Lipoprotein Signal Peptide as Adjuvants: Leveraging Lipobox-Driven TLR2 Activation in Modern Vaccine Design
Abstract
1. Introduction
| Pathogen/Condition | Antigen | SP (SP) | Immune Response | References |
|---|---|---|---|---|
| Dengue Virus | E3 protein | Neisseria meningitidis Ag473 | Higher IgG and virus-neutralizing antibodies in lipidated E3 | [17] |
| Clostridium difficile | TcdA Receptor-Binding Domains (RBD) | Ag473 | A 10-fold increase in potency; 90–100% protection against CDI | [18] |
| Staphylococcus aureus | FLIPr (Formyl Peptide Receptor-Like 1 Inhibitor Protein) | Ag473 | Boosted mucosal and systemic immunity | [19] |
| Zika Virus | Envelope Protein Domain III (rZE3) | Ag473 | Higher neutralizing antibodies with prolonged protection | [20] |
| Haemophilus influenzae | P6 and OMP26 proteins | P4 SP | Higher antibody titers and cytokine responses | [21] |
| Human Papillomavirus (HPV) | E7 protein | Ag473 | Increased anti-E7 antibodies with Th1-biased cytokine release | [22] |
| Streptococcus pneumoniae | DacB and PnrA | Signal sequence from B. burgdorferi | Increased IgG2/IgG1 subclass ratios related to Th1-type | [23] |
2. Bacterial Lipoproteins: Driving Immune Modulation and Vaccine Advancement
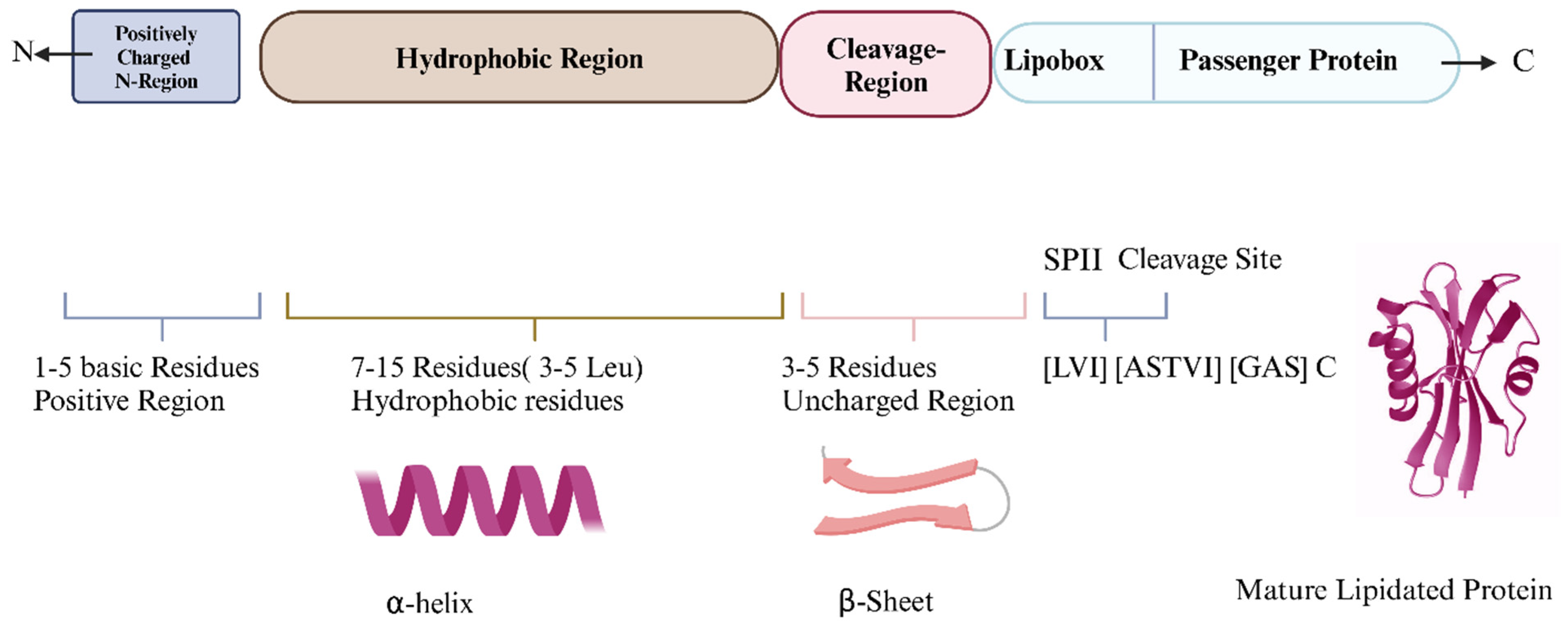
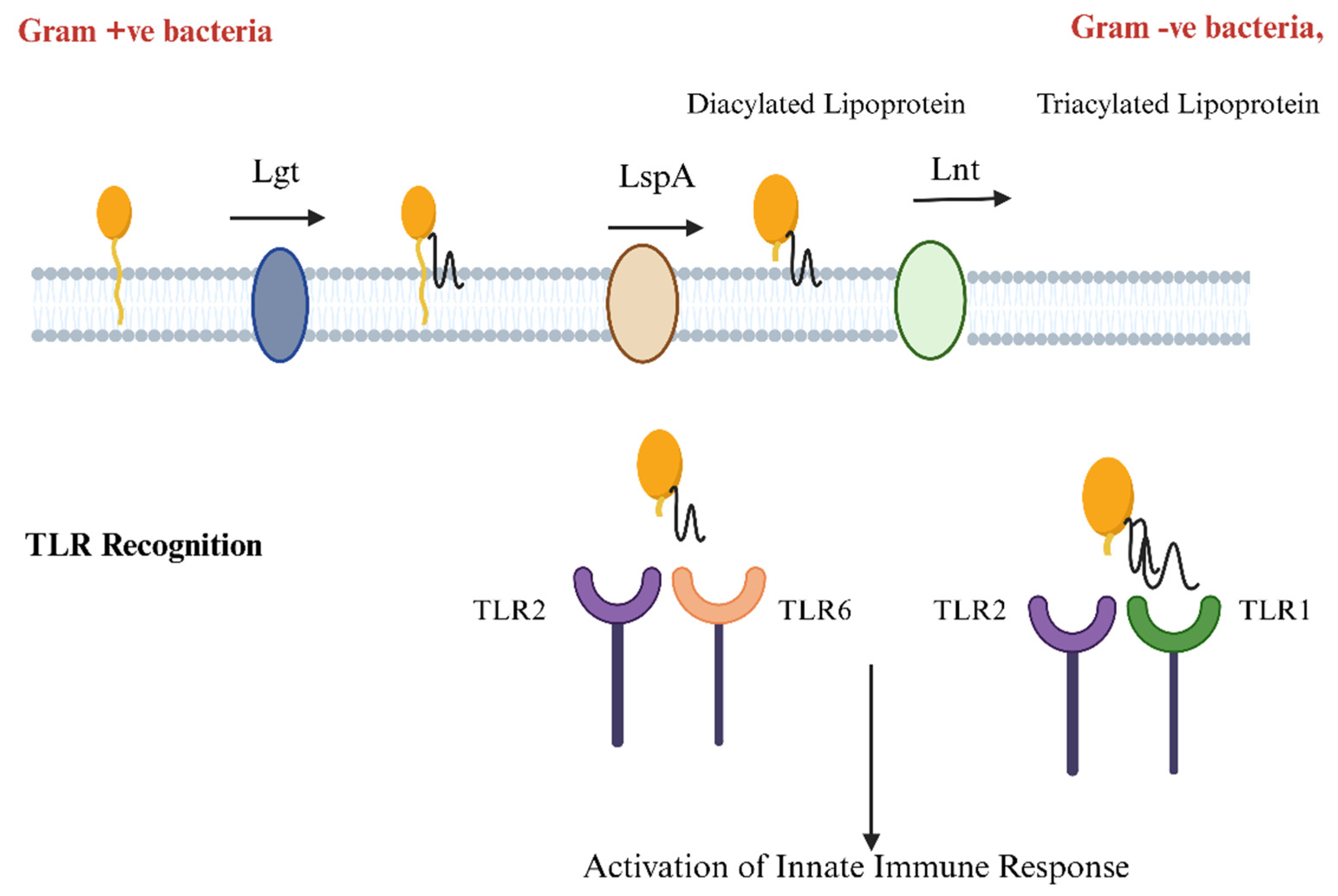
3. Bacterial SPs Enable Lipoprotein Anchoring to Cell Membranes
4. Decoding Bacterial Lipoproteins: How TLR2 Recognition Drives Immune Defense
5. The Role of SPs in Vaccine Development, Diagnostics, and Therapeutics
6. Bacterial SP Types: Structural Variations and Cleavage Pathways in Protein Targeting
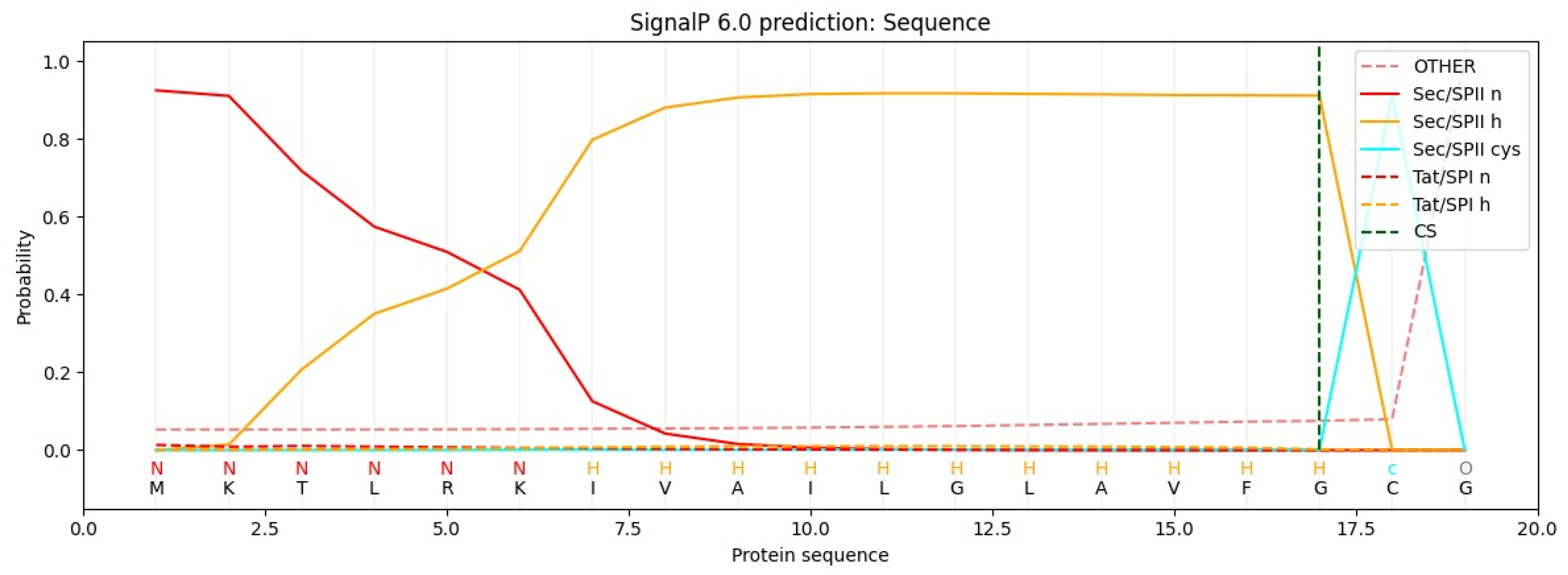
6.1. The Role of Bioinformatics: Tools for SP Prediction and Cleavage Site Detection
6.2. Using SPs for Lipidation of Other Proteins: Requirements and Validation
6.3. Lipidation by SPs: A Multifaceted Approach to Enhancing Vaccine Efficacy Across Viral, Bacterial Pathogens, and Cancer Antigens
6.4. Challenges and Solutions in Lipidated Protein Expression: Case Studies and E. coli Strain Optimization
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berche, P. Life and death of smallpox. La Presse Médicale 2022, 51, 104117. [Google Scholar] [CrossRef] [PubMed]
- Badizadegan, K.; Kalkowska, D.A.; Thompson, K.M. Polio by the Numbers—A Global Perspective. J. Infect. Dis. 2022, 226, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, K.; Ehrenfeld, E.; Agol, V.I.; Wimmer, E. Polio eradication at the crossroads. Lancet Glob. Health 2021, 9, e1172–e1175. [Google Scholar] [CrossRef] [PubMed]
- Gastañaduy, P.A.; Goodson, J.L.; Panagiotakopoulos, L.; Rota, P.A.; Orenstein, W.A.; Patel, M. Measles in the 21st Century: Progress Toward Achieving and Sustaining Elimination. J. Infect. Dis. 2021, 224 (Suppl. S4), S420–S428. [Google Scholar] [CrossRef]
- Levine, M.M.; Sztein, M.B. Vaccine development strategies for improving immunization: The role of modern immunology. Nat. Immunol. 2004, 5, 460–464. [Google Scholar] [CrossRef]
- Moyle, P.M. Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnol. Adv. 2017, 35, 375–389. [Google Scholar] [CrossRef]
- Raoufi, E.; Bahramimeimandi, B.; Salehi-Shadkami, M.; Chaosri, P.; Mozafari, M.R. Methodical Design of Viral Vaccines Based on Avant-Garde Nanocarriers: A Multi-Domain Narrative Review. Biomedicines 2021, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Azmi, F.; Ahmad Fuaad, A.A.H.; Skwarczynski, M.; Toth, I. Recent progress in adjuvant discovery for peptide-based subunit vaccines. Hum. Vaccines Immunother. 2014, 10, 778–796. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-X.; Tseng, J.-C.; Yu, G.-Y.; Luo, Y.; Huang, C.-Y.F.; Hong, Y.-R.; Chuang, T.-H. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics 2022, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, A.; Afzal, H.; Doan, T.-D.; Ke, G.-M.; Cheng, L.-T. Flagellin Improves the Immune Response of an Infectious Bursal Disease Virus (IBDV) Subunit Vaccine. Vaccines 2022, 10, 1780. [Google Scholar] [CrossRef]
- Murtaza, A.; Hoa, N.-T.; Dieu-Huong, D.; Afzal, H.; Tariq, M.H.; Cheng, L.-T.; Chung, Y.-C. Advancing PEDV Vaccination: Comparison between Inactivated and Flagellin N-Terminus-Adjuvanted Subunit Vaccines. Vaccines 2024, 12, 139. [Google Scholar] [CrossRef]
- Nguyen, M.-T.; Uebele, J.; Kumari, N.; Nakayama, H.; Peter, L.; Ticha, O.; Woischnig, A.-K.; Schmaler, M.; Khanna, N.; Dohmae, N.; et al. Lipid moieties on lipoproteins of commensal and non-commensal staphylococci induce differential immune responses. Nat. Commun. 2017, 8, 2246. [Google Scholar] [CrossRef]
- Dunne, A.; Mielke, L.A.; Allen, A.C.; Sutton, C.E.; Higgs, R.; Cunningham, C.C.; Higgins, S.C.; Mills, K.H.G. A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine. Mucosal Immunol. 2015, 8, 607–617. [Google Scholar] [CrossRef]
- Volpi, C.; Fallarino, F.; Pallotta, M.T.; Bianchi, R.; Vacca, C.; Belladonna, M.L.; Orabona, C.; De Luca, A.; Boon, L.; Romani, L.; et al. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9–TRIF pathway. Nat. Commun. 2013, 4, 1852. [Google Scholar] [CrossRef]
- Latz, E.; Schoenemeyer, A.; Visintin, A.; Fitzgerald, K.A.; Monks, B.G.; Knetter, C.F.; Lien, E.; Nilsen, N.J.; Espevik, T.; Golenbock, D.T. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 2004, 5, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.H.; Liu, S.J.; Chen, H.W.; Chong, P. Recombinant bacterial lipoproteins as vaccine candidates. Expert Rev. Vaccines 2015, 14, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-H.; Wu, C.-W.; Lien, S.-P.; Leng, C.-H.; Hsiao, K.-N.; Liu, S.-J.; Chen, H.-W.; Siu, L.-K.; Chong, P. Recombinant lipoprotein-based vaccine candidates against C. difficile infections. J. Biomed. Sci. 2015, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-S.; Chen, M.-Y.; Hsu, C.-W.; Tsai, Y.-W.; Chiu, F.-F.; Hsu, C.-L.; Lin, C.-L.; Wu, C.-C.; Tu, L.-L.; Chiang, C.-Y.; et al. Recombinant lipidated FLIPr effectively enhances mucosal and systemic immune responses for various vaccine types. npj Vaccines 2023, 8, 82. [Google Scholar] [CrossRef]
- Chen, M.Y.; Chai, K.M.; Chiang, C.Y.; Wu, C.C.; Yu, G.Y.; Liu, S.J.; Chen, H.W. Recombinant lipidated Zika virus envelope protein domain III elicits durable neutralizing antibody responses against Zika virus in mice. J. Biomed. Sci. 2020, 27, 51. [Google Scholar] [CrossRef]
- Kaur, R.; Pichichero, M. Lipidation of Haemophilus influenzae Antigens P6 and OMP26 Improves Immunogenicity and Protection against Nasopharyngeal Colonization and Ear Infection. Infect. Immun. 2022, 90, e0067821. [Google Scholar] [CrossRef]
- Song, Y.C.; Liu, H.H.; Chen, I.H.; Chen, H.W.; Chong, P.; Leng, C.H.; Liu, S.J. A purified recombinant lipopeptide as adjuvant for cancer immunotherapy. Biomed. Res. Int. 2014, 2014, 349783. [Google Scholar] [CrossRef] [PubMed]
- Voß, F.; van Beek, L.F.; Schwudke, D.; Ederveen, T.H.A.; van Opzeeland, F.J.; Thalheim, D.; Werner, S.; de Jonge, M.I.; Hammerschmidt, S. Lipidation of Pneumococcal Antigens Leads to Improved Immunogenicity and Protection. Vaccines 2020, 8, 310. [Google Scholar] [CrossRef]
- El Rayes, J.; Rodríguez-Alonso, R.; Collet, J.-F. Lipoproteins in Gram-negative bacteria: New insights into their biogenesis, subcellular targeting and functional roles. Curr. Opin. Microbiol. 2021, 61, 25–34. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Matsuo, M.; Niemann, S.; Herrmann, M.; Götz, F. Lipoproteins in Gram-Positive Bacteria: Abundance, Function, Fitness. Front. Microbiol. 2020, 11, 582582. [Google Scholar] [CrossRef] [PubMed]
- Von Heijne, G. Patterns of Amino Acids near Signal-Sequence Cleavage Sites. Eur. J. Biochem. 1983, 133, 17–21. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Niu, J.; Jarugumilli, G.K.; Wu, X. Protein Lipidation in Cell Signaling and Diseases: Function, Regulation, and Therapeutic Opportunities. Cell Chem. Biol. 2018, 25, 817–831. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Takahara, M.; Kamiya, N. Synthetic strategies for artificial lipidation of functional proteins. Chem.–A Eur. J. 2020, 26, 4645–4655. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.C.; Kriegesmann, J.; Dowman, L.J.; Becker, C.F.; Payne, R.J. Chemical synthesis and semisynthesis of lipidated proteins. Angew. Chem. Int. Ed. 2022, 61, e202111266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bulaj, G. Converting peptides into drug leads by lipidation. Curr. Med. Chem. 2012, 19, 1602–1618. [Google Scholar] [CrossRef]
- Mejuch, T.; Waldmann, H. Synthesis of Lipidated Proteins. Bioconjugate Chem. 2016, 27, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Drage, M.G.; Tsai, H.-C.; Pecora, N.D.; Cheng, T.-Y.; Arida, A.R.; Shukla, S.; Rojas, R.E.; Seshadri, C.; Moody, D.B.; Boom, W.H.; et al. Mycobacterium tuberculosis lipoprotein LprG (Rv1411c) binds triacylated glycolipid agonists of Toll-like receptor 2. Nat. Struct. Mol. Biol. 2010, 17, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, G.; Chimalapati, S.; Pollard, T.; Lapp, T.; Cohen, J.; Camberlein, E.; Stafford, S.; Periselneris, J.; Aldridge, C.; Vollmer, W.; et al. TLR-Mediated Inflammatory Responses to Streptococcus pneumoniae Are Highly Dependent on Surface Expression of Bacterial Lipoproteins. J. Immunol. 2014, 193, 3736–3745. [Google Scholar] [CrossRef]
- Yoder, A.; Wang, X.; Ma, Y.; Philipp Mario, T.; Heilbrun, M.; Weis John, H.; Kirschning Carsten, J.; Wooten, R.M.; Weis Janis, J. Tripalmitoyl-S-Glyceryl-Cysteine-Dependent OspA Vaccination of Toll-Like Receptor 2-Deficient Mice Results in Effective Protection from Borrelia burgdorferi Challenge. Infect. Immun. 2003, 71, 3894–3900. [Google Scholar] [CrossRef] [PubMed]
- Erdile, L.F.; Brandt, M.A.; Warakomski, D.J.; Westrack, G.J.; Sadziene, A.; Barbour, A.G.; Mays, J.P. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect. Immun. 1993, 61, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Thomas, V.; Schnare, M.; Lobet, Y.; Anguita, J.; Schoen, R.T.; Medzhitov, R.; Fikrig, E.; Flavell, R.A. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 2002, 8, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Friese, O.V.; Runnels, H.A.; Khandke, L.; Zlotnick, G.; Aulabaugh, A.; Gore, T.; Vidunas, E.; Raso, S.W.; Novikova, E.; et al. The Dual Role of Lipids of the Lipoproteins in Trumenba, a Self-Adjuvanting Vaccine Against Meningococcal Meningitis B Disease. AAPS J. 2016, 18, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Zlotnick, G.W.; Jones, T.R.; Liberator, P.; Hao, L.; Harris, S.; McNeil, L.K.; Zhu, D.; Perez, J.; Eiden, J.; Jansen, K.U.; et al. The Discovery and Development of a Novel Vaccine to Protect against Neisseria meningitidis Serogroup B Disease. Hum. Vaccines Immunother. 2015, 11, 5–13. [Google Scholar] [CrossRef]
- Pillai, S.; Howell, A.; Alexander, K.; Bentley, B.E.; Jiang, H.-Q.; Ambrose, K.; Zhu, D.; Zlotnick, G. Outer membrane protein (OMP) based vaccine for Neisseria meningitidis serogroup B. Vaccine 2005, 23, 2206–2209. [Google Scholar] [CrossRef] [PubMed]
- Seib, K.L.; Scarselli, M.; Comanducci, M.; Toneatto, D.; Masignani, V. Neisseria meningitidis factor H-binding protein fHbp: A key virulence factor and vaccine antigen. Expert Rev. Vaccines 2015, 14, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Kamalakkannan, S.; Murugan, V.; Jagannadham, M.V.; Nagaraj, R.; Sankaran, K. Bacterial lipid modification of proteins for novel protein engineering applications. Protein Eng. Des. Sel. 2004, 17, 721–729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tate, E.W.; Soday, L.; de la Lastra, A.L.; Wang, M.; Lin, H. Protein lipidation in cancer: Mechanisms, dysregulation and emerging drug targets. Nat. Rev. Cancer 2024, 24, 240–260. [Google Scholar] [CrossRef] [PubMed]
- Paulikat, A.D.; Schwudke, D.; Hammerschmidt, S.; Voß, F. Lipidation of pneumococcal proteins enables activation of human antigen-presenting cells and initiation of an adaptive immune response. Front. Immunol. 2024, 15, 1392316. [Google Scholar] [CrossRef] [PubMed]
- Hantke, K.; Braun, V. Covalent Binding of Lipid to Protein. Eur. J. Biochem. 1973, 34, 284–296. [Google Scholar] [CrossRef]
- Owji, H.; Nezafat, N.; Negahdaripour, M.; Hajiebrahimi, A.; Ghasemi, Y. A comprehensive review of signal peptides: Structure, roles, and applications. Eur. J. Cell Biol. 2018, 97, 422–441. [Google Scholar] [CrossRef]
- Denks, K.; Vogt, A.; Sachelaru, I.; Petriman, N.-A.; Kudva, R.; Koch, H.-G. The Sec translocon mediated protein transport in prokaryotes and eukaryotes. Mol. Membr. Biol. 2014, 31, 58–84. [Google Scholar] [CrossRef]
- Mao, G.; Zhao, Y.; Kang, X.; Li, Z.; Zhang, Y.; Wang, X.; Sun, F.; Sankaran, K.; Zhang, X.C. Crystal structure of E. coli lipoprotein diacylglyceryl transferase. Nat. Commun. 2016, 7, 10198. [Google Scholar] [CrossRef]
- Vogeley, L.; El Arnaout, T.; Bailey, J.; Stansfeld, P.J.; Boland, C.; Caffrey, M. Structural basis of lipoprotein signal peptidase II action and inhibition by the antibiotic globomycin. Science 2016, 351, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.-I.; Tokuda, H. Overexpression of LolCDE Allows Deletion of the Escherichia coli Gene Encoding Apolipoprotein N-Acyltransferase. J. Bacteriol. 2011, 193, 4832–4840. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, M.; Grau, T.; Tschumi, A.; Sander, P. Lipoprotein synthesis in mycobacteria. Microbiology 2007, 153, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Zückert, W.R. Secretion of Bacterial Lipoproteins: Through the Cytoplasmic Membrane, the Periplasm and Beyond. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Iguchi-Yokoyama, A.; Matsuyama, S.; Tokuda, H.; Narita, S. Membrane topology and functional importance of the periplasmic region of ABC transporter LolCDE. Biosci Biotechnol. Biochem. 2009, 73, 2310–2316. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seydel, A.; Gounon, P.; Pugsley, A.P. Testing the ‘+2 rule’ for lipoprotein sorting in the Escherichia coli cell envelope with a new genetic selection. Mol. Microbiol. 1999, 34, 810–821. [Google Scholar] [CrossRef]
- Terada, M.; Kuroda, T.; Matsuyama, S.-i.; Tokuda, H. Lipoprotein Sorting Signals Evaluated as the LolA-dependent Release of Lipoproteins from the Cytoplasmic Membrane of Escherichia coli. J. Biol. Chem. 2001, 276, 47690–47694. [Google Scholar] [CrossRef]
- Okuda, S.; Tokuda, H. Model of mouth-to-mouth transfer of bacterial lipoproteins through inner membrane LolC, periplasmic LolA, and outer membrane LolB. Proc. Natl. Acad. Sci. USA 2009, 106, 5877–5882. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Nan, X.; Jin, M.S.; Youn, S.-J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.-G.; Lee, J.-O. Recognition of Lipopeptide Patterns by Toll-like Receptor 2-Toll-like Receptor 6 Heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Vasselon, T.; Detmers, P.A.; Charron, D.; Haziot, A. TLR2 Recognizes a Bacterial Lipopeptide through Direct Binding. J. Immunol. 2004, 173, 7401–7405. [Google Scholar] [CrossRef]
- Schenk, M.; Belisle, J.T.; Modlin, R.L. TLR2 Looks at Lipoproteins. Immunity 2009, 31, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Mangiafesto, J.; Pryharski, K.; Rasam, S.; Zagursky, R.; Pichichero, M. Expression conditions and characterization of a novelly constructed lipoprotein intended as a vaccine to prevent human Haemophilus influenzae infections. J. Biol. Chem. 2023, 299, 105031. [Google Scholar] [CrossRef]
- de Oliviera Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef]
- Ray, A.; Karmakar, P.; Biswas, T. Up-regulation of CD80-CD86 and IgA on mouse peritoneal B-1 cells by porin of Shigella dysenteriae is Toll-like receptors 2 and 6 dependent. Mol. Immunol. 2004, 41, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Bekeredjian-Ding, I.; Jego, G. Toll-like receptors--sentries in the B-cell response. Immunology 2009, 128, 311–323. [Google Scholar] [CrossRef]
- Rawlings, D.J.; Schwartz, M.A.; Jackson, S.W.; Meyer-Bahlburg, A. Integration of B cell responses through Toll-like receptors and antigen receptors. Nat. Rev. Immunol. 2012, 12, 282–294. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, X.; Wang, Y.; Zong, Y.; Wang, Y.; Zhang, X.; Xia, X.; Sun, H. Superior adjuvanticity of the genetically fused D1 domain of Neisseria meningitides Ag473 lipoprotein among three Toll-like receptor ligands. Biosci. Rep. 2020, 40, BSR20193675. [Google Scholar] [CrossRef]
- Liaci, A.M.; Förster, F. Take Me Home, Protein Roads: Structural Insights into Signal Peptide Interactions during ER Translocation. Int. J. Mol. Sci. 2021, 22, 11871. [Google Scholar] [CrossRef] [PubMed]
- Rusch, S.L.; Kendall, D.A. Interactions that drive Sec-dependent bacterial protein transport. Biochemistry 2007, 46, 9665–9673. [Google Scholar] [CrossRef]
- Müller, M.; Klösgen, R.B. The Tat pathway in bacteria and chloroplasts (Review). Mol. Membr. Biol. 2005, 22, 113–121. [Google Scholar] [CrossRef]
- Gouridis, G.; Karamanou, S.; Gelis, I.; Kalodimos, C.G.; Economou, A. Signal peptides are allosteric activators of the protein translocase. Nature 2009, 462, 363–367. [Google Scholar] [CrossRef]
- Péterfy, M.; Gyuris, T.; Takács, L. Signal-exon trap: A novel method for the identification of signal sequences from genomic DNA. Nucleic Acids Res. 2000, 28, E26. [Google Scholar] [CrossRef] [PubMed]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Kovjazin, R.; Carmon, L. The use of signal peptide domains as vaccine candidates. Hum. Vaccin Immunother. 2014, 10, 2733–2740. [Google Scholar] [CrossRef]
- Dirican, N.; Duman, A.; Sağlam, G.; Arslan, A.; Ozturk, O.; Atalay, S.; Bircan, A.; Akkaya, A.; Cakir, M. The diagnostic significance of signal peptide-complement C1r/C1s, Uegf, and Bmp1-epidermal growth factor domain-containing protein-1 levels in pulmonary embolism. Ann. Thorac. Med. 2016, 11, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.H.; Lai, N.C.; Hammond, H.K. Signal Peptide Increases the Efficacy of Angiogenic Gene Transfer for Treatment of Myocardial Ischemia. Hum. Gene Ther. 2005, 16, 1058–1064. [Google Scholar] [CrossRef]
- Aronsohn, A.I.; Hughes, J.A. Nuclear Localization Signal Peptides Enhance Cationic Liposome-Mediated Gene Therapy. J. Drug Target. 1998, 5, 163–169. [Google Scholar] [CrossRef]
- Jarjanazi, H.; Savas, S.; Pabalan, N.; Dennis, J.W.; Ozcelik, H. Biological implications of SNPs in signal peptide domains of human proteins. Proteins 2008, 70, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, R.; Colombo, C.; Nocerino, V.; Massa, O.; Lampasona, V.; Iafusco, D.; Viscardi, M.; Chiumello, G.; Meschi, F.; Barbetti, F. Insulin gene mutations as cause of diabetes in children negative for five type 1 diabetes autoantibodies. Diabetes Care 2009, 32, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Datta, R.; Waheed, A.; Shah, G.N.; Sly, W.S. Signal sequence mutation in autosomal dominant form of hypoparathyroidism induces apoptosis that is corrected by a chemical chaperone. Proc. Natl. Acad. Sci. USA 2007, 104, 19989–19994. [Google Scholar] [CrossRef]
- Vezzoli, V.; Duminuco, P.; Vottero, A.; Kleinau, G.; Schülein, R.; Minari, R.; Bassi, I.; Bernasconi, S.; Persani, L.; Bonomi, M. A new variant in signal peptide of the human luteinizing hormone receptor (LHCGR) affects receptor biogenesis causing leydig cell hypoplasia. Hum. Mol. Genet. 2015, 24, 6003–6012. [Google Scholar] [CrossRef]
- Ng, S.Y.M.; Chaban, B.; VanDyke, D.J.; Jarrell, K.F. Archaeal signal peptidases. Microbiology 2007, 153, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Geukens, N.; De Buck, E.; Meyen, E.; Maes, L.; Vranckx, L.; Van Mellaert, L.; Anné, J.; Lammertyn, E. The type II signal peptidase of Legionella pneumophila. Res. Microbiol. 2006, 157, 836–841. [Google Scholar] [CrossRef]
- Szabó, Z.; Stahl Adriana, O.; Albers Sonja, V.; Kissinger Jessica, C.; Driessen Arnold, J.M.; Pohlschröder, M. Identification of Diverse Archaeal Proteins with Class III Signal Peptides Cleaved by Distinct Archaeal Prepilin Peptidases. J. Bacteriol. 2007, 189, 772–778. [Google Scholar] [CrossRef]
- Oman, T.J.; van der Donk, W.A. Follow the leader: The use of leader peptides to guide natural product biosynthesis. Nat. Chem. Biol. 2010, 6, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Auclair, S.M.; Bhanu, M.K.; Kendall, D.A. Signal peptidase I: Cleaving the way to mature proteins. Protein Sci. 2012, 21, 13–25. [Google Scholar] [CrossRef]
- Réglier-Poupet, H.; Frehel, C.; Dubail, I.; Beretti, J.-L.; Berche, P.; Charbit, A.; Raynaud, C. Maturation of Lipoproteins by Type II Signal Peptidase Is Required for Phagosomal Escape of Listeria monocytogenes. J. Biol. Chem. 2003, 278, 49469–49477. [Google Scholar] [CrossRef]
- Albers, S.-V.; Szabó, Z.; Arnold, J.M.D. Archaeal Homolog of Bacterial Type IV Prepilin Signal Peptidases with Broad Substrate Specificity. J. Bacteriol. 2003, 185, 3918–3925. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.; Tsirigos, K.D.; Brunak, S.; von Heijne, G. A Brief History of Protein Sorting Prediction. Protein J. 2019, 38, 200–216. [Google Scholar] [CrossRef]
- Nielsen, H.; Teufel, F.; Brunak, S.; von Heijne, G. SignalP: The Evolution of a Web Server. In Protein Bioinformatics; Lisacek, F., Ed.; Springer: New York, NY, USA, 2024; pp. 331–367. [Google Scholar]
- Rahman, O.; Cummings, S.P.; Harrington, D.J.; Sutcliffe, I.C. Methods for the bioinformatic identification of bacterial lipoproteins encoded in the genomes of Gram-positive bacteria. World J. Microbiol. Biotechnol. 2008, 24, 2377–2382. [Google Scholar] [CrossRef]
- Chong, P.; Huang, J.-H.; Leng, C.-H.; Liu, S.-J.; Chen, H.-W. Chapter Three—Recombinant Lipoproteins as Novel Vaccines with Intrinsic Adjuvant. In Advances in Protein Chemistry and Structural Biology; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 55–74. [Google Scholar]
- Leng, C.H.; Chen, H.W.; Chang, L.S.; Liu, H.H.; Liu, H.Y.; Sher, Y.P.; Chang, Y.W.; Lien, S.P.; Huang, T.Y.; Chen, M.Y.; et al. A recombinant lipoprotein containing an unsaturated fatty acid activates NF-kappaB through the TLR2 signaling pathway and induces a differential gene profile from a synthetic lipopeptide. Mol. Immunol. 2010, 47, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Li, Y.; Wang, Y.; Jin, Y.; Tong, D.; Li, Z.; Zhou, J. Bacterial ghosts engineered with lipidated antigens as an adjuvant-free vaccine for Chlamydia abortus. Int. J. Pharm. 2024, 666, 124801. [Google Scholar] [CrossRef]
- Chen, H.W.; Liu, S.J.; Liu, H.H.; Kwok, Y.; Lin, C.L.; Lin, L.H.; Chen, M.Y.; Tsai, J.P.; Chang, L.S.; Chiu, F.F.; et al. A novel technology for the production of a heterologous lipoprotein immunogen in high yield has implications for the field of vaccine design. Vaccine 2009, 27, 1400–1409. [Google Scholar] [CrossRef]
- Ding, W.; Gu, J.; Xu, W.; Wu, J.; Huang, Y.; Zhang, S.; Lin, S. The Biosynthesis and Applications of Protein Lipidation. Chem. Rev. 2024, 124, 12176–12212. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, M.; Afzal, H.; Murtaza, A.; Cheng, L.-T. Lipoprotein Signal Peptide as Adjuvants: Leveraging Lipobox-Driven TLR2 Activation in Modern Vaccine Design. Vaccines 2025, 13, 36. https://doi.org/10.3390/vaccines13010036
Umar M, Afzal H, Murtaza A, Cheng L-T. Lipoprotein Signal Peptide as Adjuvants: Leveraging Lipobox-Driven TLR2 Activation in Modern Vaccine Design. Vaccines. 2025; 13(1):36. https://doi.org/10.3390/vaccines13010036
Chicago/Turabian StyleUmar, Muhammad, Haroon Afzal, Asad Murtaza, and Li-Ting Cheng. 2025. "Lipoprotein Signal Peptide as Adjuvants: Leveraging Lipobox-Driven TLR2 Activation in Modern Vaccine Design" Vaccines 13, no. 1: 36. https://doi.org/10.3390/vaccines13010036
APA StyleUmar, M., Afzal, H., Murtaza, A., & Cheng, L.-T. (2025). Lipoprotein Signal Peptide as Adjuvants: Leveraging Lipobox-Driven TLR2 Activation in Modern Vaccine Design. Vaccines, 13(1), 36. https://doi.org/10.3390/vaccines13010036






