Characterization of the Monkeypox Virus [MPX]-Specific Immune Response in MPX-Cured Individuals Using Whole Blood to Monitor Memory Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
2.2. Study Population
2.3. Definition of MPX Disease
2.4. Peptide Pools for the Whole Blood Assay
2.5. IFN-γ Whole Blood Assay
2.6. Humoral Response
2.7. Multiplex Analysis
2.8. Statistical Analysis
3. Results
3.1. Clinical and Epidemiological Characteristics of the Study Population
3.2. MPXV-Specific T-Cell Response in Mpox-Cured Individuals
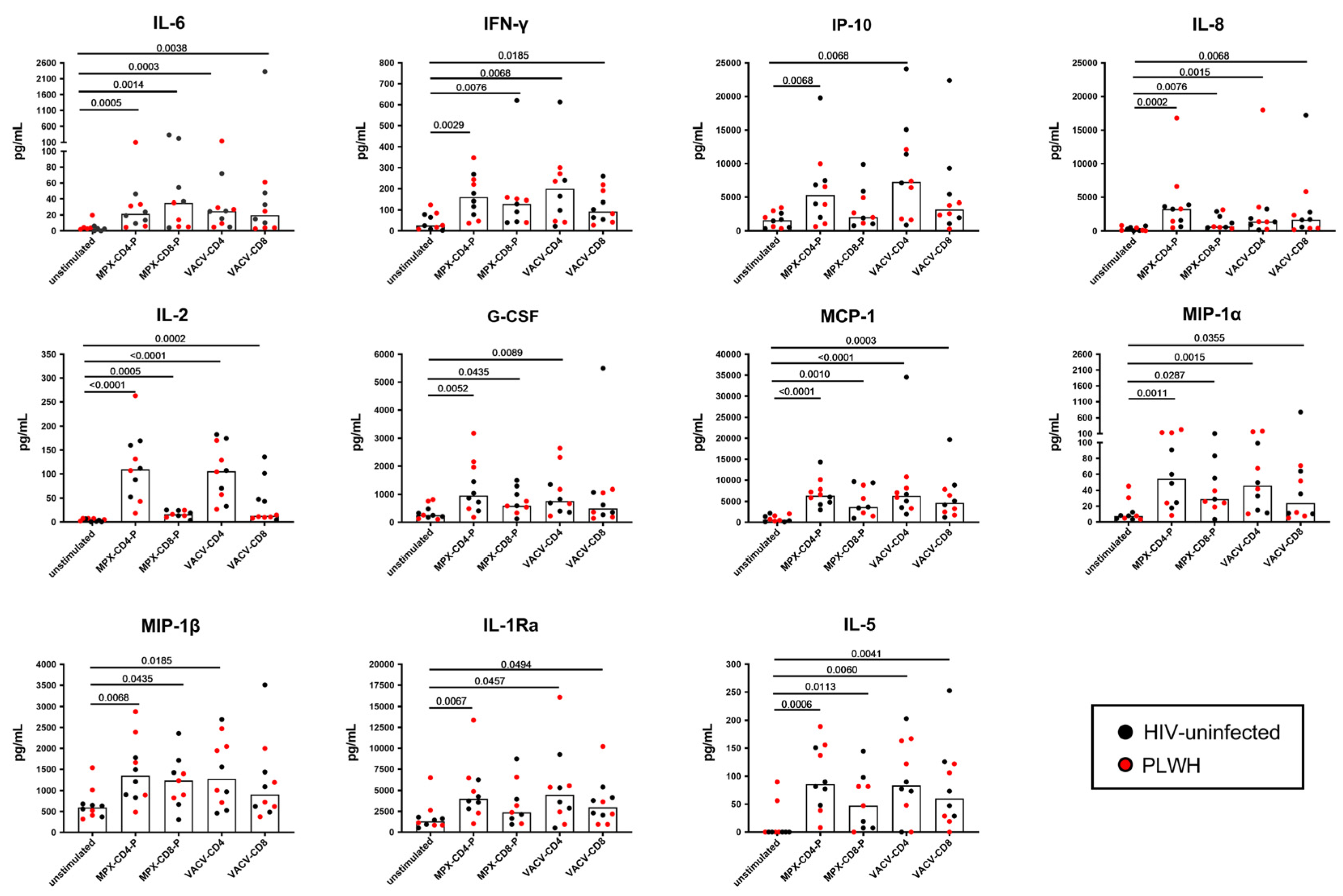
3.3. Multiplex Analysis of Immune Factors Different from IFN-γ Specifically Induced after In Vitro Mpox Peptide Stimulation in Mpox-Cured Subjects
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Multi-Country Outbreak of Mpox, External Situation Report#33-31 May 2024. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-33--31-may-2024 (accessed on 29 September 2023).
- CDC Mpox and Your Health. Available online: https://www.cdc.gov/poxvirus/mpox/your-health/index.html (accessed on 29 September 2023).
- Niu, L.; Liang, D.; Ling, Q.; Zhang, J.; Li, Z.; Zhang, D.; Xia, P.; Zhu, Z.; Lin, J.; Shi, A.; et al. Insights into Monkeypox Pathophysiology, Global Prevalence, Clinical Manifestation and Treatments. Front. Immunol. 2023, 14, 1132250. [Google Scholar] [CrossRef] [PubMed]
- Eurosurveillance editorial team Note from the Editors: WHO Declares Mpox Outbreak a Public Health Emergency of International Concern. Euro Surveill. 2024, 29, 240815v. [CrossRef]
- Zheng, J.; Zeng, J.; Long, H.; Chen, J.; Liu, K.; Chen, Y.; Du, X. Recombination and Selection Trajectory of the Monkeypox Virus during Its Adaptation in the Human Population. J. Med. Virol. 2024, 96, e29825. [Google Scholar] [CrossRef]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 Outbreak and the Pathobiology of the Monkeypox Virus. J. Autoimmun. 2022, 131, 102855. [Google Scholar] [CrossRef] [PubMed]
- Cohn, H.; Bloom, N.; Cai, G.Y.; Clark, J.J.; Tarke, A.; Bermúdez-González, M.C.; Altman, D.R.; Lugo, L.A.; Lobo, F.P.; Marquez, S.; et al. Mpox Vaccine and Infection-Driven Human Immune Signatures: An Immunological Analysis of an Observational Study. Lancet Infect. Dis. 2023, 23, 1302–1312. [Google Scholar] [CrossRef]
- Hammarlund, E.; Lewis, M.W.; Carter, S.V.; Amanna, I.; Hansen, S.G.; Strelow, L.I.; Wong, S.W.; Yoshihara, P.; Hanifin, J.M.; Slifka, M.K. Multiple Diagnostic Techniques Identify Previously Vaccinated Individuals with Protective Immunity against Monkeypox. Nat. Med. 2005, 11, 1005–1011. [Google Scholar] [CrossRef]
- Adamo, S.; Gao, Y.; Sekine, T.; Mily, A.; Wu, J.; Storgärd, E.; Westergren, V.; Filén, F.; Treutiger, C.-J.; Sandberg, J.K.; et al. Memory Profiles Distinguish Cross-Reactive and Virus-Specific T Cell Immunity to Mpox. Cell Host Microbe 2023, 31, 928–936.e4. [Google Scholar] [CrossRef]
- Matusali, G.; Mazzotta, V.; Piselli, P.; Bettini, A.; Colavita, F.; Coen, S.; Vaia, F.; Girardi, E.; Antinori, A.; Maggi, F. Asymptomatic Mpox Infection in Subjects Presenting for MVA-BN Vaccine. Clin. Infect. Dis. 2023, 77, ciad414. [Google Scholar] [CrossRef]
- Dee, K.; Manali, M.; Bissett, L.A.; Bone, J.; Magill, C.; Davis, C.; Willett, B.J.; Murcia, P.R. Smallpox Vaccination Campaigns Resulted in Age-Associated Population Cross-Immunity against Monkeypox Virus. J. Gen. Virol. 2024, 105, 001999. [Google Scholar] [CrossRef]
- Grifoni, A.; Zhang, Y.; Tarke, A.; Sidney, J.; Rubiro, P.; Reina-Campos, M.; Filaci, G.; Dan, J.M.; Scheuermann, R.H.; Sette, A. Defining Antigen Targets to Dissect Vaccinia Virus and Monkeypox Virus-Specific T Cell Responses in Humans. Cell Host Microbe 2022, 30, 1662–1670.e4. [Google Scholar] [CrossRef]
- Ennis, F.A.; Cruz, J.; Demkowicz, W.E.; Rothman, A.L.; McClain, D.J. Primary Induction of Human CD8+ Cytotoxic T Lymphocytes and Interferon-Gamma-Producing T Cells after Smallpox Vaccination. J. Infect. Dis. 2002, 185, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Isaacs, S.N. Therapeutic Vaccines and Antibodies for Treatment of Orthopoxvirus Infections. Viruses 2010, 2, 2381–2403. [Google Scholar] [CrossRef] [PubMed]
- Mack, T.M.; Noble, J.; Thomas, D.B. A Prospective Study of Serum Antibody and Protection against Smallpox. Am. J. Trop. Med. Hyg. 1972, 21, 214–218. [Google Scholar] [CrossRef]
- Chan, C.E.Z.; Wong, S.K.K.; Yazid, N.B.M.; Ng, O.T.; Marimuthu, K.; Chan, M.; Howe, H.S.; Leo, Y.-S.; Leung, B.P.; Vasoo, S.S.; et al. Residual Humoral Immunity Sustained Over Decades in a Cohort of Vaccinia-Vaccinated Individuals. J. Infect. Dis. 2023, 227, 1002–1006. [Google Scholar] [CrossRef]
- Combadiere, B.; Boissonnas, A.; Carcelain, G.; Lefranc, E.; Samri, A.; Bricaire, F.; Debre, P.; Autran, B. Distinct Time Effects of Vaccination on Long-Term Proliferative and IFN-Gamma-Producing T Cell Memory to Smallpox in Humans. J. Exp. Med. 2004, 199, 1585–1593. [Google Scholar] [CrossRef]
- Guo, L.; Song, R.; Zhang, Q.; Li, D.; Chen, L.; Fang, M.; Xiao, Y.; Wang, X.; Li, Y.; Gao, R.; et al. Profiling of Viral Load, Antibody and Inflammatory Response of People with Monkeypox during Hospitalization: A Prospective Longitudinal Cohort Study in China. eBioMedicine 2024, 106, 105254. [Google Scholar] [CrossRef]
- Yi, X.-M.; Lei, Y.-L.; Li, M.; Zhong, L.; Li, S. The Monkeypox Virus-Host Interplays. Cell Insight 2024, 3, 100185. [Google Scholar] [CrossRef]
- Golden, J.; Harryman, L.; Crofts, M.; Muir, P.; Donati, M.; Gillett, S.; Irish, C. Case of Apparent Mpox Reinfection. Sex. Transm. Infect. 2023, 99, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Agrati, C.; Cossarizza, A.; Mazzotta, V.; Grassi, G.; Casetti, R.; De Biasi, S.; Pinnetti, C.; Gili, S.; Mondi, A.; Cristofanelli, F.; et al. Immunological Signature in Human Cases of Monkeypox Infection in 2022 Outbreak: An Observational Study. Lancet Infect. Dis. 2023, 23, 320–330. [Google Scholar] [CrossRef]
- Kontsevaya, I.; Cabibbe, A.M.; Cirillo, D.M.; DiNardo, A.R.; Frahm, N.; Gillespie, S.H.; Holtzman, D.; Meiwes, L.; Petruccioli, E.; Reimann, M.; et al. Update on the Diagnosis of Tuberculosis. Clin. Microbiol. Infect. 2023. [Google Scholar] [CrossRef]
- Colavita, F.; Matusali, G.; Mazzotta, V.; Bettini, A.; Lapa, D.; Meschi, S.; Francalancia, M.; Pinnetti, C.; Bordi, L.; Mizzoni, K.; et al. Profiling the Acute Phase Antibody Response against Mpox Virus in Patients Infected during the 2022 Outbreak. J. Med. Virol. 2023, 95, e28851. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Josleyn, N.; Janosko, K.; Skinner, J.; Reeves, R.K.; Cohen, M.; Jett, C.; Johnson, R.; Blaney, J.E.; Bollinger, L.; et al. Monkeypox Virus Infection of Rhesus Macaques Induces Massive Expansion of Natural Killer Cells but Suppresses Natural Killer Cell Functions. PLoS ONE 2013, 8, e77804. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Abrahams, D.A.; Lerumo, L.; Janse van Rensburg, E.; Stone, L.; O’rie, T.; Pienaar, B.; de Kock, M.; Kaplan, G.; Mahomed, H.; et al. Functional Capacity of Mycobacterium Tuberculosis-Specific T Cell Responses in Humans Is Associated with Mycobacterial Load. J. Immunol. 2011, 187, 2222–2232. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Vita, R.; Peters, B.; Crotty, S.; Weiskopf, D.; Sette, A. SARS-CoV-2 Human T Cell Epitopes: Adaptive Immune Response against COVID-19. Cell Host Microbe 2022, 30, 1788. [Google Scholar] [CrossRef]
- Mazzotta, V.; Matusali, G.; Cimini, E.; Colavita, F.; Casetti, R.; Pinnetti, C.; Mondi, A.; Bettini, A.; Bordoni, V.; Grassi, G.; et al. Antinori ICAR 2023: Humoral and cellular immune response after eight months from mpox virus infection. In Proceedings of the OC131, Bari, Italy, 14–16 June 2023. [Google Scholar]
- Mazzotta, V.; Matusali, G.; Cimini, E.; Colavita, F.; Casetti, R.; Pinnetti, C.; Mondi, A.; Bettini, A.; Grassi, G.; Vita, S.; et al. MPOX vaccines and immunopathogenesis. In Proceedings of the CROI 2023: Humoral and Cellular Immune Response after 3 Months from MPOX Virus Infection, Seattle, WA, USA, 19–22 September 2023. [Google Scholar]
- Moraes-Cardoso, I.; Benet, S.; Carabelli, J.; Perez-Zsolt, D.; Mendoza, A.; Rivero, A.; Alemany, A.; Descalzo, V.; Alarcón-Soto, Y.; Grifoni, A.; et al. Immune Responses Associated with Mpox Viral Clearance in Men with and without HIV in Spain: A Multisite, Observational, Prospective Cohort Study. Lancet Microbe 2024, 5, 100859. [Google Scholar] [CrossRef]
- Lum, F.-M.; Torres-Ruesta, A.; Tay, M.Z.; Lin, R.T.P.; Lye, D.C.; Rénia, L.; Ng, L.F.P. Monkeypox: Disease Epidemiology, Host Immunity and Clinical Interventions. Nat. Rev. Immunol. 2022, 22, 597–613. [Google Scholar] [CrossRef]
- Parnian, R.; Heydarifard, F.; Mousavi, F.S.; Heydarifard, Z.; Zandi, M. Innate Immune Response to MPOX Infection: Mechanisms and Immune Escape. J. Innate Immun. 2024. [Google Scholar] [CrossRef]
- Saghazadeh, A.; Rezaei, N. Poxviruses and the Immune System: Implications for Monkeypox Virus. Int. Immunopharmacol. 2022, 113, 109364. [Google Scholar] [CrossRef] [PubMed]
- Al-Musa, A.; Chou, J.; LaBere, B. The Resurgence of a Neglected Orthopoxvirus: Immunologic and Clinical Aspects of Monkeypox Virus Infections over the Past Six Decades. Clin. Immunol. 2022, 243, 109108. [Google Scholar] [CrossRef]
- Johnston, S.C.; Johnson, J.C.; Stonier, S.W.; Lin, K.L.; Kisalu, N.K.; Hensley, L.E.; Rimoin, A.W. Cytokine Modulation Correlates with Severity of Monkeypox Disease in Humans. J. Clin. Virol. 2015, 63, 42–45. [Google Scholar] [CrossRef]
- Hammarlund, E.; Dasgupta, A.; Pinilla, C.; Norori, P.; Früh, K.; Slifka, M.K. Monkeypox Virus Evades Antiviral CD4+ and CD8+ T Cell Responses by Suppressing Cognate T Cell Activation. Proc. Natl. Acad. Sci. USA 2008, 105, 14567–14572. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Ahmed, R. Similarities and Differences in CD4+ and CD8+ Effector and Memory T Cell Generation. Nat. Immunol. 2003, 4, 835–842. [Google Scholar] [CrossRef]
- Howard, F.H.N.; Kwan, A.; Winder, N.; Mughal, A.; Collado-Rojas, C.; Muthana, M. Understanding Immune Responses to Viruses-Do Underlying Th1/Th2 Cell Biases Predict Outcome? Viruses 2022, 14, 1493. [Google Scholar] [CrossRef]
- Gong, J.; Zhan, H.; Liang, Y.; He, Q.; Cui, D. Role of Th22 Cells in Human Viral Diseases. Front. Med. 2021, 8, 708140. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.; Rincón, M. The Two Faces of IL-6 on Th1/Th2 Differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in Infectious Diseases Pathogenesis and Potential Therapeutic Implications. Cytokine Growth Factor Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef]
- Dorner, B.G.; Scheffold, A.; Rolph, M.S.; Hüser, M.B.; Kaufmann, S.H.E.; Radbruch, A.; Flesch, I.E.A.; Kroczek, R.A. MIP-1α, MIP-1β, RANTES, and ATAC/Lymphotactin Function Together with IFN-γ as Type 1 Cytokines. Proc. Natl. Acad. Sci. USA 2002, 99, 6181–6186. [Google Scholar] [CrossRef] [PubMed]
- Qazi, B.S.; Tang, K.; Qazi, A. Recent Advances in Underlying Pathologies Provide Insight into Interleukin-8 Expression-Mediated Inflammation and Angiogenesis. Int. J. Inflamm. 2011, 2011, e908468. [Google Scholar] [CrossRef]
- Cesta, M.C.; Zippoli, M.; Marsiglia, C.; Gavioli, E.M.; Mantelli, F.; Allegretti, M.; Balk, R.A. The Role of Interleukin-8 in Lung Inflammation and Injury: Implications for the Management of COVID-19 and Hyperinflammatory Acute Respiratory Distress Syndrome. Front. Pharmacol. 2022, 12, 808797. [Google Scholar] [CrossRef]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 Pathways in Inflammation and Human Diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef]
- Huhn, G.D.; Bauer, A.M.; Yorita, K.; Graham, M.B.; Sejvar, J.; Likos, A.; Damon, I.K.; Reynolds, M.G.; Kuehnert, M.J. Clinical Characteristics of Human Monkeypox, and Risk Factors for Severe Disease. Clin. Infect. Dis. 2005, 41, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, Q.-Z.; Zhang, H.; Liu, Z.-X.; Chen, X.-H.; Ye, L.-L.; Luo, Y. The Land-Scape of Immune Response to Monkeypox Virus. eBioMedicine 2023, 87, 104424. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Tseng, S.; Horner, R.M.; Tam, C.; Loda, M.; Rollins, B.J. Control of TH2 Polarization by the Chemokine Monocyte Chemoattractant Protein-1. Nature 2000, 404, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, K.; Onodera, A.; Kiuchi, M.; Tsuji, K.; Hirahara, K.; Nakayama, T. Conventional and Pathogenic Th2 Cells in Inflammation, Tissue Repair, and Fibrosis. Front. Immunol. 2022, 13, 945063. [Google Scholar] [CrossRef]
- Nagata, N.; Saijo, M.; Kataoka, M.; Ami, Y.; Suzaki, Y.; Sato, Y.; Iwata-Yoshikawa, N.; Ogata, M.; Kurane, I.; Morikawa, S.; et al. Pathogenesis of Fulminant Monkeypox with Bacterial Sepsis after Experimental Infection with West African Monkeypox Virus in a Cynomolgus Monkey. Int. J. Clin. Exp. Pathol. 2014, 7, 4359–4370. [Google Scholar]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- da Silva, G.B.; de Carvalho Braga, G.; Simões, J.L.B.; Kempka, A.P.; Bagatini, M.D. Cytokine Storm in Human Monkeypox: A Possible Involvement of Purinergic Signaling. Cytokine 2024, 177, 156560. [Google Scholar] [CrossRef]


| Healthy Controls | Cured-Mpox Subjects | Total | p | |
|---|---|---|---|---|
| Number (%) | 15 | 16 | 31 | |
| Median age (IQR) | 36 (30–42) | 39.5 (36–48) | 38 (32–44) | 0.1163 * |
| Male gender (%) | 4 (27) | 16 (100) | 20 (64) | <0.0001 ** |
| Origin N (%) | 0.3671 ** | |||
| Western Europe | 14 (93) | 15 (94) | 29 (94) | |
| Eastern Europe | 0 (0) | 1 (6) | 1 (3) | |
| Middle East | 1 (7) | 0 (0) | 1 (3) | |
| Vaccinated with Vaccinia virus (%) | 0 (0) | 1 (6.25) | 1 (3.2) | na |
| MPXV-SerologyN of reactive (%) | ||||
| IgM | nav | 1 (6.25) | nav | |
| IgA | nav | 7 (43.75) | nav | |
| IgG | 0 (0) | 16 (100) | nav | |
| Nabs | nav | § 14 (93) | nav | |
| HIV-infection (%) | 0 (0) | 7 (44) | 7 (23) | nav |
| CD4 count/mm3 median (IQR) | / | 754 (711–1000) | / | nav |
| HIV-RNA log10 copies/mL (plasma) | / | <30 | / | nav |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruccioli, E.; Sbarra, S.; Vita, S.; Salmi, A.; Cuzzi, G.; De Marco, P.; Matusali, G.; Navarra, A.; Pierelli, L.; Grifoni, A.; et al. Characterization of the Monkeypox Virus [MPX]-Specific Immune Response in MPX-Cured Individuals Using Whole Blood to Monitor Memory Response. Vaccines 2024, 12, 964. https://doi.org/10.3390/vaccines12090964
Petruccioli E, Sbarra S, Vita S, Salmi A, Cuzzi G, De Marco P, Matusali G, Navarra A, Pierelli L, Grifoni A, et al. Characterization of the Monkeypox Virus [MPX]-Specific Immune Response in MPX-Cured Individuals Using Whole Blood to Monitor Memory Response. Vaccines. 2024; 12(9):964. https://doi.org/10.3390/vaccines12090964
Chicago/Turabian StylePetruccioli, Elisa, Settimia Sbarra, Serena Vita, Andrea Salmi, Gilda Cuzzi, Patrizia De Marco, Giulia Matusali, Assunta Navarra, Luca Pierelli, Alba Grifoni, and et al. 2024. "Characterization of the Monkeypox Virus [MPX]-Specific Immune Response in MPX-Cured Individuals Using Whole Blood to Monitor Memory Response" Vaccines 12, no. 9: 964. https://doi.org/10.3390/vaccines12090964








