The Potential of Anti-Inflammatory DC Immunotherapy in Improving Proteinuria in Type 2 Diabetes Mellitus
Abstract
1. Introduction
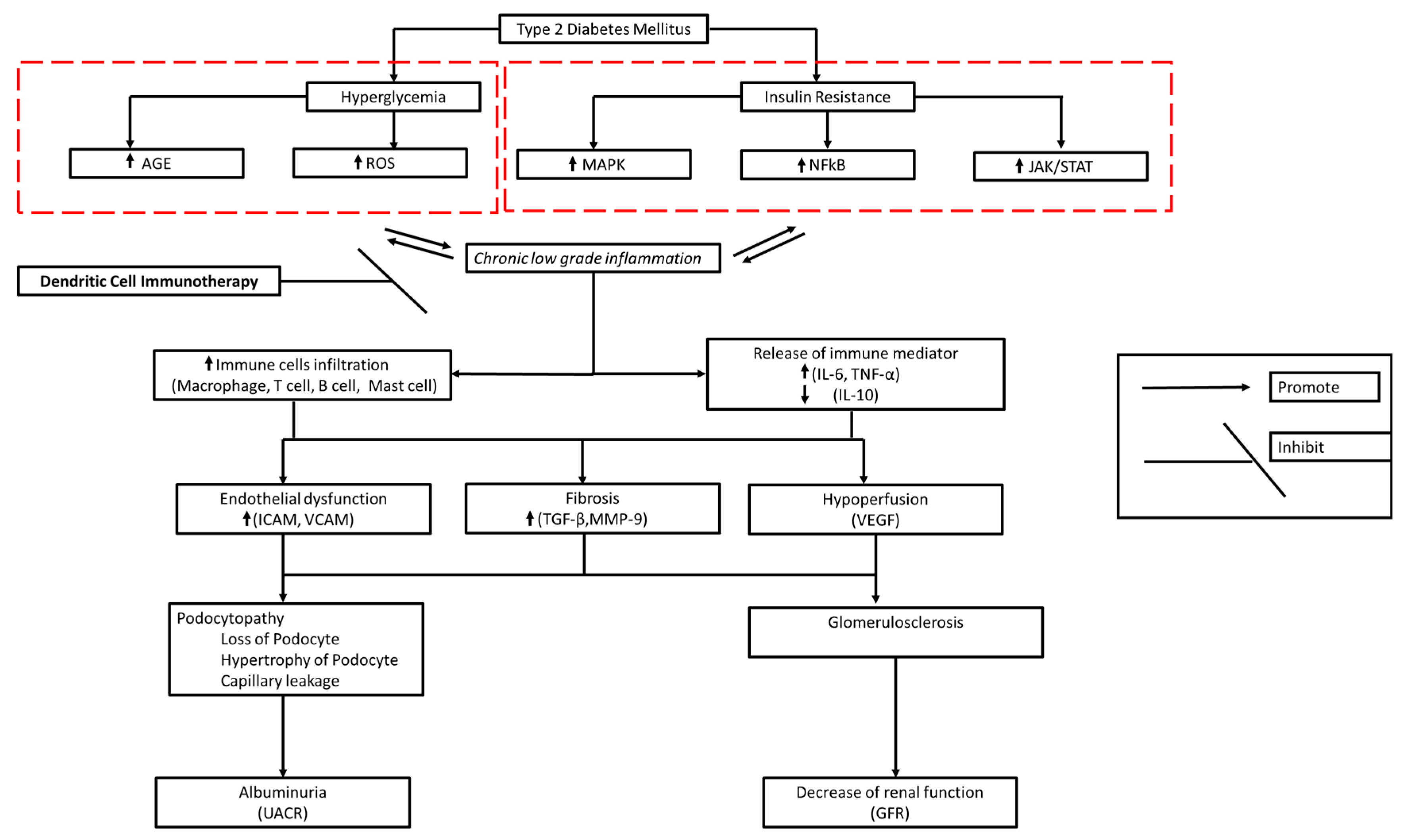
2. Diabetic Kidney Disease Immunopathology
3. Kidney Dendritic Cell Subsets
4. The Role of DCs in Inducing an Anti-Inflammatory Response
5. Ex Vivo Production of Autologous Dendritic Cells
6. Current State of Cell-Based Therapy for DKD
7. Current Treatment of DKD and Its Effect on the Immune System
8. Anti-Inflammatory DC Mechanism of Action in DKD
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selby, N.M.; Taal, M.W. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 2020, 22, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, N.; Wu, Y.; Wang, M.; Yang, S.; Zheng, Y.; Deng, X.; Xiang, D.; Zhu, Y.; Xu, P.; et al. Global, Regional, and National Burden of Diabetes-Related Chronic Kidney Disease From 1990 to 2019. Front. Endocrinol. 2021, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Wada, J.; Makino, H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. 2013, 124, 139–152. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Jaisser, F. Pathophysiologic mechanisms in diabetic kidney disease: A focus on current and future therapeutic targets. Diabetes Obes. Metab. 2020, 22, 16–31. [Google Scholar] [CrossRef]
- Kikkawa, R.; Koya, D.; Haneda, M. Progression of diabetic nephropathy. Am. J. Kidney Dis. 2003, 41, S19–S21. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Yiu, W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef]
- D’Marco, L.; Guerra-Torres, X.; Viejo, I.; Lopez-Romero, L.; Yugueros, A.; Bermúdez, V. Non-albuminuric Diabetic Kidney Disease Phenotype: Beyond Albuminuria. Eur. Endocrinol. 2022, 18, 102. [Google Scholar] [CrossRef]
- Ekinci, E.I.; Jerums, G.; Skene, A.; Crammer, P.; Power, D.; Cheong, K.Y.; Panagiotopoulos, S.; McNeil, K.; Baker, S.T.; Fioretto, P.; et al. Renal Structure in Normoalbuminuric and Albuminuric Patients with Type 2 Diabetes and Impaired Renal Function. Diabetes Care 2013, 36, 3620–3626. [Google Scholar] [CrossRef]
- Bhalla, V.; Zhao, B.; Azar, K.M.J.; Wang, E.J.; Choi, S.; Wong, E.C.; Fortmann, S.P.; Palaniappan, L.P. Racial/Ethnic Differences in the Prevalence of Proteinuric and Nonproteinuric Diabetic Kidney Disease. Diabetes Care 2013, 36, 1215–1221. [Google Scholar] [CrossRef]
- Azeem, W.; Bakke, R.M.; Appel, S.; Øyan, A.M.; Kalland, K.H. Dual Pro- and Anti-Inflammatory Features of Monocyte-Derived Dendritic Cells. Front. Immunol. 2020, 27, 11. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhou, Z. The clinical heterogeneity of diabetes challenges the accuracy of typing diagnosis. J. Chin. Physician 2022, 24, 179–183. [Google Scholar]
- Petrelli, A.; Giovenzana, A.; Insalaco, V.; Phillips, B.E.; Pietropaolo, M.; Giannoukakis, N. Autoimmune Inflammation and Insulin Resistance: Hallmarks So Far and Yet So Close to Explain Diabetes Endotypes. Curr. Diab Rep. 2021, 21, 54. [Google Scholar] [CrossRef]
- Asfandiyarova, N.S. Type 2 diabetes mellitus—An autoimmune disease? Russ. J. Immunol. 2020, 23, 9–18. [Google Scholar] [CrossRef]
- Girard, D.; Vandiedonck, C. How dysregulation of the immune system promotes diabetes mellitus and cardiovascular risk complications. Front. Cardiovasc. Med. 2022, 9, 991716. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; Georgakopoulou, V.E. Physiopathological mechanisms related to inflammation in obesity and type 2 diabetes mellitus. World J. Exp. Med. 2023, 13, 7–16. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Fontanella, A.M.; Merscher, S.; Fornoni, A. Lipid deposition and metaflammation in diabetic kidney disease. Curr. Opin. Pharmacol. 2020, 55, 60–72. [Google Scholar] [CrossRef]
- Lan, Y.; Wu, D.; Cai, Z.; Xu, Y.; Ding, X.; Wu, W.; Lan, S.; Chen, L.; Guo, Z.; Balmer, L.; et al. Supra-additive effect of chronic inflammation and atherogenic dyslipidemia on developing type 2 diabetes among young adults: A prospective cohort study. Cardiovasc. Diabetol. 2023, 22, 181. [Google Scholar] [CrossRef]
- Thimmappa, P.Y.; Vasishta, S.; Ganesh, K.; Nair, A.S.; Joshi, M.B. Neutrophil (dys)function due to altered immuno-metabolic axis in type 2 diabetes: Implications in combating infections. Hum. Cell 2023, 36, 1265–1282. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Lytrivi, M.; Igoillo-Esteve, M.; Cnop, M. Inflammatory stress in islet β-cells: Therapeutic implications for type 2 diabetes? Curr. Opin. Pharmacol. 2018, 43, 40–45. [Google Scholar] [CrossRef]
- Shahid, R.; Chu, L.M.; Arnason, T.; Pahwa, P. Association Between Insulin Resistance and the Inflammatory Marker C-reactive Protein in a Representative Healthy Adult Canadian Population: Results from the Canadian Health Measures Survey. Can. J. Diabetes 2023, 47, 428–434. [Google Scholar] [CrossRef]
- Bandawane, D.; Pujari, R.; Upaganlawar, A. High-fat diets and insulin resistance. In Everything You Need to Know about High-Fat Diets; Nova Science Publishers: Hauppauge, NY, USA, 2023; pp. 213–230. [Google Scholar]
- Li, T.; Wang, P.; Wang, X.; Liu, Z.; Zhang, Z.; Zhang, Y.; Wang, Z.; Feng, Y.; Wang, Q.; Guo, X.; et al. Inflammation and Insulin Resistance in Diabetic Chronic Coronary Syndrome Patients. Nutrients 2023, 15, 2808. [Google Scholar] [CrossRef]
- Hoorzad, P.; Mousavinasab, F.; Tofigh, P.; Kalahroud, E.M.; Aghaei-Zarch, S.M.; Salehi, A.; Fattahi, M.; Le, B.N. Understanding the lncRNA/miRNA-NFκB regulatory network in diabetes mellitus: From function to clinical translation. Diabetes Res. Clin. Pract. 2023, 202, 110804. [Google Scholar] [CrossRef] [PubMed]
- Meyerovich, K.; Ortis, F.; Cardozo, A.K. The non-canonical NF-κB pathway and its contribution to β-cell failure in diabetes. J. Mol. Endocrinol. 2018, 61, F1–F6. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Liu, W.; Wang, S.; Jiang, R.; Hu, K.; Sheng, L.; Xu, G.; Kou, X.; Song, Y. NF-κB-Inducing Kinase Provokes Insulin Resistance in Skeletal Muscle of Obese Mice. Inflammation 2023, 46, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Bhatti, R. Cellular and molecular mechanisms involved in metabolic disorders. In Drug Delivery Systems for Metabolic Disorders; Academic Press: Cambridge, MA, USA, 2022; pp. 21–29. [Google Scholar]
- Lourido, F.; Quenti, D.; Salgado-Canales, D.; Tobar, N. Domeless receptor loss in fat body tissue reverts insulin resistance induced by a high-sugar diet in Drosophila melanogaster. Sci. Rep. 2021, 11, 3263. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, X.; Zhu, J.; Liu, Y.; Jin, X.; Chen, G.; Ye, L. Current application status and structure–activity relationship of selective and non-selective JAK inhibitors in diseases. Int. Immunopharmacol. 2023, 122, 110660. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Q.; He, J.; Li, Y. Immune responses in diabetic nephropathy: Pathogenic mechanisms and therapeutic target. Front. Immunol. 2022, 13, 958790. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.; Hu, Q.; Zhou, J.; Lin, H. The landscape of immune cell infiltration in the glomerulus of diabetic nephropathy: Evidence based on bioinformatics. BMC Nephrol. 2022, 23, 303. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, Y.; Zhang, T.; Huang, T.; Lang, Y.; Sheng, Q.; Liu, Y.; Kong, Z.; Gao, Y.; Lu, S.; et al. T cells and their products in diabetic kidney disease. Front. Immunol. 2023, 14, 1084448. [Google Scholar] [CrossRef]
- Rico Fontalvo, J.; Aroca-Martínez, G.; Daza-Arnedo, R.; Raad-Sarabia, M.; Luis Torres, J.; Pajaro-Galvis, N.; Uparella-Gulfo, I.; Porto-Corbacho, D.; Sarabia-Cannepa, S.; Ramos-Clason, E. Non-proteinuric diabetic kidney disease: State of art | Enfermedad renal diabética no proteinúrica: Estado del arte. Rev. Nefrol. Dial. Traspl. 2022, 42, 330–339. [Google Scholar]
- Lis-López, L.; Bauset, C.; Seco-Cervera, M.; Cosín-Roger, J. Is the macrophage phenotype determinant for fibrosis development? Biomedicines 2021, 9, 1747. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.M.B.; Conway, B.R. Macrophages in the kidney in health, injury and repair. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2022; Volume 367, pp. 101–147. [Google Scholar]
- Chen, A.; Lee, K.; He, J.C. Treating crescentic glomerulonephritis by targeting macrophages. Kidney Int. 2022, 102, 1212–1214. [Google Scholar] [CrossRef]
- Wen, J.H.; Li, D.Y.; Liang, S.; Yang, C.; Tang, J.X.; Liu, H.F. Macrophage autophagy in macrophage polarization, chronic inflammation and organ fibrosis. Front. Immunol. 2022, 13, 946832. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Bai, X.; Chen, X. Autophagy and glomerular diseases. In Advances in Experimental Medicine and Biology; Springer Nature: Cham, Switzerland, 2020; Volume 1207, pp. 481–486. [Google Scholar]
- Moon, J.Y.; Jeong, K.H.; Lee, T.W.; Ihm, C.G.; Lim, S.J.; Lee, S.H. Aberrant Recruitment and Activation of T Cells in Diabetic Nephropathy. Am. J. Nephrol. 2012, 35, 164–174. [Google Scholar] [CrossRef]
- Bending, J.J.; Lobo-Yeo, A.; Vergani, D.; Viberti, G. Proteinuria and Activated T-Lymphocytes in Diabetic Nephropathy. Diabetes 1988, 37, 507–511. [Google Scholar] [CrossRef]
- Moriya, R.; Manivel, J.C.; Mauer, M. Juxtaglomerular apparatus T-cell infiltration affects glomerular structure in Type 1 diabetic patients. Diabetologia 2004, 47, 82–88. [Google Scholar] [CrossRef]
- Kong, L.; Andrikopoulos, S.; MacIsaac, R.J.; Mackay, L.K.; Nikolic-Paterson, D.J.; Torkamani, N.; Zafari, N.; Marin, E.C.S.; Ekinci, E.I. Role of the adaptive immune system in diabetic kidney disease. J. Diabetes Investig. 2022, 13, 213–226. [Google Scholar] [CrossRef]
- Su, H.; Lei, C.T.; Zhang, C. Interleukin-6 signaling pathway and its role in kidney disease: An update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.A.; Kim, J.Y.; Yang, S.H.; Han, S.S.; Joo, K.W.; Kim, Y.S. The role of local IL6/JAK2/STAT3 signaling in high glucose–induced podocyte hypertrophy. Kidney Res. Clin. Pract. 2016, 35, 212–218. [Google Scholar] [CrossRef]
- Yin, L.; Yu, L.; He, J.C.; Chen, A. Controversies in Podocyte Loss: Death or Detachment? Front. Cell Dev. Biol. 2021, 9, 771931. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.R. Interleukin-6 signaling in podocyte hypertrophy. Kidney Res. Clin. Pract. 2016, 35, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Coletta, I.; Soldo, L.; Polentarutti, N.; Mancini, F.; Guglielmotti, A.; Pinza, M. Selective Induction of MCP-1 in Human Mesangial Cells by the IL-6/sIL-6R Complex. Nephron Exp. Nephrol. 2000, 8, 37–43. [Google Scholar] [CrossRef]
- Thomas, H.Y.; Ford Versypt, A.N. Pathophysiology of mesangial expansion in diabetic nephropathy: Mesangial structure, glomerular biomechanics, and biochemical signaling and regulation. J. Biol. Eng. 2022, 16, 19. [Google Scholar] [CrossRef]
- Barutta, F.; Bruno, G.; Grimaldi, S.; Gruden, G. Inflammation in diabetic nephropathy: Moving toward clinical biomarkers and targets for treatment. In Endocrine; Humana Press Inc.: Totowa, NJ, USA, 2015; Volume 48, pp. 730–742. [Google Scholar]
- Idriss, H.T.; Naismith, J.H. TNF Alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Braga Gomes, K.; Fontana Rodrigues, K.; Fernandes, A.P. The Role of Transforming Growth Factor-Beta in Diabetic Nephropathy. Int. J. Med. Genet. 2014, 2014, 180270. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Liu, X.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Diverse Role of TGF-β in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef]
- Das, R.; Xu, S.; Quan, X.; Nguyen, T.T.; Kong, I.D.; Chung, C.H. Upregulation of mitochondrial Nox4 mediates TGF-β-induced apoptosis in cultured mouse podocytes. Am. J. Physiol. Ren. Physiol. 2014, 306, F155–F167. [Google Scholar] [CrossRef]
- Pestka, S.; Krause, C.D.; Sarkar, D.; Walter, M.R.; Shi, Y.; Fisher, P.B. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 2004, 22, 929–979. [Google Scholar] [CrossRef] [PubMed]
- Duran-Salgado, M.B. Diabetic nephropathy and inflammation. World J. Diabetes 2014, 5, 393. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Shafique, N.; Sharif, S.; Manzoor, F.; Saifi, S.Z.; Firasat, S. Association of Interleukin 10 (IL-10) Gene with Type 2 Diabetes Mellitus by Single Nucleotide Polymorphism of Its Promotor Region G/A 1082. Crit Rev Eukaryot Gene Expr 2020, 30, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.C.; Shakibakho, S.; Durrer, C.; Simtchouk, S.; Jawanda, K.K.; Cheung, S.T. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci. Rep. 2016, 6, 21244. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. Biomed. Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, X.; Liu, L.C.; Kim, A.Y.; Curley, S.P.; Chen, X. Interleukin-10 attenuates renal injury after myocardial infarction in diabetes. J. Investig. Med. 2022, 70, 1233–1242. [Google Scholar] [CrossRef]
- Wei, W.; Zhao, Y.; Zhang, Y.; Jin, H.; Shou, S. The role of IL-10 in kidney disease. Int. Immunopharmacol. 2022, 108, 108917. [Google Scholar] [CrossRef]
- Lenz, O.; Fornoni, A.; Ijaz, A.; Tejada, T. Role of Inflammation in Diabetic Nephropathy. Curr. Diabetes Rev. 2008, 4, 10–17. [Google Scholar] [CrossRef]
- Okada, S.; Shikata, K.; Matsuda, M.; Ogawa, D.; Usui, H.; Kido, Y.; Nagase, R.; Wada, J.; Shikata, Y.; Makino, H. Intercellular Adhesion Molecule-1–Deficient Mice Are Resistant Against Renal Injury After Induction of Diabetes. Diabetes 2003, 52, 2586–2593. [Google Scholar] [CrossRef]
- Chow, F.Y.; Nikolic-Paterson, D.J.; Ozols, E.; Atkins, R.C.; Tesch, G.H. Intercellular Adhesion Molecule-1 Deficiency Is Protective against Nephropathy in Type 2 Diabetic db/db Mice. J. Am. Soc. Nephrol. 2005, 16, 1711–1722. [Google Scholar] [CrossRef]
- Zhao, Q.; Ishibashi, M.; Hiasa K ichi Tan, C.; Takeshita, A.; Egashira, K. Essential Role of Vascular Endothelial Growth Factor in Angiotensin II–Induced Vascular Inflammation and Remodeling. Hypertension 2004, 44, 264–270. [Google Scholar] [CrossRef]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. J. Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef]
- Giri, B.; Dey, S.; Das, T.; Sarkar, M.; Banerjee, J.; Dash, S.K. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed. Pharmacother. 2018, 107, 306–328. [Google Scholar] [CrossRef] [PubMed]
- Lassén, E.; Daehn, I.S. Molecular Mechanisms in Early Diabetic Kidney Disease: Glomerular Endothelial Cell Dysfunction. Int. J. Mol. Sci. 2020, 21, 9456. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.; Rheault, M.N. Genetic Basis of Type IV Collagen Disorders of the Kidney. Clin. J. Am. Soc. Nephrol. 2021, 16, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Yabluchanskiy, A.; Ma, Y.; Iyer, R.P.; Hall, M.E.; Lindsey, M.L. Matrix Metalloproteinase-9: Many Shades of Function in Cardiovascular Disease. Physiology 2013, 28, 391–403. [Google Scholar] [CrossRef]
- Yang, H.; Chen, H.; Liu, F.; Ma, Q. Up-regulation of matrix metalloproteinases-9 in the kidneys of diabetic rats and the association with neutrophil gelatinase-associated lipocalin. BMC Nephrol. 2021, 22, 211. [Google Scholar] [CrossRef]
- Arcos-Sacramento, V.G.; Sampieri, C.L.; Sandoval-Lozano, V.H.; Orozco-Ortega, R.A.; Acuña-Hernández, M.A.; Morales-Romero, J.; Hernández-Hernández, M.E.; Rodríguez-Hernández, A. Urinary MMP-9/UCr association with albumin concentration and albumin-creatinine-ratio in Mexican patients with type 2 diabetes mellitus. PeerJ 2020, 8, e10474. [Google Scholar] [CrossRef]
- Li, Y.; Jin, L.; Yan, J.; Zhang, H.; Zhang, R.; Hu, C. CD28 Genetic Variants Increase Susceptibility to Diabetic Kidney Disease in Chinese Patients with Type 2 Diabetes: A Cross-Sectional Case Control Study. Mediat. Inflamm. 2021, 2021, 5521050. [Google Scholar] [CrossRef]
- Wang, L.; Wang, P.; Li, X.; Dong, Y.; Wu, S.; Xu, M.; Chen, X.; Wang, S.; Zheng, C.; Zou, C. Combination CTLA-4 immunoglobulin treatment and ultrasound microbubble-mediated exposure improve renal function in a rat model of diabetic nephropathy. Aging 2021, 13, 8524–8540. [Google Scholar] [CrossRef]
- Herrera, M.; Söderberg, M.; Sabirsh, A.; Valastro, B.; Mölne, J.; Santamaria, B.; Valverde, A.M.; Guionaud, S.; Heasman, S.; Bigley, A.; et al. Inhibition of T-cell activation by the CTLA4-Fc Abatacept is sufficient to ameliorate proteinuric kidney disease. Am. J. Physiol. Ren. Physiol. 2017, 312, F748–F759. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.P.; Li, X.Y.; Wei, K.N.; Yang, Y.; Zhao, Y.Z.; Wang, P.; Zheng, C.; Jiao, Y.; Zhao, Y.P. Therapeutic effects of CTLA-4-Ig on diabetic nephropathy in type 2 diabetes mellitus rats ascribed to protection of CTLA-4-Ig on podocytes. Int. J. Clin. Exp. Med. 2018, 11, 10516–10525. [Google Scholar]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xing, J.; Wang, S.; Peng, H.; Liu, Y. The role of the cGAS-STING pathway in metabolic diseases. Heliyon 2024, 10, e33093. [Google Scholar] [CrossRef] [PubMed]
- Zang, N.; Cui, C.; Guo, X.; Song, J.; Hu, H.; Yang, M.; Xu, M.; Wang, L.; Hou, X.; He, Q.; et al. cGAS-STING activation contributes to podocyte injury in diabetic kidney disease. iScience 2022, 25, 105145. [Google Scholar] [CrossRef] [PubMed]
- Yuzefovych, L.V.; Pastukh, V.M.; Ruchko, M.V.; Simmons, J.D.; Richards, W.O.; Rachek, L.I. Plasma mitochondrial DNA is elevated in obese type 2 diabetes mellitus patients and correlates positively with insulin resistance. PLoS ONE 2019, 14, e0222278. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Zheng, Z.; Zhang, H.; Wu, Y.; Shen, Y.; Chen, Q. cGAS-STING pathway mediates activation of dendritic cell sensing of immunogenic tumors. Cell. Mol. Life Sci. 2024, 81, 149. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Luo, Z.; Yang, D.; Hao, Y.; Hu, J.; Feng, J.; Zhu, Z.; Luo, Q.; Zhang, Z.; et al. STING deletion alleviates podocyte injury through suppressing inflammation by targeting NLRP3 in diabetic kidney disease. Cell Signal 2023, 109, 110777. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Li, G.; Liu, M.; Zhou, Z.; Wu, M.; Song, S.; Bian, Y.; Dong, J.; Li, X.; et al. Inhibition of PKC-δ retards kidney fibrosis via inhibiting cGAS-STING signaling pathway in mice. Cell Death Discov. 2024, 10, 314. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Fontanella, A.; Tolerico, M.; Mallela, S.; Molina David, J.; Zuo, Y.; Boulina, M.; Kim, J.-J.; Santos, J.; Ge, M.; et al. Activation of Stimulator of IFN Genes (STING) Causes Proteinuria and Contributes to Glomerular Diseases. J. Am. Soc. Nephrol. 2022, 33, 2153–2173. [Google Scholar] [CrossRef]
- Salei, N.; Rambichler, S.; Salvermoser, J.; Papaioannou, N.E.; Schuchert, R.; Pakalniškytė, D.; Li, N.; Marschner, J.A.; Lichtnekert, J.; Stremmel, C.; et al. The Kidney Contains Ontogenetically Distinct Dendritic Cell and Macrophage Subtypes throughout Development That Differ in Their Inflammatory Properties. J. Am. Soc. Nephrol. 2020, 31, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, H.; Liu, C.; Cheng, A.; Deng, Q.; Zhu, H.; Chen, J. Dendritic Cells: Versatile Players in Renal Transplantation. Front. Immunol. 2021, 12, 654540. [Google Scholar] [CrossRef]
- Li, L.; Huang, L.; Sung, S.S.J.; Vergis, A.L.; Rosin, D.L.; Rose, C.E.; Lobo, P.I.; Okusa, M.D. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia–reperfusion injury. Kidney Int. 2008, 74, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Cheng, Y.; Cao, X. Dendritic cell migration in inflammation and immunity. Cell Mol. Immunol. 2021, 18, 2461–2471. [Google Scholar] [CrossRef]
- Gottschalk, C.; Damuzzo, V.; Gotot, J.; Kroczek, R.A.; Yagita, H.; Murphy, K.M.; Knolle, P.A.; Ludwig-Portugall, I.; Kurts, C. Batf3-Dependent Dendritic Cells in the Renal Lymph Node Induce Tolerance against Circulating Antigens. J. Am. Soc. Nephrol. 2013, 24, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Tittel, A.P.; Heuser, C.; Ohliger, C.; Knolle, P.A.; Engel, D.R.; Kurts, C. Kidney Dendritic Cells Induce Innate Immunity against Bacterial Pyelonephritis. J. Am. Soc. Nephrol. 2011, 22, 1435–1441. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.; Lee, H.Y.; Park, H.Y.; Jhun, H.; Kim, S. Role of Dendritic Cell in Diabetic Nephropathy. Int. J. Mol. Sci. 2021, 22, 7554. [Google Scholar] [CrossRef]
- Chen, T.; Cao, Q.; Wang, R.; Zheng, G.; Azmi, F.; Wang, J.; Lee, V.W.; Wang, Y.M.; Yu, H.; Patel, M.; et al. Conventional Type 1 Dendritic Cells (cDC1) in Human Kidney Diseases: Clinico-Pathological Correlations. Front. Immunol. 2021, 12, 635212. [Google Scholar] [CrossRef]
- Kassianos, A.J.; Wang, X.; Sampangi, S.; Muczynski, K.; Healy, H.; Wilkinson, R. Increased tubulointerstitial recruitment of human CD141hi CLEC9A + and CD1c+ myeloid dendritic cell subsets in renal fibrosis and chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2013, 305, F1391–F1401. [Google Scholar] [CrossRef]
- Muller, D.N.; Shagdarsuren, E.; Park, J.K.; Dechend, R.; Mervaala, E.; Hampich, F.; Hampich, F.; Fiebeler, A.; Ju, X.; Finckenberg, P.; et al. Immunosuppressive Treatment Protects Against Angiotensin II-Induced Renal Damage. Am. J. Pathol. 2002, 161, 1679–1693. [Google Scholar] [CrossRef]
- Passeri, L.; Marta, F.; Bassi, V.; Gregori, S. Tolerogenic Dendritic Cell-Based Approaches in Autoimmunity. Int. J. Mol. Sci. 2021, 22, 8415. [Google Scholar] [CrossRef] [PubMed]
- Comi, M.; Avancini, D.; Santoni de Sio, F.; Villa, M.; Uyeda, M.J.; Floris, M.; Tomasoni, D.; Bulfone, A.; Roncarolo, M.G.; Gregori, S.; et al. Coexpression of CD163 and CD141 identifies human circulating IL-10-producing dendritic cells (DC-10). Cell Mol. Immunol. 2020, 17, 95–107. [Google Scholar] [CrossRef]
- Boks, M.A.; Kager-Groenland, J.R.; Haasjes, M.S.P.; Zwaginga, J.J.; van Ham, S.M.; ten Brinke, A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction—A comparative study of human clinical-applicable, DC. Clin. Immunol. 2012, 142, 332–342. [Google Scholar] [CrossRef]
- Bakdash, G.; Vogelpoel, L.T.; van Capel, T.M.; Kapsenberg, M.L.; de Jong, E.C. Retinoic acid primes human dendritic cells to induce gut-homing, IL-10-producing regulatory T cells. Mucosal Immunol. 2015, 8, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Morante-Palacios, O.; Fondelli, F.; Ballestar, E.; Martínez-Cáceres, E.M. Tolerogenic Dendritic Cells in Autoimmunity and Inflammatory Diseases. Trends Immunol. 2021, 42, 59–75. [Google Scholar] [CrossRef]
- Ochando, J.; Ordikhani, F.; Jordan, S.; Boros, P.; Thomson, A.W. Tolerogenic dendritic cells in organ transplantation. Transplant. Int. 2020, 33, 113–127. [Google Scholar] [CrossRef]
- Manicassamy, S.; Pulendran, B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011, 241, 206–227. [Google Scholar] [CrossRef]
- Choo, E.H.; Lee, J.H.; Park, E.H.; Park, H.E.; Jung, N.C.; Kim, T.H.; Koh, Y.-S.; Kim, E.; Seung, K.-B.; Park, C.; et al. Infarcted Myocardium-Primed Dendritic Cells Improve Remodeling and Cardiac Function After Myocardial Infarction by Modulating the Regulatory T Cell and Macrophage Polarization. Circulation 2017, 135, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.J.; Sharma, S.; Rangesa, M.; DeWolf, S.; Elhanati, Y.; Perica, K.; Young, J.W. Langerhans dendritic cell vaccine bearing mRNA-encoded tumor antigens induces antimyeloma immunity after autotransplant. Blood Adv. 2022, 6, 1547–1558. [Google Scholar] [CrossRef]
- Constantino, J.; Gomes, C.; Falcão, A.; Neves, B.M.; Cruz, M.T. Dendritic cell-based immunotherapy: A basic review and recent advances. Immunol. Res. 2017, 65, 798–810. [Google Scholar] [CrossRef]
- Makino, K.; Long, M.D.; Kajihara, R.; Matsueda, S.; Oba, T.; Kanehira, K.; Liu, S.; Ito, F. Generation of cDC-like cells from human induced pluripotent stem cells via Notch signaling. J. Immunother. Cancer 2022, 10, e003827. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.S.J. Monocyte-Derived Dendritic Cells as Antigen-Presenting Cells in T-Cell Proliferation and Cytokine Production. In Allergy; Humana: New York, NY, USA, 2019; pp. 131–141. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Thiruppathi, M.; Elshabrawy, H.A.; Alharshawi, K.; Kumar, P.; Prabhakar, B.S. GM-CSF: An immune modulatory cytokine that can suppress autoimmunity. Cytokine 2015, 75, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Cechim, G.; Chies, J.A. In vitro generation of human monocyte-derived dendritic cells methodological aspects in a comprehensive review. An. Acad. Bras. Cienc. 2019, 91, e20190310. [Google Scholar] [CrossRef]
- Wu, H.J.; Lo, Y.; Luk, D.; Lau, C.S.; Lu, L.; Mok, M.Y. Alternatively activated dendritic cells derived from systemic lupus erythematosus patients have tolerogenic phenotype and function. Clin. Immunol. 2015, 156, 43–57. [Google Scholar] [CrossRef][Green Version]
- Esmaeili, S.A.; Mahmoudi, M.; Rezaieyazdi, Z.; Sahebari, M.; Tabasi, N.; Sahebkar, A.; Rastin, M. Generation of tolerogenic dendritic cells using Lactobacillus rhamnosus and Lactobacillus delbrueckii as tolerogenic probiotics. J. Cell Biochem. 2018, 119, 7865–7872. [Google Scholar] [CrossRef]
- Li, C.H.; Zhang, J.; Baylink, D.J.; Wang, X.; Goparaju, N.B.; Xu, Y.; Wasnik, S.; Cheng, Y.; Berumen, E.C.; Qin, X.; et al. Dendritic cells, engineered to overexpress 25-hydroxyvitamin D 1α-hydroxylase and pulsed with a myelin antigen, provide myelin-specific suppression of ongoing experimental allergic encephalomyelitis. FASEB J. 2017, 31, 2996–3006. [Google Scholar] [CrossRef]
- Lo, J.; Xia, C.Q.; Peng, R.; Clare-Salzler, M.J. Immature dendritic cell therapy confers durable immune modulation in an antigen-dependent and antigen-independent manner in nonobese diabetic mice. J. Immunol. Res. 2018, 2018, 5463879. [Google Scholar] [CrossRef]
- Jonny, J.; Putranto, T.A.; Sitepu, E.C.; Irfon, R. Dendritic cell vaccine as a potential strategy to end the COVID-19 pandemic. Why should it be Ex Vivo? Expert. Rev. Vaccines 2022, 21, 1111–1120. [Google Scholar] [CrossRef]
- Dubský, M.; Jirkovská, A.; Bem, R.; Němcová, A.; Fejfarová, V.; Hazdrová, J.; Sutoris, K.; Chlupáč, J.; Skibová, J.; Jude, E.B. Impact of severe diabetic kidney disease on the clinical outcome of autologous cell therapy in people with diabetes and critical limb ischaemia. Diabet. Med. 2019, 36, 1133–1140. [Google Scholar] [CrossRef]
- Ying, A.F.; Tang, T.Y.; Jin, A.; Chong, T.T.; Hausenloy, D.J.; Koh, W.P. Diabetes and other vascular risk factors in association with the risk of lower extremity amputation in chronic limb-threatening ischemia: A prospective cohort study. Cardiovasc. Diabetol. 2022, 21, 7. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liu, S.; Gao, M.; Wang, W.; Chen, K.; Huang, L.; Liu, Y. Diabetic vascular diseases: Molecular mechanisms and therapeutic strategies. Signal Transduct. Target. Ther. 2023, 8, 152. [Google Scholar] [CrossRef]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef]
- Hickson, L.J.; Abedalqader, T.; Ben-Bernard, G.; Mondy, J.M.; Bian, X.; Conley, S.M.; Zhu, X.; Herrmann, S.M.; Kukla, A.; Lorenz, E.C.; et al. A Systematic Review and Meta-Analysis of Cell-Based Interventions in Experimental Diabetic Kidney Disease. Stem Cells Transl. Med. 2021, 10, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Packham, D.K.; Fraser, I.R.; Kerr, P.G.; Segal, K.R. Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine 2016, 12, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Sávio-Silva, C.; Beyerstedt, S.; Soinski-Sousa, P.E.; Casaro, E.B.; Balby-Rocha, M.T.A.; Simplício-Filho, A.; Alves-Silva, J.; Rangel, É.B. Mesenchymal Stem Cell Therapy for Diabetic Kidney Disease: A Review of the Studies Using Syngeneic, Autologous, Allogeneic, and Xenogeneic Cells. Stem Cells Int. 2020, 2020, 8833725. [Google Scholar] [CrossRef]
- Papademetriou, V.; Alataki, S.; Stavropoulos, K.; Papadopoulos, C.; Bakogiannis, K.; Tsioufis, K. Pharmacological Management of Diabetic Nephropathy. Curr. Vasc. Pharmacol. 2020, 18, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Mallik, R.; Chowdhury, T.A. Pharmacotherapy to delay the progression of diabetic kidney disease in people with type 2 diabetes: Past, present and future. Ther. Adv. Endocrinol. Metab. 2022, 13, 204201882210816. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.K.; Pinzon-Cortes, J.A.; Cooper, M.E. Novel pharmacological interventions for diabetic kidney disease. Curr. Opin. Nephrol. Hypertens. 2024, 33, 13–25. [Google Scholar] [CrossRef]
- Giglio, R.V.; Patti, A.M.; Rizvi, A.A.; Stoian, A.P.; Ciaccio, M.; Papanas, N.; Janez, A.; Sonmez, A.; Banach, M.; Sahebkar, A.; et al. Advances in the Pharmacological Management of Diabetic Nephropathy: A 2022 International Update. Biomedicines 2023, 11, 291. [Google Scholar] [CrossRef]
- Cucak, H.; Nielsen Fink, L.; Højgaard Pedersen, M.; Rosendahl, A. Enalapril treatment increases T cell number and promotes polarization towards M1-like macrophages locally in diabetic nephropathy. Int. Immunopharmacol. 2015, 25, 30–42. [Google Scholar] [CrossRef]
- Amann, B. Diabetic nephropathy and ACE inhibitors. Clin. Res. Cardiol. 2006, 95, i83–i87. [Google Scholar] [CrossRef]
- Dai, Z.C.; Chen, J.X.; Zou, R.; Liang, X.B.; Tang, J.X.; Yao, C.W. Role and mechanisms of SGLT-2 inhibitors in the treatment of diabetic kidney disease. Front. Immunol. 2023, 14, 1213473. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, Y.; Tong, J.; Yu, Y.; Yang, L.; Gu, Y.; Niu, J. Empagliflozin Ameliorates Preeclampsia and Reduces Postpartum Susceptibility to Adriamycin in a Mouse Model Induced by Angiotensin Receptor Agonistic Autoantibodies. Front. Pharmacol. 2022, 13, 826792. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.Y.; Li, X.Q.; Chen, M.; Zhao, M.H. Dapagliflozin Ameliorates Diabetic Kidney Disease via Upregulating Crry and Alleviating Complement Over-activation in db/db Mice. Front. Pharmacol. 2021, 12, 729334. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, K.; Tousoulis, D. The impact of SGLT2 inhibitors on inflammation: A systematic review and meta-analysis of studies in rodents. Int. Immunopharmacol. 2022, 111, 109080. [Google Scholar] [CrossRef] [PubMed]
- Kounatidis, D.; Vallianou, N.; Evangelopoulos, A.; Vlahodimitris, I.; Grivakou, E.; Kotsi, E.; Dimitriou, K.; Skourtis, A.; Mourouzis, I. SGLT-2 Inhibitors and the Inflammasome: What’s Next in the 21st Century? Nutrients 2023, 15, 2294. [Google Scholar] [CrossRef] [PubMed]
- Sourris, K.C.; Ding, Y.; Maxwell, S.S.; Al-Sharea, A.; Kantharidis, P.; Mohan, M.; Rosado, C.J.; Penfold, S.A.; Haase, C.; Xu, Y.; et al. Glucagon-like peptide-1 receptor signaling modifies the extent of diabetic kidney disease through dampening the receptor for advanced glycation end products–induced inflammation. Kidney Int. 2024, 105, 132–149. [Google Scholar] [CrossRef]
- Trevella, P.; Ekinci, E.I.; MacIsaac, R.J. Potential kidney protective effects of glucagon-like peptide-1 receptor agonists. Nephrology 2024, 29, 457–469. [Google Scholar] [CrossRef]
- Waisman, A.; Lukas, D.; Clausen, B.E.; Yogev, N. Dendritic cells as gatekeepers of tolerance. Semin Immunopathol. 2017, 39, 153–163. [Google Scholar] [CrossRef]
- Van Coillie, S.; Wiernicki, B.; Xu, J. Molecular and Cellular Functions of CTLA-4. In Regulation of Cancer Immune Checkpoints. Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; pp. 7–32. [Google Scholar]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 30, 15018. [Google Scholar] [CrossRef]
- Hickey, F.B.; Martin, F. Role of the Immune System in Diabetic Kidney Disease. Curr. Diab Rep. 2018, 18, 20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonny, J.; Sitepu, E.C.; Lister, I.N.E.; Chiuman, L.; Putranto, T.A. The Potential of Anti-Inflammatory DC Immunotherapy in Improving Proteinuria in Type 2 Diabetes Mellitus. Vaccines 2024, 12, 972. https://doi.org/10.3390/vaccines12090972
Jonny J, Sitepu EC, Lister INE, Chiuman L, Putranto TA. The Potential of Anti-Inflammatory DC Immunotherapy in Improving Proteinuria in Type 2 Diabetes Mellitus. Vaccines. 2024; 12(9):972. https://doi.org/10.3390/vaccines12090972
Chicago/Turabian StyleJonny, Jonny, Enda Cindylosa Sitepu, I Nyoman Ehrich Lister, Linda Chiuman, and Terawan Agus Putranto. 2024. "The Potential of Anti-Inflammatory DC Immunotherapy in Improving Proteinuria in Type 2 Diabetes Mellitus" Vaccines 12, no. 9: 972. https://doi.org/10.3390/vaccines12090972
APA StyleJonny, J., Sitepu, E. C., Lister, I. N. E., Chiuman, L., & Putranto, T. A. (2024). The Potential of Anti-Inflammatory DC Immunotherapy in Improving Proteinuria in Type 2 Diabetes Mellitus. Vaccines, 12(9), 972. https://doi.org/10.3390/vaccines12090972





