434. [Google Scholar] [CrossRef]
- Mengeling, W.L.; Brockmeier, S.L.; Lager, K.M.; Vorwald, A.C. The Role of Biotechnologically Engineered Vaccines and Diagnostics in Pseudorabies (Aujeszky’s Disease) Eradication Strategies. Vet. Microbiol. 1997, 55, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Xuan, X.; Kimura, M.; Otsuka, H. Characterization of Pseudorabies Virus Glycoprotein B Expressed by Canine Herpesvirus. J. Vet. Med. Sci. 1999, 61, 1113–1117. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.Q.; Wang, J.W.; Chen, X.H.; Wang, X.Z.; Long, Q.X. Expression of Pseudorabies Virus GE Epitopes in Pichia Pastoris and Its Utilization in an Indirect PRV GE-ELISA. J. Virol. Methods 2003, 114, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Marchioli, C.C.; Yancey, R.J.; Petrovskis, E.A.; Timmins, J.G.; Post, L.E. Evaluation of Pseudorabies Virus Glycoprotein Gp50 as a Vaccine for Aujeszky’s Disease in Mice and Swine: Expression by Vaccinia Virus and Chinese Hamster Ovary Cells. J. Virol. 1987, 61, 3977–3982. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Zarlenga, D.S.; Ren, X. An Overview of Live Attenuated Recombinant Pseudorabies Viruses for Use as Novel Vaccines. J. Immunol. Res. 2014, 2014, 824630. [Google Scholar] [CrossRef]
- Van Rooij, E.M.A.; Moonen-Leusen, H.W.; De Visser, Y.E.; Middel, W.G.J.; Boersma, W.J.A.; Bianchi, A.T.J. A DNA Vaccine Coding for GB and GD of Pseudorabies Virus (Suid Herpes Type 1) Primes the Immune System in the Presence of Maternal Immunity More Efficiently than Conventional Vaccines. Vaccine 2006, 24, 1264–1273. [Google Scholar] [CrossRef]
- Liu, Z.; Kong, Z.; Chen, M.; Shang, Y. Design of Live-Attenuated Animal Vaccines Based on Pseudorabies Virus Platform. Anim. Dis. 2022, 2, 10. [Google Scholar] [CrossRef]
- Szpara, M.L.; Tafuri, Y.R.; Parsons, L.; Shamim, S.R.; Verstrepen, K.J.; Legendre, M.; Enquist, L.W. A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses. PLoS Pathog. 2011, 7, 14–17. [Google Scholar] [CrossRef]
- Cheng, T.Y.; Henao-Diaz, A.; Poonsuk, K.; Buckley, A.; van Geelen, A.; Lager, K.; Harmon, K.; Gauger, P.; Wang, C.; Ambagala, A.; et al. Pseudorabies (Aujeszky’s Disease) Virus DNA Detection in Swine Nasal Swab and Oral Fluid Specimens Using a GB-Based Real-Time Quantitative PCR. Prev. Vet. Med. 2021, 189, 105308. [Google Scholar] [CrossRef]
- Lomniczi, B.; Blankenship, M.L.; Ben-Porat, T. Deletions in the Genomes of Pseudorabies Virus Vaccine Strains and Existence of Four Isomers of the Genomes. J. Virol. 1984, 49, 970–979. [Google Scholar] [CrossRef]
- Katz, J.B.; Pedersen, J.C. Molecular Analysis of Pseudorabies Viral Vaccines and Their Rapid Differentiation from Wild-Type Isolates Using DNA-Amplified Glycoprotein I and Thymidine Kinase Gene Segment Polymorphisms. Biologicals 1992, 20, 187–195. [Google Scholar] [CrossRef]
- Gómez-Sebastián, S.; Pérez-Filgueira, D.M.; Gómez-Casado, E.; Nuñez, M.C.; Sánchez-Ramos, I.; Tabarés, E.; Escribano, J.M. DIVA Diagnostic of Aujeszky’s Disease Using an Insect-Derived Virus Glycoprotein E. J. Virol. Methods 2008, 153, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cui, Y.; Qiu, Z.; Zhang, B.; Cui, S. A Nanoparticle-Assisted PCR Assay to Improve the Sensitivity for Rapid Detection and Differentiation of Wild-Type Pseudorabies Virus and Gene-Deleted Vaccine Strains. J. Virol. Methods 2013, 193, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, K.; Wang, X.; Wang, W. From SARS to COVID-19: What Lessons Have We Learned? J. Infect. Public. Health 2020, 13, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.; Jin, Z.; Huang, L.; Dan, H.; Xiao, W.; Liang, J.; Zou, S.; Tang, Y. Differential Diagnosis of PRV-Infected versus Vaccinated Pigs Using a Novel EuNPs-Virus Antigen Probe-Based Blocking Fluorescent Lateral Flow Immunoassay. Biosens. Bioelectron. 2020, 155, 112101. [Google Scholar] [CrossRef] [PubMed]
- Alkan, F.; Bilge-Dagalp, S.; Karapınar, Z.; Timurkan, M.O.; Coskun, N.A.; Burgu, I. Long-Term Study (2005–2010) on the Vaccination with BoHV-1 Glycoprotein E-Deleted Marker Vaccine in Selected Two Dairy Herds in Turkey. Trop. Anim. Health Prod. 2017, 50, 353–363. [Google Scholar] [CrossRef]
- Tignon, M.; De Baere, M.; Hanon, J.B.; Goolaerts, A.; Houtain, J.Y.; Delooz, L.; Cay, A.B. Characterization of Three Commercial ELISA Kits for Detection of BOHV-1 gE Specific Antibodies in Serum and Milk Samples and Applicability of BULK Milk for Determination of Herd Status. J. Virol. Methods 2017, 245, 66–72. [Google Scholar] [CrossRef]
- Petrini, S.; Iscaro, C.; Righi, C. Antibody Responses to Bovine Alphaherpesvirus 1(BoHV-1) in Passively Immunized Calves. Viruses 2019, 1, 23. [Google Scholar] [CrossRef]
- Serena, M.S.; Geisler, C.; Metz, G.E.; Corva, S.G.; Mórtola, E.C.; Larsen, A.; Jarvis, D.L.; Echeverría, M.G. Expression and Purification of Suid Herpesvirus-1 Glycoprotein e in the Baculovirus System and Its Use to Diagnose Aujeszky’s Disease in Infected Pigs. Protein Expr. Purif. 2013, 90, 1–8. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Wu, C.-W.; Liao, C.-M.; Chien, M.-S.; Huang, C. Enhancing Expression of the Pseudorabies Virus Glycoprotein E in Yeast and Its Application in an Indirect Sandwich ELISA. J. Appl. Microbiol. 2017, 123, 594–601. [Google Scholar] [CrossRef]
- Chowdhury, S.I. Identification of an Epitope within the Bovine Herpesvirus 1 Glycoprotein E Cytoplasmic Tail and Use of a Monoclonal Antibody Directed Against the Epitope for the Differentiation between Vaccinated and Infected Animals. J. Virol. Methods 2016, 233, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Chen, H.-C.; Xiao, S.-B.; Qin, Y.-L.; He, Q.-G.; Ren, Y.-Q. Development of a Latex Agglutination Test Using the Major Epitope Domain of Glycoprotein E of Pseudorabies Virus Expressed in E. Coli to Differentiate between Immune Responses in Pigs Naturally Infected or Vaccinated with Pseudorabies Virus. Vet. Res. Commun. 2005, 29, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Favoreel, H.W.; Van Minnebruggen, G.; Nauwynck, H.J.; Enquist, L.W.; Pensaert, M.B. A Tyrosine-Based Motif in the Cytoplasmic Tail of Pseudorabies Virus Glycoprotein B Is Important for Both Antibody-Induced Internalization of Viral Glycoproteins and Efficient Cell-to-Cell Spread. J. Virol. 2002, 76, 6845–6851. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhuo, M.; Zhang, X.; Xu, C.; Jiang, J.; Gao, F.; Wan, Q.; Li, Q.; Wang, T. Indium-Tin-Oxide Thin Film Transistor Biosensors for Label-Free Detection of Avian Influenza Virus H5N1. Anal. Chim. Acta 2013, 773, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xu, H.; Zhang, S.; Kang, H.; Li, C.; Sun, Q.; Zhao, J.; Li, J.; Zhou, G.; Wang, Q.; et al. Improved Detection Sensitivity of Anti-PRV Variant Antibodies through Preparation of Anti-GB and Anti-GE Monoclonal Antibodies and Development of Blocking ELISAs. Int. J. Biol. Macromol. 2024, 260, 129425. [Google Scholar] [CrossRef]
- Wu, X.; Chen, R.; Chen, Q.; Che, Y.; Yan, S.; Zhou, L.; Wang, L. Establishment of an Indirect ELISA Method for Antibody Detection of Porcine Pseudorabies by Recombinant GB, GC, and GD Proteins. J. Med. Virol. 2023, 95, e28228. [Google Scholar] [CrossRef]
- Vallbracht, M.; Brun, D.; Tassinari, M.; Vaney, M.; Pehau-arnaudet, G. Crossm Structure-Function Dissection of Pseudorabies Virus. Virology 2018, 92, e01203-17. [Google Scholar]
- Guo, Z.; Zhang, S.; Xu, H.; Li, W.; Li, C.; Zhao, J.; Gong, B.; Sun, Q.; Xiang, L.; Zhao, H.; et al. Preparation and Identification of a Monoclonal Antibody against the Pseudorabies Virus GE Glycoprotein through a Novel Strategy. Vet. Sci. 2023, 10, 133. [Google Scholar] [CrossRef]
- Serena, M.S.; Geisler, C.; Metz, G.E.; Mórtola, E.C.; Echeverría, M.G. Production of Pseudorabies Virus Recombinant Glycoprotein B and Its Use in an Agar Gel Immunodiffusion (AGID) Test for Detection of Antibodies with Sensitivity and Specificity Equal to the Virus Neutralization Assay. J. Virol. Methods 2016, 230, 9–12. [Google Scholar] [CrossRef]
- He, H.; Qi, B.; Yang, Y.; Cui, X.; Chen, T.; Cai, X.; An, T.; Wang, S. Immunogenicity Characterization of the Recombinant GI Protein Fragment from Pseudorabies Virus and an Evaluation of Its Diagnostic Use in Pigs. Vet. Sci. 2023, 10, 506. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. PrioCHECK™ PRV gE 2.0 Antibody ELISA Kit; Thermo Fisher Scientific: Waltham, MA, USA, 2024; Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0013819_7589010_UG_EN.pdf (accessed on 18 September 2024).
- Pan, J.; Li, Y.; Wang, T.; Chang, J.; Hao, L.; Chen, J.; Peng, W.; Deng, J.; Huang, B.; Tian, K. A Poly(Dimethylsiloxane)-Based Solid-Phase Microchip Platform for Dual Detection of Pseudorabies Virus GD and GE Antibodies. Front. Cell. Infect. Microbiol. 2022, 12, 912108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-Y. Contemporary Diagnostic Approaches for Pseudorabies (Aujeszky’s Disease) Virus Based on Swine Oral Fluid Specimens; Iowa State University: Ames, IA, USA, 2021. [Google Scholar]
- Cheng, T.Y.; Magtoto, R.; Henao-Díaz, A.; Poonsuk, K.; Buckley, A.; Van Geelen, A.; Lager, K.; Zimmerman, J.; Giménez-Lirola, L. Detection of Pseudorabies Virus Antibody in Swine Serum and Oral Fluid Specimens Using a Recombinant GE Glycoprotein Dual-Matrix Indirect ELISA. J. Vet. Diagn. Investig. 2021, 33, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, H.; Tang, B.; Ye, C.; Han, M.; Teng, L.; Yue, M.; Li, Y. Fast and sensitive differential diagnosis of pseudorabies virus-infected versus pseudorabies virus-vaccinated swine using CRISPR-Cas12a. Microbiol. Spectr. 2024, 12, e0261723. [Google Scholar] [CrossRef] [PubMed]
- Valones, M.A.A.; Guimarães, R.L.; Brandão, L.A.C.; De Souza, P.R.E.; De Albuquerque Tavares Carvalho, A.; Crovela, S. Principles and Applications of Polymerase Chain Reaction in Medical Diagnostic Fields: A Review. Braz. J. Microbiol. 2009, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- En, F.X.; Wei, X.; Jian, L.; Qin, C. Loop-Mediated Isothermal Amplification Establishment for Detection of Pseudorabies Virus. J. Virol. Methods 2008, 151, 35–39. [Google Scholar] [CrossRef]
- Pérez, L.J.; Arce, H.D. de Development of a Polymerase Chain Reaction Assay for the Detection of Pseudorabies Virus in Clinical Samples. Braz. J. Microbiol. 2009, 40, 433–438. [Google Scholar] [CrossRef]
- Wernike, K.; Hoffmann, B.; Kalthoff, D.; König, P.; Beer, M. Development and Validation of a Triplex Real-Time PCR Assay for the Rapid Detection and Differentiation of Wild-Type and Glycoprotein E-Deleted Vaccine Strains of Bovine Herpesvirus Type 1. J. Virol. Methods 2011, 174, 77–84. [Google Scholar] [CrossRef]
- Pawar, S.S.; Meshram, C.D.; Singh, N.K.; Saini, M.; Mishra, B.P.; Gupta, P.K. EvaGreen-Based Multiplex Real-Time PCR Assay for Rapid Differentiation of Wild-Type and Glycoprotein E-Deleted Bovine Herpesvirus-1 Strains. Anim. Biotechnol. 2017, 28, 248–252. [Google Scholar] [CrossRef]
- Nemoto, M.; Tsujimura, K.; Yamanaka, T.; Kondo, T.; Matsumura, T. Loop-Mediated Isothermal Amplification Assays for Detection of Equid Herpesvirus 1 and 4 and Differentiating a Gene-Deleted Candidate Vaccine Strain from Wild-Type Equid Herpesvirus 1 Strains. J. Vet. Diagn. Investig. 2010, 22, 30–36. [Google Scholar] [CrossRef]
- Pawar, S.S.; Meshram, C.D.; Singh, N.K.; Saini, M.; Mishra, B.P.; Gupta, P.K. Loop-Mediated Isothermal Amplification for Rapid Detection and Differentiation of Wild-Type Bovine Herpesvirus-1 and Glycoprotein E-Deleted Marker Vaccine Strain. Anim. Biotechnol. 2015, 26, 268–272. [Google Scholar] [CrossRef]
- Wernike, K.; Beer, M.; Freuling, C.M.; Klupp, B.; Mettenleiter, T.C.; Müller, T.; Hoffmann, B. Molecular Double-Check Strategy for the Identification and Characterization of Suid Herpesvirus 1. J. Virol. Methods 2014, 209, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.-Q.; Zhang, C.-L.; Tong, G.-Z.; Qiu, H.-J.; Wang, Y.-F.; Tian, Z.-J. Expression of truncated gE gene of pseudorabies virus (PRV) and primary application in differential diagnosis of PRV vaccination and infection. Chin. J. Biotechnol. 2004, 20, 526–531. [Google Scholar] [PubMed]
- Qiu, R.; Han, Z.; Liu, Y.; Tang, G.; Li, W. Development and Evalutaion of a Rapid Test Strip for GB Antibody Detection of Pseudorabies Virus. Chin. J. Vet. Sci. 2017, 37, 1463–1467. [Google Scholar]
- Li, X.; Sun, Y.; Yang, S.; Wang, Y.; Yang, J.; Liu, Y.; Jin, Q.; Li, X.; Guo, C.; Zhang, G. Development of an Immunochromatographic Strip for Antibody Detection of Pseudorabies Virus in Swine. J. Vet. Diagn. Investig. 2015, 27, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Pol, F.; Deblanc, C.; Oger, A.; Le Dimna, M.; Simon, G.; Le Potier, M.F. Validation of a Commercial Real-Time PCR Kit for Specific and Sensitive Detection of Pseudorabies. J. Virol. Methods 2013, 187, 421–423. [Google Scholar] [CrossRef]
- Shen, H.; Xie, K.; Huang, L.; Wang, L.; Ye, J.; Xiao, M.; Ma, L.; Jia, A.; Tang, Y. A Novel SERS-Based Lateral Flow Assay for Differential Diagnosis of Wild-Type Pseudorabies Virus and GE-Deleted Vaccine. Sens. Actuators B Chem. 2019, 282, 152–157. [Google Scholar] [CrossRef]
- Ren, M.; Lin, H.; Chen, S.; Yang, M.; An, W.; Wang, Y.; Xue, C.; Sun, Y.; Yan, Y.; Hu, J. Detection of Pseudorabies Virus by Duplex Droplet Digital PCR Assay. J. Vet. Diagn. Investig. 2018, 30, 105–112. [Google Scholar] [CrossRef]
- Nonaka, C.K.V.; Fonseca Junior, A.A.; Guedes, E.O.; D’Ambros, R.M.; Lima, G.K.; Camargos, M.F.; Heinemann, M.B. Diferentes Métodos de PCR Em Tempo Real Para Detecção Do Vírus Da Pseudorraiva. Cienc. Rural. 2017, 47, e20160342. [Google Scholar] [CrossRef]
- Hua, T.; Tang, B.; Huang, J.; Chang, C.; Liu, G.Y.; Zhang, X.H.; Hou, J.B.; Zhang, D.H. Establishment of real-time PCR assay of pseudorabies virus and viral load detection of PRV attenuated vaccin. Acta Agric. Jiangxi 2019, 31, 73–78. [Google Scholar]
- Fang, L.R.; Chen, H.C.; Xiao, S.B.; He, Q.G.; Wang, G.F. Expression of the gE gene of pseudorabies virus in insect cells. Chin. J. Biotechnol. 2001, 17, 449–451. [Google Scholar]
- Tu, F.; Zhang, Y.; Xu, S.; Yang, X.; Zhou, L.; Ge, X.; Han, J.; Guo, X.; Yang, H. Detection of Pseudorabies Virus with a Real-Time Recombinase-Aided Amplification Assay. Transbound. Emerg. Dis. 2022, 69, 2266–2274. [Google Scholar] [CrossRef] [PubMed]
- Jenson, T.A. Agar Gel Immunodiffusion Assay to Detect Antibodies to Type a Influenza Virus. Methods Mol. Biol. 2020, 2123, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yao, J.; Lei, L.; Xu, K.; Liao, F.; Yang, S.; Yang, L.; Shu, X.; Duan, D.; Wang, A. Emergence of a Novel Recombinant Pseudorabies Virus Derived From the Field Virus and Its Attenuated Vaccine in China. Front. Vet. Sci. 2022, 9, 872002. [Google Scholar] [CrossRef] [PubMed]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies Virus Variant in Bartha-K61-Vaccinated Pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liang, W.; Liu, Q.; Zhao, T.; Zhu, H.; Hua, L.; Peng, Z.; Tang, X.; Stratton, C.W.; Zhou, D.; et al. Epidemiological and Genetic Characteristics of Swine Pseudorabies Virus in Mainland China between 2012 and 2017. PeerJ 2018, 6, e5785. [Google Scholar] [CrossRef]
- Bo, Z.; Miao, Y.; Xi, R.; Gao, X.; Miao, D.; Chen, H.; Jung, Y.S.; Qian, Y.; Dai, J. Emergence of a Novel Pathogenic Recombinant Virus from Bartha Vaccine and Variant Pseudorabies Virus in China. Transbound. Emerg. Dis. 2021, 68, 1454–1464. [Google Scholar] [CrossRef]
- Luo, Y.; Li, N.; Cong, X.; Wang, C.H.; Du, M.; Li, L.; Zhao, B.; Yuan, J.; Liu, D.D.; Li, S.; et al. Pathogenicity and Genomic Characterization of a Pseudorabies Virus Variant Isolated from Bartha-K61-Vaccinated Swine Population in China. Vet. Microbiol. 2014, 174, 107–115. [Google Scholar] [CrossRef]
- Tan, L.; Yao, J.; Yang, Y.; Luo, W.; Yuan, X.; Yang, L.; Wang, A. Current Status and Challenge of Pseudorabies Virus Infection in China. Virol. Sin. 2021, 36, 588–607. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bude, S.A.; Lu, Z.; Zhao, Z.; Zhang, Q. Pseudorabies Virus Glycoproteins E and B Application in Vaccine and Diagnosis Kit Development. Vaccines 2024, 12, 1078. https://doi.org/10.3390/vaccines12091078
Bude SA, Lu Z, Zhao Z, Zhang Q. Pseudorabies Virus Glycoproteins E and B Application in Vaccine and Diagnosis Kit Development. Vaccines. 2024; 12(9):1078. https://doi.org/10.3390/vaccines12091078
Chicago/Turabian StyleBude, Sara Amanuel, Zengjun Lu, Zhixun Zhao, and Qiang Zhang. 2024. "Pseudorabies Virus Glycoproteins E and B Application in Vaccine and Diagnosis Kit Development" Vaccines 12, no. 9: 1078. https://doi.org/10.3390/vaccines12091078
APA StyleBude, S. A., Lu, Z., Zhao, Z., & Zhang, Q. (2024). Pseudorabies Virus Glycoproteins E and B Application in Vaccine and Diagnosis Kit Development. Vaccines, 12(9), 1078. https://doi.org/10.3390/vaccines12091078






