Neutralisation of the Immunoglobulin-Cleaving Activity of Streptococcus equi Subspecies equi IdeE by Blood Sera from Ponies Vaccinated with a Multicomponent Protein Vaccine
Abstract
1. Introduction
2. Materials and Methods
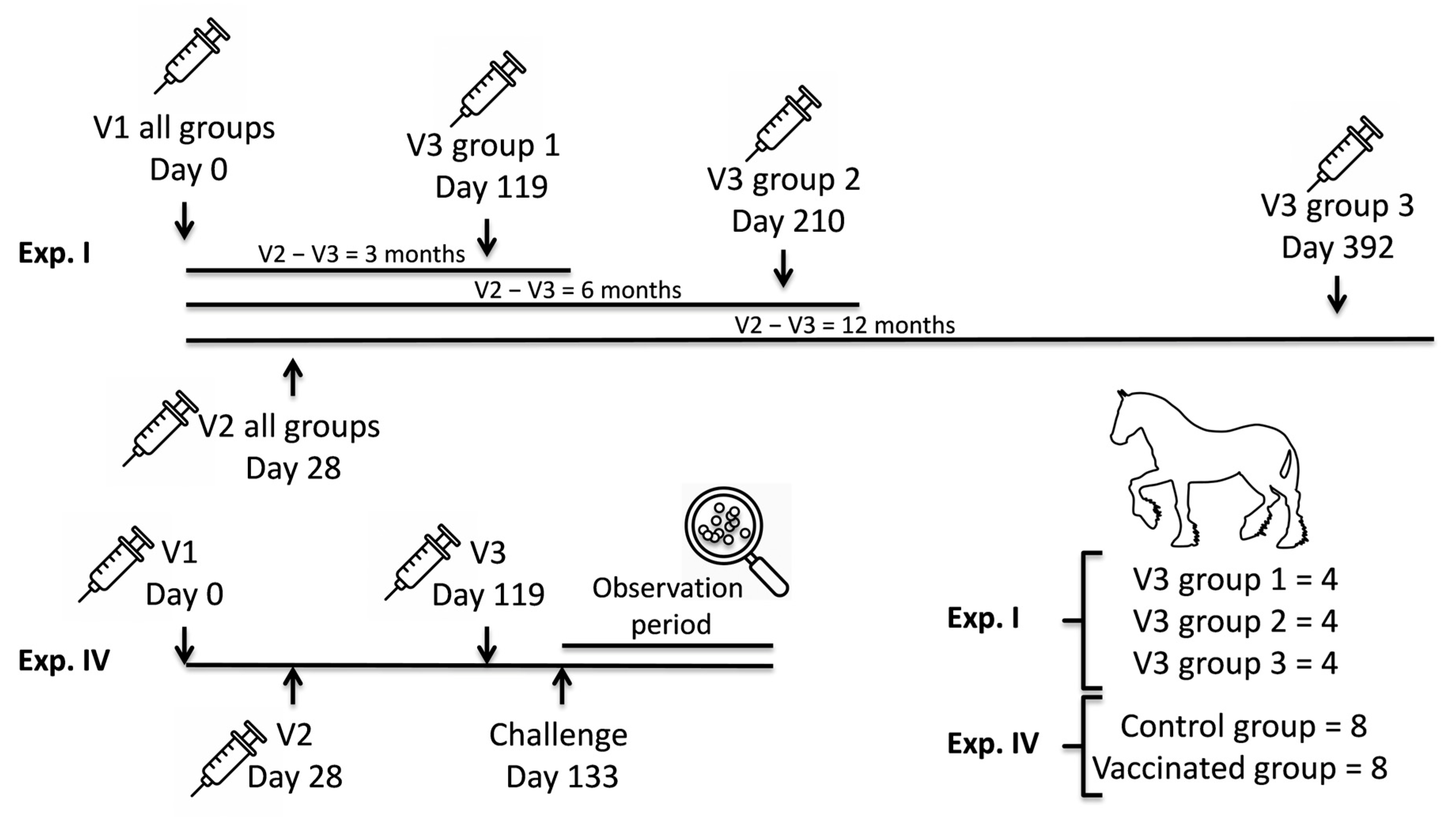
2.1. Study Design
2.2. Collection of Blood Samples
2.3. Quantification of IdeE Neutralisation
2.4. Quantification of the Antibody Response to Vaccination
2.5. Statistical Methods
3. Results
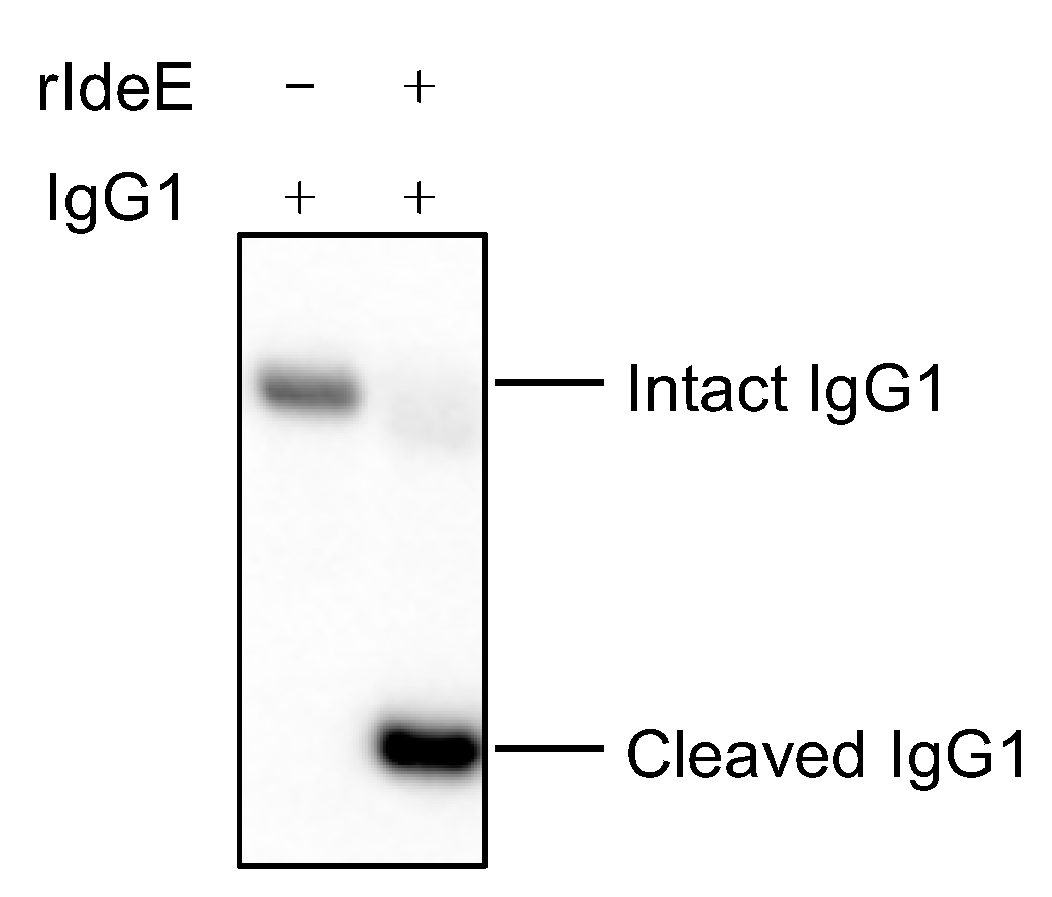
3.1. Recombinant IdeE Cleaved Human IgG1
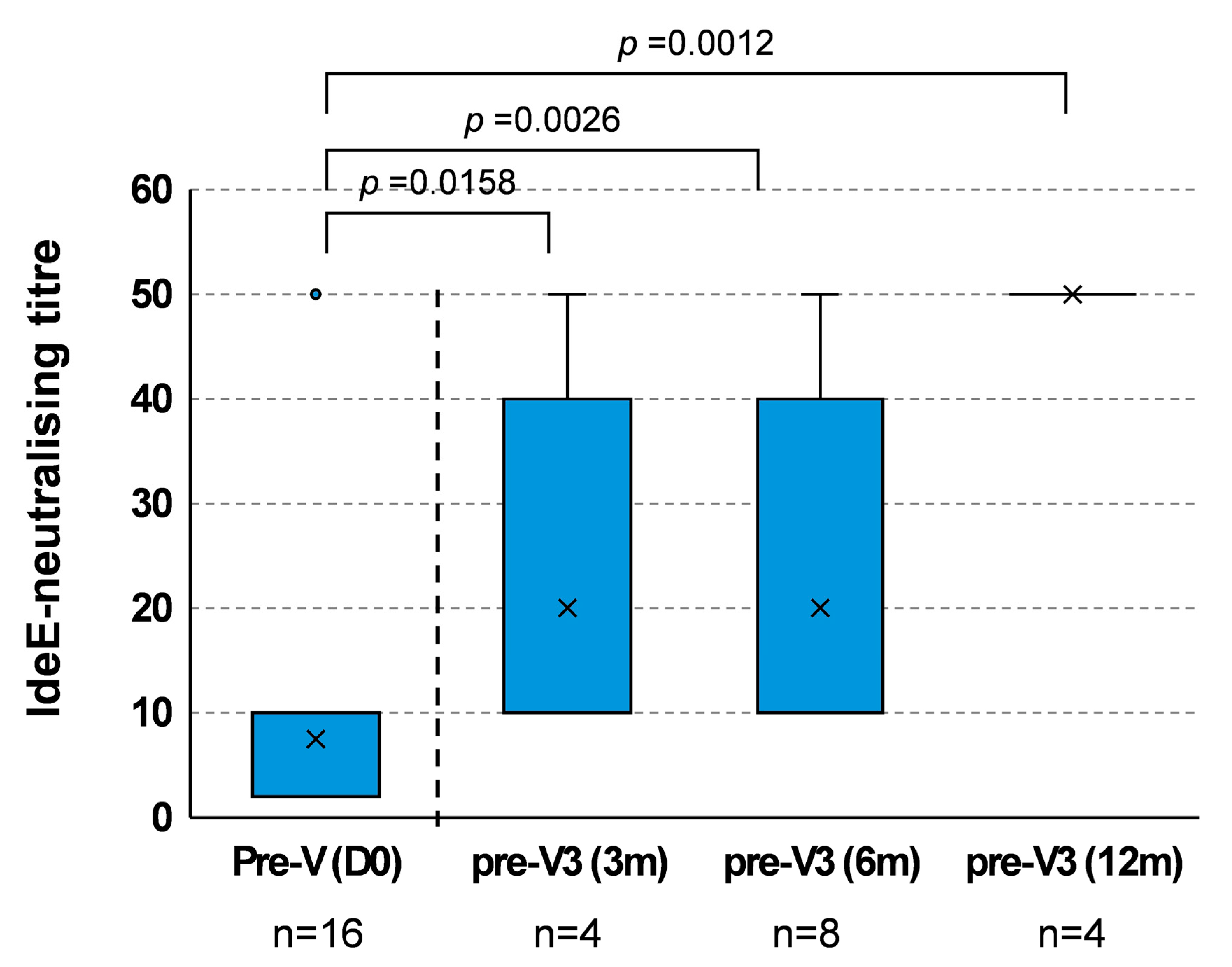
3.2. Duration of IdeE-Neutralising Activity After V2
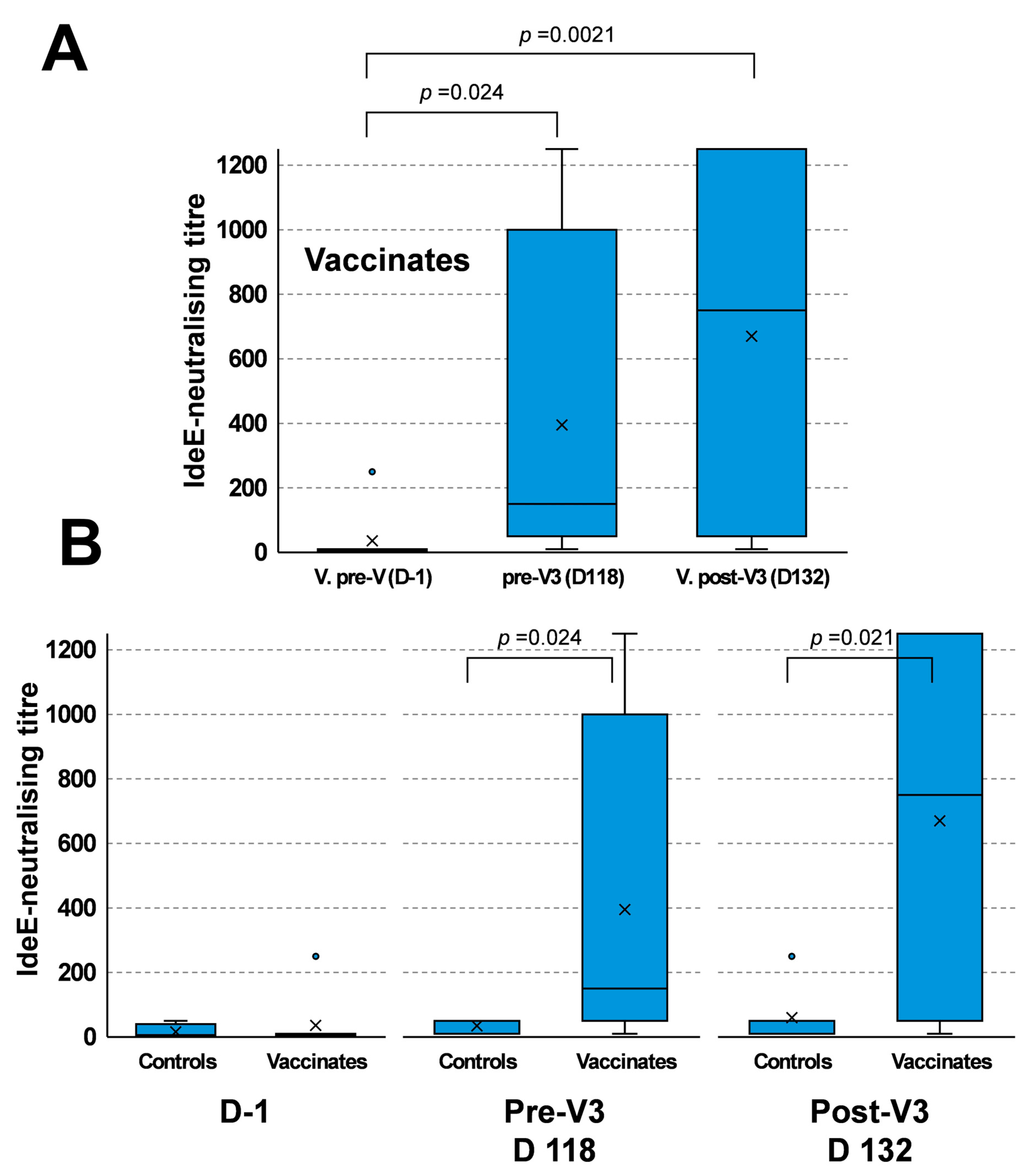
3.3. IdeE-Neutralising Activity After V3 Boost Immunisation
3.4. Comparison of IdeE-Neutralising Responses of Vaccinated and Placebo-Vaccinated Ponies
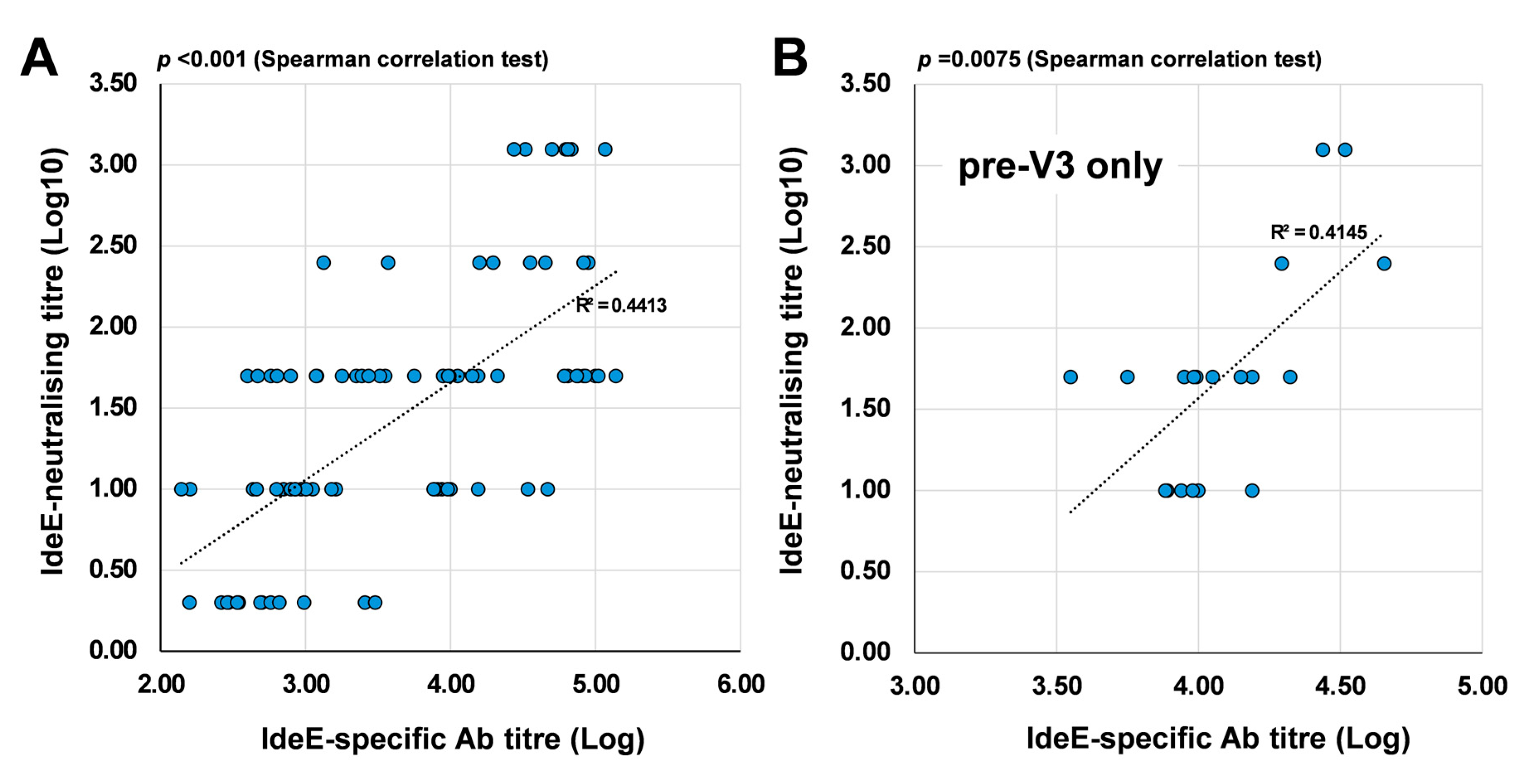
3.5. Correlation Between IdeE-Neutralising Responses and the Titre of IdeE-Specific Antibodies in Sera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Anti-Microbial Resistance |
| Exp. | Experiment |
| iELISA | indirect Enzyme-Linked Immunosorbent Assay |
| S. equi | Streptococcus equi subspecies equi |
References
- Boyle, A.G.; Timoney, J.F.; Newton, J.R.; Hines, M.T.; Waller, A.S.; Buchanan, B.R. Streptococcus equi Infections in Horses: Guidelines for Treatment, Control, and Prevention of Strangles-Revised Consensus Statement. J. Vet. Intern. Med. 2018, 32, 633–647. [Google Scholar] [CrossRef]
- Chhabra, D.; Bhatia, T.; Goutam, U.; Manuja, A.; Kumar, B. Strangles in Equines: An Overview. Microb. Pathog. 2023, 178, 106070. [Google Scholar] [CrossRef]
- Boyle, A. Streptococcus equi Subspecies equi Infection (Strangles) in Horses. Compend. Contin. Educ. Vet. 2011, 33, E1–E7. [Google Scholar] [PubMed]
- van Maanen, K.; van den Wollenberg, L.; de Haan, T.; Frippiat, T. Epidemiology of Infectious Pathogens in Horses with Acute Respiratory Disease, Abortion, and Neurological Signs: Insights Gained from the Veterinary Surveillance System for Horses in The Netherlands (SEIN). Vet. Sci. 2025, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Timoney, J.F. Strangles. Vet. Clin. N. Am. Equine Pract. 1993, 9, 365–374. [Google Scholar] [CrossRef]
- Newton, J.R.; Verheyen, K.; Talbot, N.C.; Timoney, J.F.; Wood, J.L.; Lakhani, K.H.; Chanter, N. Control of Strangles Outbreaks by Isolation of Guttural Pouch Carriers Identified Using PCR and Culture of Streptococcus equi. Equine Vet. J. 2000, 32, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.R.; Wood, J.L.; Dunn, K.A.; DeBrauwere, M.N.; Chanter, N. Naturally Occurring Persistent and Asymptomatic Infection of the Guttural Pouches of Horses with Streptococcus equi. Vet. Rec. 1997, 140, 84–90. [Google Scholar] [CrossRef]
- Verheyen, K.; Newton, J.R.; Talbot, N.C.; de Brauwere, M.N.; Chanter, N. Elimination of Guttural Pouch Infection and Inflammation in Asymptomatic Carriers of Streptococcus equi. Equine Vet. J. 2000, 32, 527–532. [Google Scholar] [CrossRef]
- Pringle, J.; Storm, E.; Waller, A.; Riihimäki, M. Influence of Penicillin Treatment of Horses with Strangles on Seropositivity to Streptococcus equi ssp. equi-Specific Antibodies. J. Vet. Intern. Med. 2020, 34, 294–299. [Google Scholar] [CrossRef]
- Morris, E.R.A.; Hillhouse, A.E.; Konganti, K.; Wu, J.; Lawhon, S.D.; Bordin, A.I.; Cohen, N.D. Comparison of Whole Genome Sequences of Streptococcus equi Subsp. equi from an Outbreak in Texas with Isolates from within the Region, Kentucky, USA, and Other Countries. Vet. Microbiol. 2020, 243, 108638. [Google Scholar] [CrossRef]
- Waller, A.S. New Perspectives for the Diagnosis, Control, Treatment, and Prevention of Strangles in Horses. Vet. Clin. N. Am. Equine Pract. 2014, 30, 591–607. [Google Scholar] [CrossRef]
- Robinson, C.; Waller, A.S.; Frykberg, L.; Flock, M.; Zachrisson, O.; Guss, B.; Flock, J.-I. Intramuscular Vaccination with Strangvac Is Safe and Induces Protection against Equine Strangles Caused by Streptococcus equi. Vaccine 2020, 38, 4861–4868. [Google Scholar] [CrossRef]
- Rendle, D.; Bowen, M.; Cavalleri, J.; De Brauwere, N.; Grondahl, G.; van Maanen, K.; Newton, J.R. Strangles Vaccination: A Current European Perspective. Equine Vet. Educ. 2025, 37, 90–97. [Google Scholar] [CrossRef]
- Robinson, C.; Frykberg, L.; Flock, M.; Guss, B.; Waller, A.S.; Flock, J.-I. Strangvac: A Recombinant Fusion Protein Vaccine That Protects Against Strangles, Caused by Streptococcus equi. Vaccine 2018, 36, 1484–1490. [Google Scholar] [CrossRef]
- Lannergård, J.; Guss, B. IdeE, an IgG-Endopeptidase of Streptococcus equi ssp. equi. FEMS Microbiol. Lett. 2006, 262, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Hulting, G.; Flock, M.; Frykberg, L.; Lannergård, J.; Flock, J.-I.; Guss, B. Two Novel IgG Endopeptidases of Streptococcus equi. FEMS Microbiol. Lett. 2009, 298, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Guss, B.; Flock, M.; Frykberg, L.; Waller, A.S.; Robinson, C.; Smith, K.C.; Flock, J.-I. Getting to Grips with Strangles: An Effective Multi-Component Recombinant Vaccine for the Protection of Horses from Streptococcus equi Infection. PLoS Pathog. 2009, 5, e1000584. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Steward, K.F.; Charbonneau, A.R.L.; Walsh, S.; Wilson, H.; Timoney, J.F.; Wernery, U.; Joseph, M.; Craig, D.; van Maanen, K.; et al. Globetrotting Strangles: The Unbridled National and International Transmission of Streptococcus equi Between Horses. Microb. Genom. 2021, 7, mgen000528. [Google Scholar] [CrossRef]
- Prion, S.; Haerling, K.A. Making Sense of Methods and Measurement: Pearson Product-Moment Correlation Coefficient. Clin. Simul. Nurs. 2014, 10, 587–588. [Google Scholar] [CrossRef]
- Galan, J.E.; Timoney, J.F. Mucosal Nasopharyngeal Immune Responses of Horses to Protein Antigens of Streptococcus equi. Infect. Immun. 1985, 47, 623–628. [Google Scholar] [CrossRef]
- Hamlen, H.J.; Timoney, J.F.; Bell, R.J. Epidemiologic and Immunologic Characteristics of Streptococcus equi Infection in Foals. J. Am. Vet. Med. Assoc. 1994, 204, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; DeLeo, F.R.; Reid, S.D.; Voyich, J.M.; Magoun, L.; Liu, M.; Braughton, K.R.; Ricklefs, S.; Hoe, N.P.; Cole, R.L.; et al. Opsonophagocytosis-Inhibiting Mac Protein of Group a Streptococcus: Identification and Characteristics of Two Genetic Complexes. Infect. Immun. 2002, 70, 6880–6890. [Google Scholar] [CrossRef]
- von Pawel-Rammingen, U.; Johansson, B.P.; Björck, L. IdeS, a Novel Streptococcal Cysteine Proteinase with Unique Specificity for Immunoglobulin G. EMBO J. 2002, 21, 1607–1615. [Google Scholar] [CrossRef]
- von Pawel-Rammingen, U.; Björck, L. IdeS and SpeB: Immunoglobulin-Degrading Cysteine Proteinases of Streptococcus pyogenes. Curr. Opin. Microbiol. 2003, 6, 50–55. [Google Scholar] [CrossRef]
- Frick, I.-M.; Happonen, L.; Wrighton, S.; Nordenfelt, P.; Björck, L. IdeS, a Secreted Proteinase of Streptococcus pyogenes, Is Bound to a Nuclease at the Bacterial Surface Where It Inactivates Opsonizing IgG Antibodies. J. Biol. Chem. 2023, 299, 105345. [Google Scholar] [CrossRef]
- Akesson, P.; Moritz, L.; Truedsson, M.; Christensson, B.; von Pawel-Rammingen, U. IdeS, a Highly Specific Immunoglobulin G (IgG)-Cleaving Enzyme from Streptococcus pyogenes, Is Inhibited by Specific IgG Antibodies Generated During Infection. Infect. Immun. 2006, 74, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Shaw, H.A.; Remmington, A.; McKenzie, G.; Winkel, C.; Mawas, F. Mucosal Immunization Has Benefits over Traditional Subcutaneous Immunization with Group A Streptococcus Antigens in a Pilot Study in a Mouse Model. Vaccines 2023, 11, 1724. [Google Scholar] [CrossRef] [PubMed]
- Shaw, H.A.; Ozanne, J.; Burns, K.; Mawas, F. Multicomponent Vaccines against Group A Streptococcus Can Effectively Target Broad Disease Presentations. Vaccines 2021, 9, 1025. [Google Scholar] [CrossRef]
- Rieckmann, K.; Seydel, A.; Klose, K.; Alber, G.; Baums, C.G.; Schütze, N. Vaccination with the Immunoglobulin M-Degrading Enzyme of Streptococcus suis, IdeSsuis, Leads to Protection against a Highly Virulent Serotype 9 Strain. Vaccine X 2019, 3, 100046. [Google Scholar] [CrossRef]
- Timoney, J.F.; Yang, J.; Liu, J.; Merant, C. IdeE Reduces the Bactericidal Activity of Equine Neutrophils for Streptococcus equi. Vet. Immunol. Immunopathol. 2008, 122, 76–82. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righetti, F.; Hentrich, K.; Flock, M.; Frosth, S.; Jacobsson, K.; Bjerketorp, J.; Pathak, A.; Ido, N.; Henriques-Normark, B.; Frykberg, L.; et al. Neutralisation of the Immunoglobulin-Cleaving Activity of Streptococcus equi Subspecies equi IdeE by Blood Sera from Ponies Vaccinated with a Multicomponent Protein Vaccine. Vaccines 2025, 13, 1061. https://doi.org/10.3390/vaccines13101061
Righetti F, Hentrich K, Flock M, Frosth S, Jacobsson K, Bjerketorp J, Pathak A, Ido N, Henriques-Normark B, Frykberg L, et al. Neutralisation of the Immunoglobulin-Cleaving Activity of Streptococcus equi Subspecies equi IdeE by Blood Sera from Ponies Vaccinated with a Multicomponent Protein Vaccine. Vaccines. 2025; 13(10):1061. https://doi.org/10.3390/vaccines13101061
Chicago/Turabian StyleRighetti, Francesco, Karina Hentrich, Margareta Flock, Sara Frosth, Karin Jacobsson, Joakim Bjerketorp, Anuj Pathak, Noela Ido, Birgitta Henriques-Normark, Lars Frykberg, and et al. 2025. "Neutralisation of the Immunoglobulin-Cleaving Activity of Streptococcus equi Subspecies equi IdeE by Blood Sera from Ponies Vaccinated with a Multicomponent Protein Vaccine" Vaccines 13, no. 10: 1061. https://doi.org/10.3390/vaccines13101061
APA StyleRighetti, F., Hentrich, K., Flock, M., Frosth, S., Jacobsson, K., Bjerketorp, J., Pathak, A., Ido, N., Henriques-Normark, B., Frykberg, L., Paillot, R., Guss, B., Wood, T., Flock, J.-I., & Waller, A. S. (2025). Neutralisation of the Immunoglobulin-Cleaving Activity of Streptococcus equi Subspecies equi IdeE by Blood Sera from Ponies Vaccinated with a Multicomponent Protein Vaccine. Vaccines, 13(10), 1061. https://doi.org/10.3390/vaccines13101061







