A Single-Component Multilayered Self-Assembling Protein Nanoparticle Vaccine Based on Extracellular Domains of Matrix Protein 2 against Both Influenza A and B
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression and Purification of Various Influenza A-B M2e Immunogens
2.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Blue Native-Polyacrylamide Gel Electrophoresis (BN-PAGE)
2.3. Dynamic Light Scattering (DLS)
2.4. Negative-Stain Electron Microscopy (nsEM) Analysis
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) for Antibody Binding
2.6. Propagation of Influenza Viruses
2.7. Influenza Virus Inactivation
2.8. Mouse Immunizations, Sample Collection, and Viral Challenge
2.9. ELISA for Serum Samples
2.10. Cell-Based ELISA
2.11. Statistical Analysis
3. Results
3.1. Design and Characterization of Influenza A-B M2e Trimer and I3-01v9a SApNPs
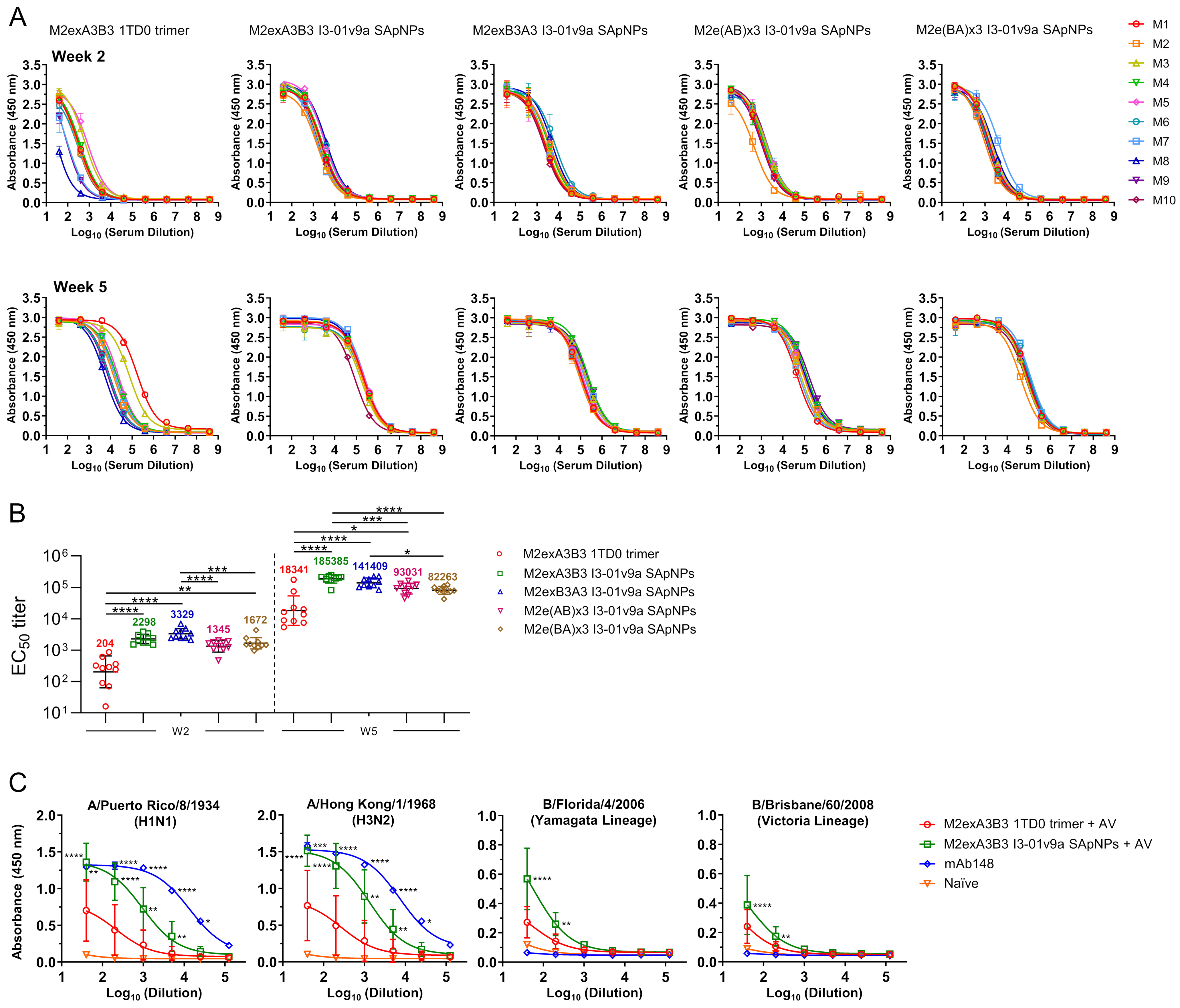
3.2. In Vivo Evaluation of Influenza A-B M2e SApNPs in Mice
3.3. Evaluation of Influenza A-B M2e Vaccine-Induced Antibody Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49–D53. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A virus cell entry, replication, virion assembly and movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, D.A.; Skehel, J.J. Genetics of influenza viruses. Annu. Rev. Genet. 2002, 36, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- Zambon, M.C. The pathogenesis of influenza in humans. Rev. Med. Virol. 2001, 11, 227–241. [Google Scholar] [CrossRef]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza virus: Dealing with a drifting and shifting pathogen. Viral Immunol. 2018, 31, 174–183. [Google Scholar] [CrossRef]
- Zanobini, P.; Bonaccorsi, G.; Lorini, C.; Haag, M.; McGovern, I.; Paget, J.; Caini, S. Global patterns of seasonal influenza activity, duration of activity and virus (sub)type circulation from 2010 to 2020. Influenza Other Respir. Viruses 2022, 16, 696–706. [Google Scholar] [CrossRef]
- Freestone, D.S.; Hamilton-Smith, S.; Schild, G.C.; Buckland, R.; Chinn, S.; Tyrrell, D.A.J. Antibody responses and resistance to challenge in volunteers vaccinated with live attenuated, detergent split and oil adjuvant A2-Hong Kong-68 (H 3 N 2) influenza vaccines. A report to the medical research council committee on influenza and other respiratory virus vaccines. J. Hyg. 1972, 70, 531–543. [Google Scholar]
- Mackenzie, J.S.; Mackenzie, I.; Lloyd, J.; Dent, V. Comparative trials of live attenuated and detergent split influenza virus vaccines. J. Hyg. 1975, 75, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Scorza, F.B.; Tsvetnitsky, V.; Donnelly, J.J. Universal influenza vaccines: Shifting to better vaccines. Vaccine 2016, 34, 2926–2933. [Google Scholar] [CrossRef]
- Wei, C.-J.; Crank, M.C.; Shiver, J.; Graham, B.S.; Mascola, J.R.; Nabel, G.J. Next-generation influenza vaccines: Opportunities and challenges. Nat. Rev. Drug Discov. 2020, 19, 239–252. [Google Scholar] [CrossRef]
- Deng, L.; Cho, K.J.; Fiers, W.; Saelens, X. M2e-based universal influenza A vaccines. Vaccines 2015, 3, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Kostolanský, F.; Tomčíková, K.; Briestenská, K.; Mikušová, M.; Varečková, E. Universal anti-influenza vaccines based on viral HA2 and M2e antigens. Acta Virol. 2020, 64, 417–426. [Google Scholar] [CrossRef]
- Mezhenskaya, D.; Isakova-Sivak, I.; Rudenko, L. M2e-based universal influenza vaccines: A historical overview and new approaches to development. J. Biomed. Sci. 2019, 26, 76. [Google Scholar] [CrossRef] [PubMed]
- Saelens, X. The role of matrix protein 2 ectodomain in the development of universal influenza vaccines. J. Infect. Dis. 2019, 219, S68–S74. [Google Scholar] [CrossRef]
- Schotsaert, M.; De Filette, M.; Fiers, W.; Saelens, X. Universal M2 ectodomain-based influenza A vaccines: Preclinical and clinical developments. Expert Rev. Vaccines 2009, 8, 499–508. [Google Scholar] [CrossRef]
- Kavishna, R.; Kang, T.Y.; Vacca, M.; Chua, B.Y.L.; Park, H.-Y.; Tan, P.S.; Chow, V.T.; Lahoud, M.H.; Alonso, S. A single-shot vaccine approach for the universal influenza A vaccine candidate M2e. Proc. Natl. Acad. Sci. USA 2022, 119, e2025607119. [Google Scholar] [CrossRef]
- Mezhenskaya, D.; Isakova-Sivak, I.; Matyushenko, V.; Donina, S.; Rekstin, A.; Sivak, K.; Yakovlev, K.; Katelnikova, A.; Kryshen, K.; Makarov, V.; et al. Universal live-attenuated influenza vaccine candidates expressing multiple M2e epitopes protect ferrets against a high-dose heterologous virus challenge. Viruses 2021, 13, 1280. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Huang, P.-H.; Lin, L.C.W.; Yao, B.-Y.; Liao, W.-T.; Pai, C.-H.; Liu, Y.-H.; Chen, H.-W.; Hu, C.-M.J. Lymph node follicle-targeting STING agonist nanoshells enable single-shot M2e vaccination for broad and durable influenza protection. Adv. Sci. 2023, 10, e2206521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deng, L.; Gonzalez, G.X.; Luthra, L.; Dong, C.; Ma, Y.; Zou, J.; Kang, S.-M.; Wang, B.-Z. Double-layered M2e-NA protein nanoparticle immunization induces broad cross-protection against different influenza viruses in mice. Adv. Healthc. Mater. 2020, 9, e1901176. [Google Scholar] [CrossRef] [PubMed]
- Neirynck, S.; Deroo, T.; Saelens, X.; Vanlandschoot, P.; Jou, W.M.; Fiers, W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 1999, 5, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Von Holle, T.A.; Moody, M.A. Influenza and antibody-dependent cellular cytotoxicity. Front. Immunol. 2019, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Schepens, B.; De Vlieger, D.; Saelens, X. Vaccine options for influenza: Thinking small. Curr. Opin. Immunol. 2018, 53, 22–29. [Google Scholar] [CrossRef]
- Kolpe, A.; Schepens, B.; Fiers, W.; Saelens, X. M2-based influenza vaccines: Recent advances and clinical potential. Expert Rev. Vaccines 2017, 16, 123–136. [Google Scholar] [CrossRef]
- Braz Gomes, K.; Zhang, Y.-N.; Lee, Y.-Z.; Eldad, M.; Lim, A.; Ward, G.; Auclair, S.; He, L.; Zhu, J. Single-component multilayered self-assembling protein nanoparticles displaying extracellular domains of matrix protein 2 as a pan-influenza A vaccine. ACS Nano 2023, 17, 23545–23567. [Google Scholar] [CrossRef]
- Lua, L.H.L.; Connors, N.K.; Sainsbury, F.; Chuan, Y.P.; Wibowo, N.; Middelberg, A.P.J. Bioengineering virus-like particles as vaccines. Biotechnol. Bioeng. 2014, 111, 425–440. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.; Yu, H.; Xia, N.; Modis, Y. Virus-like particle-based human vaccines: Quality assessment based on structural and functional properties. Trends Biotechnol. 2013, 31, 654–663. [Google Scholar] [CrossRef]
- Rodríguez-Limas, W.A.; Sekar, K.; Tyo, K.E.J. Virus-like particles: The future of microbial factories and cell-free systems as platforms for vaccine development. Curr. Opin. Biotechnol. 2013, 24, 1089–1093. [Google Scholar] [CrossRef]
- Pushko, P.; Pumpens, P.; Grens, E. Development of virus-like particle technology from small highly symmetric to large complex virus-like particle structures. Intervirology 2013, 56, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef]
- Jennings, G.T.; Bachmann, M.F. Coming of age of virus-like particle vaccines. Biol. Chem. 2008, 389, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, C.; Wagner, R. Virus-like particles—Universal molecular toolboxes. Curr. Opin. Biotechnol. 2007, 18, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Grgacic, E.V.L.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Paynter, J.; Antanasijevic, A.; Allen, J.D.; Eldad, M.; Lee, Y.-Z.; Copps, J.; Newby, M.L.; He, L.; Chavez, D.; et al. Single-component multilayered self-assembling protein nanoparticles presenting glycan-trimmed uncleaved prefusion optimized envelope trimers as HIV-1 vaccine candidates. Nat. Commun. 2022, 14, 1985. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Kumar, S.; Allen, J.D.; Huang, D.; Lin, X.; Mann, C.J.; Saye-Francisco, K.L.; Copps, J.; Sarkar, A.; Blizard, G.S.; et al. HIV-1 vaccine design through minimizing envelope metastability. Sci. Adv. 2018, 4, eaau6769. [Google Scholar] [CrossRef]
- He, L.; de Val, N.; Morris, C.D.; Vora, N.; Thinnes, T.C.; Kong, L.; Azadnia, P.; Sok, D.; Zhou, B.; Burton, D.R.; et al. Presenting native-like trimeric HIV-1 antigens with self-assembling nanoparticles. Nat. Commun. 2016, 7, 12041. [Google Scholar] [CrossRef]
- He, L.; Tzarum, N.; Lin, X.; Shapero, B.; Sou, C.; Mann, C.J.; Stano, A.; Zhang, L.; Nagy, K.; Giang, E.; et al. Proof of concept for rational design of hepatitis C virus E2 core nanoparticle vaccines. Sci. Adv. 2020, 6, eaaz6225. [Google Scholar] [CrossRef]
- He, L.; Chaudhary, A.; Lin, X.; Sou, C.; Alkutkar, T.; Kumar, S.; Ngo, T.; Kosviner, E.; Ozorowski, G.; Stanfield, R.L.; et al. Single-component multilayered self-assembling nanoparticles presenting rationally designed glycoprotein trimers as Ebola virus vaccines. Nat. Commun. 2021, 12, 2633. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Paynter, J.; Sou, C.; Fourfouris, T.; Wang, Y.; Abraham, C.; Ngo, T.; Zhang, Y.; He, L.; Zhu, J. Mechanism of a COVID-19 nanoparticle vaccine candidate that elicits a broadly neutralizing antibody response to SARS-CoV-2 variants. Sci. Adv. 2021, 7, eabj3107. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lin, X.; Wang, Y.; Abraham, C.; Sou, C.; Ngo, T.; Zhang, Y.; Wilson, I.A.; Zhu, J. Single-component, self-assembling, protien nanoparticles presenting the receptor binding domain and stabilized spike as SARS-CoV-2 vaccine candidates. Sci. Adv. 2021, 7, eabf1591. [Google Scholar] [CrossRef] [PubMed]
- van de Sandt, C.E.; Bodewes, R.; Rimmelzwaan, G.F.; de Vries, R.D. Influenza B viruses: Not to be discounted. Future Microbiol. 2015, 10, 1447–1465. [Google Scholar] [CrossRef]
- Zaraket, H.; Hurt, A.C.; Clinch, B.; Barr, I.; Lee, N. Burden of influenza B virus infection and considerations for clinical management. Antivir. Res. 2021, 185, 104970. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; Lee, Y.-N.; Kim, Y.-J.; Choi, H.-J.; Kim, K.-H.; Lee, Y.-J.; Kang, S.-M. Immunogenicity and efficacy of replication-competent recombinant influenza virus carrying multimeric M2 extracellular domains in a chimeric hemagglutinin conjugate. Antivir. Res. 2017, 148, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-C.; Song, J.-M.; Eunju, O.; Kwon, Y.-M.; Lee, Y.-J.; Compans, R.W.; Kang, S.-M. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol. Ther. 2013, 21, 485–492. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, L.; Chen, Y.-H. Immunization with high epitope density of M2e derived from 2009 pandemic H1N1 elicits protective immunity in mice. Vaccine 2012, 30, 3463–3469. [Google Scholar] [CrossRef]
- Ding, P.; Zhang, G.; Chen, Y.; Liu, H.; Liu, Y.; Jia, R.; Wang, Y.; Li, G.; Wang, A. Reasonable permutation of M2e enhances the effect of universal influenza nanovaccine. Int. J. Biol. Macromol. 2021, 173, 244–250. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Zou, P.; Wang, M.; Fu, W.; She, J.; Song, Z.; Xu, J.; Huang, J.; Wu, F. Self-assembly M2e-based peptide nanovaccine confers broad protection against influenza viruses. Front. Microbiol. 2020, 11, 1961. [Google Scholar] [CrossRef]
- Pekarek, M.J.; Petro-Turnquist, E.M.; Rubrum, A.; Webby, R.J.; Weaver, E.A. Expanding mouse-adapted Yamagata-like influenza B viruses in eggs enhances In vivo lethality in BALB/c mice. Viruses 2022, 14, 1299. [Google Scholar] [CrossRef]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Yang, J.; Hu, J.; Sun, X. On the calculation of TCID(50) for quantitation of virus infectivity. Virol. Sin. 2021, 36, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. Influenza: The once and future pandemic. Public Health Rep. 2010, 125, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Kash, J.C. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe 2010, 7, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, J.; Oh, J.; Kim, K.-H.; Shin, C.-H.; Park, B.R.; Bhatnagar, N.; Seong, B.-L.; Wang, B.-Z.; Kang, S.-M. A chimeric thermostable M2e and H3 stalk-based universal influenza A virus vaccine. npj Vaccines 2022, 7, 68. [Google Scholar] [CrossRef]
- Subbbiah, J.; Oh, J.; Kim, K.-H.; Shin, C.H.; Park, B.R.; Bhatnagar, N.; Jung, Y.-J.; Lee, Y.; Wang, B.-Z.; Seong, B.-L.; et al. Thermostable H1 hemagglutinin stem with M2e epitopes provides broad cross-protection against group1 and 2 influenza A viruses. Mol. Ther. Methods Clin. Dev. 2022, 26, 38–51. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-N.; Gomes, K.B.; Lee, Y.-Z.; Ward, G.; Xie, B.; Auclair, S.; He, L.; Zhu, J. A Single-Component Multilayered Self-Assembling Protein Nanoparticle Vaccine Based on Extracellular Domains of Matrix Protein 2 against Both Influenza A and B. Vaccines 2024, 12, 975. https://doi.org/10.3390/vaccines12090975
Zhang Y-N, Gomes KB, Lee Y-Z, Ward G, Xie B, Auclair S, He L, Zhu J. A Single-Component Multilayered Self-Assembling Protein Nanoparticle Vaccine Based on Extracellular Domains of Matrix Protein 2 against Both Influenza A and B. Vaccines. 2024; 12(9):975. https://doi.org/10.3390/vaccines12090975
Chicago/Turabian StyleZhang, Yi-Nan, Keegan Braz Gomes, Yi-Zong Lee, Garrett Ward, Bomin Xie, Sarah Auclair, Linling He, and Jiang Zhu. 2024. "A Single-Component Multilayered Self-Assembling Protein Nanoparticle Vaccine Based on Extracellular Domains of Matrix Protein 2 against Both Influenza A and B" Vaccines 12, no. 9: 975. https://doi.org/10.3390/vaccines12090975
APA StyleZhang, Y.-N., Gomes, K. B., Lee, Y.-Z., Ward, G., Xie, B., Auclair, S., He, L., & Zhu, J. (2024). A Single-Component Multilayered Self-Assembling Protein Nanoparticle Vaccine Based on Extracellular Domains of Matrix Protein 2 against Both Influenza A and B. Vaccines, 12(9), 975. https://doi.org/10.3390/vaccines12090975






