Human Papillomavirus Vaccination Coverage Estimates Among the Primary Target Cohort (9–14-Year-Old Girls) in the World (2010–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Study Variables and Measures
2.4. Statistical Analysis
2.5. Ethical Considerations
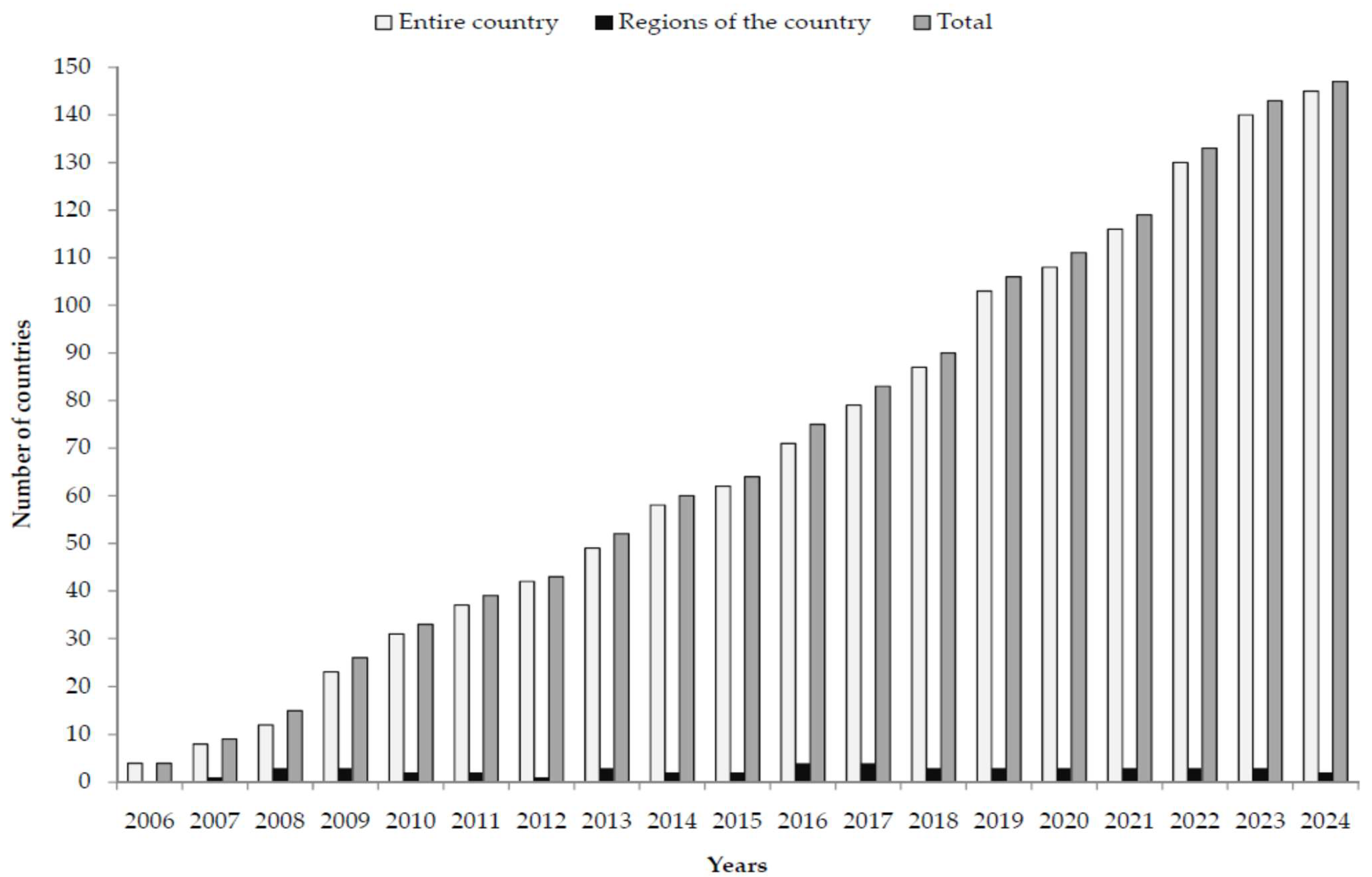
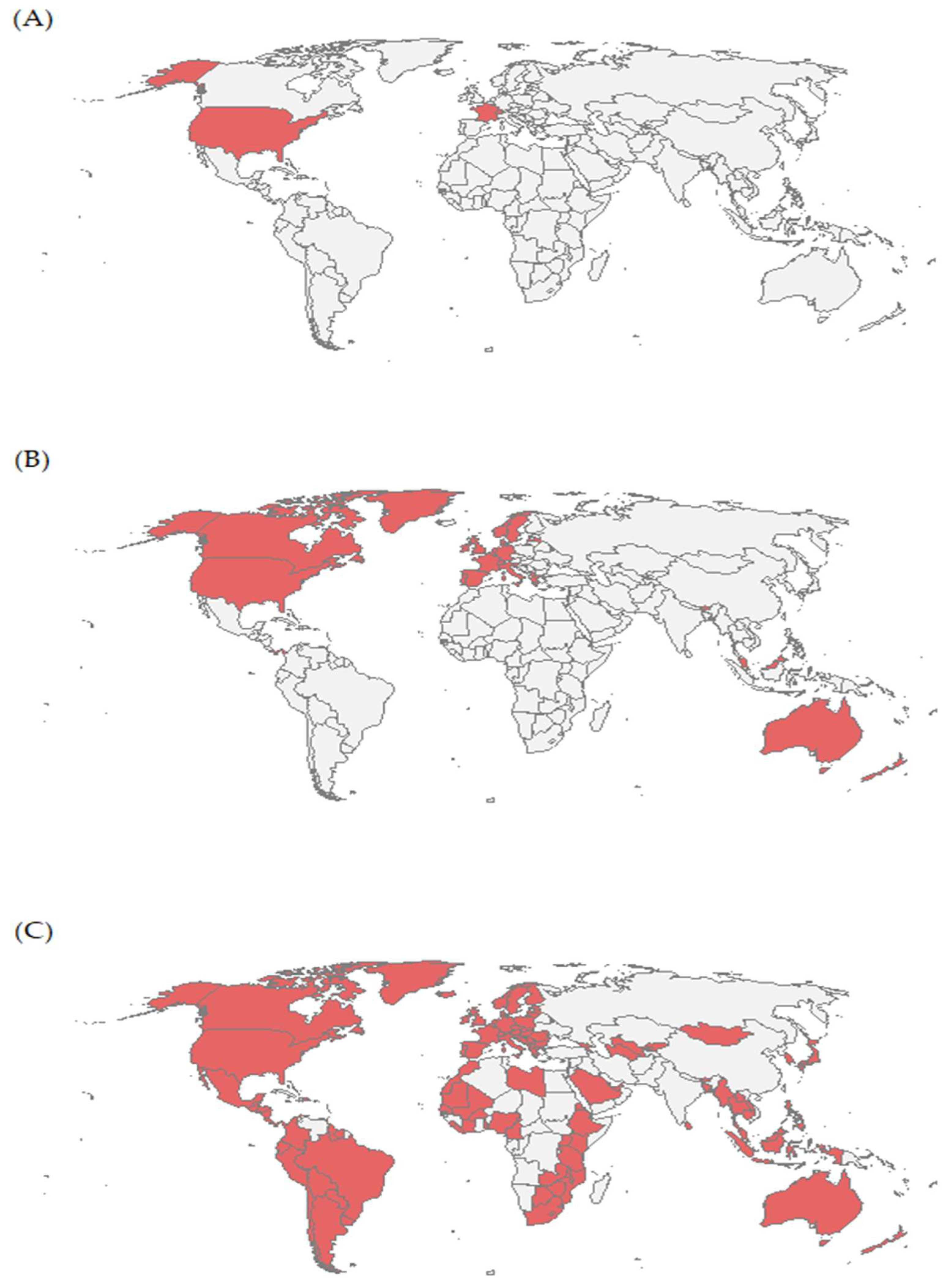
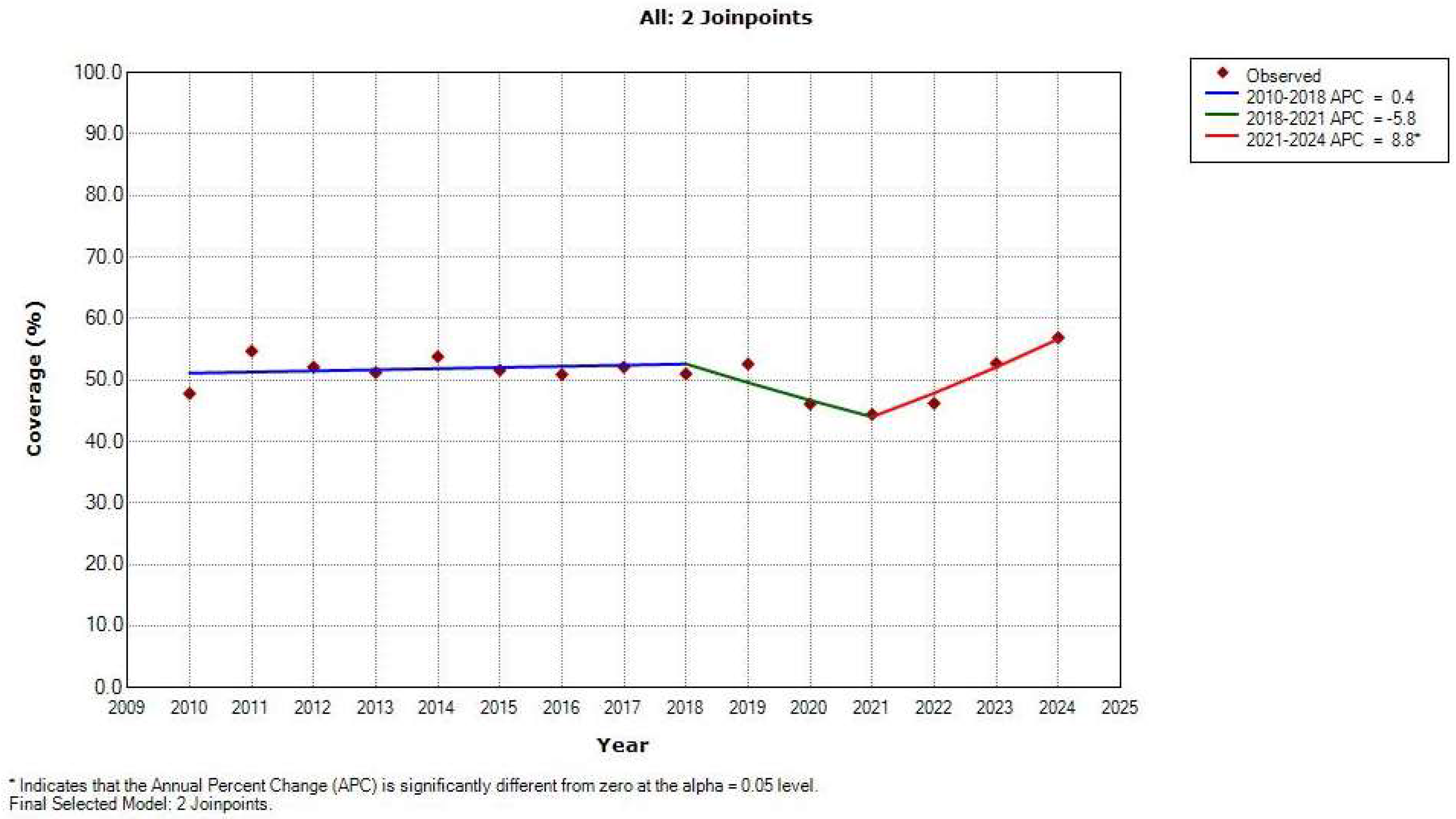
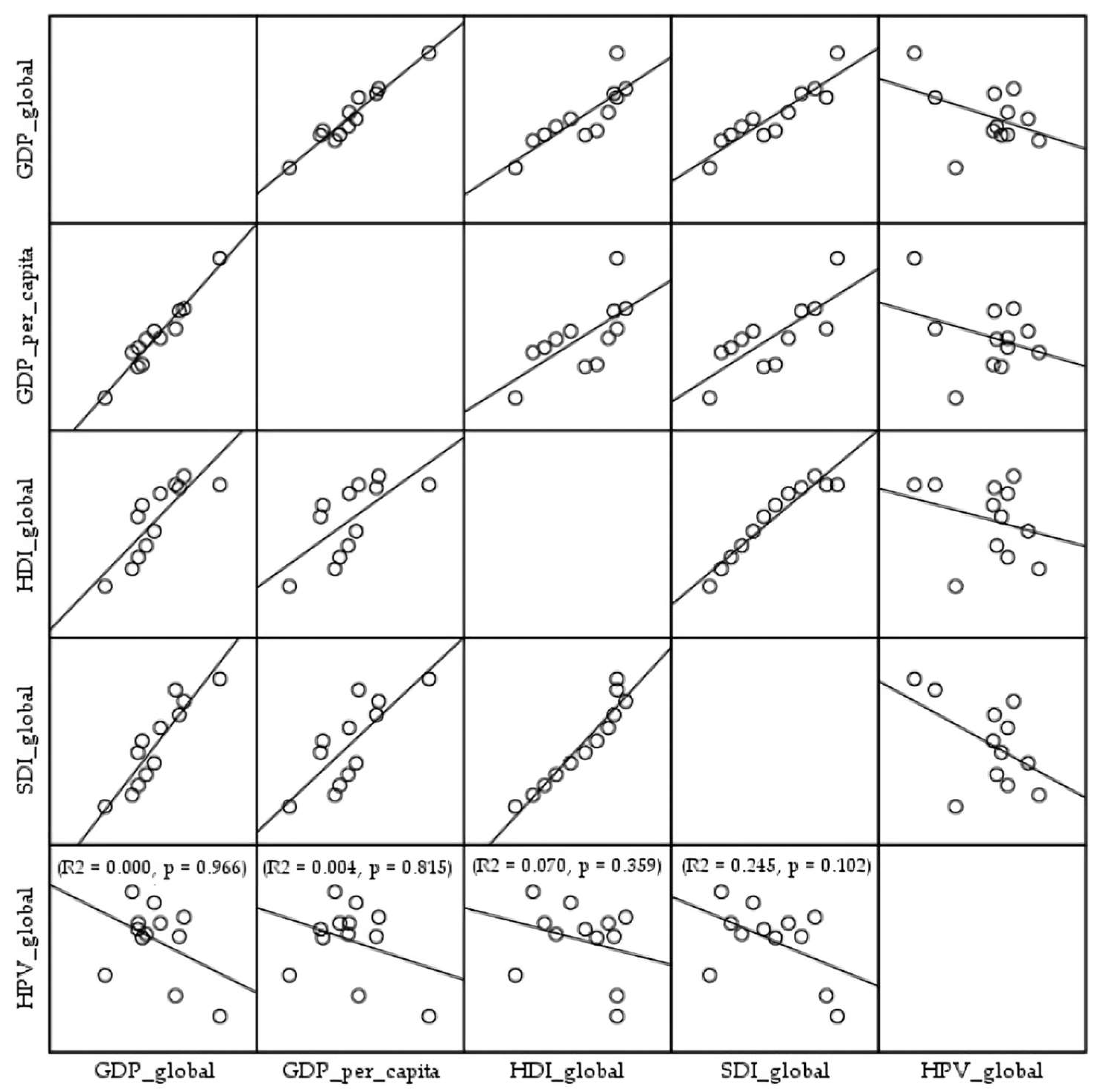
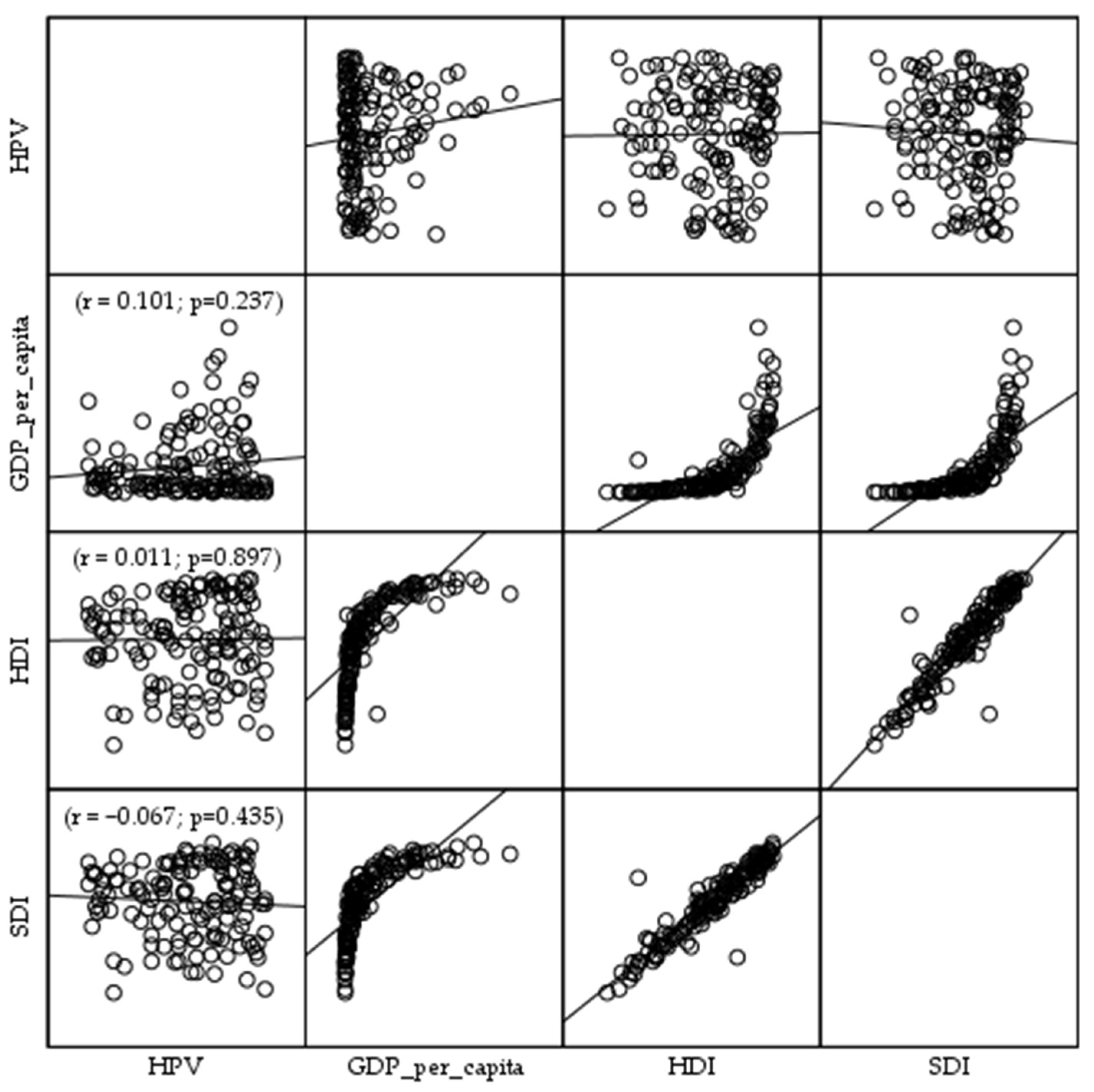
3. Results
4. Discussion
The Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F.; et al. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.fr/today (accessed on 2 July 2025).
- Singh, G.K.; Azuine, R.E.; Siahpush, M. Global inequalities in cervical cancer incidence and mortality are linked to deprivation, low socioeconomic status, and human development. Int. J. MCH AIDS 2012, 1, 17–30. [Google Scholar] [CrossRef]
- Znaor, A.; Ryzhov, A.; Losada, M.L.; Carvalho, A.; Smelov, V.; Barchuk, A.; Valkov, M.; Ten, E.; Andreasyan, D.; Zhizhilashvili, S.; et al. Breast and cervical cancer screening practices in nine countries of Eastern Europe and Central Asia: A population-based survey. J. Cancer Policy 2023, 38, 100436. [Google Scholar] [CrossRef]
- Lemp, J.M.; De Neve, J.W.; Bussmann, H.; Chen, S.; Manne-Goehler, J.; Theilmann, M.; Marcus, M.E.; Ebert, C.; Probst, C.; Tsabedze-Sibanyoni, L.; et al. Lifetime Prevalence of Cervical Cancer Screening in 55 Low- and Middle-Income Countries. JAMA 2020, 324, 1532–1542. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Priyadarshini, S.; Swain, P.K.; Agarwal, K.; Jena, D.; Padhee, S. Trends in gynecological cancer incidence, mortality, and survival among elderly women: A SEER study. Aging Med. 2024, 7, 179–188. [Google Scholar] [CrossRef]
- Pettersson, B.F.; Hellman, K.; Vaziri, R.; Andersson, S.; Hellström, A.C. Cervical cancer in the screening era: Who fell victim in spite of successful screening programs? J. Gynecol. Oncol. 2011, 22, 76–82. [Google Scholar] [CrossRef]
- Piechocki, M.; Koziołek, W.; Sroka, D.; Matrejek, A.; Miziołek, P.; Saiuk, N.; Sledzik, M.; Jaworska, A.; Bereza, K.; Pluta, E.; et al. Trends in Incidence and Mortality of Gynecological and Breast Cancers in Poland (1980–2018). Clin. Epidemiol. 2022, 14, 95–114. [Google Scholar] [CrossRef]
- Qiu, H.; Cao, S.; Xu, R. Cancer incidence, mortality, and burden in China: A time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun. 2021, 41, 1037–1048. [Google Scholar] [CrossRef]
- Yao, H.; Yan, C.; Qiumin, H.; Li, Z.; Jiao, A.; Xin, L.; Hong, L. Epidemiological Trends and Attributable Risk Burden of Cervical Cancer: An Observational Study from 1990 to 2019. Int. J. Clin. Pract. 2022, 2022, 3356431. [Google Scholar] [CrossRef]
- Benard, V.B.; Castle, P.E.; Jenison, S.A.; Hunt, W.C.; Kim, J.J.; Cuzick, J.; Lee, J.H.; Du, R.; Robertson, M.; Norville, S.; et al. Population-Based Incidence Rates of Cervical Intraepithelial Neoplasia in the Human Papillomavirus Vaccine Era. JAMA Oncol. 2017, 3, 833–837. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation (IHME). GBD Results; IHME, University of Washington: Seattle, WA, USA, 2024; Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 27 July 2025).
- World Health Organization. WHO Guidance Note: Comprehensive Cervical Cancer Prevention and Control: A Healthier Future for Girls and Women; WHO Press, World Health Organization: Geneva, Switzerland, 2013; Available online: https://www.who.int/publications/i/item/9789241505147 (accessed on 27 July 2025).
- Descamps, P.; Dixon, S.; Bosch Jose, F.X.; Kyrgiou, M.; Monsonego, J.; Neisingh, O.; Nguyen, L.; O’Connor, M.; Smith, J.S. Turning the tide-Recommendations to increase cervical cancer screening among women who are underscreened. Int. J. Gynaecol. Obstet. 2024, 166 (Suppl. S1), 3–21. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Dunne, E.F.; Saraiya, M.; Lawson, H.W.; Chesson, H.; Unger, E.R.; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP). Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2007, 56, 1–24. [Google Scholar]
- Markowitz, L.E.; Gee, J.; Chesson, H.; Stokley, S. Ten Years of Human Papillomavirus Vaccination in the United States. Acad. Pediatr. 2018, 18, S3–S10. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; WHO Press, World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240014107/ (accessed on 27 July 2025).
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development, 21 October 2015, A/RES/70/1. Available online: http://www.refworld.org/docid/57b6e3e44.html (accessed on 29 July 2025).
- Casey, R.M.; Akaba, H.; Hyde, T.B.; Bloem, P. COVID-19 pandemic and equity of global human papillomavirus vaccination: Descriptive study of World Health Organization-Unicef vaccination coverage estimates. BMJ Med. 2024, 3, e000726. [Google Scholar] [CrossRef]
- Pedersen, B.T.; Pedersen, H.; Serizawa, R.; Sonne, S.B.; Andreasen, E.K.; Bonde, J. Cervical cancer screening activity in the Capital Region of Denmark before, during and after the COVID-19 pandemic. Prev. Med. 2024, 180, 107888. [Google Scholar] [CrossRef]
- Ferreira, H.N.C.; Capistrano, G.N.; Morais, T.N.B.; Costa, K.T.D.S.; Quirino, A.L.S.; Costa, R.L.P.D.; Andrade, F.B. Screening and hospitalization of breast and cervical cancer in Brazil from 2010 to 2022: A time-series study. PLoS ONE 2023, 18, e0278011. [Google Scholar] [CrossRef]
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L.; Herrero, R.; Bray, F.; Bosch, F.X.; de Sanjosé, S.; Castellsagué, X. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef]
- Võrno, T.; Lutsar, K.; Uusküla, A.; Padrik, L.; Raud, T.; Reile, R.; Nahkur, O.; Kiivet, R.A. Cost-effectiveness of HPV vaccination in the context of high cervical cancer incidence and low screening coverage. Vaccine 2017, 35, 6329–6335. [Google Scholar] [CrossRef]
- Tobaiqy, M.; MacLure, K. A Systematic Review of Human Papillomavirus Vaccination Challenges and Strategies to Enhance Uptake. Vaccines 2024, 12, 746. [Google Scholar] [CrossRef]
- World Health Organization. Immunizing Against HPV; WHO Press, World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/activities/immunizing-against-hpv/ (accessed on 27 July 2025).
- Burton, A.; Monasch, R.; Lautenbach, B.; Gacic-Dobo, M.; Neill, M.; Karimov, R.; Wolfson, L.; Jones, G.; Birmingham, M. WHO and UNICEF estimates of national infant immunization coverage: Methods and processes. Bull. World Health Organ. 2009, 87, 535–541. [Google Scholar] [CrossRef]
- Rau, C.; Lüdecke, D.; Dumolard, L.B.; Grevendonk, J.; Wiernik, B.M.; Kobbe, R.; Gacic-Dobo, M.; Danovaro-Holliday, M.C. Data quality of reported child immunization coverage in 194 countries between 2000 and 2019. PLoS Glob. Public Health 2022, 2, e0000140. [Google Scholar] [CrossRef]
- Burton, A.; Kowalski, R.; Gacic-Dobo, M.; Karimov, R.; Brown, D. A formal representation of the WHO and UNICEF estimates of national immunization coverage: A computational logic approach. PLoS ONE 2012, 7, e47806. [Google Scholar] [CrossRef]
- Immunization and Vaccine related Implementation Research Advisory Committee (IVIR-AC) recommendations—March 2019. Wkly. Epidemiol. Rec. 2019, 94, 225–232.
- United Nations (UN). National Accounts Main Aggregates Database. Available online: https://unstats.un.org/unsd/snaama (accessed on 27 July 2025).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Lerman, P.M. Fitting segmented regression models by grid search. Appl. Stat. 1980, 29, 77–84. [Google Scholar] [CrossRef]
- Clegg, L.X.; Hankey, B.F.; Tiwari, R.; Feuer, E.J.; Edwards, B.K. Estimating average annual per cent change in trend analysis. Stat. Med. 2009, 28, 3670–3682. [Google Scholar] [CrossRef]
- Rohenkohl, B.; Arriagada, P. How Does the World Bank Classify Countries by Income? OurWorldinData.org: Oxford, UK, 2025; Available online: https://ourworldindata.org/organization (accessed on 14 September 2025).
- Han, J.; Zhang, L.; Chen, Y.; Zhang, Y.; Wang, L.; Cai, R.; Li, M.; Dai, Y.; Dang, L.; Chen, H.; et al. Global HPV vaccination programs and coverage rates: A systematic review. eClinicalMedicine 2025, 84, 103290. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Z.; Pan, W.; Song, Y.; Zheng, L.; Li, L.; Ye, J.; Cao, L.; Yu, W. Estimated Human Papillomavirus Vaccine Coverage Among Females 9–45 Years of Age—China, 2017–2022. China CDC Wkly. 2024, 6, 413–417. [Google Scholar] [CrossRef]
- Tran, N.T.; Phan, T.N.T.; Pham, T.T.; Le, T.T.; Le, H.M.; Nguyen, D.T.; Lam, A.N.; Pham, T.T.; Le, H.T.; Dang, N.B.; et al. Urban-rural disparities in acceptance of human papillomavirus vaccination among women in Can Tho, Vietnam. Ann. Ig. 2023, 35, 641–659. [Google Scholar] [CrossRef]
- Burki, T.K. India rolls out HPV vaccination. Lancet Oncol. 2023, 24, e147. [Google Scholar] [CrossRef]
- Altobelli, E.; Rapacchietta, L.; Profeta, V.F.; Fagnano, R. HPV-vaccination and cancer cervical screening in 53 WHO European Countries: An update on prevention programs according to income level. Cancer Med. 2019, 8, 2524–2534. [Google Scholar] [CrossRef]
- Valdecantos, R.L.; Sorrentino, M.; Mercogliano, M.; Giordano, V.; Trama, U.; Triassi, M.; Palladino, R. The structural and organizational aspects of human papillomavirus vaccine affecting immunization coverage in Europe: A systematic review. BMC Public Health 2025, 25, 1254. [Google Scholar] [CrossRef]
- Elfström, K.M.; Dillner, J.; Arnheim-Dahlström, L. Organization and quality of HPV vaccination programs in Europe. Vaccine 2015, 33, 1673–1681. [Google Scholar] [CrossRef]
- Jankowski, M.; Grudziąż-Sękowska, J.; Wrześniewska-Wal, I.; Tyszko, P.; Sękowski, K.; Ostrowski, J.; Gujski, M.; Pinkas, J. National HPV Vaccination Program in Poland-Public Awareness, Sources of Knowledge, and Willingness to Vaccinate Children against HPV. Vaccines 2023, 11, 1371. [Google Scholar] [CrossRef]
- Mihretie, G.N.; Liyeh, T.M.; Ayele, A.D.; Belay, H.G.; Yimer, T.S.; Miskr, A.D. Knowledge and willingness of parents towards child girl HPV vaccination in Debre Tabor Town, Ethiopia: A community-based cross-sectional study. Reprod. Health 2022, 19, 136. [Google Scholar] [CrossRef]
- Achimaș-Cadariu, T.; Pașca, A.; Jiboc, N.M.; Puia, A.; Dumitrașcu, D.L. Vaccine Hesitancy among European Parents-Psychological and Social Factors Influencing the Decision to Vaccinate against HPV: A Systematic Review and Meta-Analysis. Vaccines 2024, 12, 127. [Google Scholar] [CrossRef]
- Rehn, M.; Uhnoo, I.; Kühlmann-Berenzon, S.; Wallensten, A.; Sparén, P.; Netterlid, E. Highest Vaccine Uptake after School-Based Delivery—A County-Level Evaluation of the Implementation Strategies for HPV Catch-Up Vaccination in Sweden. PLoS ONE 2016, 11, e0149857. [Google Scholar] [CrossRef]
- McClure, C.A.; MacSwain, M.A.; Morrison, H.; Sanford, C.J. Human papillomavirus vaccine uptake in boys and girls in a school-based vaccine delivery program in Prince Edward Island, Canada. Vaccine 2015, 33, 1786–1790. [Google Scholar] [CrossRef]
- Lefevere, E.; Hens, N.; De Smet, F.; Beutels, P. The impact of non-financial and financial encouragements on participation in non school-based human papillomavirus vaccination: A retrospective cohort study. Eur. J. Health Econ. 2016, 17, 305–315. [Google Scholar] [CrossRef]
- Wang, J.; Ploner, A.; Sparén, P.; Lepp, T.; Roth, A.; Arnheim-Dahlström, L.; Sundström, K. Mode of HPV vaccination delivery and equity in vaccine uptake: A nationwide cohort study. Prev. Med. 2019, 120, 26–33. [Google Scholar] [CrossRef]
- Felsher, M.; Shumet, M.; Velicu, C.; Chen, Y.T.; Nowicka, K.; Marzec, M.; Skowronek, G.; Pieniążek, I. A systematic literature review of human papillomavirus vaccination strategies in delivery systems within national and regional immunization programs. Hum. Vaccines Immunother. 2024, 20, 2319426. [Google Scholar] [CrossRef]
- Lefevere, E.; Theeten, H.; Hens, N.; De Smet, F.; Top, G.; Van Damme, P. From non school-based, co-payment to school-based, free Human Papillomavirus vaccination in Flanders (Belgium): A retrospective cohort study describing vaccination coverage, age-specific coverage and socio-economic inequalities. Vaccine 2015, 33, 5188–5195. [Google Scholar] [CrossRef]
- Dema, E.; Osman, R.; Soldan, K.; Field, N.; Sonnenberg, P. Are there any sociodemographic factors associated with non-uptake of HPV vaccination of girls in high-income countries with school-based vaccination programmes? A systematic review. J. Epidemiol. Community Health 2025, 79, 388–396. [Google Scholar] [CrossRef]
- Wemrell, M.; Vicente, R.P.; Merlo, J. Mapping sociodemographic and geographical differences in human papillomavirus non-vaccination among young girls in Sweden. Scand. J. Public Health 2023, 51, 288–295. [Google Scholar] [CrossRef]
- Melo, M.S.; Minuzzi-Souza, T.T.C.E.; Soares, L.M.; Santos, A.D.D.; Raiol, T.; Ribeiro, A. Human papillomavirus vaccination access, coverage and dropout in the Federal District: A time series study, 2013–2023. Epidemiol. Serv. Saude 2025, 34, e20240006. [Google Scholar] [CrossRef]
- Owsianka, B.; Gańczak, M. Evaluation of human papilloma virus (HPV) vaccination strategies and vaccination coverage in adolescent girls worldwide. Przegl. Epidemiol. 2015, 69, 53–88. [Google Scholar]
- Blakely, T.; Kvizhinadze, G.; Karvonen, T.; Pearson, A.L.; Smith, M.; Wilson, N. Cost-effectiveness and equity impacts of three HPV vaccination programmes for school-aged girls in New Zealand. Vaccine 2014, 32, 2645–2656. [Google Scholar] [CrossRef]
- Gountas, I.; Favre-Bulle, A.; Saxena, K.; Wilcock, J.; Collings, H.; Salomonsson, S.; Skroumpelos, A.; Sabale, U. Impact of the COVID-19 Pandemic on HPV Vaccinations in Switzerland and Greece: Road to Recovery. Vaccines 2023, 11, 258. [Google Scholar] [CrossRef]
- Moura, C.; Truche, P.; Sousa Salgado, L.; Meireles, T.; Santana, V.; Buda, A.; Bentes, A.; Botelho, F.; Mooney, D. The impact of COVID-19 on routine pediatric vaccination delivery in Brazil. Vaccine 2022, 40, 2292–2298. [Google Scholar] [CrossRef]
- Daniels, V.; Saxena, K.; Roberts, C.; Kothari, S.; Corman, S.; Yao, L.; Niccolai, L. Impact of reduced human papillomavirus vaccination coverage rates due to COVID-19 in the United States: A model based analysis. Vaccine 2021, 39, 2731–2735. [Google Scholar] [CrossRef]
- World Health Organization. Human papillomavirus vaccines: WHO position paper (2022 update). Wkly. Epidemiol. Rec. 2022, 97, 645–672. [Google Scholar]
- Khalid, K.; Lee, K.Y.; Mukhtar, N.F.; Warijo, O. Recommended Interventions to Improve Human Papillomavirus Vaccination Uptake among Adolescents: A Review of Quality Improvement Methodologies. Vaccines 2023, 11, 1390. [Google Scholar] [CrossRef]
- Tsu, V.D.; LaMontagne, D.S.; Atuhebwe, P.; Bloem, P.N.; Ndiaye, C. National implementation of HPV vaccination programs in low-resource countries: Lessons, challenges, and future prospects. Prev. Med. 2021, 144, 106335. [Google Scholar] [CrossRef]
- Xu, M.A.; Choi, J.; Capasso, A.; DiClemente, R.J. Improving HPV Vaccination Uptake Among Adolescents in Low Resource Settings: Sociocultural and Socioeconomic Barriers and Facilitators. Adolesc. Health Med. Ther. 2024, 15, 73–82. [Google Scholar] [CrossRef]
- Chidebe, R.C.W.; Osayi, A.; Torode, J.S. The Global Fund, Cervical Cancer, and HPV infections: What can low- and middle-income countries do to accelerate progress by 2030? eClinicalMedicine 2025, 81, 103127. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Kaul, S.; Do, T.Q.N.; Hsu, E.; Schmeler, K.M.; Montealegre, J.R.; Rodriguez, A.M. School-based human papillomavirus vaccination program for increasing vaccine uptake in an underserved area in Texas. Papillomavirus Res. 2019, 8, 100189. [Google Scholar] [CrossRef]
- Mengistie, B.A.; Yirsaw, A.N.; Lakew, G.; Mekonnen, G.B.; Shibabaw, A.A.; Chereka, A.A.; Kitil, G.W.; Wondie, W.T.; Abuhay, A.E.; Getachew, E. Human papillomavirus vaccine uptake and its determinants among women in Africa: An umbrella review. Front. Public Health 2025, 13, 1537250. [Google Scholar] [CrossRef]
- Theotonio Dos Santos, L.F.; Marques Fidalgo, T.; Cordeiro Mattos, A.J.; Albuquerque Ribeiro, G.; Rizzo, L.V.; Andrade Rodrigues Fonseca, H. Education and social determinants shaping HPV vaccine uptake: Insights from a nationwide cross-sectional study. Hum. Vaccines Immunother. 2025, 21, 2517488. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Krog, L.; Lycke, K.D.; Kahlert, J.; Randrup, T.H.; Jensen, P.T.; Rositch, A.F.; Hammer, A. Risk of progression of cervical intraepithelial neoplasia grade 2 in human papillomavirus-vaccinated and unvaccinated women: A population-based cohort study. Am. J. Obstet. Gynecol. 2024, 230, 430.e1–430.e11. [Google Scholar] [CrossRef]
- Mix, J.M.; Van Dyne, E.A.; Saraiya, M.; Hallowell, B.D.; Thomas, C.C. Assessing Impact of HPV Vaccination on Cervical Cancer Incidence among Women Aged 15–29 Years in the United States, 1999–2017: An Ecologic Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 30–37. [Google Scholar] [CrossRef]
- Pei, J.; Shu, T.; Wu, C.; Li, M.; Xu, M.; Jiang, M.; Zhu, C. Impact of human papillomavirus vaccine on cervical cancer epidemic: Evidence from the surveillance, epidemiology, and end results program. Front. Public Health 2023, 10, 998174. [Google Scholar] [CrossRef]
- Arroyo Mühr, L.S.; Gini, A.; Yilmaz, E.; Hassan, S.S.; Lagheden, C.; Hultin, E.; Garcia Serrano, A.; Ure, A.E.; Andersson, H.; Merino, R.; et al. Concomitant human papillomavirus (HPV) vaccination and screening for elimination of HPV and cervical cancer. Nat. Commun. 2024, 15, 3679. [Google Scholar] [CrossRef]
- Castle, P.E. Looking Back, Moving Forward: Challenges and Opportunities for Global Cervical Cancer Prevention and Control. Viruses 2024, 16, 1357. [Google Scholar] [CrossRef] [PubMed]
- Baisley, K.; Kemp, T.J.; Mugo, N.R.; Whitworth, H.; Onono, M.A.; Njoroge, B.; Indangasi, J.; Bukusi, E.A.; Prabhu, P.R.; Mutani, P.; et al. Comparing one dose of HPV vaccine in girls aged 9–14 years in Tanzania (DoRIS) with one dose in young women aged 15–20 years in Kenya (KEN SHE): An immunobridging analysis of randomised controlled trials. Lancet Glob. Health 2024, 12, e491–e499. [Google Scholar] [CrossRef] [PubMed]
- Barnabas, R.V.; Brown, E.R.; Onono, M.A.; Bukusi, E.A.; Njoroge, B.; Winer, R.L.; Galloway, D.A.; Pinder, L.F.; Donnell, D.; Wakhungu, I.; et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evid. 2022, 1, EVIDoa2100056. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, J.M.; Budd, A.; Rompotis, C.; Bartlett, N.; Malloy, M.J.; Andersen, R.L.; Coulter, K.A.; Couvee, P.W.; Steel, N.; Ward, G.H.; et al. Is one dose of human papillomavirus vaccine as effective as three?: A national cohort analysis. Papillomavirus Res. 2019, 8, 100177. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Yousefi, Z.; Khosravi, G.; Malayeri, F.E.; Golabi, M.; Askarzadeh, M.; Shams, M.H.; Ghezelbash, B.; Eskandari, N. Human papillomavirus vaccination in low- and middle-income countries: Progression, barriers, and future prospective. Front. Immunol. 2023, 14, 1150238. [Google Scholar] [CrossRef]
- Waheed, D.E.; Bolio, A.; Guillaume, D.; Sidibe, A.; Morgan, C.; Karafillakis, E.; Holloway, M.; Van Damme, P.; Limaye, R.; Vorsters, A. Planning, implementation, and sustaining high coverage of human papillomavirus (HPV) vaccination programs: What works in the context of low-resource countries? Front. Public Health 2023, 11, 1112981. [Google Scholar] [CrossRef]
- Casey, R.M.; Adrien, N.; Badiane, O.; Diallo, A.; Loko Roka, J.; Brennan, T.; Doshi, R.; Garon, J.; Loharikar, A. National introduction of HPV vaccination in Senegal-Successes, challenges, and lessons learned. Vaccine 2022, 40 (Suppl. S1), A10–A16. [Google Scholar] [CrossRef] [PubMed]

| Global | WHO Region | ||||||
|---|---|---|---|---|---|---|---|
| African Region | Region of the Americas | Eastern Mediterranean Region | European Region | South-East Asia Region | Western Pacific Region | ||
| Countries with HPV vaccination in schedule, no. (%) | 147/194 (75.8) | 29/47 (61.7) | 32/35 (91.4) | 8/21 (38.1) | 47/53 (88.7) | 7/11 (63.6) | 24/27 (88.9) |
| HPV vaccination coverage (%) | 56.9 | 57.9 | 55.4 | 19.5 | 55.6 | 63.9 | 58.2 |
| Locations | 2010/ First Available | 2024/ Last Available | AAPC (95%CI) | p Value | Income Group *** | Delivery Strategy |
|---|---|---|---|---|---|---|
| Global | 47.8 | 56.9 | −0.2 (−1.1 to 0.7) | 0.621 | ||
| Countries ** | ||||||
| Afghanistan | LIC | |||||
| Albania | 18 | 43 | - | - | UMIC | Facility-based |
| Algeria | UMIC | |||||
| Andorra | 49 | 82 | 8.1 * (1.8 to 14.9) | 0.021 | HIC | School-based |
| Angola | LMIC | |||||
| Antigua and Barbuda | 2 | 1 | - | - | HIC | Facility-based |
| Argentina | 36 | 55 | 0.44 (−2.2 to 3.1) | 0.725 | UMIC | Mixed |
| Armenia | 2 | 31 | 48.0 * (27.9 to 71.2) | <0.001 | UMIC | Facility-based |
| Australia | 67 | 73 | 0.4 (−0.6 to 1.5) | 0.397 | HIC | School-based |
| Austria | 1 | 31 | 54.4 * (21.6 to 95.9) | 0.003 | HIC | School-based |
| Azerbaijan | UMIC | |||||
| Bahamas | 1 | 3 | −6.7 (−23.7 to 14.0) | 0.447 | HIC | School-based |
| Bahrain | / | 74 | - | - | HIC | Not available |
| Bangladesh | 19 | 90 | - | - | LMIC | Not available |
| Barbados | 8 | 43 | 9.3 (3.9 to 24.2) | 0.152 | HIC | School-based |
| Belarus | UMIC | |||||
| Belgium | 59 | 72 | 1.6 * (1.3 to 1.8) | <0.001 | HIC | School-based |
| Belize | 51 | 62 | −5.6 (−37.9 to 43.4) | 0.747 | UMIC | School-based |
| Benin | LMIC | |||||
| Bhutan | 96 | 92 | 0.3 (−1.3 to 1.9) | 0.715 | LMIC | School-based |
| Bolivia | 65 | 78 | 1.4 (−14.5 to 20.2) | 0.849 | LMIC | School-based |
| Bosnia and Herzegovina | 5 | 9 | - | - | UMIC | Mixed |
| Botswana | 65 | 34 | - | - | UMIC | School-based |
| Brazil | 61 | 79 | 2.2 (−1.1 to 5.5) | 0.169 | UMIC | Facility-based |
| Brunei Darussalam | 88 | 88 | 0.7 (−0.1 to 1.4) | 0.066 | HIC | Mixed |
| Bulgaria | 21 | 9 | −8.7 (−19.8 to 3.9) | 0.147 | HIC | Facility-based |
| Burkina Faso | 16 | 99 | - | - | LIC | School-based |
| Burundi | LIC | |||||
| Cabo Verde | 99 | 99 | - | - | UMIC | Not available |
| Cambodia | 87 | 85 | - | - | LMIC | Not available |
| Cameroon | 5 | 36 | - | - | LMIC | School-based |
| Canada | 73 | 86 | 1.7 * (1.1 to 2.2) | <0.001 | HIC | School-based |
| Central African Republic | LIC | |||||
| Chad | LIC | |||||
| Chile | 76 | 87 | 0.2 (−2.8 to 3.3) | 0.883 | HIC | School-based |
| China | UMIC | |||||
| Colombia | 86 | 60 | 1.3 (−16.7 to 23.3) | 0.886 | UMIC | School-based |
| Comoros | LMIC | |||||
| Congo, Republic of the | LMIC | |||||
| Cook Islands | 74 | 37 | −8.3 (−17.3 to 1.7) | 0.092 | School-based | |
| Costa Rica | 56 | 84 | −2.8 (−21.3 to 20.2) | 0.730 | HIC | School-based |
| Côte d’Ivoire | 12 | 61 | - | - | LMIC | School-based |
| Croatia | 3 | 53 | 40.4 * (23.1 to 60.1) | <0.001 | HIC | School-based |
| Cuba | UMIC | |||||
| Cyprus | 57 | 89 | 6.8 * (3.6 to 10.1) | 0.003 | HIC | School-based |
| Czechia | 74 | 75 | 0.2 (−0.8 to 1.3) | 0.628 | HIC | Facility-based |
| Korea (South) | HIC | |||||
| DR Congo | LIC | |||||
| Denmark | 68 | 81 | 2.6 (−3.8 to 9.5) | 0.402 | HIC | Facility-based |
| Djibouti | LMIC | |||||
| Dominica | 74 | 77 | - | - | UMIC | School-based |
| Dominican Republic | 34 | 45 | 8.2 (−26.0 to 58.4) | 0.629 | UMIC | Facility-based |
| Ecuador | 81 | 89 | −10.5 (−29.5 to 13.6) | 0.319 | UMIC | School-based |
| Egypt | LMIC | |||||
| El Salvador | 24 | 86 | - | - | UMIC | School-based |
| Equatorial Guinea | UMIC | |||||
| Eritrea | 84 | 49 | - | - | LIC | Not available |
| Estonia | 46 | 63 | 1.8 (−5.0 to 9.0) | 0.533 | HIC | School-based |
| Eswatini | 61 | 30 | - | - | LMIC | School-based |
| Ethiopia | 24 | 58 | - | - | LIC | School-based |
| Fiji | 53 | 71 | 0.4 (−3.2 to 4.1) | 0.812 | UMIC | School-based |
| Finland | 55 | 62 | 1.6 * (0.2 to 3.0) | 0.034 | HIC | School-based |
| France | 25 | 45 | 8.0 * (4.2 to 11.9) | 0.001 | HIC | Facility-based |
| Gabon | UMIC | |||||
| Gambia | 24 | 15 | - | - | LIC | School-based |
| Georgia | 11 | 29 | - | - | UMIC | Facility-based |
| Germany | 27 | 55 | 6.5 * (5.7 to 7.2) | <0.001 | HIC | Facility-based |
| Ghana | LMIC | |||||
| Greece | HIC | |||||
| Grenada | 42 | 5 | - | - | UMIC | School-based |
| Greenland | HIC | |||||
| Guatemala | 31 | 57 | - | - | UMIC | School-based |
| Guinea | LMIC | |||||
| Guinea-Bissau | LIC | |||||
| Guyana | 2 | 71 | 18.9 * (0.7 to 40.4) | HIC | School-based | |
| Haiti | LMIC | |||||
| Honduras | 52 | 72 | 1.5 (−1.8 to 4.9) | 0.310 | LMIC | School-based |
| Hungary | 75 | 75 | 0.6 (−0.5 to 1.8) | 0.241 | HIC | School-based |
| Iceland | 92 | 89 | −0.2 (−0.8 to 0.3) | 0.328 | HIC | School-based |
| India | LMIC | |||||
| Indonesia | 3 | 79 | 58.4 * (14.6 to 118.9) | 0.013 | HIC | School-based |
| Iran | UMIC | |||||
| Iraq | UMIC | |||||
| Ireland | 82 | 73 | −1.0 (−2.8 to 0.9) | 0.263 | HIC | School-based |
| Israel | 51 | 58 | 1.1 * (0.3 to 1.9) | 0.012 | HIC | School-based |
| Italy | 44 | 51 | −2.1 (−4.6 to 0.5) | 0.102 | HIC | Mixed |
| Jamaica | 8 | 7 | −8.3 (−33.9 to 27.1) | 0.526 | UMIC | School-based |
| Japan | 12 | 17 | - | - | HIC | Facility-based |
| Jordan | LMIC | |||||
| Kazakhstan | / | 38 | - | - | UMIC | School-based |
| Kenya | 16 | 36 | - | - | LMIC | Facility-based |
| Kiribati | 55 | 71 | - | - | LMIC | Not available |
| Kuwait | HIC | |||||
| Kyrgyzstan | 48 | 88 | - | - | LMIC | School-based |
| Lao People’s Republic | 54 | 95 | - | - | LMIC | School-based |
| Latvia | 58 | 51 | −0.5 (−3.7 to 2.8) | 0.721 | HIC | Facility-based |
| Lebanon | LMIC | |||||
| Lesotho | 55 | 70 | - | - | LMIC | Not available |
| Liberia | 17 | 71 | - | - | LIC | Mixed |
| Libya | 30 | 28 | - | - | UMIC | Not available |
| Lithuania | 28 | 59 | 4.2 (−7.3 to 17.0) | 0.425 | HIC | Facility-based |
| Luxembourg | 16 | 79 | 15.4 * (10.6 to 20.4) | <0.001 | HIC | Facility-based |
| Madagascar | LIC | |||||
| Malawi | 74 | 21 | - | - | LIC | School-based |
| Malaysia | 83 | 78 | - | - | UMIC | School-based |
| Maldives | 66 | 55 | - | - | UMIC | School-based |
| Mali | / | 15 | - | - | LIC | Mixed |
| Malta | 93 | 80 | −0.1 (−1.1. to 0.9) | 0.852 | HIC | Facility-based |
| Marshall Islands | 37 | 50 | 7.2 (−1.1 to 16.2) | 0.086 | UMIC | Mixed |
| Mauritania | 24 | 51 | - | - | LMIC | Not available |
| Mauritius | 74 | 92 | −9.0 (−25.4 to 11.0) | 0.288 | UMIC | School-based |
| Mexico | 82 | 82 | −11.0 (−29.3 to 12.1) | 0.291 | UMIC | School-based |
| Micronesia | 32 | 40 | −6.4 (−14.7 to 2.8) | 0.137 | LMIC | School-based |
| Monaco | / | 14 | - | - | HIC | Facility-based |
| Mongolia | / | 25 | - | - | UMIC | School-based |
| Montenegro | 18 | 5 | - | - | UMIC | Facility-based |
| Morocco | / | 3 | - | - | LMIC | Not available |
| Mozambique | 32 | 89 | - | - | LIC | School-based |
| Myanmar | 44 | 83 | - | - | LMIC | School-based |
| Namibia | LMIC | |||||
| Nauru | 40 | 6 | - | - | HIC | Not available |
| Nepal | LMIC | |||||
| Netherlands | 52 | 63 | 0.8 (−0.6 to 2.3) | 0.241 | HIC | Facility-based |
| New Zealand | 45 | 52 | 1.3 (−0.1 to 2.7) | 0.070 | HIC | Mixed |
| Nicaragua | LMIC | |||||
| Niger | LIC | |||||
| Nigeria | 27 | 60 | - | - | LMIC | Mixed |
| Niue | 76 | 99 | - | - | School-based | |
| North Macedonia | 31 | 40 | 0.7 (−2.4 to 3.8) | 0.650 | UMIC | School-based |
| Norway | 58 | 91 | 2.5 * (1.7 to 3.3) | <0.001 | HIC | School-based |
| Oman | HIC | |||||
| Pakistan | LMIC | |||||
| Palau | 9 | 43 | 18.7 * (12.4 to 25.4) | <0.001 | HIC | School-based |
| Panama | 65 | 54 | −2.1 * (−4.2 to −0.0) | 0.048 | HIC | Mixed |
| Papua New Guinea | LMIC | |||||
| Paraguay | 79 | 47 | −10.8 * (−16.3 to −4.9) | 0.003 | UMIC | Mixed |
| Peru | 64 | 97 | - | - | UMIC | School-based |
| Philippines | 3 | 5 | - | - | LMIC | School-based |
| Poland | 2 | 13 | - | - | HIC | Facility-based |
| Portugal | 88 | 92 | 0.2 (−0.6 to 1.0) | 0.596 | HIC | Facility-based |
| Qatar | / | 1 | - | - | HIC | Not available |
| Republic of Korea | 50 | 69 | 2.1 (−2.3 to 6.7) | 0.296 | LIC | Facility-based |
| Republic of Moldova | 47 | 42 | 0.3 (−5.1 to 6.0) | 0.893 | UMIC | School-based |
| Romania | 23 | 17 | - | - | HIC | Facility-based |
| Russian Federation | HIC | |||||
| Rwanda | 34 | 69 | - | - | LIC | School-based |
| Saint Kitts and Nevis | 98 | 78 | - | - | HIC | School-based |
| Saint Lucia | 42 | 71 | - | - | UMIC | School-based |
| Saint Vincent/Grenadines | 4 | 8 | - | - | UMIC | School-based |
| Samoa | 87 | 64 | - | - | UMIC | School-based |
| San Marino | 21 | 56 | 13.3 * (6.4 to 20.5) | 0.001 | HIC | Facility-based |
| Sao Tome and Principe | 57 | 75 | - | - | LMIC | Not available |
| Saudi Arabia | 47 | / | - | - | HIC | School-based |
| Senegal | 26 | 51 | - | - | LMIC | Facility-based |
| Serbia | 0 | 4 | - | - | UMIC | Not available |
| Seychelles | 77 | 23 | −9.7 * (−17.9 to −0.8) | 0.036 | HIC | School-based |
| Sierra Leone | 24 | 61 | - | - | LIC | Not available |
| Singapore | 1 | 70 | - | - | HIC | School-based |
| Slovakia | 29 | 24 | - | - | HIC | Not available |
| Slovenia | 44 | 43 | 0.2 (−1.4 to 1.8) | 0.801 | HIC | School-based |
| Solomon Islands | 17 | 78 | - | - | LMIC | Mixed |
| Somalia | LIC | |||||
| South Africa | 66 | 79 | - | - | UMIC | School-based |
| South Sudan | LIC | |||||
| Spain | 61 | 90 | 1.6 * (0.7 to 2.6) | 0.003 | HIC | Mixed |
| Sri Lanka | 12 | 12 | −21.1 * (−36.6 to −1.9) | 0.038 | LMIC | Mixed |
| Sudan | LIC | |||||
| Suriname | 36 | 13 | −26.3 * (−42.7 to −5.2) | 0.023 | UMIC | School-based |
| Sweden | 73 | 87 | 2.8 * (2.0 to 3.6) | <0.001 | HIC | School-based |
| Switzerland | 20 | 70 | 5.2 * (2.3 to 8.2) | 0.002 | HIC | Mixed |
| Syrian Arab Republic | LIC | |||||
| Tajikistan | LMIC | |||||
| Thailand | 66 | 36 | - | - | UMIC | School-based |
| Timor-Leste | / | 99 | - | - | LMIC | School-based |
| Togo | 45 | 36 | - | - | LIC | School-based |
| Tonga | 12 | 67 | - | - | UMIC | Not available |
| Trinidad and Tobago | 8 | 16 | 5.2 (−1.1 to 12.0) | 0.097 | HIC | Mixed |
| Tunisia | LMIC | |||||
| Türkiye | UMIC | |||||
| Turkmenistan | 90 | 99 | 0.9 (−0.0 to 1.9) | 0.055 | UMIC | Mixed |
| Tuvalu | 27 | 70 | - | - | UMIC | Not available |
| Uganda | 30 | 95 | 7.4 (−6.2 to 23.1) | 0.253 | LIC | School-based |
| Ukraine | UMIC | |||||
| United Arab Emirates | 25 | 46 | 9.3 * (5.2 to 13.6) | 0.002 | HIC | School-based |
| United Kingdom | 77 | 75 | −1.4 * (−2.6 to −0.2) | 0.026 | HIC | School-based |
| Tanzania | 19 | 94 | 24.9 * (10.3 to 41.4) | 0.006 | LMIC | Mixed |
| United States of America | 23 | 52 | 7.7 * (6.2 to 9.4) | <0.001 | HIC | Facility-based |
| Uruguay | 26 | 54 | 3.5 (−0.9 to 8.2) | 0.106 | HIC | Facility-based |
| Uzbekistan | 97 | 99 | - | - | LMIC | School-based |
| Vanuatu | 45 | 43 | - | - | LMIC | School-based |
| Venezuela | UMIC | |||||
| Vietnam | LMIC | |||||
| Yemen | LIC | |||||
| Zambia | 68 | 60 | - | - | LMIC | School-based |
| Zimbabwe | 64 | 51 | - | - | LMIC | Mixed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilic, I.; Ilic, M. Human Papillomavirus Vaccination Coverage Estimates Among the Primary Target Cohort (9–14-Year-Old Girls) in the World (2010–2024). Vaccines 2025, 13, 1010. https://doi.org/10.3390/vaccines13101010
Ilic I, Ilic M. Human Papillomavirus Vaccination Coverage Estimates Among the Primary Target Cohort (9–14-Year-Old Girls) in the World (2010–2024). Vaccines. 2025; 13(10):1010. https://doi.org/10.3390/vaccines13101010
Chicago/Turabian StyleIlic, Irena, and Milena Ilic. 2025. "Human Papillomavirus Vaccination Coverage Estimates Among the Primary Target Cohort (9–14-Year-Old Girls) in the World (2010–2024)" Vaccines 13, no. 10: 1010. https://doi.org/10.3390/vaccines13101010
APA StyleIlic, I., & Ilic, M. (2025). Human Papillomavirus Vaccination Coverage Estimates Among the Primary Target Cohort (9–14-Year-Old Girls) in the World (2010–2024). Vaccines, 13(10), 1010. https://doi.org/10.3390/vaccines13101010






