Advancing the Fight Against Cervical Cancer: The Promise of Therapeutic HPV Vaccines
Abstract
:1. Introduction
2. Prophylactic HPV Vaccine
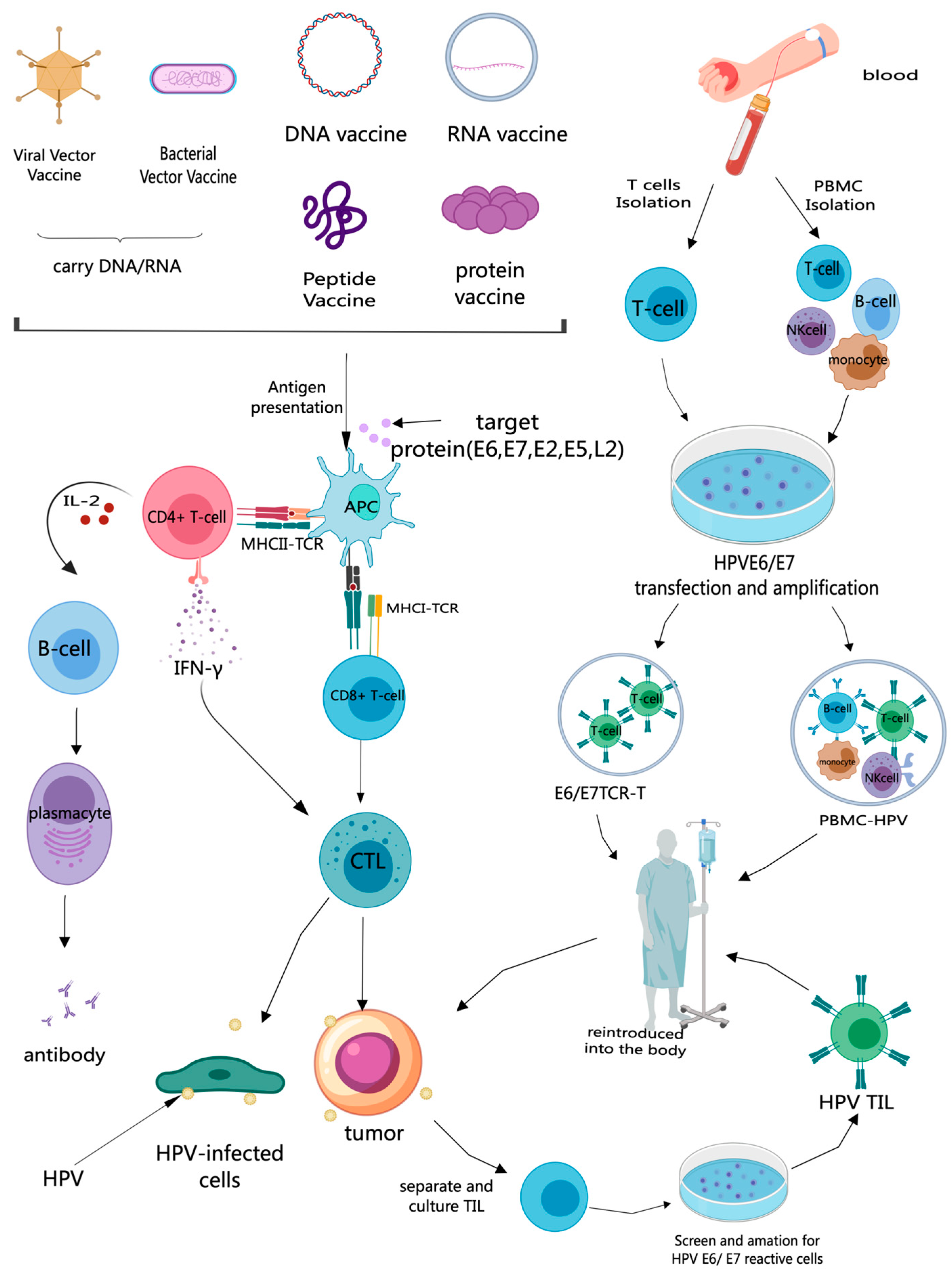
3. Therapeutic HPV Vaccine
3.1. Mechanism of HPV Therapeutic Vaccines
3.2. Treatment of Persistent High-Risk HPV Infection and CIN
3.2.1. Clearance of High-Risk HPV Infection and Low-Grade Lesions (CIN1)
3.2.2. Induce Regression of VIN
3.2.3. Induce Regression of CIN2/3
| Indications | Combined Treatment | Vaccine Platform | Vaccine Name | HPV Types | Clinical Trial | Research Phase | Current State | Year | Number of Patients | Efficacy | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal cytology or ASCUS/LSIL | no | protein vaccine | TA-CIN | HPV16-E6/E7/L2 | NCT01957878 | Phase 2, RCT, placebo-controlled | Completed | 2013–2016 | 239 | At 12 months, the HPV clearance rate was 58.7% compared to 37.5% in the placebo group. | [38] |
| ASC-US, ASC-H or LSIL | no | protein vaccine and DNA vaccine | TA-CIN and pNGVL4a Sig/E7(detox)/HSP70 | HPV16-E6/E7/L2 | NCT03911076 | Phase 2, single-arm | Terminated | 2019–2022 | 16 | At 12 month, 64% (7/11) were HPV16-negative. In total, 36% (4/11) had normal cytology. | [39] |
| ASCUS or LSIL | no | protein vaccine | SGN-00101 | HPV16-E7 | NCT00091130 | Phase 2, RCT, placebo-controlled | Completed | 2004–2007 | 139 | Unknown. | [47] |
| Persistent ASC-US/LSI L (>6 month period) | no | protein vaccine and DNA vaccine | TA-CIN and pNGVL4aCRTE6E7L2 | HPV16-E6/E7/L2 | NCT03913117 | Phase 1, single-arm | Recruiting | Started in 2021, until now | Target: 30 | Recruiting. | [48] |
| CIN2/3 | no | DNA vaccine | VGX-3100 | HPV16/18-E6/E7 | NCT03721978 REVEAL 2 | Phase 3, RCT, placebo-controlled | Completed | 2019–2022 | 203 | At week 36, lesion regression with viral clearance: vaccinated group = 27.6% (37/134), placebo group = 8.7% (6/69). | [45] |
| CIN2/3 | no | bacterial vector vaccine | ADXS11-001 | HPV 16-E7 | NCT01116245 | Phase 2, RCT, placebo-controlled | Terminated | 2010–2016 | 81 | Unknown. | [49] |
| CIN 2/3 | no | viral vector vaccine | TG4001 | HPV 16- E6/E7 | NCT01022346 | Phase 2, RCT, placebo-controlled | Completed | 2009–2013 | 206 | Follow-up results after 2.5-years: CIN two-thirds complete regression rate: vaccine group = 24%, placebo group = 10%; CIN 3 complete regression rate: vaccine group = 21%, placebo group = 0%. | [43] |
| CIN2/3 | no | DNA vaccine | VB10.16 | HPV16-E6/E7 | NCT02529930 | Phase 1/phase 2, single-arm | Completed | 2015–2019 | 34 | At 12 months, HPV16 clearance = 47% (8/17), lesions became smaller = 94% (16/17), lesion regression to CIN1 or normal = 59% (10/17). | [50] |
| CIN1/2/3 | no | viral vector vaccine | MVA E2 | HPV-E2 | no | Phase 3, non-randomized, two-arm | Completed | Published in 2014 | 1176 | Experimental group: complete lesion regression = 89% (1051/1184), lesion regression to CIN 1 = 2.4% (28/1184). Control group (surgery and physical/pharmacological treatments): 89% had new lesion recurrence within 24 months. | [21] |
| CIN 3 | no | protein vaccine | SGN-00101(HspE7) | HPV16-E7 | NCT00075569 | Phase 2, non-placebo-controlled | Completed | 2004–2005 | 64 | The results of the 1–2 month follow-up: lesion regression to CIN1 or normal = 22.5% (13/58), >50% regression of lesion size = 55% (32/58), stable disease = 19% (11/58) | [51] |
| HSIL | no | peptide vaccine | PepCan | HPV16-E6 | NCT02481414 | Phase 2, RCT | Completed | 2015–2022 | 81 | At 15 months, pathological regression rate (PepCan group) = 30.8%, pathological regression rate (Candin group) = 47.6%. | [52,53] |
| CIN 3 | no | DNA vaccine | GX-188E | HPV16/18-E6/E7 | NCT02139267 | Phase 2, non-placebo-controlled | Completed | 2014–2016 | 72 | At 20 weeks, histopathologic regression rate = 67% (35/52). | [54] |
| CIN 3 | no | DNA vaccine | GX-188E | HPV16/18-E6/E7 | NCT02411019 | Phase 2, non-placebo-controlled | Unknown | Started in 2015 | 67 | Unknown. | [55] |
| CIN 3 | no | DNA vaccine | GX-188E | HPV16/18-E6/E7 | NCT02596243 | Phase 2, RCT, placebo-controlled | Unknown | Started in 2015 | 124 | Unknown. | [56] |
| CIN3 | GX-I7 or Imiquimod | DNA vaccine | GX-188E | HPV16/18-E6/E7 | NCT03206138 | unknown, non-placebo-controlled | Unknown | Started in 2017 | 50 | Unknown. | [57] |
| CIN3 | no | viral vector vaccine | Vvax001 | HPV16-E6/E7 | NCT06015854 | Phase 2, single-arm | Recruiting | Starting in 2021, until now | Target: 18 | Recruiting. | [58] |
| HSIL | no | DNA vaccine | VGX-3100 | HPV16/18-E6/E7 | NCT01304524 | Phase 2, RCT, placebo-controlled | Completed | 2011–2015 | 107 | Histopathological regression at 36 weeks: VGX-3100 group = 49.5% (53/107), placebo group = 30.6% (11/36). At 18 months: cytological improvement—vaccine group (no surgery) = 100% (32/32), placebo group (underwent surgery) = 100% (45/45), HPV clearance rate. Vaccine group = 91%, placebo group = 88%. | [46,59] |
| HSIL | no | DNA vaccine | VGX-3100 | HPV16/18-E6/E7 | NCT03185013 | Phase 3, RCT, placebo-controlled | Completed | 2017–2021 | 201 | HSIL histopathologic regression and HPV-16/18 clearance at week 36: treatment group = 23.7% (31/131), placebo group = 11.3% (7/62) | [44] |
| VIN 2/3 | Imiquimod | DNA vaccine | VGX-3100 | HPV16/18-E6/E7 | NCT03180684 | Phase 2, non-placebo-controlled | Completed | 2017–2020 | 33 | After six months of treatment, 63% (12/19) experienced ≥25% reduction in lesion area. | [42] |
3.3. Treatment of Cervical Cancer
3.3.1. Second-Line Treatment for Recurrent, Metastatic, Refractory CC Alone
3.3.2. Combination with Chemotherapy or Chemoradiotherapy for First- and Second-Line Treatment of CC
3.3.3. Utilization in Conjunction with Other Immunological Agents for the Second-Line Management of Advanced, Recurrent, Metastatic, and Refractory CC
| Indications | Combined Treatment | Vaccine Platform | Vaccine Name | HPV Types | Clinical Trial | Research Phase | CURRENT STATE | Year | Number of Patients | Efficacy | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Early CC | Surgery | Viral vector vaccine | TA-HPV | HPV16/18-E6/E7 | NCT00002916 | Phase 2, two-arm, non-randomized | Completed | Started in 2017 | Target 44 | Unknown | [79] |
| Stage IB3-IVA CC | Radiotherapy | Peptide vaccine | PDS0101 | HPV 16-E6/E7 | NCT04580771 | Phase 2, single-arm | Recruitment completed | 2022 | 17 | ORR = 100% (8 CR, 1 PR) | [70] |
| Stage IIB-IVA CC | No | Cell-based vaccine | E7 T-Cell Receptor (TCR-T) | HPV16-E7 | NCT04476251 | Phase 1, single-arm | Terminated | 2021 | 1 | One participant was enrolled but not treated | [80] |
| HPV16+ R/M solid tumors with HLA-A*02+ | No | Cell-based vaccine | SQZ-PBMC-HPV | HPV16-E6/E7 | NCT04084951 | Phase 1, single-arm | Completed | 2020–2023 | 18 | Overall DCR 33.3% | [28] |
| HPV-associated R/M solid tumors | Combined or not with M7824 (MSB0011359C) (dual PD-L1 and TGF- beta inhibitor) | Viral vector vaccine | PRGN-2009 | HPV 16/18-E6/E7 | NCT04432597 | Phase 1/phase 2, multi-armed, non-randomized | Recruitment completed | Starting in 2020, until now | 39 | Phase 1 results: ORR = 30.0%, OS (PRGN-2009) = 7.4 months, OS (combination) = 12.5 months | [81] |
| Pembrolizumab-resistant R/M CC | Pembrolizumab (PD-1 inhibitor) | Viral vector vaccine | PRGN-2009 | HPV 16/18 -E6/E7 | NCT06157151 | Phase 2, RCT, placebo-controlled | Recruiting | Starting in 2024, until now | Target 46 | Recruiting | [82] |
| HPV-associated cancers | Atezolizumab (PD-L1 inhibitor) | Viral vector vaccine | MG1-E6E7 | HPV16/18-E6/E7 | NCT03618953 | Phase 1, non-placebo-controlled | Terminated | 2018–2021 | 8 | Terminated | [83] |
| HPV 16+ cancer with HLA-A*02+ | Cyclophosphamide | Peptide vaccine | DPX-E7 | HPV16-E7 | NCT02865135 | Phase 1/phase 2, single-arm | Completed | 2017–2023 | 11 | 7 out of 11 PD, 3 SD. | [67] |
| Metastatic HPV+cancers | No | Cell-based vaccine | E7 TCR-T | HPV16-E7 | NCT02858310 | Phase 1/phase 2, single-arm | Phase 1 completed, phase 2 recruiting | Starting in 2018, until now | 12,(5 cervix,1 vulva) | Objective clinical responses: 6/12 patients, including 4 of 8 patients with anti-PD-1 refractory disease | [61] |
| IB1-IVA CC or persistent/ recurrent CC | After chemoradiation | DNA vaccine | NO-3112 (MEDI0457) | HPV 16/18- E6/E7 | NCT02172911 | Phase 1, single-arm | Completed | 2014–2020 | 10 | Anti-HPV antibody responses: 6/10 patients, IFNγ-producing T cell responses: 6/10 patients, treatment-related adverse events: all grade 1 | [84] |
| R/M HPV-16+ cancers | Avelumab (PD-L1 inhibitor) | Viral vector vaccine | TG4001 | HPV16-E6/E7 | NCT03260023 | Phase 1/phase 2, single-arm | Recruitment completed | Starting in 2017, until now | 43(18 cervix) | ORR = 22% (1 CR, 7 PR), DCR at 12 weeks = 42% (15/36), PFS = 2.8 months, OS = 11.0 months | [85] |
| Recurrent CC with disease progression after first-line chemotherapy | Cemiplimab (PD-1 inhibitor) | Peptide vaccine | ISA101b | HPV16-E6/E7 | NCT04646005 | Phase 2, single-arm | Recruitment completed | Starting in 2021, until now | 113 | ORR = 16.8%, ORR (PD-L1 <1%) = 12.5%, ORR (PD-L1 ≥1%) = 22.4%, OS = 13.3 months, PFS = 3.0 months | [77] |
| HPV-16 + incurable solid tumors | Nivolumab(PD-1 inhibitor) | Peptide vaccine | ISA101 | HPV16-E6/E7 | NCT02426892 | Phase 2, single-arm | Completed | 2015–2021 | 33 | Median DOR = 11.2 months, PFS = 2.66 months, OS = 15.3 months, 2-year OS rate = 33% | [86] |
| HPV16+ R/M CC | Pegylated interferon alpha (IFN α) | Peptide vaccine | ISA101/ISA101b | HPV16-E6/E7 | NCT02128126 CervISA | Phase 1/phase 2, single-arm | Completed | 2013–2018 | 93 | Tumor regressions = 43%, stable disease = 43% (62/72 patients), OS (low response) = 11.2 months, OS (strong response) = 16.8 months | [68] |
| CC refractory after platinum-based first-line chemotherapy | Durvalumab (PD-L1 inhibitor) | Cell-based vaccine | BVAC-C | HPV16/18-E6/E7 | NCT04800978 | Phase 2, single-arm | In progress | Starting in 2021, until now | Target 37 | Interim analysis: 6-month PFS rate = 50%, ORR = 38%, CR = 14%, PR = 24%, DOR = 13.3 months, median PFS = 8.7 months | [78] |
| Advanced or recurrent non-resectable CC with failure of standard therapy | Atezolizumab (PD-L1 inhibitor) | DNA vaccine | VB10.16 | HPV16- E6/7 | NCT04405349 | Phase 2, single-arm | Completed | 2020–2023 | 52 | Interim results (39 patients, median follow-up 6 months): ORR = 21% (2 CR, 6 PR), DCR = 64%, ORR (PD-L1+) = 27%, DCR (PD-L1+) = 77%, ORR (PD-L1-) = 17%, DCR (PD-L1-) = 58% | [75] |
| R/M HPV16+, PD-L1+ CC | Combined or not with atezolizumab (PD-L1 inhibitor) | DNA vaccine | VB10.16 | HPV16- E6/E7 | NCT06099418 | Phase 2, single-arm | Withdrawn | Starting in 2024, until now | Target 130 | Withdrawn | [87] |
| Advanced, non-resectable CC | Pembrolizumab (PD-1 inhibitor) | DNA vaccine | GX-188E | HPV16/18-E6/E7 | NCT03444376 | Phase 1/phase 2, single-arm | Completed | 2018–2023 | 65 | 24-week ORR = 35.0%, CR = 8.3%, PR = 26.7%, median PFS = 4.4 months, median OS = 23.8 months | [73] |
| HPV16+ cancer | No | RNA vaccine | HARE-40 (HPV Anti-CD40 RNA Vaccine) | HPV16-E7 | NCT03418480 | Phase 1/phase 2, non-placebo-controlled | Completed | 2017–2024 | 32 | Not yet reported | [88] |
| R/M CC with failure of conventional therapy | No | Bacterial vector vaccine | ADXS11-001 | HPV 16-E7 | NCT02164461 | Phase 1, single-arm | Completed | 2015–2017 | 12 | The increased dosage of ADXS11-001 demonstrated good tolerability, establishing an RP2D at 1 × 1010 CFU | [89] |
| Platinum- refractory CC | No | Bacterial vector vaccine | ADXS-HPV | HPV 16-E7 | NCT01266460 | Phase 2, single-arm | Completed | 2011–2018 | 50 | 12-month OS = 38% (n = 19), median OS = 6.1 months, median PFS = 2.8 months | [60] |
| Advanced CC after first-line treatment | Combined or not with cisplatin | Bacterial vector vaccine | ADXS11-001 | HPV 16-E7 | CTRI/2010/091/001232 | Phase 2, RCT, placebo-controlled | Completed | 2018 | 109 | Median OS (ADXS11-001) = 8.28 months (95% CI: 5.85–10.5), median OS (ADXS11-001 + cisplatin) = 8.78 months, median PFS (both groups) = 6.09 months, ORR (ADXS11-001) = 17.1%, ORR (ADXS11-001 + cisplatin) = 14.7% | [64] |
| Checkpoint inhibitor (CPI) primary and CPI-refractory advanced HPV-positive cancers | M9241, bintrafusp alfa | Peptide vaccine | PDS0101 | HPV 16-E6/E7 | NCT04287868 | Phase 2, single-arm | In progress | Starting in 2022, until now | 29 | Median OS (PDS0101 + M9241 + bintrafusp alfa) = 21 months (n = 29), ORR (CPI-refractory) = 63%, ORR (CPI-primary) = 88% | [90] |
| Recurrent/Metastatic HPV-associated cancers | Durvalumab (PD-L1 inhibitor) | DNA vaccine | INO-3112 (MEDI0457) | HPV 16/18-E6/E7 | NCT03439085 | Phase 2, single-arm | Completed | 2018–2022 | 21 (12 cervix) | ORR = 21%, DCR = 37%, DOR = 21.8 months, PFS = 4.6 months, OS = 17.7 months, grades 3–4 AE = 23% | [91] |
| Metastatic or locally advanced refractory or recurrent CC | No | Cell-based vaccine | HPV-TILs | HPV 16/18-E6/E7 | NCT01585428 | Phase 2, single-arm | Completed | 2012–2016 | 29 | Objective tumor responses = 3/9 patients (2 CR, 1 PR), duration of CR = 22 months and 15 months | [31] |
3.4. Prophylaxis Following Completion of Treatment
3.4.1. Prophylaxis After Local Surgery for CIN
3.4.2. Prophylaxis Following the Completion of Cervical Cancer Treatment
| Indications | Combined Treatment | Vaccine Platform | Vaccine Name | HPV Types | Clinical Trial | Research Phase | Current State | Year | Number of Patients | Efficacy | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A history of CIN 2.3 | no | Viral vector vaccine | Vvax001 | HPV16-E6/E7 | NCT03141463 | Phase 1 | Completed | 2017–2017 | 12 | Vvax001 is immunologically safe and well tolerated, Vvax001 causes CD4+ and CD8+ T cell response against E6 and E7 antigens, and Vvax001 induces strong HPV16 E6- and E7-specific IFN-γ responses. | [93] |
| Stage IB or IIA CC after undergoing radical surgery | no | Cell-based vaccine | HPV DC vaccine | HPV16/18-E7 | no | Phase 1 | Completed | 2004–2006 | 10 | All patients developed CD4(+) T cell and antibody responses to DC vaccination, and 8 out of 10 patients demonstrated levels of E7-specific CD8(+) T cell counts after immunization, which were increased compared to prevaccination baseline levels. | [30] |
| IB1-IV CC having completed treatment within 12 months | no | Protein vaccine | TA-CIN | HPV16-E6/E7/L2 | NCT02405221 | Phase 1 | Recruitment completed | 2019–2024 | 14 | Not yet reported. | [94] |
4. Characteristics and Limitations of Therapeutic HPV Vaccine Development
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Lindsay, L.; Hoots, B.; Keys, J.; Franceschi, S.; Winer, R.; Clifford, G.M. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int. J. Cancer 2007, 121, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: A register-based observational study. Lancet 2021, 398, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, M.; Soldan, K.; Ndlela, B.; Sasieni, P. Effect of the HPV vaccination programme on incidence of cervical cancer and grade 3 cervical intraepithelial neoplasia by socioeconomic deprivation in England: Population based observational study. BMJ-Brit Med. J. 2024, 385, e77341. [Google Scholar] [CrossRef]
- Hu, S.; Xu, X.; Zhang, Y.; Liu, Y.; Yang, C.; Wang, Y.; Wang, Y.; Yu, Y.; Hong, Y.; Zhang, X.; et al. A nationwide post-marketing survey of knowledge, attitude and practice toward human papillomavirus vaccine in general population: Implications for vaccine roll-out in mainland China. Vaccine 2021, 39, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Farmer, E.; Wu, T.C.; Hung, C.-F. Perspectives for therapeutic HPV vaccine development. J. Biomed. Sci. 2016, 23, 75. [Google Scholar] [CrossRef]
- Nardelli-Haefliger, D.; Wirthner, D.; Schiller, J.T.; Lowy, D.R.; Hildesheim, A.; Ponci, F.; De Grandi, P. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. Jnci-J. Natl. Cancer I. 2003, 95, 1128–1137. [Google Scholar] [CrossRef]
- WHO. Considerations for Human Papillomavirus (HPV) Vaccine Product Choice. Available online: https://www.who.int/publications/i/item/9789240100930 (accessed on 6 December 2024).
- Mac Eochagain, C.; Power, R.; Parker, I.; Brennan, D. HPV vaccination among seropositive, DNA negative cohorts: A systematic review & meta-analysis. J. Gynecol. Oncol. 2022, 33, e24. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Joura, E.A.; Garland, S.M.; Huh, W.K.; Iversen, O.-E.; Kjaer, S.K.; Ferenczy, A.; Kurman, R.J.; Ronnett, B.M.; Stoler, M.H.; et al. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: Comparison with historic placebo population. Gynecol. Oncol. 2019, 154, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Zarchi, M.; Allahqoli, L.; Nehmati, A.; Kashi, A.M.; Taghipour-Zahir, S.; Alkatout, I. Can the prophylactic quadrivalent HPV vaccine be used as a therapeutic agent in women with CIN? A randomized trial. BMC Public Health 2020, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Akhatova, A.; Azizan, A.; Atageldiyeva, K.; Ashimkhanova, A.; Marat, A.; Iztleuov, Y.; Suleimenova, A.; Shamkeeva, S.; Aimagambetova, G. Prophylactic Human Papillomavirus Vaccination: From the Origin to the Current State. Vaccines 2022, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Available online: https://iris.who.int/handle/10665/336583 (accessed on 6 December 2024).
- WHO. One-dose Human Papillomavirus (HPV) Vaccine Offers Solid Protection Against Cervical Cancer. Available online: https://www.who.int/news/item/11-04-2022-one-dose-human-papillomavirus-(hpv)-vaccine-offers-solid-protection-against-cervical-cancer (accessed on 25 September 2024).
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef]
- Chow, E.P.; Phillips, T.R.; Bowesman, H.; Ong, J.J.; Tran, J.; Aung, E.T.; Chen, M.Y.; Fairley, C.K. Human papillomavirus vaccine coverage in male-male partnerships attending a sexual health clinic in Melbourne, Australia. Hum. Vacc. Immunother. 2022, 18, 2068929. [Google Scholar] [CrossRef]
- Pan, X.-F.; Li, R.; Pan, A.; Larson, H. Human papillomavirus vaccine approval in China: A major step forward but challenges ahead. Lancet Infect. Dis. 2016, 16, 1322–1323. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Preferred Product Characteristics for Therapeutic HPV Vaccines. Available online: https://www.who.int/publications/i/item/9789240092174 (accessed on 7 December 2024).
- Rosales, R.; López-Contreras, M.; Rosales, C.; Magallanes-Molina, J.-R.; Gonzalez-Vergara, R.; Arroyo-Cazarez, J.M.; Ricardez-Arenas, A.; del Follo-Valencia, A.; Padilla-Arriaga, S.; Guerrero, M.V.; et al. Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Hum. Gene Ther. 2014, 25, 1035–1049. [Google Scholar] [CrossRef]
- Yang, A.; Jeang, J.; Cheng, K.; Cheng, T.; Yang, B.; Wu, T.-C.; Hung, C.-F. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev. Vaccines 2016, 15, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges, and prospects. Signal Transduct. Tar. 2023, 8, 450. [Google Scholar] [CrossRef]
- Mo, Y.; Ma, J.; Zhang, H.; Shen, J.; Chen, J.; Hong, J.; Xu, Y.; Qian, C. Prophylactic and Therapeutic HPV Vaccines: Current Scenario and Perspectives. Front. Cell Infect. Microbiol. 2022, 12, 909223. [Google Scholar] [CrossRef] [PubMed]
- Hancock, G.; Blight, J.; Lopez-Camacho, C.; Kopycinski, J.; Pocock, M.; Byrne, W.; Price, M.J.; Kemlo, P.; Evans, R.I.; Bloss, A.; et al. A multi-genotype therapeutic human papillomavirus vaccine elicits potent T cell responses to conserved regions of early proteins. Sci. Rep. 2019, 9, 18713. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, C.S.; Kissick, H.T.; Patel, M.R.; Cardenas, M.A.; Prokhnevska, N.; Obeng, R.C.; Nasti, T.H.; Griffith, C.C.; Im, S.J.; Wang, X.; et al. Functional HPV-specific PD-1(+) stem-like CD8 T cells in head and neck cancer. Nature 2021, 597, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Draper, L.M.; Kwong, M.L.M.; Gros, A.; Stevanović, S.; Tran, E.; Kerkar, S.; Raffeld, M.; Rosenberg, S.A.; Hinrichs, C.S. Targeting of HPV-16+ Epithelial Cancer Cells by TCR Gene Engineered T Cells Directed against E6. Clin. Cancer Res. 2015, 21, 4431–4439. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.N.; Iams, W.T.; Park, J.C.; Mita, M.; Holtick, U.; Gordon, M.S.; Rodabaugh, K.J.; Dhani, N.; Neupane, P.; Taylor, M.; et al. Final outcomes analysis of the cell product SQZ-PBMC-HPV Phase 1 trial in incurable HPV16+ solid tumors shows improved overall survival in patients with increased CD8+ T cell tumor infiltration. Mol. Carcinog. 2024, 63, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Lee, J.-W.; Bae, D.-S.; Kang, E.-S.; Cho, D.; Kim, Y.-M.; Kim, K.; Kim, J.-W.; Kim, H.S.; Kim, Y.-T.; et al. Efficacy and safety of BVAC-C in HPV type 16- or 18-positive cervical carcinoma who failed 1st platinum-based chemotherapy: A phase I/IIa study. Front. Immunol. 2024, 15, 1371353. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Palmieri, M.; Zanolini, A.; Ravaggi, A.; Siegel, E.R.; Roman, J.J.; Pecorelli, S.; Cannon, M.J. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: A phase I escalating-dose trial. J. Virol. 2008, 82, 1968–1979. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, S.; Draper, L.M.; Langhan, M.M.; Campbell, T.E.; Kwong, M.L.; Wunderlich, J.R.; Dudley, M.E.; Yang, J.C.; Sherry, R.M.; Kammula, U.S.; et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J. Clin. Oncol. 2015, 33, 1543–1550. [Google Scholar] [CrossRef]
- McCredie, M.R.; Sharples, K.J.; Paul, C.; Baranyai, J.; Medley, G.; Jones, R.W.; Skegg, D.C. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: A retrospective cohort study. Lancet Oncol. 2008, 9, 425–434. [Google Scholar] [CrossRef]
- Tainio, K.; Athanasiou, A.; Tikkinen, K.; Aaltonen, R.; Cárdenas, J.; Glazer-Livson, S.; Jakobsson, M.; Joronen, K.; Kiviharju, M.; Louvanto, K.; et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: Systematic review and meta-analysis. BMJ-Brit. Med. J. 2018, 360, k499. [Google Scholar] [CrossRef]
- Santesso, N.; Mustafa, R.A.; Wiercioch, W.; Kehar, R.; Gandhi, S.; Chen, Y.; Cheung, A.; Hopkins, J.; Khatib, R.; Bin Ma, B.; et al. Systematic reviews and meta-analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. Int. J. Gynecol. Obstet. 2016, 132, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Kyrgiou, M.; Athanasiou, A.; Paraskevaidi, M.; Mitra, A.; Kalliala, I.; Martin-Hirsch, P.; Arbyn, M.; Bennett, P.; Paraskevaidis, E. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: Systematic review and meta-analysis. BMJ-Brit. Med. J. 2016, 354, i3633. [Google Scholar] [CrossRef] [PubMed]
- Reuschenbach, M.; Valente, S.; Takyar, J.; Dhawan, A.; Hall, A.; Agrawal, N.; Ghelardi, A.; del Pino, M.; Nowakowski, A.; Sabale, U. Treatment characteristics, HPV genotype distribution and risk of subsequent disease among women with high-grade cervical intraepithelial neoplasia in Europe: A systematic literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 300, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Alouini, S.; Pichon, C. Therapeutic Vaccines for HPV-Associated Cervical Malignancies: A Systematic Review. Vaccines 2024, 12, 428. [Google Scholar] [CrossRef] [PubMed]
- Genticel Reports Additional Results at 12 Months From Phase 2 Trial of HPV Immunotherapeutic Candidate, GTL001. Available online: https://www.globenewswire.com/news-release/2016/04/20/830923/35562/en/Genticel-Reports-Additional-Results-at-12-Months-From-Phase-2-Trial-of-HPV-Immunotherapeutic-Candidate-GTL001.html (accessed on 7 December 2024).
- Einstein, M.H.; Roden, R.B.; Ferrall, L.; Akin, M.; Blomer, A.; Wu, T.-C.; Chang, Y.-N. Safety Run-in of Intramuscular pNGVL4a-Sig/E7(detox)/HSP70 DNA and TA-CIN Protein Vaccination as Treatment for HPV16+ ASC-US, ASC-H, or LSIL/CIN1. Cancer Prev. Res. 2023, 16, 219–227. [Google Scholar] [CrossRef]
- Thuijs, N.B.; van Beurden, M.; Bruggink, A.H.; Steenbergen, R.D.M.; Berkhof, J.; Bleeker, M.C.G. Vulvar intraepithelial neoplasia: Incidence and long-term risk of vulvar squamous cell carcinoma. Int. J. Cancer 2021, 148, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.J.; Van Der Burg, S.H.; Boswell, C.M.; Offringa, R.; Hickling, J.K.; Dobson, J.; Roberts, J.S.C.; A Latimer, J.; Moseley, R.P.; Coleman, N.; et al. Vaccinia-expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin. Cancer Res. 2003, 9, 5205–5213. [Google Scholar] [PubMed]
- INOVIO’s VGX-3100 Demonstrates Positive Phase 2 Efficacy In Treatment of Precancerous Vulvar Dysplasia Caused by HPV-16/18. Available online: https://ir.inovio.com/news-releases/news-releases-details/2021/INOVIOs-VGX-3100-Demonstrates-Positive-Phase-2-Efficacy-In-Treatment-of-Precancerous-Vulvar-Dysplasia-Caused-by-HPV-1618/default.aspx (accessed on 7 December 2024).
- Harper, D.M.; Nieminen, P.; Donders, G.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Stoler, M.H.; Glavini, K.; Attley, G.; Limacher, J.-M.; et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: Randomized controlled phase II trial with 2.5 years of follow-up. Gynecol. Oncol. 2019, 153, 521–529. [Google Scholar] [CrossRef]
- INOVIO Announces Positive Results from REVEAL 1, a Phase 3 Pivotal Trial Evaluating VGX-3100, its DNA-based HPV Immunotherapy for the Treatment of High-grade Precancerous Cervical Dysplasia Caused by HPV-16 and/or HPV-18. Available online: https://ir.inovio.com/news-releases/news-releases-details/2021/INOVIO-Announces-Positive-Results-from-REVEAL-1-a-Phase-3-Pivotal-Trial-Evaluating-VGX-3100-its-DNA-based-HPV-Immunotherapy-for-the-Treatment-of-High-grade-Precancerous-Cervical-Dysplasia-Caused-by-HPV-16-andor-HPV-18/default.aspx (accessed on 25 September 2024).
- INOVIO Reports Fourth Quarter and Full Year 2022 Financial Results and Clinical Highlights. Available online: https://ir.inovio.com/news-releases/news-releases-details/2023/INOVIO-Reports-Fourth-Quarter-and-Full-Year-2022-Financial-Results-and-Clinical-Highlights/default.aspx (accessed on 7 December 2024).
- Bhuyan, P.K.; Dallas, M.; Kraynyak, K.; Herring, T.; Morrow, M.; Boyer, J.; Duff, S.; Kim, J.; Weiner, D.B. Durability of response to VGX-3100 treatment of HPV16/18 positive cervical HSIL. Hum. Vaccines Immunother. 2021, 17, 1288–1293. [Google Scholar] [CrossRef]
- SGN-00101 Vaccine in Treating Human Papillomavirus in Patients Who Have Abnormal Cervical Cells. Available online: https://clinicaltrials.gov/study/NCT00091130?term=NCT00091130&rank=1 (accessed on 25 September 2024).
- Study of Treatment for HPV16+ ASC-US or LSIL (PVX-6). Available online: https://clinicaltrials.gov/study/NCT03913117 (accessed on 7 December 2024).
- An Assessment of an Attenuated Live Listeria Vaccine in CIN 2+ (ADXS11-001). Available online: https://www.clinicaltrials.gov/study/NCT01116245?term=ADXS11-001&rank=7&tab=results (accessed on 7 December 2024).
- Hillemanns, P.; Denecke, A.; Woelber, L.; Böhmer, G.; Jentschke, M.; Schjetne, K.W.; Slot, K.M.B.; Fredriksen, A.B. A Therapeutic Antigen-Presenting Cell-Targeting DNA Vaccine VB10.16 in HPV16-Positive High-Grade Cervical Intraepithelial Neoplasia: Results from a Phase I/IIa Trial. Clin. Cancer Res. 2022, 28, 4885–4892. [Google Scholar] [CrossRef] [PubMed]
- Einstein, M.H.; Kadish, A.S.; Burk, R.D.; Kim, M.Y.; Wadler, S.; Streicher, H.; Goldberg, G.L.; Runowicz, C.D. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecol. Oncol. 2007, 106, 453–460. [Google Scholar] [CrossRef] [PubMed]
- A Clinical Trial of PepCan to Two Therapy Arms for Treating Cervical High-Grade Squamous Intraepithelial Lesions. Available online: https://clinicaltrials.gov/study/NCT02481414?term=NCT02481414.&rank=1&tab=results#outcome-measures (accessed on 7 December 2024).
- Nakagawa, M.; Evans, T.; Bimali, M.; Coleman, H.; Crane, J.; Darwish, N.; Lu, Y.-C.; Nookaew, I.; Marsh, K.; Robeson, M.S.; et al. Immune responses in a phase 2 clinical trial of peptide-based therapeutic human papillomavirus vaccine, PepCan, versus Candida adjuvant alone in treating cervical intraepithelial neoplasia 2/3. J. Clin. Oncol. 2024, 42, 2634. [Google Scholar] [CrossRef]
- Choi, Y.J.; Hur, S.Y.; Kim, T.-J.; Hong, S.R.; Lee, J.K.; Cho, C.-H.; Park, K.S.; Woo, J.W.; Sung, Y.C.; Suh, Y.S.; et al. A Phase II, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clin. Cancer Res. 2020, 26, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Safety and Efficacy of GX-188E DNA Therapeutic Vaccine Administered by Electroporation After Observation (GX-188E). Available online: https://clinicaltrials.gov/study/NCT02411019?term=NCT02411019&rank=1 (accessed on 7 December 2024).
- Phase 2 Clinical Trial to Evaluate the Safety and Efficacy of Plasmid DNA Therapeutic Vaccine(GX-188E). Available online: https://clinicaltrials.gov/study/NCT02596243 (accessed on 7 December 2024).
- Safety and Efficacy of GX-188E Administered Via EP Plus GX-I7 or Imiquimod. Available online: https://clinicaltrials.gov/study/NCT03206138 (accessed on 7 December 2024).
- Vvax001 Cancer Vaccine in Premalignant Cervical Lesions—Phase II (Vvax). Available online: https://clinicaltrials.gov/study/NCT06015854 (accessed on 7 December 2024).
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef]
- Huh, W.K.; Brady, W.E.; Fracasso, P.M.; Dizon, D.S.; Powell, M.A.; Monk, B.J.; Leath, C.A.; Landrum, L.M.; Tanner, E.J.; Crane, E.K.; et al. Phase II study of axalimogene filolisbac (ADXS-HPV) for platinum-refractory cervical carcinoma: An NRG oncology/gynecologic oncology group study. Gynecol. Oncol. 2020, 158, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Nagarsheth, N.B.; Norberg, S.M.; Sinkoe, A.L.; Adhikary, S.; Meyer, T.J.; Lack, J.B.; Warner, A.C.; Schweitzer, C.; Doran, S.L.; Korrapati, S.; et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 2021, 27, 419–425. [Google Scholar] [CrossRef]
- Ramakrishnan, R.; Assudani, D.; Nagaraj, S.; Hunter, T.; Cho, H.-I.; Antonia, S.; Altiok, S.; Celis, E.; Gabrilovich, D.I. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J. Clin. Investg. 2010, 120, 1111–1124. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kang, T.H.; Knoff, J.; Huang, Z.; Soong, R.-S.; Alvarez, R.D.; Hung, C.-F.; Wu, T.-C. Intratumoral injection of therapeutic HPV vaccinia vaccine following cisplatin enhances HPV-specific antitumor effects. Cancer Immunol. Immunother. 2013, 62, 1175–1185. [Google Scholar] [CrossRef]
- Basu, P.; Mehta, A.; Jain, M.; Gupta, S.; Nagarkar, R.V.; John, S.; Petit, R. A Randomized Phase 2 Study of ADXS11-001 Listeria monocytogenes-Listeriolysin O Immunotherapy With or Without Cisplatin in Treatment of Advanced Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 764–772. [Google Scholar] [CrossRef]
- Welters, M.J.; van der Sluis, T.C.; van Meir, H.; Loof, N.M.; van Ham, V.J.; van Duikeren, S.; Santegoets, S.J.; Arens, R.; de Kam, M.L.; Cohen, A.F.; et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci. Transl. Med. 2016, 8, 334ra52. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-M.; Shin, K.-S.; Koh, C.-H.; Song, B.; Jeon, I.; Park, M.H.; Kim, B.-S.; Chung, Y.; Kang, C.-Y. Inhibition of topoisomerase I shapes antitumor immunity through the induction of monocyte-derived dendritic cells. Cancer Lett. 2021, 520, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Trial To Test Safety And Efficacy Of Vaccination For Incurable HPV 16-Related Oropharyngeal, Cervical And Anal Cancer. Available online: https://clinicaltrials.gov/study/NCT02865135?term=NCT02865135&rank=1&tab=results (accessed on 7 December 2024).
- Melief, C.J.M.; Welters, M.J.P.; Vergote, I.; Kroep, J.R.; Kenter, G.G.; Ottevanger, P.B.; Tjalma, W.A.A.; Denys, H.; van Poelgeest, M.I.E.; Nijman, H.W.; et al. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci. Transl. Med. 2020, 12, eaaz8235. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Sato, H.; Multhoff, G.; Shibata, A. Novel Approaches to Improve the Efficacy of Immuno-Radiotherapy. Front. Oncol. 2019, 9, 156. [Google Scholar] [CrossRef]
- Yoshida-Court, K.; Gjyshi, O.; O’hara, M.; Lin, L.; Jhingran, A.; Joyner, M.; Napravnik, T.C.; Lynn, E.; Colbert, L.; Sastry, J.K.; et al. 674 IMMUNOCERV, an ongoing phase II trial combining PDS0101, an HPV-specific T cell immunotherapy, with chemotherapy and radiation for treatment of locally advanced cervical cancers. J. Immunother. Cancer 2022, 10, A704. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol.-Mech. 2021, 16, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.W.; Hur, S.-Y.; Woo, J.W.; Kim, Y.-M.; Lim, M.C.; Park, S.Y.; Seo, S.S.; No, J.H.; Kim, B.-G.; Lee, J.-K.; et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: Interim results of a single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.C.; Choi, Y.J.; Hur, S.-Y.; Kim, Y.-M.; No, J.H.; Kim, B.-G.; Cho, C.H.; Kim, S.H.; Jeong, D.H.; Lee, J.-K.; et al. GX-188E DNA vaccine plus pembrolizumab in HPV 16- and/or 18-positive recurrent or advance cervical cancer: A phase 2 trial. Eclinicalmedicine 2024, 74, 102716. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Andre, F.; Blay, J.-Y.; Bustillos, A.; Fear, S.; Ganta, S.; Jaeger, D.; Maio, M.; Mileshkin, L.; Melero, I. Phase II multicohort study of atezolizumab monotherapy in multiple advanced solid cancers. ESMO Open 2022, 7, 100419. [Google Scholar] [CrossRef]
- Nykode Therapeutics Announces Positive Interim Results From its Phase 2 Trial with VB10.16 in Combination with Immune Checkpoint Inhibitor Atezolizumab in Advanced Cervical Cancer. Available online: https://www.globenewswire.com/news-release/2022/05/09/2438179/0/en/Nykode-Therapeutics-announces-positive-interim-results-from-its-Phase-2-trial-with-VB10-16-in-combination-with-immune-checkpoint-inhibitor-atezolizumab-in-advanced-cervical-cancer.html (accessed on 7 December 2024).
- Tewari, K.S.; Monk, B.J.; Vergote, I.; Miller, A.; de Melo, A.C.; Kim, H.-S.; Kim, Y.M.; Lisyanskaya, A.; Samouëlian, V.; Lorusso, D.; et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N. Engl. J. Med. 2022, 386, 544–555. [Google Scholar] [CrossRef]
- Lorusso, D.; Oaknin, A.; Borges, G.S.; Damian, F.; Ottevanger, P.B.; Van Gorp, T.; Paiva, C.E.; Kroep, J.R.; Kim, Y.M.; Kim, H.S.; et al. Combination of cemiplimab and ISA101b vaccine for the treatment of recurrent/metastatic HPV16 cervical cancer. J. Clin. Oncol. 2024, 42, 5522. [Google Scholar] [CrossRef]
- Choi, C.H.; Kim, B.-G.; Lee, J.-W.; Kim, T.-J.; Lee, Y.-Y.; Cho, D.; Park, B.-K.H.; Song, S.Y.; Kim, D.-Y.; Kim, K.; et al. An open label, single arm phase II, multicenter trial of durvalumab and BVAC-C, in patients with HPV 16- or 18-positive cervical cancer and failure of first-line platinum-based chemotherapy (DURBAC). J. Clin. Oncol. 2024, 42, 5513. [Google Scholar] [CrossRef]
- Surgery and Vaccine Therapy in Treating Patients With Early Cervical Cancer. Available online: https://clinicaltrials.gov/study/NCT00002916?term=NCT00002916&rank=1 (accessed on 7 December 2024).
- E7 TCR T Cell Induction Immunotherapy for Stage IIB-IVA Cervical Cancer. Available online: https://clinicaltrials.gov/study/NCT04476251?term=NCT04476251&rank=1&tab=results (accessed on 7 December 2024).
- Floudas, C.S.; Strauss, J.; Redman, J.M.; Pastor, D.M.; Turkbey, E.B.; Donahue, R.N.; Jochems, C.; McMahon, S.; Lamping, E.; Cordes, L.M.; et al. Phase I evaluation of PRGN-2009 alone and in combination with bintrafusp alfa in patients (pts) with recurrent/metastatic (R/M) HPV-associated cancers (HPV-C). J. Clin. Oncol. 2023, 41, 2628. [Google Scholar] [CrossRef]
- PRGN-2009 in Combination With Pembrolizumab Versus Pembrolizumab in Patients With Recurrent or Metastatic Cervical Cancer. Available online: https://clinicaltrials.gov/study/NCT06157151?term=NCT06157151&rank=1 (accessed on 7 December 2024).
- This is a Trial of MG1-E6E7 With Ad-E6E7 and Atezolizumab in Patients With HPV Associated Cancers (Kingfisher). Available online: https://clinicaltrials.gov/study/NCT03618953?term=NCT03618953&rank=1 (accessed on 7 December 2024).
- Hasan, Y.; Furtado, L.; Tergas, A.; Lee, N.; Brooks, R.; McCall, A.; Golden, D.; Jolly, S.; Fleming, G.; Morrow, M.; et al. A Phase 1 Trial Assessing the Safety and Tolerability of a Therapeutic DNA Vaccination Against HPV16 and HPV18 E6/E7 Oncogenes After Chemoradiation for Cervical Cancer. Int. J. Radiat. Oncol. 2020, 107, 487–498. [Google Scholar] [CrossRef]
- Borcoman, E.; Lalanne, A.; Delord, J.-P.; Cassier, P.A.; Rolland, F.; Salas, S.; Limacher, J.-M.; Capitain, O.; Lantz, O.; Ekwegbara, C.; et al. Phase Ib/II trial of tipapkinogene sovacivec, a therapeutic human papillomavirus16-vaccine, in combination with avelumab in patients with advanced human papillomavirus16-positive cancers. Eur. J. Cancer 2023, 191, 112981. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, L.G.; Rajapakshe, K.; Canales, J.R.; Chin, R.L.; Feng, L.; Wang, Q.; Barrese, T.Z.; Massarelli, E.; William, W.; Johnson, F.M.; et al. ISA101 and nivolumab for HPV-16(+) cancer: Updated clinical efficacy and immune correlates of response. J. Immunother. Cancer 2022, 10, e004232. [Google Scholar] [CrossRef] [PubMed]
- Efficacy and Safety of VB10.16 Alone or in Combination With Atezolizumab in Patients With Advanced Cervical Cancer. Available online: https://clinicaltrials.gov/study/NCT06099418?term=NCT06099418&rank=1 (accessed on 7 December 2024).
- HARE-40: HPV Anti-CD40 RNA vaccinE (HARE-40). Available online: https://clinicaltrials.gov/study/NCT03418480?term=NCT03418480&rank=1 (accessed on 7 December 2024).
- Axalimogene Filolisbac (ADXS11-001) High Dose in Women With Human Papillomavirus (HPV) + Cervical Cancer. Available online: https://clinicaltrials.gov/study/NCT02164461?term=NCT02164461&rank=1 (accessed on 7 December 2024).
- PDS Biotech Reports Median Overall Survival (OS) of 21 Months in Advanced, Refractory Cancer Patients Having Few Remaining Treatment Options and with Reported Historical Survival of 3-4 months. Available online: https://www.pdsbiotech.com/index.php/investors/news-center/press-releases/press-releases1/118-2022-news/758-pds-biotech-reports-median-overall-survival-os-of-21-months-in-advanced-refractory-cancer-patients-having-few-remaining-treatment-options-and-with-reported-historical-survival-of-3-4-months (accessed on 7 December 2024).
- Morris, V.K.; Jazaeri, A.; Westin, S.N.; Pettaway, C.; George, S.; Huey, R.W.; Grinsfelder, M.; Shafer, A.; Johnson, B.; Vining, D.; et al. Phase II Trial of MEDI0457 and Durvalumab for Patients With Recurrent/Metastatic Human Papillomavirus-Associated Cancers. Oncologist 2023, 28, 618–623. [Google Scholar] [CrossRef]
- Lundstrom, K. Replicon RNA Viral Vectors as Vaccines. Vaccines 2016, 4, 39. [Google Scholar] [CrossRef]
- Komdeur, F.L.; Singh, A.; van de Wall, S.; Meulenberg, J.J.M.; Boerma, A.; Hoogeboom, B.N.; Paijens, S.T.; Oyarce, C.; de Bruyn, M.; Schuuring, E.; et al. First-in-Human Phase I Clinical Trial of an SFV-Based RNA Replicon Cancer Vaccine against HPV-Induced Cancers. Mol. Ther. 2021, 29, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Safety and Feasibility of TA-CIN Vaccine in HPV16 Associated Cervical Cancer. Available online: https://clinicaltrials.gov/study/NCT02405221 (accessed on 7 December 2024).
- Ramos da Silva, J.; Bitencourt Rodrigues, K.; Formoso Pelegrin, G.; Silva Sales, N.; Muramatsu, H.; de Oliveira Silva, M.; Porchia, B.F.M.M.; Moreno, A.C.R.; Aps, L.R.M.M.; de Souza Ferreira, L.C.; et al. Single immunizations of self-amplifying or non-replicating mRNA-LNP vaccines control HPV-associated tumors in mice. Sci. Transl. Med. 2023, 15, eabn3464. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Y.; Peng, S.; Han, L.; Qiu, J.; Song, L.; Tsai, Y.; Yang, B.; Roden, R.B.; Trimble, C.L.; Hung, C.-F.; et al. Local HPV Recombinant Vaccinia Boost Following Priming with an HPV DNA Vaccine Enhances Local HPV-Specific CD8+ T-cell-Mediated Tumor Control in the Genital Tract. Clin. Cancer Res. 2016, 22, 657–669. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Li, X.; Li, J.; Zhao, C.; Zhao, Y.; Zhang, X.; He, P.; Wu, X.; Jiang, S.; et al. Lipid Nanoparticles Outperform Electroporation in Delivering Therapeutic HPV DNA Vaccines. Vaccines 2024, 12, 666. [Google Scholar] [CrossRef]
| Vaccine Types | Representative Vaccines | Advantages | Disadvantages |
|---|---|---|---|
| DNA vaccine | - VB10.16 | - Simple production. | - Low transfection efficiency. |
| - GX-188E | - Repeatable vaccination. | - Low immunogenicity. | |
| - VGX-3100 | - Low cost. | - Risk of autoimmune reactions. | |
| - NO-3112(MEDI0457) | - Good stability. | - Risk of integration with the human genome. | |
| - Long-lasting immune response. | |||
| -Multiple antigens can be delivered. | |||
| RNA vaccine | - HARE-40 | - No risk of integration into host genome. | - Poor stability. |
| - Quick degradation. | |||
| - Quick to develop, easy to modify. - High immunogenicity. | - May cause inflammatory reactions. | ||
| Peptide vaccine | - DPX-E7 | - High specificity and safety | - Complex manufacture. |
| - PepCan | - Low risk of autoimmunity. | - High cost. | |
| - ISA101/ISA101b | - Safe and tolerable. | - Limitation of MHC. | |
| - PDS0101 | - Low immunogenicity. | ||
| Protein vaccine | - SGN-00101 | - Safe and tolerable. | - Low immunogenicity. |
| - TA-CIN | - No limitation of MHC. | - Easier to mediate humoral immunity. | |
| Viral and bacterial vector vaccine | - PRGN-2009 | - High transfection efficiency. | - Potentially pathogenic. |
| - MG1-E6E7 | - High immunogenicity. | - Immune response to vectors may exceed HPV antigens. | |
| -ADXS11-001 | - Long immune response times. | ||
| - TG4001 | - Intrinsic adjuvant properties. | - Antibodies against the vectors may be pre-existing. | |
| - Vvax001 | |||
| - Neutralizing antibodies produced limit repeat treatments. | |||
| Cell-based vaccine | - HPV-TIL | - Strong immune stimulation. | - High cost. |
| - E7 TCR T Cells | - Multi-form antigen loading. | - Highly demanding cell culture technology. | |
| - SQZ-PBMC-HPV | |||
| - BVAC-C | - Need for patient-specific customization. |
| Feature | Prophylactic HPV Vaccine | Therapeutic HPV Vaccine |
|---|---|---|
| Indications | Prevention of new HPV infections | Clearance of existing HPV infections |
| Main Antigenic Epitopes | L1, L2 capsid proteins | E6, E7 oncogenic protein epitopes |
| Type of Immunity | Humoral immunity | Cellular immunity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; He, M.; Mao, Z.; Huang, Y.; Li, X.; Long, L.; Guo, M.; Zou, D. Advancing the Fight Against Cervical Cancer: The Promise of Therapeutic HPV Vaccines. Vaccines 2025, 13, 92. https://doi.org/10.3390/vaccines13010092
Zheng Q, He M, Mao Z, Huang Y, Li X, Long L, Guo M, Zou D. Advancing the Fight Against Cervical Cancer: The Promise of Therapeutic HPV Vaccines. Vaccines. 2025; 13(1):92. https://doi.org/10.3390/vaccines13010092
Chicago/Turabian StyleZheng, Qian, Misi He, Zejia Mao, Yue Huang, Xiuying Li, Ling Long, Mingfang Guo, and Dongling Zou. 2025. "Advancing the Fight Against Cervical Cancer: The Promise of Therapeutic HPV Vaccines" Vaccines 13, no. 1: 92. https://doi.org/10.3390/vaccines13010092
APA StyleZheng, Q., He, M., Mao, Z., Huang, Y., Li, X., Long, L., Guo, M., & Zou, D. (2025). Advancing the Fight Against Cervical Cancer: The Promise of Therapeutic HPV Vaccines. Vaccines, 13(1), 92. https://doi.org/10.3390/vaccines13010092







