Single Dose of Attenuated Vaccinia Viruses Expressing H5 Hemagglutinin Affords Rapid and Long-Term Protection Against Lethal Infection with Highly Pathogenic Avian Influenza A H5N1 Virus in Mice and Monkeys
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Cells
2.3. Viruses
2.4. Generation of Recombinant Vaccinia Viruses Encoding H5 HA
2.5. Mouse Study
2.6. Macaque Study
2.7. ELISA
2.8. In Vitro Neutralization Titer Assay for VACV
2.9. Hemagglutination Inhibition (HI) Assay
2.10. ELISpot Assay
2.11. Lung Histopathology and Inflammation Scores
2.12. Viral RNA Quantification
2.13. Statistical Analyses
3. Results
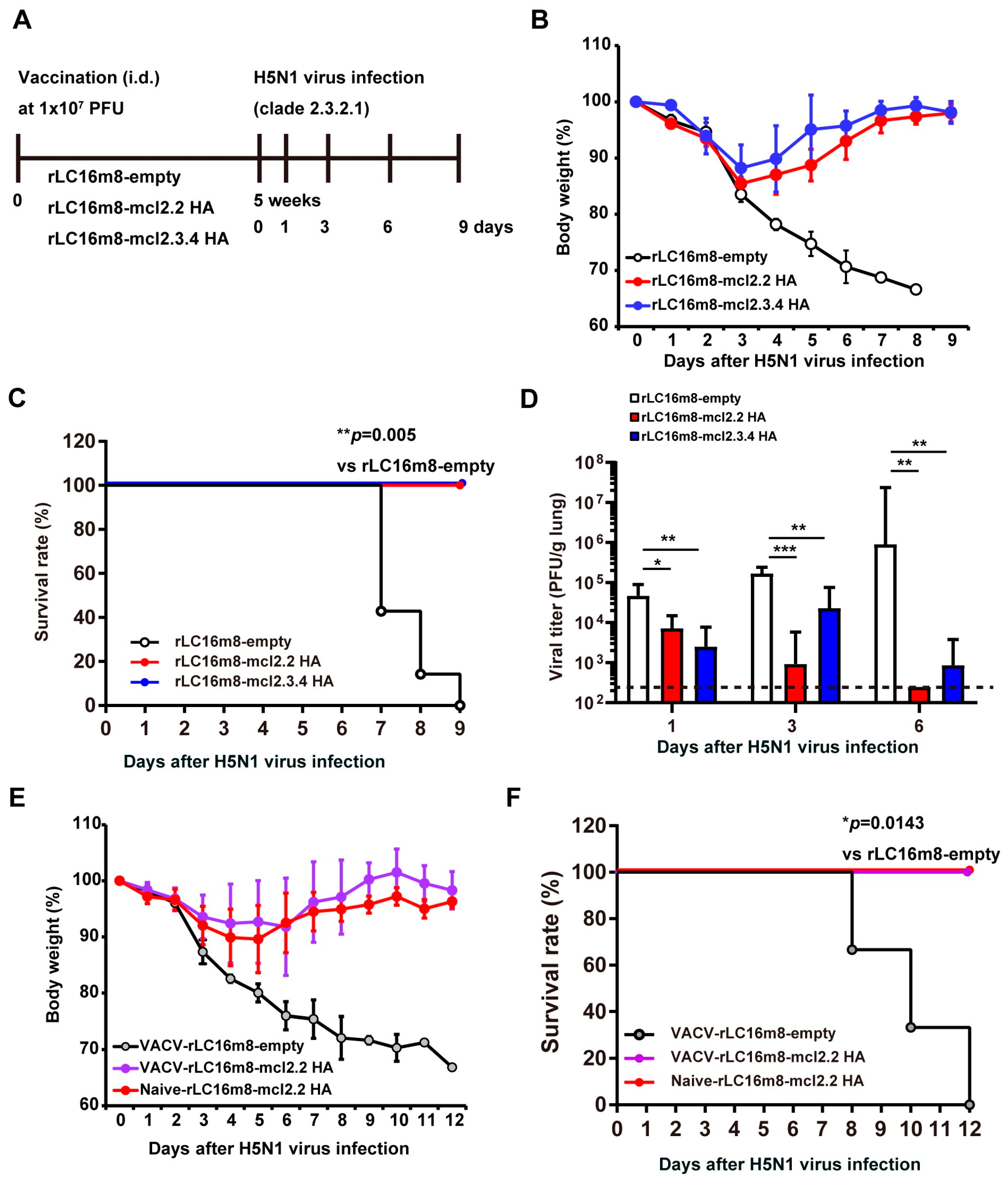
3.1. Single Dose of Recombinant VACV LC16m8 Encoding H5 Hemagglutinin Protein Protects Mice from Lethal Challenges with the HPAI H5N1 Virus
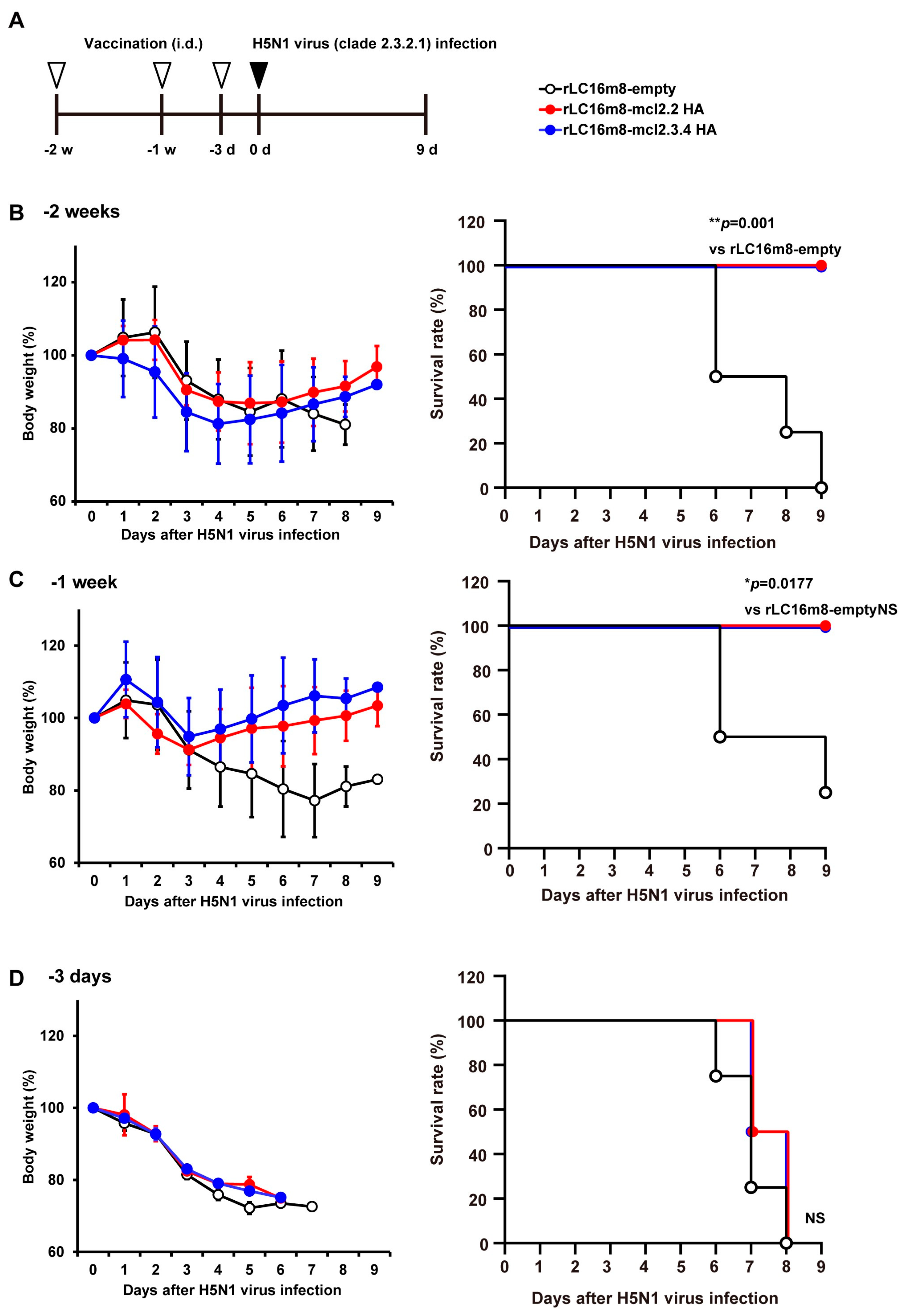
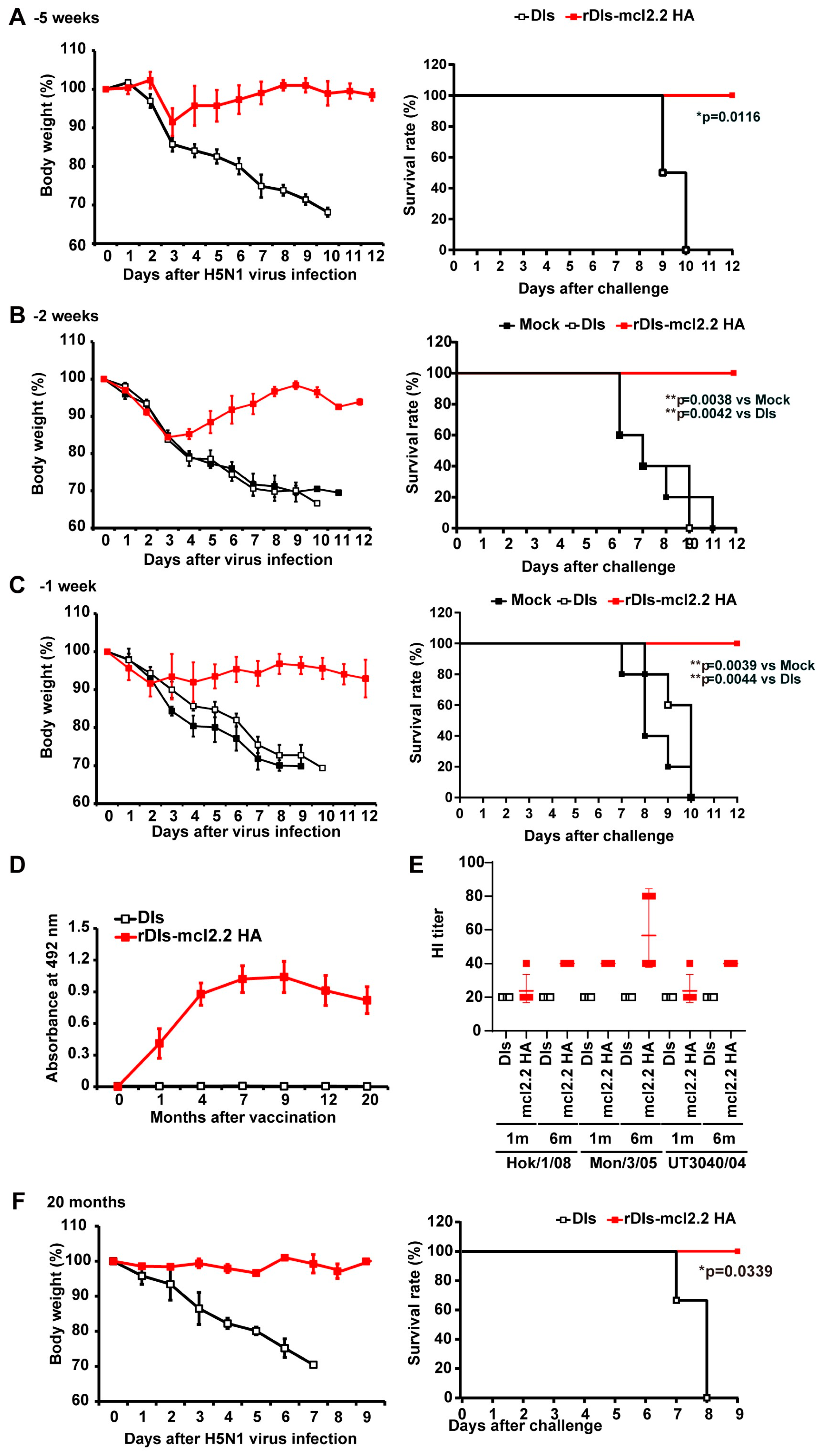
3.2. Rapid Protection in Mice by rLC16m8-H5 HA Vaccine Candidates
3.3. Long-Term Protection in Mice by rLC16m8-H5 HA Vaccine Candidates
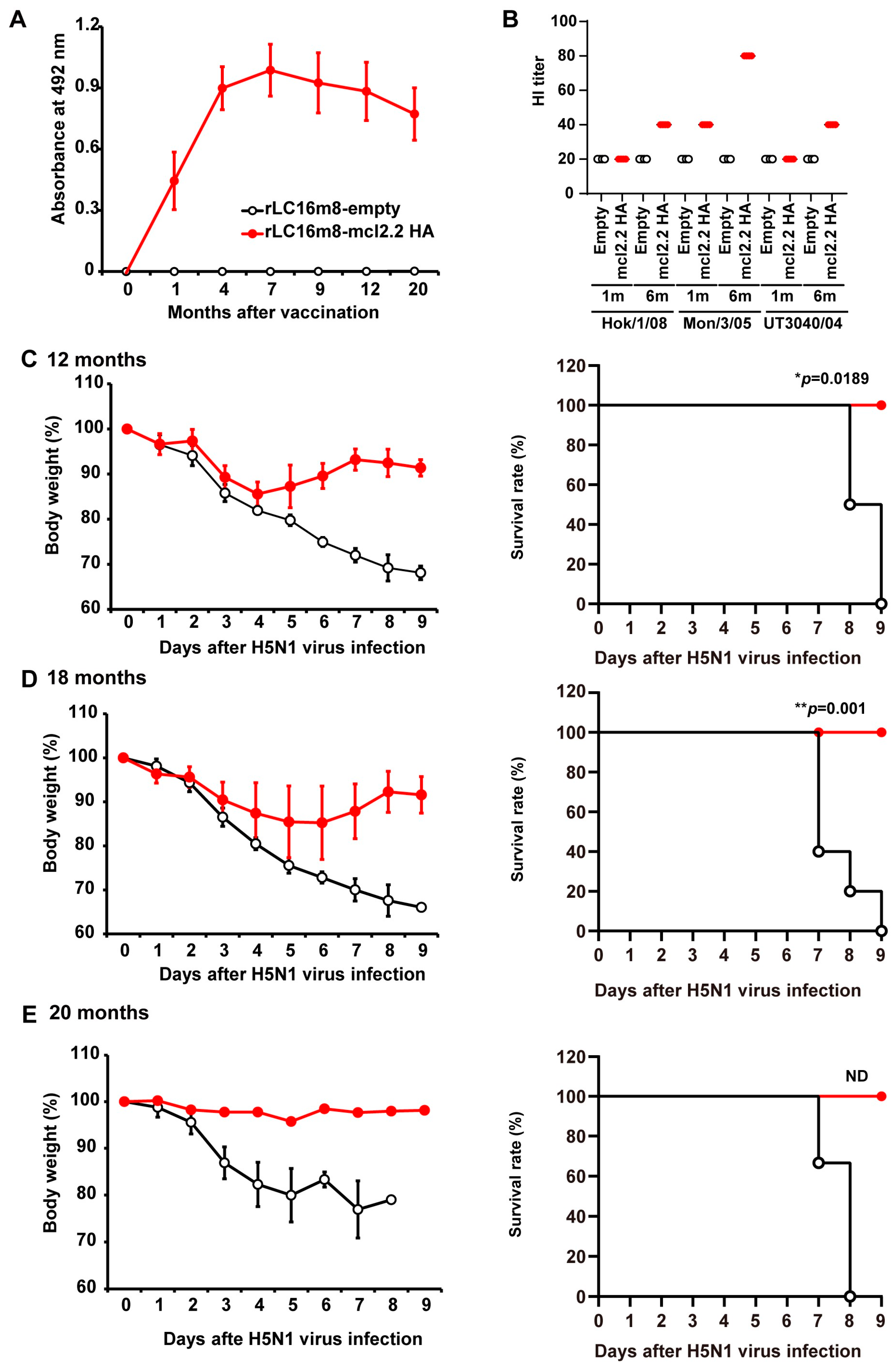
3.4. Long-Term Protection in Non-Human Primates by rLC16m8-mcl2.2 HA Vaccine Candidates
3.5. Rapid Protection and Long-Term Protection Against HPAI H5N1 Virus Induced by a Single Dose of rDIs-mcl2.2 HA in Mice
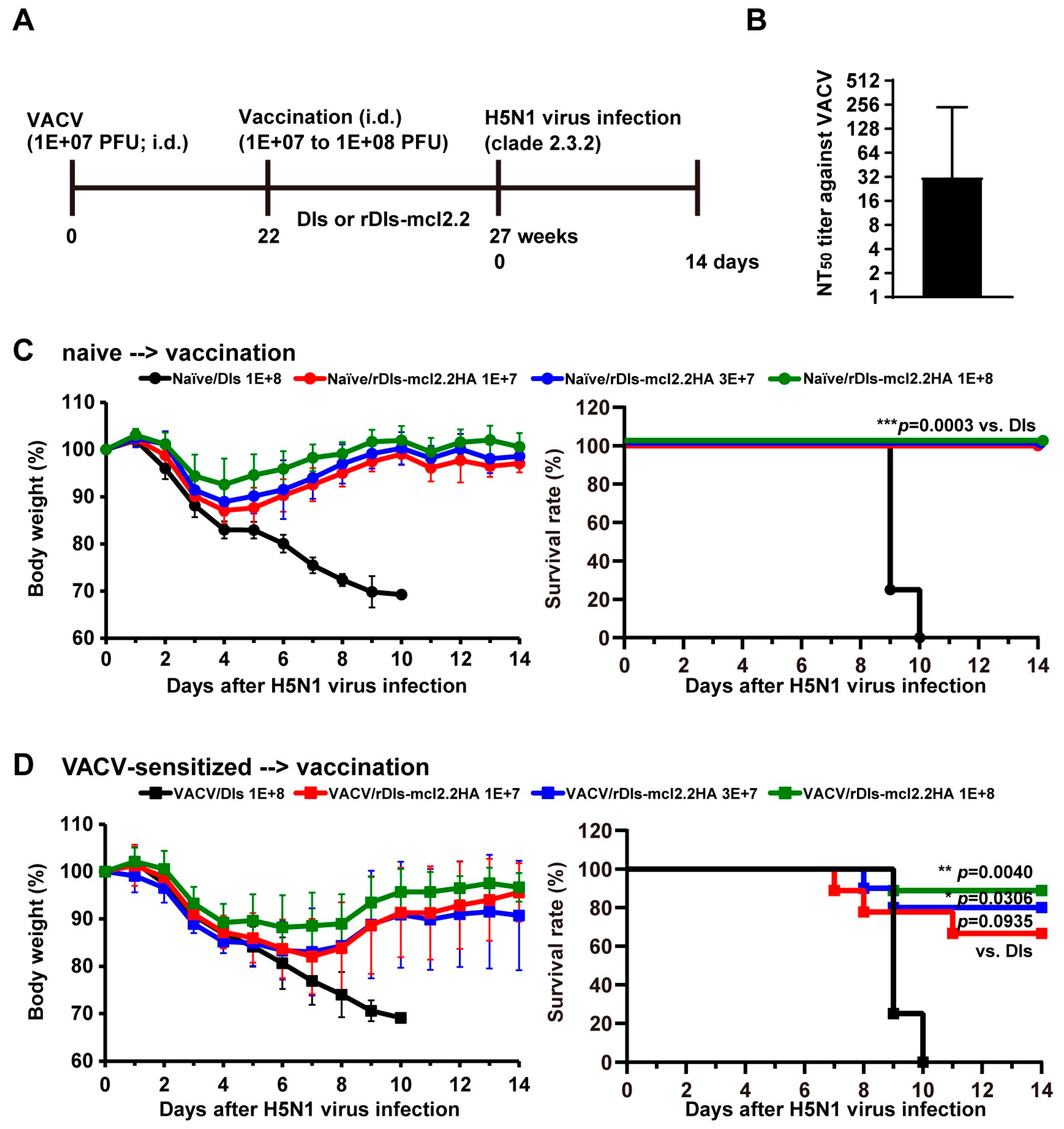
3.6. Protective Efficacy of rDIs-mcl2.2 HA Against HPAI H5N1 Virus in VACV-Sensitized Mice
3.7. Th1/Th2 Immune Response to rVACV-mcl2.2 HA Vaccine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cumulative Number of Confirmed Human Cases for Avian Influenza A(H5N1) Reported to WHO, 2003–2024, 27 September 2024. Available online: https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who--2003-2024--27-september-2024 (accessed on 30 October 2024).
- Puryear, W.; Sawatzki, K.; Hill, N.; Foss, A.; Stone, J.J.; Doughty, L.; Walk, D.; Gilbert, K.; Murray, M.; Cox, E.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in New England Seals, United States. Emerg. Infect. Dis. 2023, 29, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Tomás, G.; Marandino, A.; Panzera, Y.; Rodríguez, S.; Wallau, G.L.; Dezordi, F.Z.; Pérez, R.; Bassetti, L.; Negro, R.; Williman, J.; et al. Highly pathogenic avian influenza H5N1 virus infections in pinnipeds and seabirds in Uruguay: Implications for bird–mammal transmission in South America. Virus Evol. 2024, 10, veae031. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Milton, S.; Hamid, C.A.; Webb, C.R.; Presley, S.M.; Shetty, V.; Rollo, S.N.; Martinez, D.L.; Rai, S.; Gonzales, E.R.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Infection in a Dairy Farm Worker. N. Engl. J. Med. 2024, 390, 2028–2029. [Google Scholar] [CrossRef] [PubMed]
- USDA Animal and Plant Health Inspection Service. Available online: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/livestock (accessed on 30 October 2024).
- Gao, W.; Soloff, A.C.; Lu, X.; Montecalvo, A.; Nguyen, D.C.; Matsuoka, Y.; Robbins, P.D.; Swayne, D.E.; Donis, R.O.; Katz, J.M.; et al. Protection of Mice and Poultry from Lethal H5N1 Avian Influenza Virus through Adenovirus-Based Immunization. J. Virol. 2006, 80, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Deng, G.; Wen, Z.; Tian, G.; Wang, Y.; Shi, J.; Wang, X.; Li, Y.; Hu, S.; Jiang, Y.; et al. Newcastle Disease Virus-Based Live Attenuated Vaccine Completely Protects Chickens and Mice from Lethal Challenge of Homologous and Heterologous H5N1 Avian Influenza Viruses. J. Virol. 2007, 81, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Kreijtz, J.H.C.M.; Suezer, Y.; van Amerongen, G.; de Mutsert, G.; Schnierle, B.S.; Wood, J.M.; Kuiken, T.; Fouchier, R.A.M.; Löwer, J.; Osterhaus, A.D.M.E.; et al. Recombinant Modified Vaccinia Virus Ankara–Based Vaccine Induces Protective Immunity in Mice against Infection with Influenza Virus H5N1. J. Infect. Dis. 2007, 195, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.J.; Nuismer, S.L.; Antia, R. Recombinant vector vaccine evolution. PLoS Comput. Biol. 2019, 15, e1006857. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, I.R.; Sebastian, S. Novel viral vectors in infectious diseases. Immunology 2018, 153, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Saijo, M.; Ami, Y.; Suzaki, Y.; Nagata, N.; Iwata, N.; Hasegawa, H.; Ogata, M.; Fukushi, S.; Mizutani, T.; Sata, T.; et al. LC16m8, a Highly Attenuated Vaccinia Virus Vaccine Lacking Expression of the Membrane Protein B5R, Protects Monkeys from Monkeypox. J. Virol. 2006, 80, 5179–5188. [Google Scholar] [CrossRef]
- Morikawa, S.; Sakiyama, T.; Hasegawa, H.; Saijo, M.; Maeda, A.; Kurane, I.; Maeno, G.; Kimura, J.; Hirama, C.; Yoshida, T.; et al. An Attenuated LC16m8 Smallpox Vaccine: Analysis of Full-Genome Sequence and Induction of Immune Protection. J. Virol. 2005, 79, 11873–11891. [Google Scholar] [CrossRef]
- Saito, T.; Fujii, T.; Kanatani, Y.; Saijo, M.; Morikawa, S.; Yokote, H.; Takeuchi, T.; Kuwabara, N. Clinical and Immunological Response to Attenuated Tissue-Cultured Smallpox Vaccine LC16m8. JAMA 2009, 301, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Kenner, J.; Cameron, F.; Empig, C.; Jobes, D.V.; Gurwith, M. LC16m8: An attenuated smallpox vaccine. Vaccine 2006, 24, 7009–7022. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.S.; Gurwith, M.; Dekker, C.L.; Frey, S.E.; Edwards, K.M.; Kenner, J.; Lock, M.; Empig, C.; Morikawa, S.; Saijo, M.; et al. Safety and Immunogenicity of LC16m8, an Attenuated Smallpox Vaccine in Vaccinia-Naive Adults. J. Infect. Dis. 2011, 204, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Yasui, F.; Kai, C.; Kitabatake, M.; Inoue, S.; Yoneda, M.; Yokochi, S.; Kase, R.; Sekiguchi, S.; Morita, K.; Hishima, T.; et al. Prior Immunization with Severe Acute Respiratory Syndrome (SARS)-Associated Coronavirus (SARS-CoV) Nucleocapsid Protein Causes Severe Pneumonia in Mice Infected with SARS-CoV. J. Immunol. 2008, 181, 6337–6348. [Google Scholar] [CrossRef]
- Yasui, F.; Itoh, Y.; Ikejiri, A.; Kitabatake, M.; Sakaguchi, N.; Munekata, K.; Shichinohe, S.; Hayashi, Y.; Ishigaki, H.; Nakayama, M.; et al. Sensitization with vaccinia virus encoding H5N1 hemagglutinin restores immune potential against H5N1 influenza virus. Sci. Rep. 2016, 6, 37915. [Google Scholar] [CrossRef] [PubMed]
- Tagaya, I.; Kitamura, T.; Sano, Y. A New Mutant of Dermovaccinia Virus. Nature 1961, 192, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Ishiia, K.; Uedaa, Y.; Matsuobc, K.; Matsuurad, Y.; Kitamuraa, T.; Katoa, K.; Izumibc, Y.; Someyabc, K.; Ohsubc, T.; Hondabc, M.; et al. Structural Analysis of Vaccinia Virus DIs Strain: Application as a New Replication-Deficient Viral Vector. Virology 2002, 302, 433–444. [Google Scholar] [CrossRef]
- Ishii, K.; Hasegawa, H.; Nagata, N.; Mizutani, T.; Morikawa, S.; Suzuki, T.; Taguchi, F.; Tashiro, M.; Takemori, T.; Miyamura, T.; et al. Induction of protective immunity against severe acute respiratory syndrome coronavirus (SARS-CoV) infection using highly attenuated recombinant vaccinia virus DIs. Virology 2006, 351, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.M.; Ito, M.; Muramoto, Y.; Hoang, P.V.M.; Vuong, C.D.; Sakai-Tagawa, Y.; Kiso, M.; Ozawa, M.; Takano, R.; Kawaoka, Y. Pathogenicity of highly pathogenic avian H5N1 influenza A viruses isolated from humans between 2003 and 2008 in northern Vietnam. J. Gen. Virol. 2010, 91, 2485–2490. [Google Scholar] [CrossRef] [PubMed]
- Fujiyuki, T.; Yoneda, M.; Yasui, F.; Kuraishi, T.; Hattori, S.; Kwon, H.-J.; Munekata, K.; Kiso, Y.; Kida, H.; Kohara, M.; et al. Experimental Infection of Macaques with a Wild Water Bird-Derived Highly Pathogenic Avian Influenza Virus (H5N1). PLoS ONE 2013, 8, e83551. [Google Scholar] [CrossRef]
- Tumpey, T.M.; García-Sastre, A.; Taubenberger, J.K.; Palese, P.; Swayne, D.E.; Basler, C.F. Pathogenicity and immunogenicity of influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. USA 2004, 101, 3166–3171. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Nomura, N.; Nanba, R.; Sinkai, T.; Iwaki, T.; Obayashi, T.; Hashimoto, K.; Hasegawa, M.; Sakoda, Y.; Naito, A.; et al. Rapid typing of influenza viruses using super high-speed quantitative real-time PCR. J. Virol. Methods 2011, 178, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, E.; Lewis, M.W.; Hansen, S.G.; I Strelow, L.; A Nelson, J.; Sexton, G.J.; Hanifin, J.M.; Slifka, M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003, 9, 1131–1137. [Google Scholar] [CrossRef]
- Taub, D.D.; Ershler, W.B.; Janowski, M.; Artz, A.; Key, M.L.; McKelvey, J.; Muller, D.; Moss, B.; Ferrucci, L.; Duffey, P.L.; et al. Immunity from Smallpox Vaccine Persists for Decades: A Longitudinal Study. Am. J. Med. 2008, 121, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Antigua, K.J.C.; Choi, W.-S.; Baek, Y.H.; Song, M.-S. The Emergence and Decennary Distribution of Clade 2.3.4.4 HPAI H5Nx. Microorganisms 2019, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- WHO/OIE/FAO/H5N1 Evolution Working Group. Toward a Unified Nomenclature System for Highly Pathogenic Avian Influenza Virus (H5N1). Emerg. Infect. Dis. 2008, 14, e1. [Google Scholar] [CrossRef]
- Li, J.; Gu, M.; Liu, K.; Gao, R.; Sun, W.; Liu, D.; Jiang, K.; Zhong, L.; Wang, X.; Hu, J.; et al. Amino acid substitutions in antigenic region B of hemagglutinin play a critical role in the antigenic drift of subclade 2.3.4.4 highly pathogenic H5NX influenza viruses. Transbound. Emerg. Dis. 2019, 67, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Evolution of a Pandemic A(H1N1) 2009. Available online: https://iris.who.int/handle/10665/78414 (accessed on 30 October 2024).
- Langley, J.M.; Risi, G.; Caldwell, M.; Gilderman, L.; Berwald, B.; Fogarty, C.; Poling, T.; Riff, D.; Baron, M.; Frenette, L.; et al. Dose-Sparing H5N1 A/Indonesia/05/2005 Pre-pandemic Influenza Vaccine in Adults and Elderly Adults: A Phase III, Placebo-Controlled, Randomized Study. J. Infect. Dis. 2011, 203, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Rümke, H.C.; Bayas, J.-M.; de Juanes, J.-R.; Caso, C.; Richardus, J.H.; Campins, M.; Rombo, L.; Duval, X.; Romanenko, V.; Schwarz, T.F.; et al. Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine 2008, 26, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Baz, M.; Luke, C.J.; Cheng, X.; Jin, H.; Subbarao, K. H5N1 vaccines in humans. Virus Res. 2013, 178, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Keitel, W.A.; Dekker, C.L.; Mink, C.; Campbell, J.D.; Edwards, K.M.; Patel, S.M.; Ho, D.Y.; Talbot, H.K.; Guo, K.; Noah, D.L.; et al. Safety and immunogenicity of inactivated, Vero cell culture-derived whole virus influenza A/H5N1 vaccine given alone or with aluminum hydroxide adjuvant in healthy adults. Vaccine 2009, 27, 6642–6648. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.; Chan, P.; Peiris, M.; Tsang, D.; Que, T.; Shortridge, K.; Cheung, P.; To, W.; Ho, E.; Sung, R.; et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998, 351, 467–471. [Google Scholar] [CrossRef]
- Acharya, K.P.; Acharya, N.; Phuyal, S.; Subramanya, S.H. Human infection with Avian influenza A virus in Nepal: Requisite for timely management and preparedness. VirusDisease 2020, 31, 244–248. [Google Scholar] [CrossRef]
- Combadiere, B.; Boissonnas, A.; Carcelain, G.; Lefranc, E.; Samri, A.; Bricaire, F.; Debre, P.; Autran, B. Distinct Time Effects of Vaccination on Long-Term Proliferative and IFN-γ–producing T Cell Memory to Smallpox in Humans. J. Exp. Med. 2004, 199, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Meisinger-Henschel, C.; Schmidt, M.; Lukassen, S.; Linke, B.; Krause, L.; Konietzny, S.; Goesmann, A.; Howley, P.; Chaplin, P.; Suter, M.; et al. Genomic sequence of chorioallantois vaccinia virus Ankara, the ancestor of modified vaccinia virus Ankara. J. Gen. Virol. 2007, 88, 3249–3259. [Google Scholar] [CrossRef] [PubMed]
- Kreijtz, J.H.C.M.; Suezer, Y.; de Mutsert, G.; van Amerongen, G.; Schwantes, A.; Brand, J.M.A.v.D.; Fouchier, R.A.M.; Löwer, J.; Osterhaus, A.D.M.E.; Sutter, G.; et al. MVA-Based H5N1 Vaccine Affords Cross-Clade Protection in Mice against Influenza A/H5N1 Viruses at Low Doses and after Single Immunization. PLoS ONE 2009, 4, e7790. [Google Scholar] [CrossRef] [PubMed]
- Kreijtz, J.H.C.M.; Goeijenbier, M.; Moesker, F.M.; Dries, L.v.D.; Goeijenbier, S.; De Gruyter, H.L.M.; Lehmann, M.H.; de Mutsert, G.; Vijver, D.A.M.C.v.d.; Volz, A.; et al. Safety and immunogenicity of a modified-vaccinia-virus-Ankara-based influenza A H5N1 vaccine: A randomised, double-blind phase 1/2a clinical trial. Lancet Infect. Dis. 2014, 14, 1196–1207. [Google Scholar] [CrossRef]
- de Vries, R.D.; Altenburg, A.F.; Nieuwkoop, N.J.; de Bruin, E.; E van Trierum, S.; Pronk, M.R.; Lamers, M.M.; Richard, M.; Nieuwenhuijse, D.F.; Koopmans, M.P.G.; et al. Induction of Cross-Clade Antibody and T-Cell Responses by a Modified Vaccinia Virus Ankara–Based Influenza A(H5N1) Vaccine in a Randomized Phase 1/2a Clinical Trial. J. Infect. Dis. 2018, 218, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Fenner, F.; Henderso, D.A.; Atira, I.; Jezek, Z.; Ladnyi, I.D. Smallpox and It Eradication; World Health Organization: Geneva, Switzerland, 1988; pp. 1–68. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasui, F.; Munekata, K.; Fujiyuki, T.; Kuraishi, T.; Yamaji, K.; Honda, T.; Gomi, S.; Yoneda, M.; Sanada, T.; Ishii, K.; et al. Single Dose of Attenuated Vaccinia Viruses Expressing H5 Hemagglutinin Affords Rapid and Long-Term Protection Against Lethal Infection with Highly Pathogenic Avian Influenza A H5N1 Virus in Mice and Monkeys. Vaccines 2025, 13, 74. https://doi.org/10.3390/vaccines13010074
Yasui F, Munekata K, Fujiyuki T, Kuraishi T, Yamaji K, Honda T, Gomi S, Yoneda M, Sanada T, Ishii K, et al. Single Dose of Attenuated Vaccinia Viruses Expressing H5 Hemagglutinin Affords Rapid and Long-Term Protection Against Lethal Infection with Highly Pathogenic Avian Influenza A H5N1 Virus in Mice and Monkeys. Vaccines. 2025; 13(1):74. https://doi.org/10.3390/vaccines13010074
Chicago/Turabian StyleYasui, Fumihiko, Keisuke Munekata, Tomoko Fujiyuki, Takeshi Kuraishi, Kenzaburo Yamaji, Tomoko Honda, Sumiko Gomi, Misako Yoneda, Takahiro Sanada, Koji Ishii, and et al. 2025. "Single Dose of Attenuated Vaccinia Viruses Expressing H5 Hemagglutinin Affords Rapid and Long-Term Protection Against Lethal Infection with Highly Pathogenic Avian Influenza A H5N1 Virus in Mice and Monkeys" Vaccines 13, no. 1: 74. https://doi.org/10.3390/vaccines13010074
APA StyleYasui, F., Munekata, K., Fujiyuki, T., Kuraishi, T., Yamaji, K., Honda, T., Gomi, S., Yoneda, M., Sanada, T., Ishii, K., Sakoda, Y., Kida, H., Hattori, S., Kai, C., & Kohara, M. (2025). Single Dose of Attenuated Vaccinia Viruses Expressing H5 Hemagglutinin Affords Rapid and Long-Term Protection Against Lethal Infection with Highly Pathogenic Avian Influenza A H5N1 Virus in Mice and Monkeys. Vaccines, 13(1), 74. https://doi.org/10.3390/vaccines13010074




