Bactofection, Bacterial-Mediated Vaccination, and Cancer Therapy: Current Applications and Future Perspectives
Abstract
1. Introduction
2. Bacterial Vectors Used as Vaccine Candidates
2.1. Main Efforts Using Bacterial-Based Experimental Vaccines
2.2. Deterioration of Immunity Due to Previous Infections with the Bacterial Vector
2.3. Induction of the Immunity in the Presence or Absence of Phagosomal Escape Genes
3. Bacterial-Mediated Cancer Therapy
3.1. Main Efforts to Treat Cancer with Bacterial Vectors
3.2. Clinical Trials Treating Cancer with Bacterial Vectors
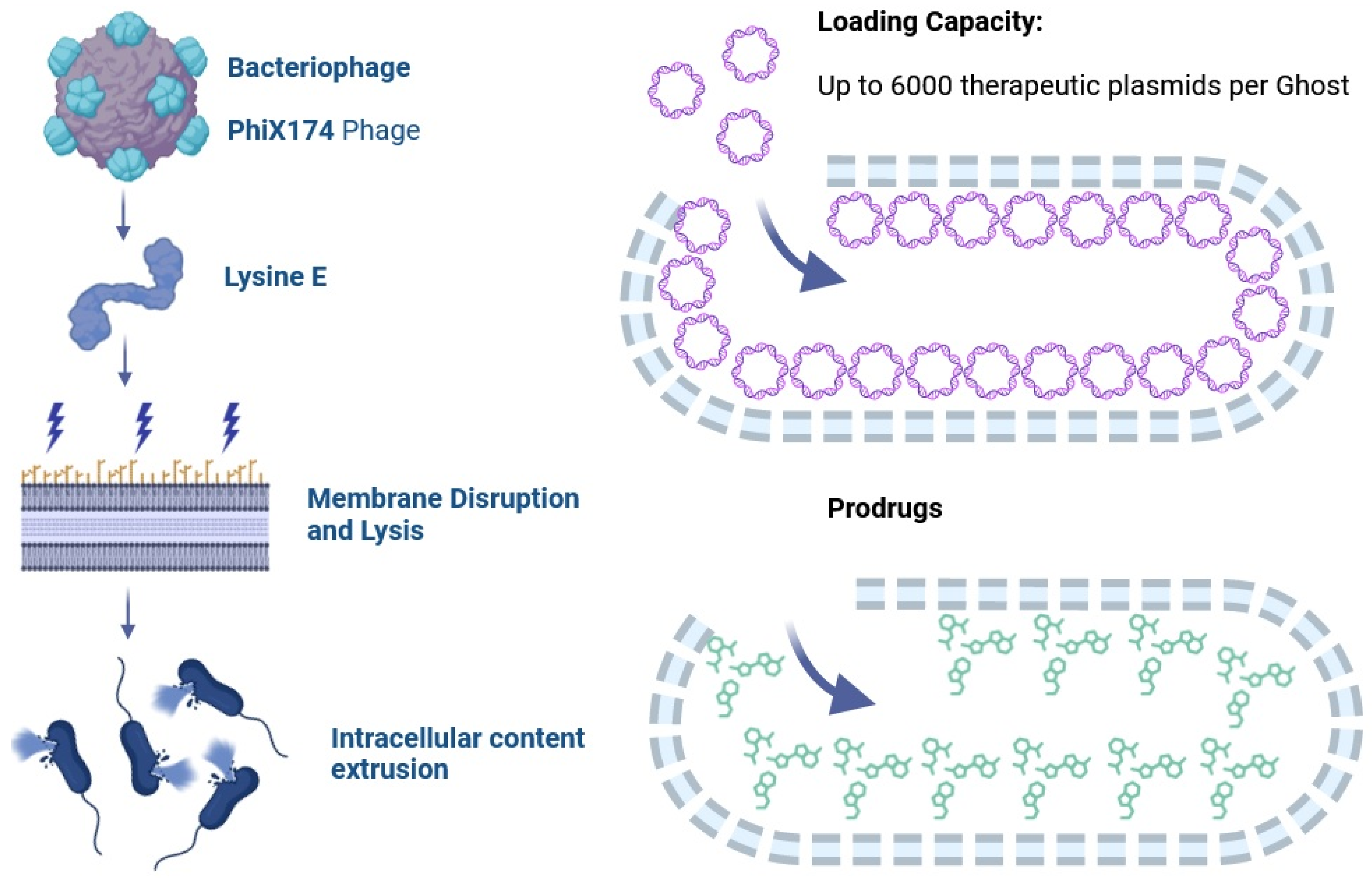
4. Vaccination Based on Bacterial Ghosts
4.1. Effectiveness of Bacterial Ghosts Loaded with Therapeutic Nucleic Acids or Drugs
4.2. Cancer Therapy Based on Bacterial Ghosts Harboring DNA
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pilgrim, S.; Stritzker, J.; Schoen, C.; Kolb-Mäurer, A.; Geginat, G.; Loessner, M.J.; Gentschev, I.; Goebel, W. Bactofection of mammalian cells by Listeria monocytogenes: Improvement and mechanism of DNA delivery. Gene Ther. 2003, 10, 2036–2045. [Google Scholar] [CrossRef][Green Version]
- Schaffner, W. Direct Transfer of Cloned Genes from Bacteria to Mammalian Cells. Proc. Natl. Acad. Sci. USA 1980, 77, 2163–2167. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Krusch, S. Bacteria-Mediated Transfer of Eukaryotic Expression Plasmids into Mammalian Host Cells. Biol. Chem. 2001, 382, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Roland, K.L.; Brenneman, K.E. Salmonellaas a Vaccine Delivery Vehicle. Expert Rev. Vaccines 2013, 12, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Park, Y.; Kim, S.; Bang, I.S. Development of an Oral Salmonella-Based Vaccine Platform against SARS-CoV-2. Vaccines 2022, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Courvalin, P.; Goussard, S.; Grillot-Courvalin, C. Gene transfer from bacteria to mammalian cells. C. R. De L’academie Des Sci. Ser. III Sci. De La Vie 1995, 318, 1207–1212. [Google Scholar] [PubMed]
- Darji, A.; Lage, S.; Garbe, A.I.; Chakraborty, T.; Weiss, S. Oral Delivery of DNA Vaccines Using attenuated Salmonella Typhimuriumas Carrier. FEMS Immunol. Med. Mic. 2000, 27, 341–349. [Google Scholar] [CrossRef]
- Dietrich, G.; Bubert, A.; Gentschev, I.; Sokolovic, Z.; Simm, A.; Catic, A.; Kaufmann, S.H.E.; Hess, J.; Szalay, A.A.; Goebel, W. Delivery of Antigen-Encoding Plasmid DNA into the Cytosol of Macrophages by Attenuated Suicide Listeria Monocytogenes. Nat. Biotechnol. 1998, 16, 181–185. [Google Scholar] [CrossRef]
- Sizemore, D.R.; Branstrom, A.A.; Sadoff, J.C. Attenuated Shigella as a DNA Delivery Vehicle for DNA-Mediated Immunization. Science 1995, 270, 299–302. [Google Scholar] [CrossRef]
- Yuhua, L.; Kunyuan, G.; Hui, C.; Yongmei, X.; Chaoyang, S.; Xun, T.; Daming, R. Oral cytokine gene therapy against murine tumor using attenuated Salmonella typhimurium. Int. J. Cancer. 2001, 94, 438–443. [Google Scholar] [CrossRef]
- Krusch, S.; Domann, E.; Frings, M.; Zelmer, A.; Diener, M.; Chakraborty, T.; Weiss, S. Listeria monocytogenes mediated CFTR transgene transfer to mammalian cells. J. Genet. Med. 2002, 4, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Kopecko, D.J.; Sieber, H.; Ures, J.A.; Fürer, A.; Schlup, J.; Knof, U.; Collioud, A.; Xu, D.Q.; Colburn, K.; Dietrich, G. Genetic stability of vaccine strain Salmonella Typhi Ty21a over 25 years. Int. J. Med. Microbiol. 2009, 299, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Robbe-saule, V.; Coynault, C.; Norel, F. The live oral typhoid vaccine Ty21a is a rpoS mutant and is susceptible to various environmental stresses. FEMS Microbiol. Lett. 1995, 126, 171–176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Witte, A.; Bläsi, U.; Halfmann, G.; Szostak, M.; Wanner, G.; Lubitz, W. Phi X174 protein E-mediated lysis of Escherichia coli. Biochimie 1990, 72, 191–200. [Google Scholar] [CrossRef]
- Montanaro, J.; Inic-Kanada, A.; Ladurner, A.; Stein, E.; Belij, S.; Bintner, N.; Schlacher, S.; Schuerer, N.; Mayr, U.B.; Lubitz, W.; et al. Escherichia coli Nissle 1917 bacterial ghosts retain crucial surface properties and express chlamydial antigen: An imaging study of a delivery system for the ocular surface. Drug. Des. Dev. Ther. 2015, 9, 3741–3754. [Google Scholar] [CrossRef][Green Version]
- Daudel, D.; Weidinger, G.; Spreng, S. Use of attenuated bacteria as delivery vectors for DNA vaccines. Expert Rev. Vaccines 2007, 6, 97–110. [Google Scholar] [CrossRef]
- Verdecia, M.; Kokai-Kun, J.F.; Kibbey, M.; Acharya, S.; Venema, J.; Atouf, F. COVID-19 vaccine platforms: Delivering on a promise? Hum. Vaccin. Immunother. 2021, 17, 2873–2893. [Google Scholar] [CrossRef]
- Weiss, S.; Chakraborty, T. Transfer of eukaryotic expression plasmids to mammalian host cells by bacterial carriers. Curr. Opin. Biotechnol. 2001, 12, 467–472. [Google Scholar] [CrossRef]
- Karpenko, L.I.; Danilenko, A.V.; Bazhan, S.I.; Danilenko, E.D.; Sysoeva, G.M.; Kaplina, O.N.; Volkova, O.Y.; Oreshkova, S.F.; Ilyichev, A.A. Attenuated Salmonella enteritidis E23 as a vehicle for the rectal delivery of DNA vaccine coding for HIV-1 polyepitope CTL immunogen. Microb. Biotechnol. 2012, 5, 241–250. [Google Scholar] [CrossRef]
- Li, Y.A.; Sun, Y.; Zhang, Y.; Wang, S.; Shi, H. Live attenuated Salmonella enterica serovar Choleraesuis vector delivering a virus-like particles induces a protective immune response against porcine circovirus type 2 in mice. Vaccine 2022, 40, 4732–4741. [Google Scholar] [CrossRef]
- Jawalagatti, V.; Kirthika, P.; Park, J.Y.; Hewawaduge, C.; Lee, J.H. Highly feasible immunoprotective multicistronic SARS-CoV-2 vaccine candidate blending novel eukaryotic expression and Salmonella bactofection. J. Adv. Res. 2022, 36, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Mengyue, M.; Qimuge, A.; Bilige, B.; Baiyin, T.; Temuqile, T.; Chen, S.; Borjigen, S.; Baigude, H.; Yang, D. Oral Delivery of SARS-CoV-2 DNA Vaccines Using Attenuated Salmonella typhimurium as a Carrier in Rat. Mol. Gen. Microbiol. Virol. 2022, 37, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Kanoh, M.; Liu, F.; Maruyama, S.; Asano, Y. Modulation of the Immune System by Listeria monocytogenes-Mediated Gene Transfer into Mammalian Cells. J. Microbiol. Immunol. 2004, 48, 329–337. [Google Scholar] [CrossRef]
- Gengenbacher, M.; Kaiser, P.; Schuerer, S.; Lazar, D.; Kaufmann, S.H.E. Post-exposure vaccination with the vaccine candidate Bacillus Calmette–Guérin ΔureC::hly induces superior protection in a mouse model of subclinical tuberculosis. Microbes Infect. 2016, 18, 364–368. [Google Scholar] [CrossRef]
- Nardelli-Haefliger, D.; Roden, R.B.S.; Benyacoub, J.; Sahli, R.; Kraehenbuhl, J.P.; Schiller, J.T.; Lachat, P.; Potts, A.; De Grandi, P. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect. Immun. 1997, 65, 3328–3336. [Google Scholar] [CrossRef]
- Herrington, D.A.; Van De Verg, L.; Formal, S.B.; Hale, T.L.; Tall, B.D.; Cryz, S.J.; Tramont, E.C.; Levine, M.M. Studies in volunteers to evaluate candidate Shigella vaccines: Further experience with a bivalent Salmonella typhi-Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine 1990, 8, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.Q.; Cisar, J.O.; Osorio, M.; Wai, T.T.; Kopecko, D.J. Core-linked LPS expression of Shigella dysenteriae serotype 1 O-antigen in live Salmonella Typhi vaccine vector Ty21a: Preclinical evidence of immunogenicity and protection. Vaccine 2007, 25, 6167–6175. [Google Scholar] [CrossRef]
- Dharmasena, M.N.; Hanisch, B.W.; Wai, T.T.; Kopecko, D.J. Stable expression of Shigella sonnei form I O-polysaccharide genes recombineered into the chromosome of live Salmonella oral vaccine vector Ty21a. Int. J. Med. Microbiol. 2013, 303, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Osorio, M.; Wu, Y.; Singh, S.; Merkel, T.J.; Bhattacharyya, S.; Blake, M.S.; Kopecko, D.J. Anthrax protective antigen delivered by Salmonella enterica serovar typhi Ty21a protects mice from a lethal anthrax spore challenged. Infect. Immun. 2009, 77, 1475–1482. [Google Scholar] [CrossRef]
- Fraillery, D.; Baud, D.; Pang, S.Y.Y.; Schiller, J.; Bobst, M.; Zosso, N.; Ponci, F.; Nardelli-Haefliger, D. Salmonella enterica serovar Typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin. Vaccine Immunol. 2007, 14, 1285–1295. [Google Scholar] [CrossRef][Green Version]
- Zhao, S.H.; Zhao, F.; Zhang, L.-Y.; Zhao, L.-J.; Zheng, J.-Y.; Cui, M.-H. Attenuated Salmonella carrying plasmid HPV18-L1 for prevention of human Papillomavirus (HPV) infection. Acta Med. 2013, 29, 201–205. [Google Scholar]
- Upton, J.W.; Kaiser, W.J.; Mocarski, E.S. Cytomegalovirus M45 Cell Death Suppression Requires Receptor-interacting Protein (RIP) Homotypic Interaction Motif (RHIM)-dependent Interaction with RIP1. J. Biol. Chem. 2008, 283, 16966. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Yan, F.; Doronina, V.A.; Escuin-Ordinas, H.; Ryan, M.D.; Brown, J.D. 2A peptides provide distinct solutions to driving stop-carry on translational recoding. Nucleic Acids Res. 2012, 40, 3143–3151. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Harding, E. WHO global progress report on tuberculosis elimination. Lancet Respir. Med. 2020, 8, 19. [Google Scholar] [CrossRef]
- Roberts, L. How COVID is derailing the fight against HIV, TB and malaria. Nature 2021, 597, 314. [Google Scholar] [CrossRef]
- Toosky, M.; Javid, B. Novel diagnostics and therapeutics for drug-resistant tuberculosis. Br. Med. Bull. 2014, 110, 129–140. [Google Scholar] [CrossRef]
- Dramsi, S.; Cossart, P. Listeriolysin O a genuine cytolysin optimized for an intracellular parasite. J. Cell Biol. 2002, 156, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.E.; Kulkarni, P.S.; Shaligram, U.; Cotton, M.F.; Rentsch, C.A.; Eisele, B.; Grode, L.; Kaufmann, S.H.E. The recombinant bacille Calmette-Guérin vaccine VPM1002: Ready for clinical efficacy testing. Front. Immunol. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Blossey, A.M.; Brückner, S.; May, M.; Parzmair, G.P.; Sharma, H.; Shaligram, U.; Grode, L.; Kaufmann, S.H.E.; Netea, M.G.; Schindler, C. VPM1002 as Prophylaxis Against Severe Respiratory Tract Infections Including COVID-19 in the Elderly: A phase III randomised, double-blind, placebo-controlled, multicenter clinical study. Clin. Infect. Dis. 2023, 76, 1304–1310. [Google Scholar] [CrossRef]
- Cotton, M.F.; Madhi, S.A.; Luabeya, A.K.; Tameris, M.; Hesseling, A.C.; Shenje, J.; Schoeman, E.; Hatherill, M.; Desai, S.; Kapse, D.; et al. Safety and immunogenicity of VPM1002 versus BCG in South African newborn babies: A randomised, phase 2 non-inferiority double-blind controlled trial. Lancet Infect. Dis. 2022, 22, 1472–1483. [Google Scholar] [CrossRef]
- Grode, L.; Ganoza, C.A.; Brohm, C.; Weiner, J.; Eisele, B.; Kaufmann, S.H.E. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine 2013, 31, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Loxton, A.G.; Knaul, J.K.; Grode, L.; Gutschmidt, A.; Meller, C.; Eisele, B.; Johnstone, H.; Van Der Spuy, G.; Maertzdorf, J.; Kaufmann, S.H.E.; et al. Safety and Immunogenicity of the Recombinant Mycobacterium bovis BCG Vaccine VPM1002 in HIV-Unexposed Newborn Infants in South Africa. Clin. Vaccine Immunol. 2017, 24, e00439-16. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M.; Ferreccio, C.; Abrego, P.; Martin, O.S.; Ortiz, E.; Cryz, S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine 1999, 17 (Suppl. 2), S22–S27. [Google Scholar] [CrossRef]
- Dharmasena, M.N.; Feuille, C.M.; Elizabeth Starke, C.C.; Bhagwat, A.A.; Stibitz, S.; Kopecko, D.J. Development of an Acid-Resistant Salmonella Typhi Ty21a Attenuated Vector for Improved Oral Vaccine Delivery. PLoS ONE 2016, 11, e0163511. [Google Scholar] [CrossRef]
- Metzger, W.G.; Mansouri, E.; Kronawitter, M.; Diescher, S.; Soerensen, M.; Hurwitz, R.; Bumann, D.; Aebischer, T.; Von Specht, B.U.; Meyer, T.F. Impact of vector-priming on the immunogenicity of a live recombinant Salmonella enterica serovar typhi Ty21a vaccine expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine 2004, 22, 2273–2277. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jeza, V.T.; Pan, Q. Salmonella Typhi: From a Human Pathogen to a Vaccine Vector. Cell. Mol. Immunol. 2008, 5, 91–97. [Google Scholar] [CrossRef][Green Version]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Nakakana, U.N.; Scorza, F.B. Towards a Four-Component GMMA-Based Vaccine against Shigella. Vaccines 2022, 10, 328. [Google Scholar] [CrossRef]
- Black, R.E.; Levine, M.M.; Clements, M.; Lou Losonsky, G.; Herrington, D.; Berman, S.; Formal, S.B. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J. Infect. Dis. 1987, 155, 1260–1265. [Google Scholar] [CrossRef]
- Bao, J.X.; Clements, J.D. Prior immunologic experience potentiates the subsequent antibody response when Salmonella strains are used as vaccine carriers. Infect. Immun. 1991, 59, 3841–3845. [Google Scholar] [CrossRef]
- Whittle, B.L.; Verma, N.K. The immune response to a B-cell epitope delivered by Salmonella is enhanced by prior immunological experience. Vaccine 1997, 15, 1737–1740. [Google Scholar] [CrossRef]
- Simanjuntak, C.H.; Totosudirjo, H.; Haryanto, P.; Suprijanto, E.; Paleologo, F.P.; Punjabi, N.H.; Witham, N.D.; Darmowigoto, R.; Soeprawoto; Hoffman, S.L. Oral immunization against typhoid fever in Indonesia with Ty21a vaccine. Lancet 1991, 338, 1055–1059. [Google Scholar] [CrossRef]
- Attridge, S.R.; Davies, R.; LaBrooy, J.T. Oral delivery of foreign antigens by attenuated Salmonella: Consequences of prior exposure to the vector strain. Vaccine 1997, 15, 155–162. [Google Scholar] [CrossRef]
- Roberts, M.; Bacon, A.; Li, J.; Chatfield, S. Prior immunity to homologous and heterologous Salmonella serotypes suppresses local and systemic anti-fragment C antibody responses and protection from tetanus toxin in mice immunized with Salmonella strains expressing fragment C. Infect. Immun. 1999, 67, 3810–3815. [Google Scholar] [CrossRef]
- Van Pijkeren, J.P.; Morrissey, D.; Monk, I.R.; Cronin, M.; Rajendran, S.; O’Sullivan, G.C.; Gahan, C.G.; Tangney, M. A novel Listeria monocytogenes-based DNA delivery system for cancer gene therapy. Hum. Gene Ther. 2010, 21, 405–416. [Google Scholar] [CrossRef]
- Darji, A.; Guzmán, C.A.; Gerstel, B.; Wachholz, P.; Timmis, K.N.; Wehland, J.; Chakraborty, T.; Weiss, S. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell 1997, 91, 765–775. [Google Scholar] [CrossRef]
- Schnupf, P.; Sansonetti, P.J. Shigella Pathogenesis: New Insights through Advanced Methodologies. Microbiol. Spectr. 2019, 7, 15–39. [Google Scholar] [CrossRef]
- Michael, A.; Stratford, R.; Khan, S.; Dalgleish, A.; Pandha, H. Novel strains of Salmonella typhimurium as potential vectors for gene delivery. FEMS Microbiol. Lett. 2004, 238, 345–351. [Google Scholar] [CrossRef]
- Coley, W.B., II. Contribution to the Knowledge of Sarcoma. Ann. Surg. 1891, 14, 199. [Google Scholar] [CrossRef]
- McCarthy, E.F. The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. Iowa Orthop. J. 2006, 26, 154. Available online: https://pubmed.ncbi.nlm.nih.gov/16789469 (accessed on 23 September 2023).
- Kramer, M.G.; Masner, M.; Ferreira, F.A.; Hoffman, R.M. Bacterial therapy of cancer: Promises, limitations, and insights for future directions. Front. Microbiol. 2018, 9, 16. [Google Scholar] [CrossRef]
- Yam, C.; Zhao, M.; Hayashi, K.; Ma, H.; Kishimoto, H.; McElroy, M.; Bouvet, M.; Hoffman, R.M. Monotherapy with a tumor-targeting mutant of S. typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J. Surg. Res. 2010, 164, 248–255. [Google Scholar] [CrossRef]
- Lahiri, A.; Eswarappa, S.M.; Das, P.; Chakravortty, D. Altering the balance between pathogen containing vacuoles and lysosomes: A lesson from Salmonella. Virulence 2010, 1, 325–329. [Google Scholar] [CrossRef]
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 2007, 67, 3201–3209. [Google Scholar] [CrossRef]
- Fu, W.; Lan, H.; Li, S.; Han, X.; Gao, T.; Ren, D.; Daming, R. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2′-deoxyriboside via Salmonella against murine tumors. Cancer Gene Ther. 2008, 15, 474–484. [Google Scholar] [CrossRef]
- Silva-Valenzuela, C.A.; Desai, P.T.; Molina-Quiroz, R.C.; Pezoa, D.; Zhang, Y.; Porwollik, S.; Zhao, M.; Hoffman, R.M.; Contreras, I.; Santiviago, C.A.; et al. Solid tumors provide niche-specific conditions that lead to preferential growth of Salmonella. Oncotarget 2016, 7, 35169. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Kale, V.; Chen, M. Gene-Directed Enzyme Prodrug Therapy. AAPS J. 2015, 17, 102. [Google Scholar] [CrossRef]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. IL-18-producing Salmonella inhibit tumor growth. Cancer Gene Ther. 2008, 15, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Xiang, R.; Mizutani, N.; Luo, Y.; Chiodoni, C.; Zhou, H.; Mizutani, M.; Ba, Y.; Becker, J.C.; Reisfeld, R.A. A DNA Vaccine Targeting Survivin Combines Apoptosis with Suppression of Angiogenesis in Lung Tumor Eradication. Cancer Res. 2005, 65, 553–561. [Google Scholar] [CrossRef]
- Chen, G.; Tang, B.; Yang, B.Y.; Chen, J.X.; Zhou, J.H.; Li, J.H.; Hua, Z.C. Tumor-targeting Salmonella typhimurium, a natural tool for activation of prodrug 6MePdR and their combination therapy in murine melanoma model. Appl. Microbiol. Biotechnol. 2013, 97, 4393–4401. [Google Scholar] [CrossRef] [PubMed]
- Manuel, E.R.; Blache, C.A.; Paquette, R.; Kaltcheva, T.I.; Ishizaki, H.; Ellenhorn, J.D.I.; Hensel, M.; Metelitsa, L.; Diamond, D.J. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella-based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer Res. 2011, 71, 4183–4191. [Google Scholar] [CrossRef]
- Crull, K.; Bumann, D.; Weiss, S. Influence of infection route and virulence factors on colonization of solid tumors by Salmonella enterica serovar Typhimurium. FEMS Immunol Med. Mic. 2011, 62, 75–83. [Google Scholar] [CrossRef]
- Kim, J.E.; Phan, T.X.; Nguyen, V.H.; Dinh-Vu, H.-V.; Zheng, J.H.; Yun, M.; Park, S.-G.; Hong, Y.; Choy, H.E.; Szardenings, M.; et al. Salmonella typhimurium Suppresses Tumor Growth via the Pro-Infammatory Cytokine Interleukin-1 beta. Theranostics 2018, 5, 1328–1342. [Google Scholar] [CrossRef]
- King, I.; Lang, W.; Runyan, J.D.; Luo, X.; Li, Z.; Zheng, L.M.; Bermudes, D.; Lin, S.; Belcourt, M.; Pike, J.; et al. Tumor-Targeted Salmonella Expressing Cytosine Deaminase as an Anticancer Agent. Hum. Gene Ther. 2004, 13, 1225–1233. [Google Scholar] [CrossRef]
- Loeffler, M.; Le’Negrate, G.; Krajewska, M.; Reed, J.C. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunol. Immun. 2009, 58, 769–775. [Google Scholar] [CrossRef]
- Xiang, R.; Luo, Y.; Niethammer, A.G.; Reisfeld, R.A. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol. Rev. 2008, 222, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.; Soldati, R.; Huebener, N.; Hohn, O.; Stermann, A.; Durmus, T.; Lobitz, S.; Zenclussen, A.C.; Christiansen, H.; Lode, H.N.; et al. Salmonella SL7207 application is the most effective DNA vaccine delivery method for successful tumor eradication in a murine model for neuroblastoma. Cancer Lett. 2013, 331, 167–173. [Google Scholar] [CrossRef]
- Choe, E.; Kazmierczak, R.A.; Eisenstark, A. Phenotypic evolution of therapeutic Salmonella enterica serovar Typhimurium after invasion of TRAMP mouse prostate tumor. mBio 2014, 5, 10–1128. [Google Scholar] [CrossRef]
- Gu, J.; Li, Y.; Zeng, J.; Wang, B.; Ji, K.; Tang, Y.; Sun, Q. Knockdown of HIF-1α by siRNA-expressing plasmid delivered by attenuated Salmonella enhances the antitumor effects of cisplatin on prostate cancer. Sci. Rep. 2017, 7, 7546. [Google Scholar] [CrossRef]
- Murakami, T.; Hiroshima, Y.; Miyak, K.; Kiyuna, T.; Endo, I.; Zha, M.; Hoffman, R.M. Efficacy of Tumor-Targeting Salmonella typhimurium A1-R against Malignancies in Patient-Derived Orthotopic Xenograft (PDOX) Murine Models. Cells 2019, 8, 599. [Google Scholar] [CrossRef] [PubMed]
- Grille, S.; Moreno, M.; Bascuas, T.; Marqués, J.M.; Muñoz, N.; Lens, D.; Chabalgoity, J.A. Salmonella enterica serovar Typhimurium immunotherapy for B-cell lymphoma induces broad anti-tumour immunity with therapeutic effect. Immunology 2014, 143, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Urashima, M.; Suzuki, H.; Yuza, Y.; Akiyama, M.; Ohno, N.; Eto, Y. An oral CD40 ligand gene therapy against lymphoma using attenuated Salmonella typhimurium. Blood 2000, 95, 1258–1263. [Google Scholar] [CrossRef]
- Vendrell, A.; Gravisaco, M.J.; Goin, J.C.; Pasetti, M.F.; Herschllik, L.; Toro J de Rodríguez, C.; Larotonda, G.; Mongini, C.; Waldner, C.I. Therapeutic effects of Salmonella typhi in a mouse model of t-cell lymphoma. J. Immunotherap. 2013, 36, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Friedlos, F.; Lehouritis, P.; Ogilvie, L.; Hedley, D.; Davies, L.; Bermudes, D.; King, I.; Martin, J.; Marais, R.; Springer, C.J. Attenuated Salmonella Targets Prodrug Activating Enzyme Carboxypeptidase G2 to Mouse Melanoma and Human Breast and Colon Carcinomas for Effective Suicide Gene Therapy. Clin. Cancer Res. 2008, 14, 4259–4266. [Google Scholar] [CrossRef]
- Ganai, S.; Arenas, R.B.; Forbes, N.S. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br. J. Cancer. 2009, 101, 1683–1691. [Google Scholar] [CrossRef]
- Li, Z.; Yin, P.H.; Yang, S.S.; Li, Q.Y.; Chang, T.; Fang, L.; Shi, L.X.; Fang, G.E. Recombinant attenuated Salmonella typhimurium carrying a plasmid co-expressing ENDO-VEGI151 and survivin siRNA inhibits the growth of breast cancer in vivo. Mol. Med. Rep. 2013, 7, 1215–1222. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, M.; Ma, H.; Li, X.; Tan, X.; Li, S.; Yang, Z.; Hoffman, R.M. Targeted Therapy with a Salmonella typhimurium Leucine-Arginine Auxotroph Cures Orthotopic Human Breast Tumors in Nude Mice. Cancer Res. 2006, 66, 7647–7652. [Google Scholar] [CrossRef]
- Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Miyake, K.; Miyake, M.; Li, S.; Han, Q.; Tan, Y.; Zhao, M.; Li, Y.; et al. Tumor-targeting Salmonella typhimurium A1-R combined with recombinant methioninase and cisplatinum eradicates an osteosarcoma cisplatinum-resistant lung metastasis in a patient-derived orthotopic xenograft (PDOX) mouse model: Decoy, trap and kill chemotherapy moves toward the clinic. Cell Cycle 2018, 17, 801–809. [Google Scholar] [CrossRef]
- Murakami, T.; Igarashi, K.; Kawaguchi, K.; Kiyuna, T.; Zhang, Y.; Zhao, M.; Hiroshima, Y.; Nelson, S.D.; Dry, S.M.; Li, Y.; et al. Tumor-targeting Salmonella typhimurium A1-R regresses an osteosarcoma in a patient-derived xenograft model resistant to a molecular-targeting drug. Oncotarget 2017, 8, 8035. [Google Scholar] [CrossRef]
- Hoffman, R.M. Tumor-targeting amino acid auxotrophic Salmonella typhimurium. Amino Acids 2009, 37, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Manuel, E.R.; Chen, J.; D’Apuzzo, M.; Lampa, M.G.; Kaltcheva, T.I.; Thompson, C.B.; Ludwig, T.; Chung, V.; Diamond, D.J. Salmonella-based therapy targeting indoleamine 2,3-dioxygenase coupled with enzymatic depletion of tumor hyaluronan induces complete regression of aggressive pancreatic tumors. Cancer Immun. Res. 2015, 3, 1096–1107. [Google Scholar] [CrossRef]
- Momiyama, M.; Zhao, M.; Kimura, H.; Tran, B.; Chishima, T.; Bouvet, M.; Endo, I.; Hoffman, R.M. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle 2012, 11, 628–632. [Google Scholar] [CrossRef]
- Baird, J.R.; Byrne, K.T.; Lizotte, P.H.; Toraya-Brown, S.; Scarlett, U.K.; Alexander, M.P.; Sheen, M.R.; Fox, B.A.; Bzik, D.J.; Bosenberg, M.; et al. Immune-mediated regression of established B16F10 melanoma by intratumoral injection of attenuated Toxoplasma gondii protects against rechallenge. J. Immun. 2013, 190, 469. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Wu, C.L.; Shiau, A.L. Systemic administration of attenuated Salmonella choleraesuis carrying thrombospondin-1 gene leads to tumor-specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gen. Ther. 2004, 12, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sasaki, T.; Fujimori, M.; Yazawa, K.; Kano, Y.; Amano, J.; Taniguchi, S. Cloned cytosine deaminase gene expression of Bifidobacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci. Biotechnol. Biochem. 2002, 66, 2362–2366. [Google Scholar] [CrossRef]
- Yi, C.; Huang, Y.; Guo, Z.Y.; Wang, S.R. Antitumor effect of cytosine deaminase/5-fluorocytosine suicide gene therapy system mediated by Bifidobacterium infantis on melanoma 1. Acta Pharmacol. Sin. 2005, 26, 629–634. [Google Scholar] [CrossRef]
- Fu, G.F.; Li, X.; Hou, Y.Y.; Fan, Y.R.; Liu, W.H.; Xu, G.X. Bifidobacterium longum as an oral delivery system of endostatin for gene therapy on solid liver cancer. Cancer Gen. Ther. 2005, 12, 133–140. [Google Scholar] [CrossRef]
- Li, X.; Fu, G.F.; Fan, Y.R.; Liu, W.H.; Liu, X.J.; Wang, J.J.; Xu, G.X. Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: Selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gen. Ther. 2003, 10, 105–111. [Google Scholar] [CrossRef]
- Heppner, F.; Möse, J.R. The liquefaction (oncolysis) of malignant gliomas by a nonpathogenic Clostridium. Acta Neurochir. 1978, 42, 123–125. [Google Scholar] [CrossRef]
- Xiang, S.; Fruehauf, J.; Li, C.J. Short hairpin RNA–expressing bacteria elicit RNA interference in mammals. Nat. Biotechnol. 2006, 24, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Seow, S.W.; Cai, S.; Rahmat, J.N.; Bay, B.H.; Lee, Y.K.; Chan, Y.H.; Mahendran, R. Lactobacillus rhamnosus GG induces tumor regression in mice bearing orthotopic bladder tumors. Cancer Sci. 2010, 101, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997, 57, 4537–4544. Available online: https://pubmed.ncbi.nlm.nih.gov/9377566/ (accessed on 23 September 2023).
- Avogadri, F.; Martinoli, C.; Petrovska, L.; Chiodoni, C.; Transidico, P.; Bronte, V.; Longhi, R.; Colombo, M.P.; Dougan, G.; Rescigno, M. Cancer Immunotherapy Based on Killing of Salmonella-Infected Tumor Cells. Cancer Res. 2005, 65, 3920–3927. [Google Scholar] [CrossRef]
- Patyar, S.; Joshi, R.; Byrav, D.S.P.; Prakash, A.; Medhi, B.; Das, B.K. Bacteria in cancer therapy: A novel experimental strategy. J. Biomed. Sci. 2010, 17, 1–9. [Google Scholar] [CrossRef]
- Saccheri, F.; Pozzi, C.; Avogadri, F.; Barozzi, S.; Faretta, M.; Fusi, P.; Rescigno, M. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci. Transl. Med. 2010, 2, 44ra57. [Google Scholar] [CrossRef]
- Knodler, L.A.; Vallance, B.A.; Celli, J.; Winfree, S.; Hansen, B.; Montero, M.; Steele-Mortimer, O. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl. Acad. Sci. USA 2010, 107, 17733–17738. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Pyroptosis and host cell death responses during Salmonella infection. Cell. Microbiol. 2007, 9, 2562–2570. [Google Scholar] [CrossRef]
- Mesa-Pereira, B.; Medina, C.; Camacho, E.M.; Flores, A.; Santero, E. Novel tools to analyze the function of Salmonella effectors show that SvpB ectopic expression induces cell cycle arrest in tumor cells. PLoS ONE 2013, 8, e78458. [Google Scholar] [CrossRef]
- Wang, Q.; Imamura, R.; Motani, K.; Kushiyama, H.; Nagata, S.; Suda, T. Pyroptotic cells externalize eat-me and release find-me signals and are efficiently engulfed by macrophages. Inter. Immunol. 2013, 25, 363–372. [Google Scholar] [CrossRef]
- Zhao, M.; Suetsugu, A.; Ma, H.; Zhang, L.; Liu, F.; Zhang, Y.; Tran, B.; Hoffman, R.M. Efficacy against lung metastasis with a tumor-targeting mutant of Salmonella typhimurium in immunocompetent mice. Cell Cycle 2012, 11, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Tu, D.G.; Chang, W.W.; Lin, S.T.; Kuo, C.Y.; Tsao, Y.T.; Lee, C.H. Salmonella inhibits tumor angiogenesis by downregulation of vascular endothelial growth factor. Oncotarget 2016, 7, 37513. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Chen, M.C.; Leong, H.F.; Kuo, C.Y.; Kuo, C.Y.; Lee, C.H. Connexin 43 Suppresses Tumor Angiogenesis by Down-Regulation of Vascular Endothelial Growth Factor via Hypoxic-Induced Factor-1α. Int. J. Mol. Sci. 2015, 16, 439–451. [Google Scholar] [CrossRef]
- Chen, Y.E.; Bousbaine, D.; Veinbachs, A.; Atabakhsh, K.; Dimas, A.; Yu, V.K.; Zhao, A.; Enright, N.J.; Nagashima, K.; Belkaid, Y.; et al. Engineered skin bacteria induce antitumor T cell responses against melanoma. Science 2023, 380, 203–210. [Google Scholar] [CrossRef]
- Siddiqui, N.A.; Ventrola, A.J.; Hartman, A.R.; Konare, T.; Kamble, N.S.; Thomas, S.C.; Madaan, T.; Kharofa, J.; Sertorio, M.G.; Kotagiri, N. An Engineered Probiotic Platform for Cancer Epitope-Independent Targeted Radionuclide Therapy of Solid Tumors. Adv. Healthc. Mater. 2023, 12, e2202870. [Google Scholar] [CrossRef]
- Komor, U.; Bielecki, P.; Loessner, H.; Rohde, M.; Wolf, K.; Westphal, K.; Weiss, S.; Häussler, S. Biofilm formation by Pseudomonas aeruginosa in solid murine tumors—A novel model system. Microbes Infect. 2012, 14, 951–958. [Google Scholar] [CrossRef]
- Johnson, C.H.; Dejea, C.M.; Edler, D.; Hoang, L.T.; Santidrian, A.F.; Felding, B.H.; Ivanisevic, J.; Cho, K.; Wick, E.C.; Hechenbleikner, E.M.; et al. Metabolism links bacterial biofilms and colon carcinogenesis. Cell Metab. 2015, 21, 891–897. [Google Scholar] [CrossRef]
- de Lima Fragelli, B.D.; Camillo, L.; de Almeida Rodolpho, J.M.; de Godoy, K.F.; de Castro, C.A.; Brassolatti, P.; da Silva, A.J.; Borra, R.C.; de Freitas Anibal, F. Antitumor Effect of IL-2 and TRAIL Proteins Expressed by Recombinant Salmonella in Murine Bladder Cancer Cells. Cell Physiol Biochem. 2021, 55, 460–476. [Google Scholar] [CrossRef]
- Saltzman, D. Abstract LB161: Microbial based immunotherapy: Saltikva as a novel therapeutic for solid tumors. Cancer Res. 2021, 81 (Suppl. 13), LB161. [Google Scholar] [CrossRef]
- Schmitz-Winnenthal, F.H.; Hohmann, N.; Schmidt, T.; Podola, L.; Friedrich, T.; Lubenau, H.; Springer, M.; Wieckowski, S.; Breiner, K.M.; Mikus, G.; et al. A phase 1 trial extension to assess immunologic efficacy and safety of prime-boost vaccination with VXM01, an oral T cell vaccine against VEGFR2, in patients with advanced pancreatic cancer. Oncoimmunology 2018, 7, e1303584. [Google Scholar] [CrossRef] [PubMed]
- Toso, J.F.; Gill, V.J.; Hwu, P.; Marincola, F.M.; Restifo, N.P.; Schwartzentruber, D.J.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Stock, F.; et al. Phase I Study of the Intravenous Administration of Attenuated Salmonella typhimurium to Patients with Metastatic Melanoma. J. Clin. Oncol. 2002, 20, 142. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Cunningham, C.; Senzer, N.; Kuhn, J.; Cramm, J.; Litz, C.; Cavagnolo, R.; Cahill, A.; Clairmont, C.; Sznol, M. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003, 10, 737–744. [Google Scholar] [CrossRef]
- Amro, A.A.; Salem-Bekhit, M.M.; Alanazi, F.K. Plackett–Burman randomization method for Bacterial Ghosts preparation form E. coli JM109. Saudi Pharm. J. 2014, 22, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, A.; Hewawaduge, C.; Lee, J.H. Salmonella enterica serovar Enteritidis ghosts displaying a surface FliC adjuvant elicit a robust immune response and effective protection against virulent challenge. Vet. Microbiol. 2020, 243, 108633. [Google Scholar] [CrossRef]
- Senevirathne, A.; Hewawaduge, C.; Lee, J.H. Immunization of chicken with flagellin adjuvanted Salmonella enteritidis bacterial ghosts confers complete protection against chicken salmonellosis. Poult. Sci. 2021, 100, 101205. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; Batah, A.M.; Ghazy, A.A.; Hussein, A.; Amara, A.A. A new strain of Acinetobacter baumannii and characterization of its ghost as a candidate vaccine. J. Infect. Public Health 2019, 12, 831–842. [Google Scholar] [CrossRef]
- Piperaki, E.T.; Syrogiannopoulos, G.A.; Tzouvelekis, L.S.; Daikos, G.L. Klebsiella pneumoniae: Virulence, Biofilm and Antimicrobial Resistance. Pediatr. Infect. Dis. J. 2017, 36, 1002–1005. [Google Scholar] [CrossRef]
- Haslberger, A.G.; Kohl, G.; Felnerova, D.; Mayr, U.B.; Fürst-Ladani, S.; Lubitz, W. Activation, stimulation and uptake of bacterial ghosts in antigen presenting cells. J. Biotechnol. 2000, 83, 57–66. [Google Scholar] [CrossRef]
- Scheerlinck, J.P.Y. Genetic adjuvants for DNA vaccines. Vaccine 2001, 19, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Mendel, S.; Holbourn, J.M.; Schouten, J.A.; Bugg, T.D.H. Interaction of the transmembrane domain of lysis protein E from bacteriophage φX174 with bacterial translocase MraY and peptidyl-prolyl isomerase SlyD. Microbiology 2006, 152, 2959–2967. [Google Scholar] [CrossRef]
- Langemann, T.; Koller, V.J.; Muhammad, A.; Kudela, P.; Mayr, U.B.; Lubitz, W. The bacterial ghost platform system. Bioeng. Bugs 2010, 1, 326–336. [Google Scholar] [CrossRef]
- Kudela, P.; Paukner, S.; Mayr, U.B.; Cholujova, D.; Schwarczova, Z.; Sedlak, J.; Bizik, J.; Lubitz, W. Bacterial ghosts as novel efficient targeting vehicles for DNA delivery to the human monocyte-derived dendritic cells. J. Immunotherap. 2005, 28, 136–143. [Google Scholar] [CrossRef]
- Huter, V.; Szostak, M.P.; Gampfer, J.; Prethaler, S.; Wanner, G.; Gabor, F.; Lubitz, W. Bacterial ghosts as drug carrier and targeting vehicles. J. Control. Release 1999, 61, 51–63. [Google Scholar] [CrossRef]
- Paukner, S.; Kohl, G.; Jalava, K.; Lubitz, W. Sealed Bacterial Ghosts—Novel Targeting Vehicles for Advanced Drug Delivery of Water-soluble Substances. J. Drug Target 2010, 11, 151–161. [Google Scholar] [CrossRef]
- Lubitz, W. Bacterial ghosts as carrier and targeting systems. Expert Opin. Biol. Ther. 2005, 1, 765–771. [Google Scholar] [CrossRef]
- Paukner, S.; Kudela, P.; Kohl, G.; Schlapp, T.; Friedrichs, S.; Lubitz, W. DNA-Loaded Bacterial Ghosts Efficiently Mediate Reporter Gene Transfer and Expression in Macrophages. Mol. Ther. 2005, 11, 215–223. [Google Scholar] [CrossRef]
- Jechlinger, W.; Tabrizi, C.A.; Lubitz, W.; Mayrhofer, P. Minicircle DNA Immobilized in Bacterial Ghosts: In vivo Production of Safe Non-Viral DNA Delivery Vehicles. Microb. Physiol. 2004, 8, 222–231. [Google Scholar] [CrossRef]
- Mayrhofer, P.; Tabrizi, C.A.; Walcher, P.; Haidinger, W.; Jechlinger, W.; Lubitz, W. Immobilization of plasmid DNA in bacterial ghosts. J. Control. Release 2005, 102, 725–735. [Google Scholar] [CrossRef]
- Ebensen, T.; Paukner, S.; Link, C.; Kudela, P.; de Domenico, C.; Lubitz, W.; Guzmán, C.A. Bacterial Ghosts Are an Efficient Delivery System for DNA Vaccines. J. Immunol. 2004, 172, 6858–6865. [Google Scholar] [CrossRef]
- Ekong, E.E.; Okenu, D.N.; Mania-Pramanik, J.; He, Q.; Igietseme, J.U.; Ananaba, G.A.; Lyn, D.; Black, C.; Eko, F.O. A Vibrio cholerae ghost-based subunit vaccine induces cross-protective chlamydial immunity that is enhanced by CTA2B, the nontoxic derivative of cholera toxin. FEMS Microbiol. Immunol. 2009, 55, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Jechlinger, W.; Haller, C.; Resch, S.; Hofmann, A.; Szostak, M.P.; Lubitz, W. Comparative immunogenicity of the Hepatitis B virus core 149 antigen displayed on the inner and outer membrane of bacterial ghosts. Vaccine 2005, 23, 3609–3617. [Google Scholar] [CrossRef]
- Panthel, K.; Jechlinger, W.; Matis, A.; Rohde, M.; Szostak, M.; Lubitz, W.; Haas, R. Generation of Helicobacter pylori ghosts by PhiX protein E-mediated inactivation and their evaluation as vaccine candidates. Infect. Immun. 2003, 71, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kudela, P.; Paukner, S.; Mayr, U.B.; Cholujova, D.; Kohl, G.; Schwarczova, Z.; Bizik, J.; Sedlak, J.; Lubitz, W. Effective gene transfer to melanoma cells using bacterial ghosts. Cancer Lett. 2008, 262, 54–63. [Google Scholar] [CrossRef]
- Paukner, S.; Kohl, G.; Lubitz, W. Bacterial ghosts as novel advanced drug delivery systems: Antiproliferative activity of loaded doxorubicin in human Caco-2 cells. J. Control. Release 2004, 94, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Fürst-Ladani, S.; Redl, H.; Haslberger, A.; Lubitz, W.; Messner, P.; Sleytr, U.B.; Schlag, G. Bacterial cell envelopes (ghosts) but not S-layers activate human endothelial cells (HUVECs) through sCD14 and LBP mechanism. Vaccine 1999, 18, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Kudela, P.; Koller, V.J.; Mayr, U.B.; Nepp, J.; Lubitz, W.; Barisani-Asenbauer, T. Bacterial Ghosts as antigen and drug delivery system for ocular surface diseases: Effective internalization of Bacterial Ghosts by human conjunctival epithelial cells. J. Biotechnol. 2011, 153, 167–175. [Google Scholar] [CrossRef]
- Kudela, P.; Koller, V.J.; Lubitz, W. Bacterial ghosts (BGs)—advanced antigen and drug delivery system. Vaccine 2010, 28, 5760–5767. [Google Scholar] [CrossRef]
- Jiao, H.; Yang, H.; Zheng, W.; Zhang, Q.; Zhao, D.; Li, G. Enhancement of immune responses by co-administration of bacterial ghosts-mediated Neisseria gonorrhoeae DNA vaccines. J. Appl. Microbiol. 2021, 130, 1770–1777. [Google Scholar] [CrossRef]
- Zhou, P.; Wu, H.; Chen, S.; Bai, Q.; Chen, X.; Chen, L.; Zeng, X.; Liu, L.; Chen, L. MOMP and MIP DNA-loaded bacterial ghosts reduce the severity of lung lesions in mice after Chlamydia psittaci respiratory tract infection. Immunobiology 2019, 224, 739–746. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, X.C.; Liu, X.Y.; Yuan, K.; Zhang, J.J.; Gao, H.H.; Li, J.N. An oral double-targeted DNA vaccine induces systemic and intestinal mucosal immune responses and confers high protection against Vibrio mimicus in grass carps. Aquaculture 2019, 504, 248–259. [Google Scholar] [CrossRef]
- Eko, F.O.; Lubitz, W.; McMillan, L.; Ramey, K.; Moore, T.T.; Ananaba, G.A.; Lyn, D.; Black, C.M.; Igietseme, J.U. Recombinant Vibrio cholerae ghosts as a delivery vehicle for vaccinating against Chlamydia trachomatis. Vaccine 2003, 21, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Eko, F.O.; He, Q.; Brown, T.; McMillan, L.; Ifere, G.O.; Ananaba, G.A.; Lyn, D.; Lubitz, W.; Kellar, K.L.; Black, C.M.; et al. A Novel Recombinant Multisubunit Vaccine against Chlamydia. J. Immun. 2004, 173, 3375–3382. [Google Scholar] [CrossRef]
- Katinger, A.; Lubitz, W.; Szostak, M.P.; Stadler, M.; Klein, R.; Indra, A.; Huter, V.; Hensel, A. Pigs aerogenously immunized with genetically inactivated (ghosts) or irradiated Actinobacillus pleuropneumoniae are protected against a homologous aerosol challenge despite differing in pulmonary cellular and antibody responses. J. Biotechnol. 1999, 73, 251–260. [Google Scholar] [CrossRef]
- Riedmann, E.M.; Lubitz, W.; McGrath, J.; Kyd, J.M.; Cripps, A.W. Effectiveness of engineering the nontypeable Haemophilus influenzae antigen Omp26 as an S-layer fusion in bacterial ghosts as a mucosal vaccine delivery. Hum. Vaccines 2011, 99–107. [Google Scholar] [CrossRef]
- Szostak, M.P.; Hensel, A.; Eko, F.O.; Klein, R.; Auer, T.; Mader, H.; Haslberger, A.; Bunka, S.; Wanner, G.; Lubitz, W. Bacterial ghosts: Non-living candidate vaccines. J. Biotechnol. 1996, 44, 161–170. [Google Scholar] [CrossRef]
- Walcher, P.; Cui, X.; Arrow, J.A.; Scobie, S.; Molinia, F.C.; Cowan, P.E.; Lubitz, W.; Duckworth, J.A. Bacterial ghosts as a delivery system for zona pellucida-2 fertility control vaccines for brushtail possums (Trichosurus vulpecula). Vaccine 2008, 26, 6832–6838. [Google Scholar] [CrossRef]
- Wang, X.; Lu, C. Mice orally vaccinated with Edwardsiella tarda ghosts are significantly protected against infection. Vaccine 2009, 27, 1571–1578. [Google Scholar] [CrossRef]
- Parmiani, G.; Rivoltini, L.; Andreola, G.; Carrabba, M. Cytokines in cancer therapy. Immunol. Lett. 2000, 74, 41–44. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Spanò, S.; Liu, X.; Galán, J.E. Proteolytic targeting of Rab29 by an effector protein distinguishes the intracellular compartments of human-adapted and broad-host Salmonella. Proc. Natl. Acad. Sci. USA 2011, 108, 18418–18423. [Google Scholar] [CrossRef]
- Rabiei, P.; Mohabatkar, H.; Behbahani, M. Studying the effects of several heat-inactivated bacteria on colon and breast cancer cells. Mol. Biol. Res. Commun. 2019, 8, 91–98. [Google Scholar] [CrossRef] [PubMed]
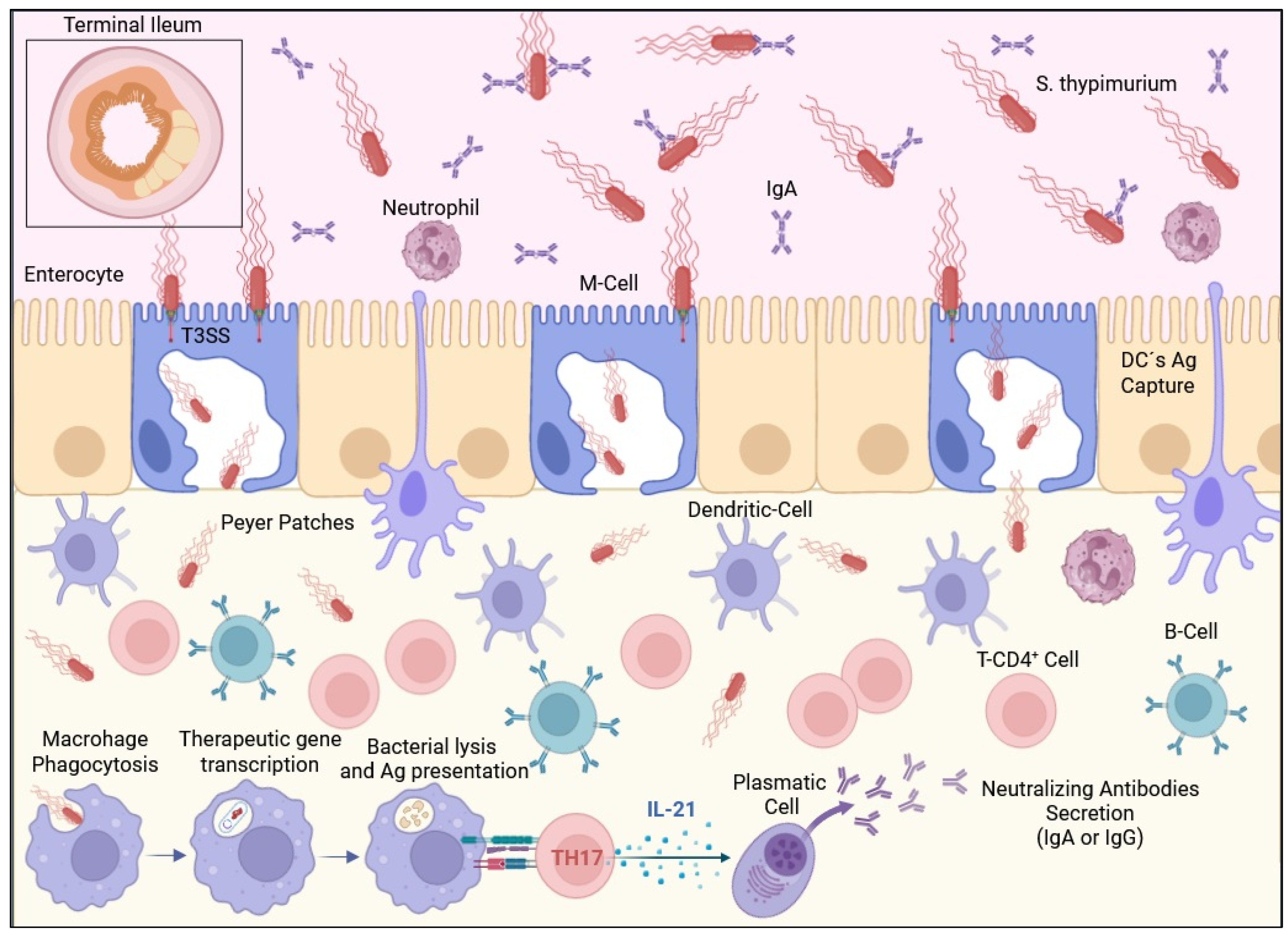
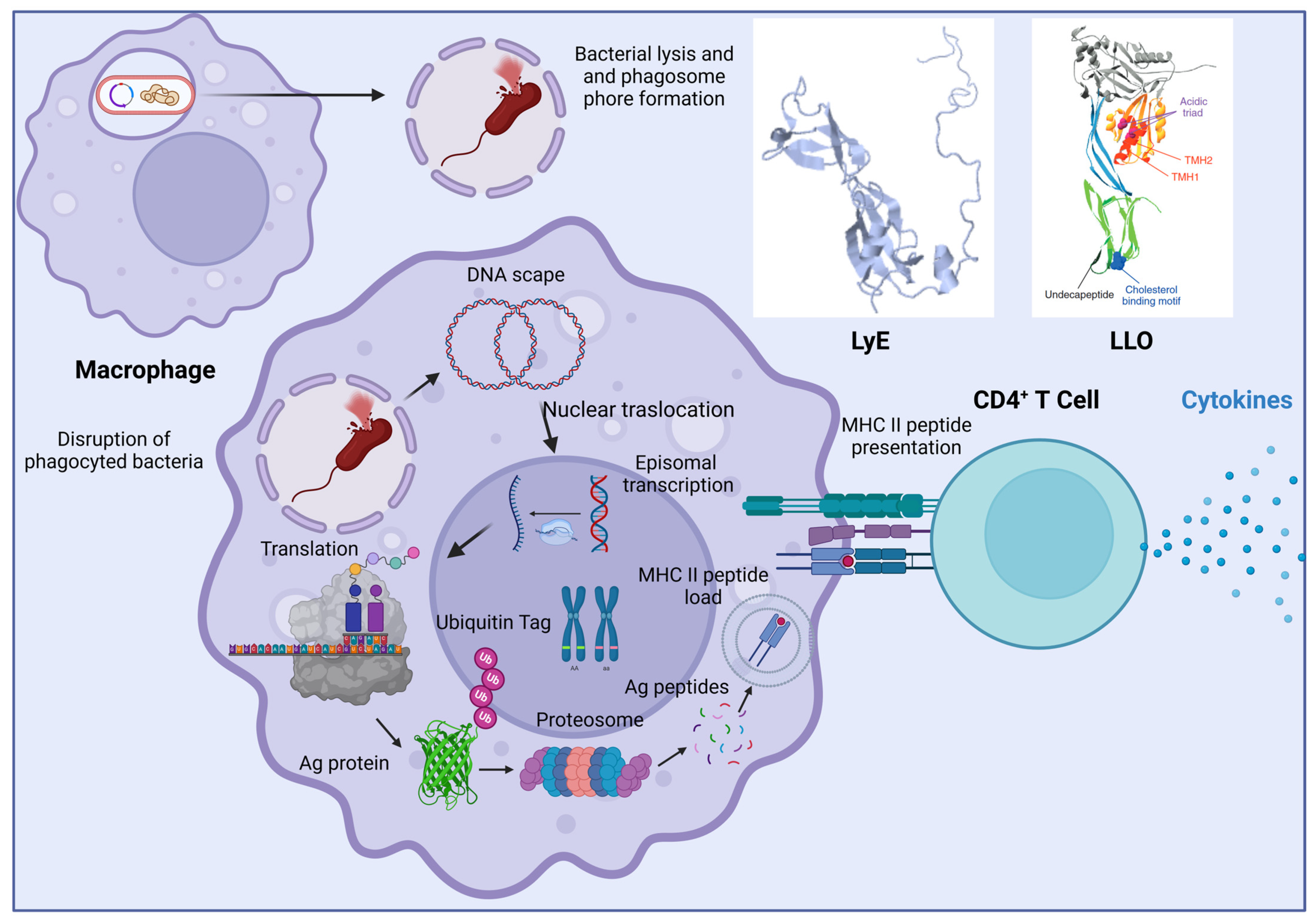
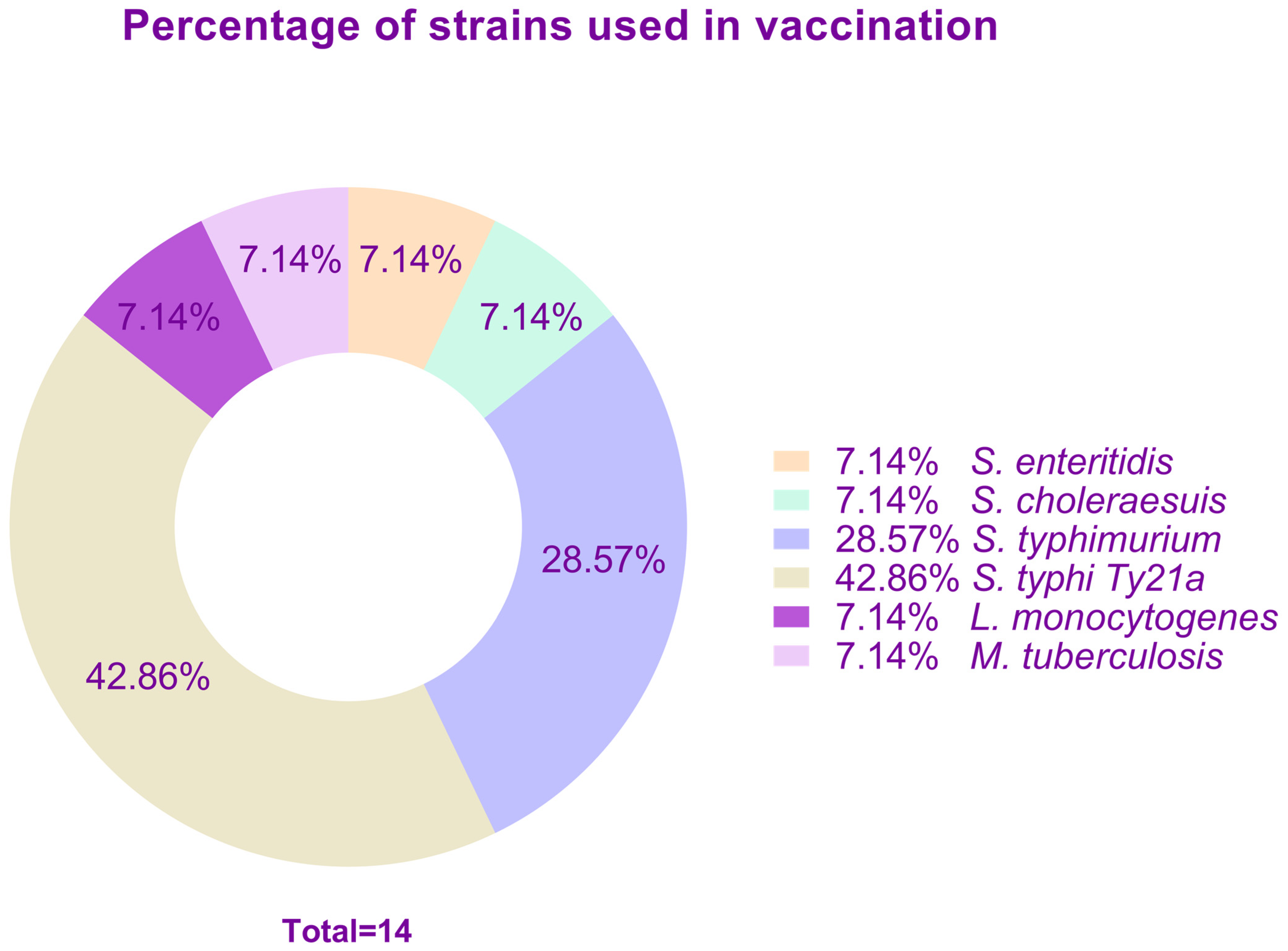
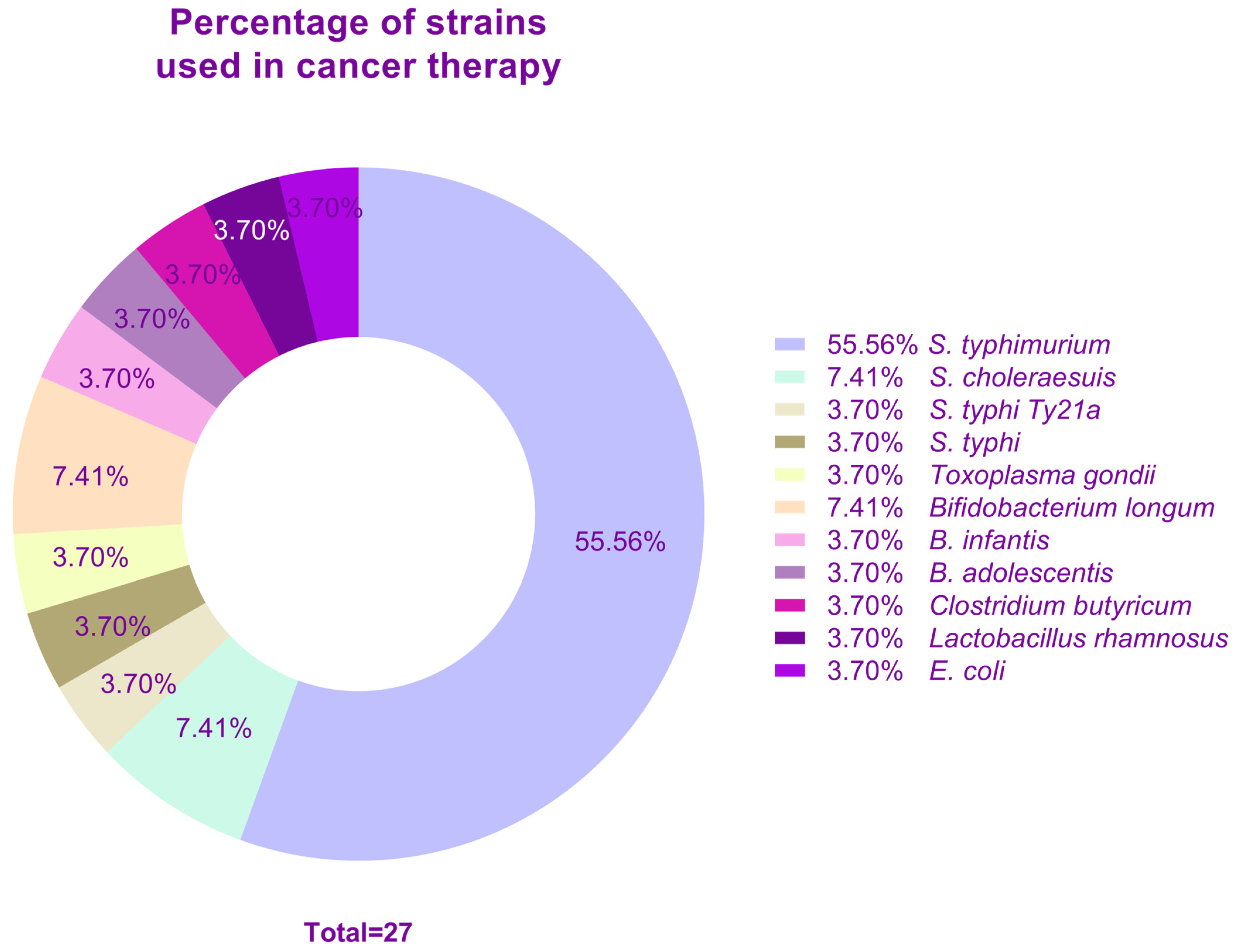

| Disease | Strain | Encoded Antigen or Strategy | Dosage | Model | Reference |
|---|---|---|---|---|---|
| Human immunodeficiency virus (HIV-1) | S. enteritidis | Polyepitope protein comprising 80 CTL epitopes from subtype A, B and C HIV-1 proteins | Two rectal doses of 108 CFU with a 4-week interval | Murine | [19] |
| Porcine circovirus type 2 (PCV2) | S. choleraesuis | Cap protein to produce functional VLPs | Two oral doses of 109 CFU with a 3-week interval | Murine | [20] |
| SARS-CoV-2 | S. typhimurium | Fusion of SipB160 signal peptide with spike protein to secrete it via TTSS into APC cytosol | Three oral doses of 107 CFU with a 2-week interval | Murine | [5] |
| SARS-CoV-2 | S. typhimurium | RBD, HR, M, nsp1, nsp2, nsp3 and nsp4 (nsp13) proteins | Single intramuscular dose of 107 CFU | Murine | [21] |
| SARS-CoV-2 | S. typhimurium | Spike protein | Three oral doses of 107 CFU in one-week interval | Murine | [22] |
| Leishmania | L. monocytogenes | IL-12 | Single dose of 2 × 106 CFU intraperitoneally or 2 × 105 CFU intravenously | Murine | [23] |
| Mycobacterium tuberculosis TB | M. tuberculosis | BCG-∆ureC::hly Deletion of urease C gene and insertion of listeriolysin O from L. monocytogenes | Two subcutaneous doses of 106 CFU | Murine | [24] |
| Human papillomavirus 16 (HPV16) | S. typhimurium | L1 major capsid protein inducing the assembly of VLPs | Nasal administration of 5 × 107 at week 0 and 5 × 108 at week 14 | Murine | [25] |
| Shigella sonnei Shigellosis | S. typhi Ty21a | S. sonnei LPS | Three oral doses of 109 CFU within one week | Human | [26] |
| S. dysenteriae Shigellosis | S. typhi Ty21a | S. dysenteriae LPS | Two doses of intraperitoneal 5 × 107 CFU | Murine | [27] |
| Shigella sonnei Shigellosis | S. typhi Ty21a | O polysaccharide (O-Ps) | Single dose of 5 × 107 CFU | Murine | [28] |
| Bacillus anthracis Anthrax | S. typhi Ty21a | PA gene fused to the secretion signal hly | Three intranasal or intraperitoneal doses of 5 × 108 CFU within two weeks | Murine | [29] |
| HPV16 | S. typhi Ty21a | L1 major capsid protein inducing the assembly of VLPs | Oral and intranasal single dose of 109 CFU | Murine | [30] |
| HPV18 | S. typhi Ty21a | L1 major capsid protein | Single intranasal dose of 5 × 108 CFU | Murine | [31] |
| Type of Tumor | Strain | Encoded Gene or Strategy | Vector | Model | Reference |
|---|---|---|---|---|---|
| Lung cancer | S. typhimurium | CCL21 and survivin | pBudCE4.1 | Murine | [70] |
| Melanoma | S. typhimurium | 6MePdR | pEZZ-EGFP | Murine | [71] |
| Melanoma | S. choleraesuis | Thrombospondin-1 (TSP-1) | pTCYTSP-1 | Murine | [95] |
| Melanoma | S. typhimurium | YS1646-shSTAT3 and survivin 3342Max | pWSK29 | Murine | [72] |
| Melanoma | S. typhimurium | HSV TK | - | Murine | [103] |
| Colorectal carcinoma | S. typhimurium | ΔinvG and ΔphoP | Chromosomal deletion | Murine | [73] |
| Colorectal carcinoma | S. typhimurium | ΔppGpp | Chromosomal deletion | Murine | [74] |
| Breast and colon carcinoma | S. typhimurium | IL-18 & FasL | pGEN206 | Murine | [69] |
| Melanoma, colon, breast, and lung carcinoma | S. typhimurium | IL-18 & Fra-1 | pIRES | Murine | [77] |
| Neuroblastoma | S. typhimurium | Survivin | pPRIEG7 | Murine | [78] |
| Prostate cancer | S. typhi Ty21a | siRNA & si-HIF-1α | - | Murine | [80] |
| B-cell lymphoma | S. typhimurium | aroC mutant | Chromosomal deletion | Murine | [82] |
| T-cell lymphoma | S. typhi | guaBA mutant | Chromosomal deletion | Murine | [84] |
| Melanoma, breast, and colon carcinoma | S. typhimurium | CPG2 | pTrc99A | Murine | [85] |
| Type of Tumor | Clinical Trial Number | Objective | Phase | Status | Reference |
|---|---|---|---|---|---|
| Metastatic pancreatic cancer | NCT04589234 | S. typhimurium harboring IL-2 gene will prolong survival and disease progression. | II | Active, not recruiting | https://clinicaltrials.gov/ct2/show/NCT04589234 (accessed on 5 June 2024) |
| Metastatic cancer | NCT00004988 | Stabilize maximum tolerated dose of attenuated S. typhimurium in a manner that increases tumor localization. | I | Completed Positive results | [103] |
| Advanced solid tumors | NCT00006254 | Determine the maximum tolerated dose and minimum effective dose of recombinant S. typhimurium. | I | Completed | https://www.clinicaltrials.gov/ct2/show/NCT00006254 (accessed on 5 June 2024) |
| Hepatic metastasis | NCT01099631 | Determine effective dose and maximum tolerated dose of S. typhimurium harboring IL-2 gene. | I | Completed | https://www.clinicaltrials.gov/ct2/show/NCT01099631 (accessed on 5 June 2024) |
| Refractory solid tumors | NCT05038150 | Assess safety, tolerability and efficacy of S. typhimurium harboring L-methioninase gene. | I and II | Recruiting | https://www.clinicaltrials.gov/ct2/show/NCT05038150 (accessed on 5 June 2024) |
| Refractory superficial solid tumors | NCT00004216 | Determine the maximum tolerated dose, safety and efficacy of S. typhimurium in solid tumors. | I | Completed | https://www.clinicaltrials.gov/ct2/show/NCT00004216 (accessed on 5 June 2024) |
| Advanced solid tumors | NCT05103345 | Assess safety, tolerability and efficacy of S. typhimurium harboring L-methioninase gene. | I and II | Recruiting | https://clinicaltrials.gov/ct2/show/NCT05103345 (accessed on 5 June 2024) |
| Multiple myeloma | NCT03762291 | Assess safety and tolerability of S. typhimurium harboring survivin gene. | I | Active, not recruiting | https://clinicaltrials.gov/ct2/show/NCT03762291 (accessed on 5 June 2024) |
| Neuroblastoma | NCT04049864 | Evaluate the safety and immunogenicity of S. typhimurium harboring neuroblastoma-associated antigen and potato virus X coat protein (PVXCP) genes. | I | Unknown | https://clinicaltrials.gov/ct2/show/NCT04049864 (accessed on 5 June 2024) |
| Refractory solid tumors | NCT01924689 | Evaluate safety and efficacy of Clostridium novyi-NT spores in patients with treatment-refractory solid tumor malignancies. | I | Completed | https://www.clinicaltrials.gov/study/NCT01924689?cond=NCT01924689%20&rank=1 (accessed on 5 June 2024) |
| Metastatic cancer | NCT00004988 | Evaluate safety and tolerability of S. typhimurium VNP20009 with deletions in the msbB and purI loci in patients with metastatic cancer. | I | Completed | https://www.clinicaltrials.gov/study/NCT00004988?cond=NCT00004988%20%20&rank=1 (accessed on 5 June 2024) |
| Tumors expressing NY-ESO-1 antigen (cancer-testis Ag) | NCT00623831 | Determine the safety and tolerability of a mixed bacterial vaccine candidate that induced a pyrogenic effect in in subjects with malignant tumors that expressed the NY-ESO-1 antigen. | I | Completed | https://www.clinicaltrials.gov/study/NCT00623831?cond=NCT00623831%20%20%20&rank=1 (accessed on 5 June 2024) |
| Rectal cancer | NCT03420443 | Evaluate safety, tolerability and efficacy of Lactobacillus plantarum to reduce inflammation and to diminish tissue damage caused by radiation therapy in patients diagnosed with rectal cancer. | II | Completed | https://www.clinicaltrials.gov/study/NCT03420443?cond=NCT03420443%20&rank=1 (accessed on 5 June 2024) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renteria-Flores, F.I.; García-Chagollán, M.; Jave-Suárez, L.F. Bactofection, Bacterial-Mediated Vaccination, and Cancer Therapy: Current Applications and Future Perspectives. Vaccines 2024, 12, 968. https://doi.org/10.3390/vaccines12090968
Renteria-Flores FI, García-Chagollán M, Jave-Suárez LF. Bactofection, Bacterial-Mediated Vaccination, and Cancer Therapy: Current Applications and Future Perspectives. Vaccines. 2024; 12(9):968. https://doi.org/10.3390/vaccines12090968
Chicago/Turabian StyleRenteria-Flores, Francisco Israel, Mariel García-Chagollán, and Luis Felipe Jave-Suárez. 2024. "Bactofection, Bacterial-Mediated Vaccination, and Cancer Therapy: Current Applications and Future Perspectives" Vaccines 12, no. 9: 968. https://doi.org/10.3390/vaccines12090968
APA StyleRenteria-Flores, F. I., García-Chagollán, M., & Jave-Suárez, L. F. (2024). Bactofection, Bacterial-Mediated Vaccination, and Cancer Therapy: Current Applications and Future Perspectives. Vaccines, 12(9), 968. https://doi.org/10.3390/vaccines12090968








