The Major Role of T Regulatory Cells in the Efficiency of Vaccination in General and Immunocompromised Populations: A Review
Abstract
:1. Introduction
2. General Description of Memory T Cell Immune Response during Vaccination
3. General Description of Different T Regulatory Cells
4. Regulatory T Cells Downregulate the Immune Response to Vaccines
4.1. Pre-Vaccination Treg Level Affects Vaccination Efficacy
4.2. Presence of Tregs Affects Efficacy after Vaccination against Infectious Diseases
4.3. Presence of Tregs Affects Efficacy after Vaccination against Cancer
4.4. Depletion of Treg Cells Improves Vaccination Efficacy
5. Role of Tr1 Cells in Vaccination of Transplant Recipients
6. Methods to Overcome Treg/Tr1 to Improve Vaccination Efficacy
6.1. Treg/Tr1 Modulation to Improve Vaccine Immunogenicity
6.2. Other Methods to Improve Vaccination’s Efficacy and Overcome Strong Treg/Tr1 Function
7. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Baxby, D. Jenner and the control of smallpox. Trans. Med. Soc. Lond. 1996, 113, 18–22. [Google Scholar] [PubMed]
- Barquet, N.; Domingo, P. Smallpox: The triumph over the most terrible of the ministers of death. Ann. Intern. Med. 1997, 127 Pt 1, 635–642. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Polio vaccines: WHO position paper—June 2022. Wkly. Epidemiol. Rec. 2022, 97, 277–300. [Google Scholar]
- World Health Organization. Measles vaccines: WHO position paper—April 2017. Wkly. Epidemiol. Rec. 2017, 92, 205–228. [Google Scholar]
- Pleguezuelos, O.; James, E.; Fernandez, A.; Lopes, V.; Rosas, L.A.; Cervantes-Medina, A.; Cleath, J.; Edwards, K.; Neitzey, D.; Gu, W.; et al. Efficacy of FLU-v, a broad-spectrum influenza vaccine, in a randomized phase IIb human influenza challenge study. NPJ Vaccines 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atsmon, J.; Kate-Ilovitz, E.; Shaikevich, D.; Singer, Y.; Volokhov, I.; Haim, K.Y.; Ben-Yedidia, T. Safety and immunogenicity of multimeric-001--a novel universal influenza vaccine. J. Clin. Immunol. 2012, 32, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Kasten-Jolly, J.; Lawrence, D.A. Cellular and Molecular Immunity to Influenza Viruses and Vaccines. Vaccines 2024, 12, 389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, C.; Bai, Y.; Liu, J.; Wang, Y.; He, Q.; Zhang, X.; Cheng, F.; Xu, M.; Mao, Q.; Liang, Z. Research progress on the quality control of mRNA vaccines. Expert. Rev. Vaccines 2024, 23, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lagana, A.; Visalli, G.; Di Pietro, A.; Facciola, A. Vaccinomics and adversomics: Key elements for a personalized vaccinology. Clin. Exp. Vaccine Res. 2024, 13, 105–120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clinic, M. Vaccine Guidance from Mayo Clinic 2024 [Updated 13 March 2024]. Available online: https://www.mayoclinic.org/diseases-conditions/infectious-diseases/in-depth/vaccine-guidance/art-20536857 (accessed on 17 June 2024).
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, 1128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geckin, B.; Konstantin Fohse, F.; Dominguez-Andres, J.; Netea, M.G. Trained immunity: Implications for vaccination. Curr. Opin. Immunol. 2022, 77, 102190. [Google Scholar] [CrossRef] [PubMed]
- Danziger-Isakov, L.; Kumar, D.; Practice, A.I.C.o. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019, 33, e13563. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Zapien, G.J.; Martinez-Cuazitl, A.; Sanchez-Brito, M.; Delgado-Macuil, R.J.; Atriano-Colorado, C.; Garibay-Gonzalez, F.; Sanchez-Monroy, V.; Lopez-Reyes, A.; Mata-Miranda, M.M. Comparison of the Immune Response in Vaccinated People Positive and Negative to SARS-CoV-2 Employing FTIR Spectroscopy. Cells 2022, 11, 3884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindenstrom, T.; Woodworth, J.; Dietrich, J.; Aagaard, C.; Andersen, P.; Agger, E.M. Vaccine-induced th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect. Immun. 2012, 80, 3533–3544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roncarolo, M.G.; Gregori, S.; Battaglia, M.; Bacchetta, R.; Fleischhauer, K.; Levings, M.K. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 2006, 212, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, J.M.; Masopust, D. Tissue-resident memory T cells. Immunity 2014, 41, 886–897. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sallusto, F.; Lenig, D.; Forster, R.; Lipp, M.; Lanzavecchia, A. Pillars article: Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999. 401: 708–712. J. Immunol. 2014, 192, 840–844. [Google Scholar] [PubMed]
- von Andrian, U.H.; Mackay, C.R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000, 343, 1020–1034. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lanzavecchia, A.; Araki, K.; Ahmed, R. From vaccines to memory and back. Immunity 2010, 33, 451–463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Butcher, E.C.; Picker, L.J. Lymphocyte homing and homeostasis. Science 1996, 272, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Mackay, C.R.; Marston, W.L.; Dudler, L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 1990, 171, 801–817. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yenyuwadee, S.; Sanchez-Trincado Lopez, J.L.; Shah, R.; Rosato, P.C.; Boussiotis, V.A. The evolving role of tissue-resident memory T cells in infections and cancer. Sci. Adv. 2022, 8, eabo5871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karin, N.; Razon, H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine 2018, 109, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Gershon, R.K.; Kondo, K. Cell interactions in the induction of tolerance: The role of thymic lymphocytes. Immunology 1970, 18, 723–737. [Google Scholar] [PubMed] [PubMed Central]
- Stepkowski, S.M.; Bitter-Suermann, H.; Duncan, W.R. Induction of transplantation tolerance in rats by spleen allografts. II. Evidence that W3/25+ T suppressor/inducer and OX8+ T suppressor/effector cells are required to mediate specific unresponsiveness. Transplantation 1987, 44, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Brown, J.A.; Freeman, G.J.; Hafler, D.A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001, 167, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Taams, L.S.; Smith, J.; Rustin, M.H.; Salmon, M.; Poulter, L.W.; Akbar, A.N. Human anergic/suppressive CD4(+)CD25(+) T cells: A highly differentiated and apoptosis-prone population. Eur. J. Immunol. 2001, 31, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Jonuleit, H.; Schmitt, E.; Stassen, M.; Tuettenberg, A.; Knop, J.; Enk, A.H. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001, 193, 1285–1294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hori, S.; Nomura, T.; Sakaguchi, S. Pillars Article: Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003. 299: 1057–1061. J. Immunol. 2017, 198, 981–985. [Google Scholar] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Pillars Article: Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. Nat. Immunol. 2003. 4: 330–336. J. Immunol. 2017, 198, 986–992. [Google Scholar] [PubMed]
- Luo, Y.; Xue, Y.; Wang, J.; Dang, J.; Fang, Q.; Huang, G.; Olsen, N.; Zheng, S.G. Negligible Effect of Sodium Chloride on the Development and Function of TGF-beta-Induced CD4(+) Foxp3(+) Regulatory T Cells. Cell Rep. 2019, 26, 1869–1879.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, S.G.; Wang, J.; Horwitz, D.A. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J. Immunol. 2008, 180, 7112–7116. [Google Scholar] [CrossRef] [PubMed]
- Halim, L.; Romano, M.; McGregor, R.; Correa, I.; Pavlidis, P.; Grageda, N.; Hoong, S.J.; Yuksel, M.; Jassem, W.; Hannen, R.F.; et al. An Atlas of Human Regulatory T Helper-like Cells Reveals Features of Th2-like Tregs that Support a Tumorigenic Environment. Cell Rep. 2017, 20, 757–770. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romagnani, S.; Maggi, E.; Liotta, F.; Cosmi, L.; Annunziato, F. Properties and origin of human Th17 cells. Mol. Immunol. 2009, 47, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Tanaka, S.; Chu, F.; Nurieva, R.I.; Martinez, G.J.; Rawal, S.; Wang, Y.H.; Lim, H.; Reynolds, J.M.; Zhou, X.H.; et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011, 17, 983–988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suhrkamp, I.; Scheffold, A.; Heine, G. T-cell subsets in allergy and tolerance induction. Eur. J. Immunol. 2023, 53, e2249983. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Dong, L.; Zhong, J. Immunomodulatory effects of iTr35 cell subpopulation and its research progress. Clin. Exp. Med. 2024, 24, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koizumi, S.I.; Ishikawa, H. Transcriptional Regulation of Differentiation and Functions of Effector T Regulatory Cells. Cells 2019, 8, 939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, P.; Liu, C.; Yu, Z.; Wu, M. New Insights into Regulatory T Cells: Exosome- and Non-Coding RNA-Mediated Regulation of Homeostasis and Resident Treg Cells. Front. Immunol. 2016, 7, 574. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korn, T.; Muschaweckh, A. Stability and Maintenance of Foxp3(+) Treg Cells in Non-lymphoid Microenvironments. Front. Immunol. 2019, 10, 2634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, A.; Rudra, D. Emerging Functions of Regulatory T Cells in Tissue Homeostasis. Front. Immunol. 2018, 9, 883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Najaf Khosravi, H.; Razi, S.; Rezaei, N. The role of interleukin-2 in graft-versus-host disease pathogenesis, prevention and therapy. Cytokine 2024, 183, 156723. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, D.A.; Wang, J.H.; Kim, D.; Kang, C.; Brion, K.; Bickerton, S.; La Cava, A. Nanoparticles loaded with IL-2 and TGF-beta promote transplantation tolerance to alloantigen. Front. Immunol. 2024, 15, 1429335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boehm, F.; Martin, M.; Kesselring, R.; Schiechl, G.; Geissler, E.K.; Schlitt, H.J.; Fichtner-Feigl, S. Deletion of Foxp3+ regulatory T cells in genetically targeted mice supports development of intestinal inflammation. BMC Gastroenterol. 2012, 12, 97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Collison, L.W.; Pillai, M.R.; Chaturvedi, V.; Vignali, D.A. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J. Immunol. 2009, 182, 6121–6128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, K.; Kitani, A.; Strober, W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 2001, 194, 629–644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pandiyan, P.; Zheng, L.; Ishihara, S.; Reed, J.; Lenardo, M.J. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 2007, 8, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Tekguc, M.; Wing, J.B.; Osaki, M.; Long, J.; Sakaguchi, S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2023739118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cross, A.R.; Lion, J.; Poussin, K.; Glotz, D.; Mooney, N. Inflammation Determines the Capacity of Allogenic Endothelial Cells to Regulate Human Treg Expansion. Front. Immunol. 2021, 12, 666531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kazanova, A.; Rudd, C.E. Programmed cell death 1 ligand (PD-L1) on T cells generates Treg suppression from memory. PLoS Biol. 2021, 19, e3001272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kondelkova, K.; Vokurkova, D.; Krejsek, J.; Borska, L.; Fiala, Z.; Ctirad, A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica 2010, 53, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Rocamora-Reverte, L.; Melzer, F.L.; Wurzner, R.; Weinberger, B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front. Immunol. 2020, 11, 616949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Workman, C.J.; Szymczak-Workman, A.L.; Collison, L.W.; Pillai, M.R.; Vignali, D.A. The development and function of regulatory T cells. Cell. Mol. Life Sci. 2009, 66, 2603–2622. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Russler-Germain, E.V.; Rengarajan, S.; Hsieh, C.S. Antigen-specific regulatory T-cell responses to intestinal microbiota. Mucosal Immunol. 2017, 10, 1375–1386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duhen, T.; Duhen, R.; Lanzavecchia, A.; Sallusto, F.; Campbell, D.J. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 2012, 119, 4430–4440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koch, M.A.; Thomas, K.R.; Perdue, N.R.; Smigiel, K.S.; Srivastava, S.; Campbell, D.J. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity 2012, 37, 501–510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, Y.; Chaudhry, A.; Kas, A.; deRoos, P.; Kim, J.M.; Chu, T.T.; Corcoran, L.; Treuting, P.; Klein, U.; Rudensky, A.Y. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature 2009, 458, 351–356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gregori, S.; Goudy, K.S.; Roncarolo, M.G. The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front. Immunol. 2012, 3, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Geginat, J.; Vasco, C.; Gruarin, P.; Bonnal, R.; Rossetti, G.; Silvestri, Y.; Carelli, E.; Pulvirenti, N.; Scantamburlo, M.; Moschetti, G.; et al. Eomesodermin-expressing type 1 regulatory (EOMES(+) Tr1)-like T cells: Basic biology and role in immune-mediated diseases. Eur. J. Immunol. 2023, 53, e2149775. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, N.; Li, H.; Bian, Y.; Wen, W.; Kong, X.; Wang, F. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: A crosstalk between ancient “Yin-Yang” theory and modern immunology. Cell Commun. Signal. 2024, 22, 99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, X.; Zhang, J.; Cui, J.; Xu, W.; Zhao, G.; Guo, C.; Yuan, W.; Zhou, X.; Ma, J. Adaptive plasticity of natural interleukin-35-induced regulatory T cells (Tr35) that are required for T-cell immune regulation. Theranostics 2024, 14, 2897–2914. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anastassopoulou, C.; Ferous, S.; Medic, S.; Siafakas, N.; Boufidou, F.; Gioula, G.; Tsakris, A. Vaccines for the Elderly and Vaccination Programs in Europe and the United States. Vaccines 2024, 12, 566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palatella, M.; Guillaume, S.M.; Linterman, M.A.; Huehn, J. The dark side of Tregs during aging. Front. Immunol. 2022, 13, 940705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrero-Fernandez, I.; Rosado-Sanchez, I.; Alvarez-Rios, A.I.; Galva, M.I.; De Luna-Romero, M.; Sanbonmatsu-Gamez, S.; Perez-Ruiz, M.; Navarro-Mari, J.M.; Carrillo-Vico, A.; Sanchez, B.; et al. Effect of homeostatic T-cell proliferation in the vaccine responsiveness against influenza in elderly people. Immun. Ageing 2019, 16, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bodey, B.; Bodey, B., Jr.; Siegel, S.E.; Kaiser, H.E. Involution of the mammalian thymus, one of the leading regulators of aging. In Vivo 1997, 11, 421–440. [Google Scholar] [PubMed]
- Chougnet, C.A.; Tripathi, P.; Lages, C.S.; Raynor, J.; Sholl, A.; Fink, P.; Plas, D.R.; Hildeman, D.A. A major role for Bim in regulatory T cell homeostasis. J. Immunol. 2011, 186, 156–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raynor, J.; Sholl, A.; Plas, D.R.; Bouillet, P.; Chougnet, C.A.; Hildeman, D.A. IL-15 Fosters Age-Driven Regulatory T Cell Accrual in the Face of Declining IL-2 Levels. Front. Immunol. 2013, 4, 161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jagger, A.; Shimojima, Y.; Goronzy, J.J.; Weyand, C.M. Regulatory T cells and the immune aging process: A mini-review. Gerontology 2014, 60, 130–137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lages, C.S.; Suffia, I.; Velilla, P.A.; Huang, B.; Warshaw, G.; Hildeman, D.A.; Belkaid, Y.; Chougnet, C. Functional regulatory T cells accumulate in aged hosts and promote chronic infectious disease reactivation. J. Immunol. 2008, 181, 1835–1848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenkranz, D.; Weyer, S.; Tolosa, E.; Gaenslen, A.; Berg, D.; Leyhe, T.; Gasser, T.; Stoltze, L. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J. Neuroimmunol. 2007, 188, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Agius, E.; Lacy, K.E.; Vukmanovic-Stejic, M.; Jagger, A.L.; Papageorgiou, A.P.; Hall, S.; Reed, J.R.; Curnow, S.J.; Fuentes-Duculan, J.; Buckley, C.D.; et al. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J. Exp. Med. 2009, 206, 1929–1940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gregg, R.; Smith, C.M.; Clark, F.J.; Dunnion, D.; Khan, N.; Chakraverty, R.; Nayak, L.; Moss, P.A. The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin. Exp. Immunol. 2005, 140, 540–546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D.; et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B. Vaccines for the elderly: Current use and future challenges. Immun. Ageing 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batista-Duharte, A.; Pera, A.; Alino, S.F.; Solana, R. Regulatory T cells and vaccine effectiveness in older adults. Challenges and prospects. Int. Immunopharmacol. 2021, 96, 107761. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Rabaan, A.A.; Alwarthan, S.; Alhajri, M.; Halwani, M.A.; Alshengeti, A.; Najim, M.A.; Alwashmi, A.S.S.; Alshehri, A.A.; Alshamrani, S.A.; et al. Regulatory T Cells (Tregs) and COVID-19: Unveiling the Mechanisms, and Therapeutic Potentialities with a Special Focus on Long COVID. Vaccines 2023, 11, 699. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Franco, A.; Song, J.; Chambers, C.; Sette, A.; Grifoni, A. SARS-CoV-2 spike-specific regulatory T cells (Treg) expand and develop memory in vaccine recipients suggesting a role for immune regulation in preventing severe symptoms in COVID-19. Autoimmunity 2023, 56, 2259133. [Google Scholar] [CrossRef] [PubMed]
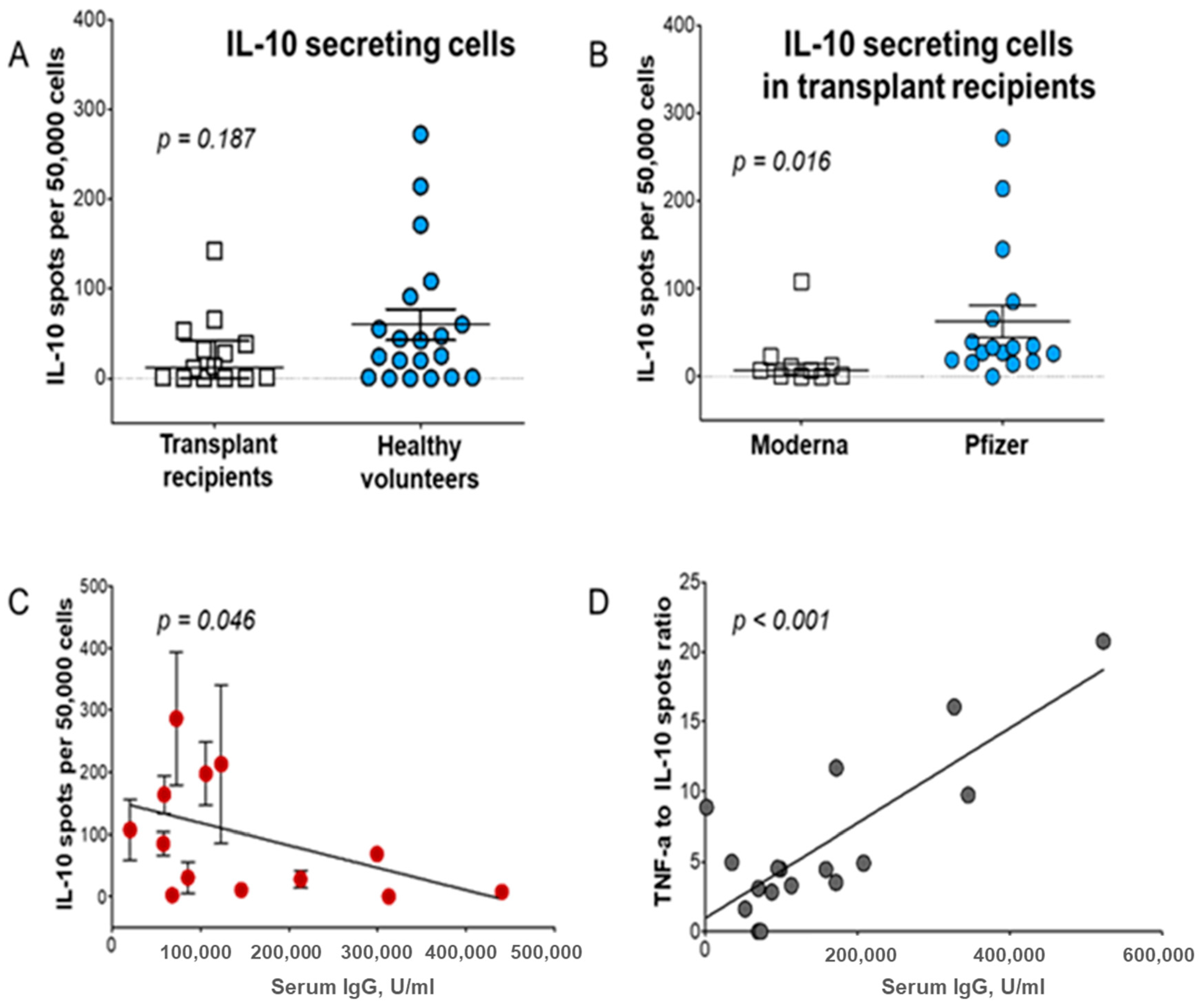
- Bekbolsynov, D.; Waack, A.; Buskey, C.; Bhadkamkar, S.; Rengel, K.; Petersen, W.; Brown, M.L.; Sparkle, T.; Kaw, D.; Syed, F.J.; et al. Differences in Responses of Immunosuppressed Kidney Transplant Patients to Moderna mRNA-1273 versus Pfizer-BioNTech. Vaccines 2024, 12, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, H.; Peng, X.; Gan, L.; Feng, J.; Gao, Y.; Yang, S.; Hu, X.; Zhang, L.; Yin, Y.; Wang, H.; et al. Protective Regulatory T Cell Immune Response Induced by Intranasal Immunization With the Live-Attenuated Pneumococcal Vaccine SPY1 via the Transforming Growth Factor-beta1-Smad2/3 Pathway. Front. Immunol. 2018, 9, 1754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amr, A.E.E.; Abo-Ghalia, M.H.; Moustafa, G.O.; Al-Omar, M.A.; Nossier, E.S.; Elsayed, E.A. Design, Synthesis and Docking Studies of Novel Macrocyclic Pentapeptides as Anticancer Multi-Targeted Kinase Inhibitors. Molecules 2018, 23, 2416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, M.L.; Chen, Z.H.; Teng, Y.Y.; Liu, S.Y.; Jia, Y.; Zhang, K.W.; Sun, Z.L.; Wu, J.J.; Yuan, Z.D.; Feng, Y.; et al. The Smad Dependent TGF-beta and BMP Signaling Pathway in Bone Remodeling and Therapies. Front. Mol. Biosci. 2021, 8, 593310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brezar, V.; Godot, V.; Cheng, L.; Su, L.; Levy, Y.; Seddiki, N. T-Regulatory Cells and Vaccination “Pay Attention and Do Not Neglect Them”: Lessons from HIV and Cancer Vaccine Trials. Vaccines 2016, 4, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappellano, G.; Abreu, H.; Casale, C.; Dianzani, U.; Chiocchetti, A. Nano-Microparticle Platforms in Developing Next-Generation Vaccines. Vaccines 2021, 9, 606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, A.C.; Zhang, A.J.; Li, C.; Chen, Y.; Liu, F.; Zhao, Y.; Chu, H.; Fong, C.H.; Wang, P.; Lau, S.Y.; et al. Intradermal vaccination of live attenuated influenza vaccine protects mice against homologous and heterologous influenza challenges. NPJ Vaccines 2021, 6, 95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, P.H.; Wong, W.I.; Wang, Y.L.; Hsieh, M.P.; Lu, C.W.; Liang, C.Y.; Jui, S.H.; Wu, F.Y.; Chen, P.J.; Yang, H.C. Vaccine-induced antigen-specific regulatory T cells attenuate the antiviral immunity against acute influenza virus infection. Mucosal Immunol. 2018, 11, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Dinc, R. Leishmania Vaccines: The Current Situation with Its Promising Aspect for the Future. Korean J. Parasitol. 2022, 60, 379–391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muller, A.; Solnick, J.V. Inflammation, immunity, and vaccine development for Helicobacter pylori. Helicobacter 2011, 16 (Suppl. S1), 26–32. [Google Scholar] [CrossRef] [PubMed]
- Dyck, L.; Wilk, M.M.; Raverdeau, M.; Misiak, A.; Boon, L.; Mills, K.H. Anti-PD-1 inhibits Foxp3(+) Treg cell conversion and unleashes intratumoural effector T cells thereby enhancing the efficacy of a cancer vaccine in a mouse model. Cancer Immunol. Immunother. 2016, 65, 1491–1498. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weinberg, A.; Canniff, J.; Rouphael, N.; Mehta, A.; Mulligan, M.; Whitaker, J.A.; Levin, M.J. Varicella-Zoster Virus-Specific Cellular Immune Responses to the Live Attenuated Zoster Vaccine in Young and Older Adults. J. Immunol. 2017, 199, 604–612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sellars, M.C.; Wu, C.J.; Fritsch, E.F. Cancer vaccines: Building a bridge over troubled waters. Cell 2022, 185, 2770–2788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strum, S.; Andersen, M.H.; Svane, I.M.; Siu, L.L.; Weber, J.S. State-Of-The-Art Advancements on Cancer Vaccines and Biomarkers. Am. Soc. Clin. Oncol. Educ. Book. 2024, 44, e438592. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.G.; Chattopadhyay, S.; Mehrotra, S.; Chhabra, A.; Mukherji, B. Regulatory T-cell response and tumor vaccine-induced cytotoxic T lymphocytes in human melanoma. Hum. Immunol. 2004, 65, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Lahl, K.; Sparwasser, T. In vivo depletion of FoxP3+ Tregs using the DEREG mouse model. Methods Mol. Biol. 2011, 707, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Klages, K.; Mayer, C.T.; Lahl, K.; Loddenkemper, C.; Teng, M.W.; Ngiow, S.F.; Smyth, M.J.; Hamann, A.; Huehn, J.; Sparwasser, T. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010, 70, 7788–7799. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Solomon, I.; Amann, M.; Goubier, A.; Arce Vargas, F.; Zervas, D.; Qing, C.; Henry, J.Y.; Ghorani, E.; Akarca, A.U.; Marafioti, T.; et al. CD25-T(reg)-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat. Cancer 2020, 1, 1153–1166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nicolini, A.; Ferrari, P. Involvement of tumor immune microenvironment metabolic reprogramming in colorectal cancer progression, immune escape, and response to immunotherapy. Front. Immunol. 2024, 15, 1353787. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Franco, F.; Tsui, Y.C.; Xie, X.; Trefny, M.P.; Zappasodi, R.; Mohmood, S.R.; Fernandez-Garcia, J.; Tsai, C.H.; Schulze, I.; et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 2020, 21, 298–308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021, 33, 1001–1012.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, X.; Hartman, C.L.; Li, L.; Albert, C.J.; Si, F.; Gao, A.; Huang, L.; Zhao, Y.; Lin, W.; Hsueh, E.C.; et al. Reprogramming lipid metabolism prevents effector T cell senescence and enhances tumor immunotherapy. Sci. Transl. Med. 2021, 13, aaz6314. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kumar, S.; Dahiya, S.; Wang, F.; Wu, J.; Newick, K.; Han, R.; Samanta, A.; Beier, U.H.; Akimova, T.; et al. Ubiquitin-specific Protease-7 Inhibition Impairs Tip60-dependent Foxp3+ T-regulatory Cell Function and Promotes Antitumor Immunity. EBioMedicine 2016, 13, 99–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harbecke, R.; Cohen, J.I.; Oxman, M.N. Herpes Zoster Vaccines. J. Infect. Dis. 2021, 224, S429–S442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wen, Z.; Wang, X.; Dong, K.; Zhang, H.; Bu, Z.; Ye, L.; Yang, C. Blockage of regulatory T cells augments induction of protective immune responses by influenza virus-like particles in aged mice. Microbes Infect. 2017, 19, 626–634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romeli, S.; Hassan, S.S.; Yap, W.B. Multi-Epitope Peptide-Based and Vaccinia-Based Universal Influenza Vaccine Candidates Subjected to Clinical Trials. Malays. J. Med. Sci. 2020, 27, 10–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rutigliano, J.A.; Sharma, S.; Morris, M.Y.; Oguin, T.H., 3rd; McClaren, J.L.; Doherty, P.C.; Thomas, P.G. Highly pathological influenza A virus infection is associated with augmented expression of PD-1 by functionally compromised virus-specific CD8+ T cells. J. Virol. 2014, 88, 1636–1651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caselli, E.; Boni, M.; Di Luca, D.; Salvatori, D.; Vita, A.; Cassai, E. A combined bovine herpesvirus 1 gB-gD DNA vaccine induces immune response in mice. Comp. Immunol. Microbiol. Infect. Dis. 2005, 28, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Toka, F.N.; Suvas, S.; Rouse, B.T. CD4+ CD25+ T cells regulate vaccine-generated primary and memory CD8+ T-cell responses against herpes simplex virus type 1. J. Virol. 2004, 78, 13082–13089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furuichi, Y.; Tokuyama, H.; Ueha, S.; Kurachi, M.; Moriyasu, F.; Kakimi, K. Depletion of CD25+CD4+T cells (Tregs) enhances the HBV-specific CD8+ T cell response primed by DNA immunization. World J. Gastroenterol. 2005, 11, 3772–3777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaron, B.; Maranghi, E.; Leclerc, C.; Majlessi, L. Effect of attenuation of Treg during BCG immunization on anti-mycobacterial Th1 responses and protection against Mycobacterium tuberculosis. PLoS ONE 2008, 3, e2833. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quinn, K.M.; McHugh, R.S.; Rich, F.J.; Goldsack, L.M.; de Lisle, G.W.; Buddle, B.M.; Delahunt, B.; Kirman, J.R. Inactivation of CD4+ CD25+ regulatory T cells during early mycobacterial infection increases cytokine production but does not affect pathogen load. Immunol. Cell Biol. 2006, 84, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Espinoza Mora, M.R.; Steeg, C.; Tartz, S.; Heussler, V.; Sparwasser, T.; Link, A.; Fleischer, B.; Jacobs, T. Depletion of regulatory T cells augments a vaccine-induced T effector cell response against the liver-stage of malaria but fails to increase memory. PLoS ONE 2014, 9, e104627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garner-Spitzer, E.; Wagner, A.; Paulke-Korinek, M.; Kollaritsch, H.; Heinz, F.X.; Redlberger-Fritz, M.; Stiasny, K.; Fischer, G.F.; Kundi, M.; Wiedermann, U. Tick-borne encephalitis (TBE) and hepatitis B nonresponders feature different immunologic mechanisms in response to TBE and influenza vaccination with involvement of regulatory T and B cells and IL-10. J. Immunol. 2013, 191, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Litjens, N.H.; Boer, K.; Betjes, M.G. Identification of circulating human antigen-reactive CD4+ FOXP3+ natural regulatory T cells. J. Immunol. 2012, 188, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, L.; Niu, W.; Wu, Y.; Zhang, J.; Meng, G. Increased CD4+CD25+FoxP3+ regulatory T cells in the blood of nonresponders after standard hepatitis B surface antigen vaccine immunization. Clin. Immunol. 2008, 127, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, S.; Zhang, J.; Wang, L.; Wu, Y. Expression of PD-1 is up-regulated in CD4+CD25+ FoxP3+ regulatory T cell of non-responders after hepatitis B surface antigen vaccine immunization. Clin. Immunol. 2008, 129, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Farez, M.; Razzitte, G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann. Neurol. 2008, 64, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Duddy, M.; Niino, M.; Adatia, F.; Hebert, S.; Freedman, M.; Atkins, H.; Kim, H.J.; Bar-Or, A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 2007, 178, 6092–6099. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.A.; Norena, L.Y.; Flores-Borja, F.; Rawlings, D.J.; Isenberg, D.A.; Ehrenstein, M.R.; Mauri, C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010, 32, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Anolik, J.H.; Barnard, J.; Owen, T.; Zheng, B.; Kemshetti, S.; Looney, R.J.; Sanz, I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007, 56, 3044–3056. [Google Scholar] [CrossRef] [PubMed]
- Newell, K.A.; Asare, A.; Kirk, A.D.; Gisler, T.D.; Bourcier, K.; Suthanthiran, M.; Burlingham, W.J.; Marks, W.H.; Sanz, I.; Lechler, R.I.; et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J. Clin. Investig. 2010, 120, 1836–1847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Das, A.; Ellis, G.; Pallant, C.; Lopes, A.R.; Khanna, P.; Peppa, D.; Chen, A.; Blair, P.; Dusheiko, G.; Gill, U.; et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. J. Immunol. 2012, 189, 3925–3935. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lemoine, S.; Morva, A.; Youinou, P.; Jamin, C. Human T cells induce their own regulation through activation of B cells. J. Autoimmun. 2011, 36, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Macatangay, B.J.; Szajnik, M.E.; Whiteside, T.L.; Riddler, S.A.; Rinaldo, C.R. Regulatory T cell suppression of Gag-specific CD8 T cell polyfunctional response after therapeutic vaccination of HIV-1-infected patients on ART. PLoS ONE 2010, 5, e9852. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asseman, C.; Mauze, S.; Leach, M.W.; Coffman, R.L.; Powrie, F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999, 190, 995–1004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Durando, P.; Iudici, R.; Alicino, C.; Alberti, M.; de Florentis, D.; Ansaldi, F.; Icardi, G. Adjuvants and alternative routes of administration towards the development of the ideal influenza vaccine. Hum. Vaccin. 2011, 7 (Suppl. S1), 29–40. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence: A systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 2019, 124, 110632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Batista-Duharte, A.; Tellez-Martinez, D.; Fuentes, D.L.P.; Carlos, I.Z. Molecular adjuvants that modulate regulatory T cell function in vaccination: A critical appraisal. Pharmacol. Res. 2018, 129, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Ndure, J.; Flanagan, K.L. Targeting regulatory T cells to improve vaccine immunogenicity in early life. Front. Microbiol. 2014, 5, 477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curreri, A.; Sankholkar, D.; Mitragotri, S.; Zhao, Z. RNA therapeutics in the clinic. Bioeng. Transl. Med. 2023, 8, e10374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bartkowiak, T.; Curran, M.A. 4-1BB Agonists: Multi-Potent Potentiators of Tumor Immunity. Front. Oncol. 2015, 5, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bartkowiak, T.; Singh, S.; Yang, G.; Galvan, G.; Haria, D.; Ai, M.; Allison, J.P.; Sastry, K.J.; Curran, M.A. Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proc. Natl. Acad. Sci. USA 2015, 112, E5290–E5299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bullock, T.N.J. CD40 stimulation as a molecular adjuvant for cancer vaccines and other immunotherapies. Cell Mol. Immunol. 2022, 19, 14–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, Y.; Li, R.; Qian, L.; Liu, F.; Xu, R.; Meng, F.; Ke, Y.; Shao, J.; Yu, L.; Liu, Q.; et al. Tumor eradicated by combination of imiquimod and OX40 agonist for in situ vaccination. Cancer Sci. 2021, 112, 4490–4500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jacobs, J.F.; Punt, C.J.; Lesterhuis, W.J.; Sutmuller, R.P.; Brouwer, H.M.; Scharenborg, N.M.; Klasen, I.S.; Hilbrands, L.B.; Figdor, C.G.; de Vries, I.J.; et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: A phase I/II study in metastatic melanoma patients. Clin. Cancer Res. 2010, 16, 5067–5078. [Google Scholar] [CrossRef] [PubMed]
- Li, A.P.Y.; Cohen, C.A.; Leung, N.H.L.; Fang, V.J.; Gangappa, S.; Sambhara, S.; Levine, M.Z.; Iuliano, A.D.; Perera, R.; Ip, D.K.M.; et al. Immunogenicity of standard, high-dose, MF59-adjuvanted, and recombinant-HA seasonal influenza vaccination in older adults. NPJ Vaccines 2021, 6, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winokur, P.; El Sahly, H.M.; Mulligan, M.J.; Frey, S.E.; Rupp, R.; Anderson, E.J.; Edwards, K.M.; Bernstein, D.I.; Schmader, K.; Jackson, L.A.; et al. Immunogenicity and safety of different dose schedules and antigen doses of an MF59-adjuvanted H7N9 vaccine in healthy adults aged 65 years and older. Vaccine 2021, 39, 1339–1348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelton, S.I.; Divino, V.; Shah, D.; Mould-Quevedo, J.; DeKoven, M.; Krishnarajah, G.; Postma, M.J. Evaluating the Relative Vaccine Effectiveness of Adjuvanted Trivalent Influenza Vaccine Compared to High-Dose Trivalent and Other Egg-Based Influenza Vaccines among Older Adults in the US during the 2017-2018 Influenza Season. Vaccines 2020, 8, 446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kodali, L.; Budhiraja, P.; Gea-Banacloche, J. COVID-19 in kidney transplantation-implications for immunosuppression and vaccination. Front. Med. 2022, 9, 1060265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tylicki, L.; Debska-Slizien, A.; Muchlado, M.; Slizien, Z.; Golebiewska, J.; Dabrowska, M.; Biedunkiewicz, B. Boosting Humoral Immunity from mRNA COVID-19 Vaccines in Kidney Transplant Recipients. Vaccines 2021, 10, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Icardi, G.; Orsi, A.; Ceravolo, A.; Ansaldi, F. Current evidence on intradermal influenza vaccines administered by Soluvia licensed micro injection system. Hum. Vaccin. Immunother. 2012, 8, 67–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verma, S.K.; Mahajan, P.; Singh, N.K.; Gupta, A.; Aggarwal, R.; Rappuoli, R.; Johri, A.K. New-age vaccine adjuvants, their development, and future perspective. Front. Immunol. 2023, 14, 1043109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webster, R.G.; Govorkova, E.A. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014, 1323, 115–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pavlicevic, M.; Marmiroli, N.; Maestri, E. Immunomodulatory peptides-A promising source for novel functional food production and drug discovery. Peptides 2022, 148, 170696. [Google Scholar] [CrossRef] [PubMed]
- Du, P.Y.; Gandhi, A.; Bawa, M.; Gromala, J. The ageing immune system as a potential target of senolytics. Oxf. Open Immunol. 2023, 4, iqad004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



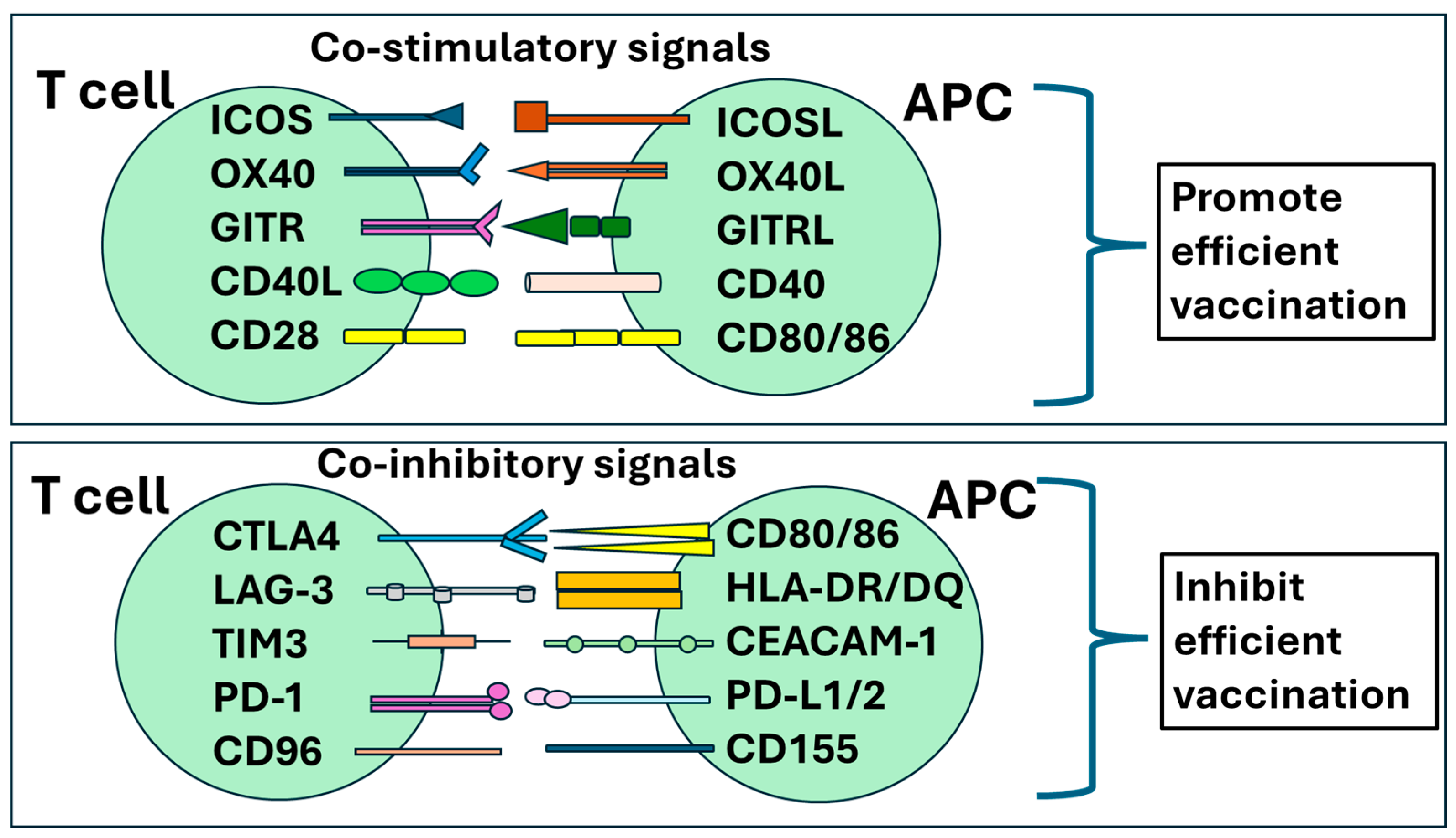
| T Regulatory Cells | Origin | Markers | References |
|---|---|---|---|
| tTreg | Thymus (central) | CCR7, CD45RA, CD31, SELL, NRP1, Foxp3+ | Luo, Xue, Wang et al., 2019 [34] |
| pTreg | Peripheral | GATA3, IRF4, RORC, TBX21, HELIOS, Foxp3+ | Zheng, Wang, Horwitz et al., 2008 [35] |
| iTreg | Peripheral | Treg-specific demethylation region (TSDR), Foxp3+ | Zheng, Wang, Horwitz et al., 2008 [35] |
| Th1-Treg | Peripheral | CXCR, Tbet, Foxp3+ | Halim et al., 2017 [36] |
| Th2-Treg | Peripheral | IL-4, IL-13, IRF4, Foxp3+ | Halim et al., 2017 [36] |
| Th17-Treg | Peripheral | CD161, CCR6+, IL17A, IL23R, IL-12Rβ2, Fox3p+ | Romagnani et al., 2009 [37] |
| Tfh-Treg | Peripheral | CXCR5, Bcl-6, IL-21, Foxp3+ | Chung et al., 2011 [38] |
| Tr1 | Peripheral | IL-10, CD49b, Lag3, Foxp3– | Suhrkamp et al., 2023 [39] |
| Tr35 | Peripheral | IL-35, IL-10, p35, EB13, IL-12b2, gp130, Foxp3- | Yang, Dong, Zhong et al., 2024 [40] |
| Group of Individuals | High Treg/Tr1 Ratio | Normal Treg/Tr1 Ratio | Low Treg/Tr1 Ratio |
|---|---|---|---|
| Prior to vaccination: healthy individuals | Good/deficient vaccination effect | Excellent vaccination effect | Excellent/good vaccination effect |
| After vaccination: healthy individuals | Good/deficient vaccination effect | Excellent/good vaccination effect | Excellent/good/deficient vaccination effect |
| After vaccination: healthy older individuals (>60 years-old) | Lack of/deficient vaccination effect; require higher and repeated doses ± adjuvant | Lack of/deficient/good vaccination effect; require higher and repeated doses ± adjuvant | Deficient/good vaccination effect; require higher and repeated doses ± adjuvant |
| After vaccination: cancer patients | Require anti-PD-1 mAb/addition of adjuvant + active removal of Tregs | Require anti-PD-1 mAb/addition of adjuvant ± active removal of Tregs | Require anti-PD-1 mAb/addition of adjuvant |
| After vaccination: transplant recipients | Lack of/deficient vaccination effect; require higher and repeated doses ± adjuvant | Lack of/deficient vaccination effect; require higher and repeated doses ± adjuvant | Lack of/deficient/good vaccination effect; require higher and repeated doses ±adjuvant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepkowski, S.; Bekbolsynov, D.; Oenick, J.; Brar, S.; Mierzejewska, B.; Rees, M.A.; Ekwenna, O. The Major Role of T Regulatory Cells in the Efficiency of Vaccination in General and Immunocompromised Populations: A Review. Vaccines 2024, 12, 992. https://doi.org/10.3390/vaccines12090992
Stepkowski S, Bekbolsynov D, Oenick J, Brar S, Mierzejewska B, Rees MA, Ekwenna O. The Major Role of T Regulatory Cells in the Efficiency of Vaccination in General and Immunocompromised Populations: A Review. Vaccines. 2024; 12(9):992. https://doi.org/10.3390/vaccines12090992
Chicago/Turabian StyleStepkowski, Stanislaw, Dulat Bekbolsynov, Jared Oenick, Surina Brar, Beata Mierzejewska, Michael A. Rees, and Obi Ekwenna. 2024. "The Major Role of T Regulatory Cells in the Efficiency of Vaccination in General and Immunocompromised Populations: A Review" Vaccines 12, no. 9: 992. https://doi.org/10.3390/vaccines12090992





