Abstract
Background: Solid organ transplant (SOT) recipients are at risk of severe COVID-19. Vaccination is an important preventive measure but may have side effects, including decreased leukocyte counts. We aimed to describe the prevalence and relative incidence of decreased leukocyte counts and changes in leukocyte counts before and after SARS-CoV-2 mRNA vaccination and SARS-CoV-2 infection in SOT recipients. Methods: Changes in leukocyte counts from before to after each vaccine dose were investigated using linear mixed models. We determined the prevalence of decreased leukocyte counts before and after each vaccine dose and before and after SARS-CoV-2 infection. Self-controlled case series analysis was used to investigate whether the period after either vaccination or infection was associated with risk of decreased leukocyte count. Results: We included 228 adult kidney, lung, and liver transplant recipients. Prior to the first vaccine dose, the mean leukocyte count was 7.3 × 109 cells/L (95% CI 6.9–7.6). Both the leukocyte counts, and the prevalence of decreased leukocyte counts remained unchanged from before to after vaccination regardless of the number of vaccine doses provided. There was no association between vaccination and decreased leukocyte counts (incidence rate ratio (IRR): 0.6; 95% CI: 0.2–2.1; p = 0.461). In contrast, SARS-CoV-2 infection was associated with increased risk of a decreased leukocyte count (IRR: 7.1; 95% CI: 2.8–18.1; p < 0.001). Conclusions: SARS-CoV-2 mRNA vaccination was not associated with risk of decreased leukocyte count and did not affect the prevalence of decreased leukocyte counts in SOT recipients. In contrast, SARS-CoV-2 infection was associated with a higher risk of a decreased leukocyte count.
1. Introduction
Solid organ transplant (SOT) recipients remain at increased risk of severe COVID-19 [1,2]. Vaccination is an effective and important strategy to mitigate this risk [3,4,5]. SARS-CoV-2 mRNA vaccines have been tested in SOT populations and shown safety profiles similar to those in the background population [3,6,7,8,9,10]. Furthermore, vaccination with BNT162b2 has also been reported to be safe in immunocompromised populations other than SOT recipients [10,11,12,13]. However, some studies in healthy individuals have reported adverse hematological events following vaccination. Most of these studies are case series of thrombocytopenia, but one case–control study reported an increased risk of leukopenia after the second dose of the BNT162b2 vaccine [14,15,16,17]. Additionally, two randomized controlled trials of the BNT162b1 vaccine found decreased lymphocyte counts in up to 50% of participants after the first dose [18,19]. Furthermore, a decrease in leukocyte count after influenza vaccination in people aged over 65 has previously been reported [20].
Leukopenia is highly prevalent in SOT recipients due to the use of immunosuppressive drugs and prophylaxis against opportunistic infections [21,22]. Furthermore, leukopenia following SARS-CoV-2 infection in kidney transplant recipients has been reported [23]. In kidney transplant recipients, an increased number of adverse outcomes, including infectious complications, have been reported after a mild degree of decreased leukocyte count [24], and a leukocyte count of <3 × 109 cells/L has been associated with an increased risk of invasive aspergillosis [25]. Furthermore, in SOT recipients, a mild degree of decreased leukocyte count has been found to be associated with an increased hazard of blood stream infection [26] making a decreased leukocyte count a clinically relevant outcome when monitoring vaccine side effects. At present, there are no studies on a decreased leukocyte count as a potential side effect of SARS-CoV-2 mRNA vaccines in SOT recipients. As the beneficial effects of vaccination greatly outweigh the risk of serious side effects [15,27,28], and as vaccines against new variants emerge, vaccination continues to be strongly recommended for high-risk groups like SOT recipients [29,30]. Hence, investigations into the side effects and potential harmful effects of vaccinations are necessary to inform both future vaccination guidelines and transplant clinicians.
We aim to explore whether SARS-CoV-2 mRNA vaccination and SARS-CoV-2 infection are associated with risk of a decreased leukocyte count. Furthermore, we aim to describe the prevalence of decreased leukocyte counts and to describe leukocyte counts in SOT recipients before and after SARS-CoV-2 mRNA vaccination and SARS-CoV-2 infection.
2. Methods
2.1. Study Design
This prospective observational cohort study is based on a study of immune responses to SARS-CoV-2 vaccinations and infections in adult SOT recipients [31,32,33]. From January 2021 through April 2021, adult kidney, lung, and liver transplant recipients followed at Copenhagen University Hospital, Rigshospitalet, were invited to participate in the VACCIM study at the time of their first SARS-CoV-2 vaccination. From July 2021, adult kidney, lung, and liver transplant recipients were invited to participate regardless of vaccination status. All participants were followed until March 2023. In this study we included SOT recipients who lived in the Capital Region of Denmark or Region Zealand throughout the study period and were at least one year from transplantation at the start of follow-up (these participants were considered to be in a stable phase with regard to immunosuppressive maintenance therapy). SOT recipients who received SARS-CoV-2 vaccines other than monovalent or bivalent BNT162b2 vaccines as their first SARS-CoV-2 vaccine dose were excluded. In Denmark, SOT recipients were prioritized to receive BNT162b2 vaccines as their first SARS-CoV-2 vaccine. The first SARS-CoV-2 vaccines in Denmark were administered on 27 December 2020. To start observation at least two months prior to the administration of the first vaccine doses follow-up started on 27 October 2020. Follow-up ended in March 2023, after administration of SARS-CoV-2 vaccines other than monovalent or bivalent BNT162b2 vaccines, death, or re-transplantation, whichever came first. Data were available until March 2023, and participants were followed for the longest time possible to allow for the longest possible follow-up and the administration of as many vaccine doses and leukocyte samples as possible.
In Denmark, SOT recipients are monitored closely with routine biochemistry controls, including leukocyte counts, every 3–6 months. Leukocyte count is a part of the routine hematological panel. This study did not interfere with the Danish COVID-19 vaccination strategy or the sampling frequency of clinical samples from study participants. If a participant followed recommendations by the Danish health authorities during the study, the participant would receive five vaccine doses by the end of follow-up. Additionally, it was possible for clinicians to refer patients to receive extra vaccinations.
All participants provided written and oral informed consent. The study was conducted in accordance with the Declaration of Helsinki and approved by the Regional Scientific Ethics Committee of the Capital Region of Denmark (H-20079890).
2.2. Data Retrieval
Data on vaccinations were collected from the Danish Vaccination Registry (DDV). Since 2015, it has been mandatory to register all vaccines administered in Denmark in the DDV [34]. Data on leukocyte count were retrieved from the laboratory module of the electronic medical records system that covers all hospitals in the Capital Region of Denmark and Region Zealand. Data on demographics, immunosuppressive maintenance therapy, and transplantation-related clinical characteristics were collected from the electronic medical records system. Data on SARS-CoV-2 infections were collected from the Danish Microbiology Database (MiBa), which is a national database including information on all SARS-CoV-2 PCR samples from the primary sector, hospitals, and SARS-CoV-2 test centers in Denmark [35].
2.3. Definitions
We defined a decreased leukocyte count as a leukocyte count below <3.5 × 109 cell/L following Common Terminology for Adverse Events (CTCAE) 5.0 [36]. According to CTCAE 5.0, a decreased leukocyte count can be classified as follows: grade 1 (lower limit normal to 3.0 × 109 cell/L), grade 2 (<3.0–2.0 × 109 cells/L), grade 3 (<2.0–1.0 × 109 cell/L), and grade 4 (<1.0 × 109 cells/L).
2.4. Self-Controlled Case Series Analysis
For the self-controlled case series (SCCS) analysis, we included all participants with a decreased leukocyte count during the study period. Risk periods were defined as days 1–21 after the first vaccine dose and days 1–60 after each SARS-CoV-2 mRNA vaccine dose. The shorter period after the first vaccine dose was due to the recommended interval of 21 days between the first and second vaccine doses. Control periods were defined as time outside of risk periods.
To investigate the relative incidence of decreased leukocyte counts after SARS-CoV-2 infection, we fitted a model with risk periods defined as days 0–60 after positive SARS-CoV-2 PCR tests.
2.5. Statistics
Continuous data were reported as medians with interquartile ranges (IQRs). Categorical data were reported as counts and percentages. Normality of data was assessed by quantile–quantile plots. To report the leukocyte counts before and after each vaccine dose, geometric mean concentrations with 95% confidence intervals (CIs) were calculated. To investigate whether there was a change in leukocyte count from before to after each vaccine dose, a linear mixed model with a compound symmetry covariance structure, random intercept, and slope was fitted using the lmm function from the LMMstar package [37]. The dependent variable of the model was the log-transformed leukocyte count; the independent variable was the time of sampling in relation to vaccine doses. The model was chosen to account for repeated measures and missing data, with the latter being handled by maximum likelihood estimation. Prevalence of decreased leukocyte count was calculated based on number of individuals with decreased leukocyte count in the population two months before and after SARS-CoV-2 infections and after each vaccine dose. Only participants with paired samples available from before and after a vaccine dose or SARS-CoV-2 infection were included in prevalence calculations for the respective vaccine dose or infection. The 95% CIs were calculated using the exact method, and differences in prevalence were tested using McNemar’s test. As the time between the first and second vaccine doses was 21 days in most cases, prevalence was not calculated for the period between the first and second vaccine doses. Instead, prevalences two months before first vaccine dose and two months after second vaccine dose were compared. To explore if the number of vaccine doses administered modified the effect of vaccination and SARS-CoV-2 infection on the prevalence of decreased leukocyte count, a generalized estimating equation (GEE) model with a compound symmetry covariance structure was fitted. The dependent variable was the decreased leukocyte count (binary), the independent variables were the time of sampling relative to vaccination (binary, pre-, or post-vaccine/infection), the number of vaccine doses (continuous), and an interaction term between the timing of sample and number of vaccine doses. To explore whether the relative incidence of the first episode of decreased leukocyte count was higher after the administration of SARS-CoV-2 mRNA vaccines, an extension of the SCCS model that allows for event-dependent exposure was fitted. To accommodate a possible violation of the SCCS model’s assumption that future exposures do not depend on a prior event, where future vaccination may be deferred due to the occurrence of a decreased leukocyte count, the extension of the model was applied. Furthermore, we fitted a standard SCCS model to explore whether the relative incidence of first episode of decreased leukocyte count was higher after SARS-CoV-2 infections than in control periods.
3. Results
3.1. Cohort Characteristics
Out of the 277 SOT recipients included in the VACCIM cohort, we excluded 9 who did not live in the Capital Region of Denmark or Region Zealand throughout the study period, 1 who did not receive BNT162b2 as the first SARS-CoV-2 vaccine, and 39 who were transplanted less than one year prior to the start of follow-up. The study cohort thus consisted of 228 adult kidney, lung, and liver transplant recipients. The median age at baseline was 56 years (IQR 46–64), and 59.6% of participants were male. Of the 228 SOT recipients, 107 (46.9%) were kidney transplant recipients, 87 (38.2%) were liver transplant recipients, and 34 (14.9%) were lung transplant recipients. For more information on comorbidities and immunosuppressive maintenance therapy at the beginning of the follow-up, see Table 1.

Table 1.
The baseline characteristics of the study cohort.
At the end of the study, 6 participants (2.6%) had received six doses of the SARS-CoV-2 mRNA vaccine, while 143 (62.7%) received five doses, 54 (23.7%) received four doses, 22 (9.6%) received three doses, and 3 participants (1.3%) received two doses.
During follow-up, five participants (2.2%) underwent re-transplantation, and seven participants (3.1%) died (Table 1).
3.2. Relative Incidence and Prevalence of Decreased Leukocyte Count Before and After SARS-CoV-2 mRNA Vaccination
During 484 person-years of follow-up, we observed 28 first cases of a decreased leukocyte count, corresponding to an incidence rate of 5.8 per 100 person-years of follow-up (95% CI: 3.8–8.4). For the SCCS analysis, all 28 cases were included (64 person-years of follow-up, 47 person-years of control time, and 17 person-years of risk time). The risk of a decreased leukocyte count was not increased after vaccination compared to the control periods (incidence rate ratio (IRR): 0.6; 95% CI: 0.2–2.1; p = 0.461).
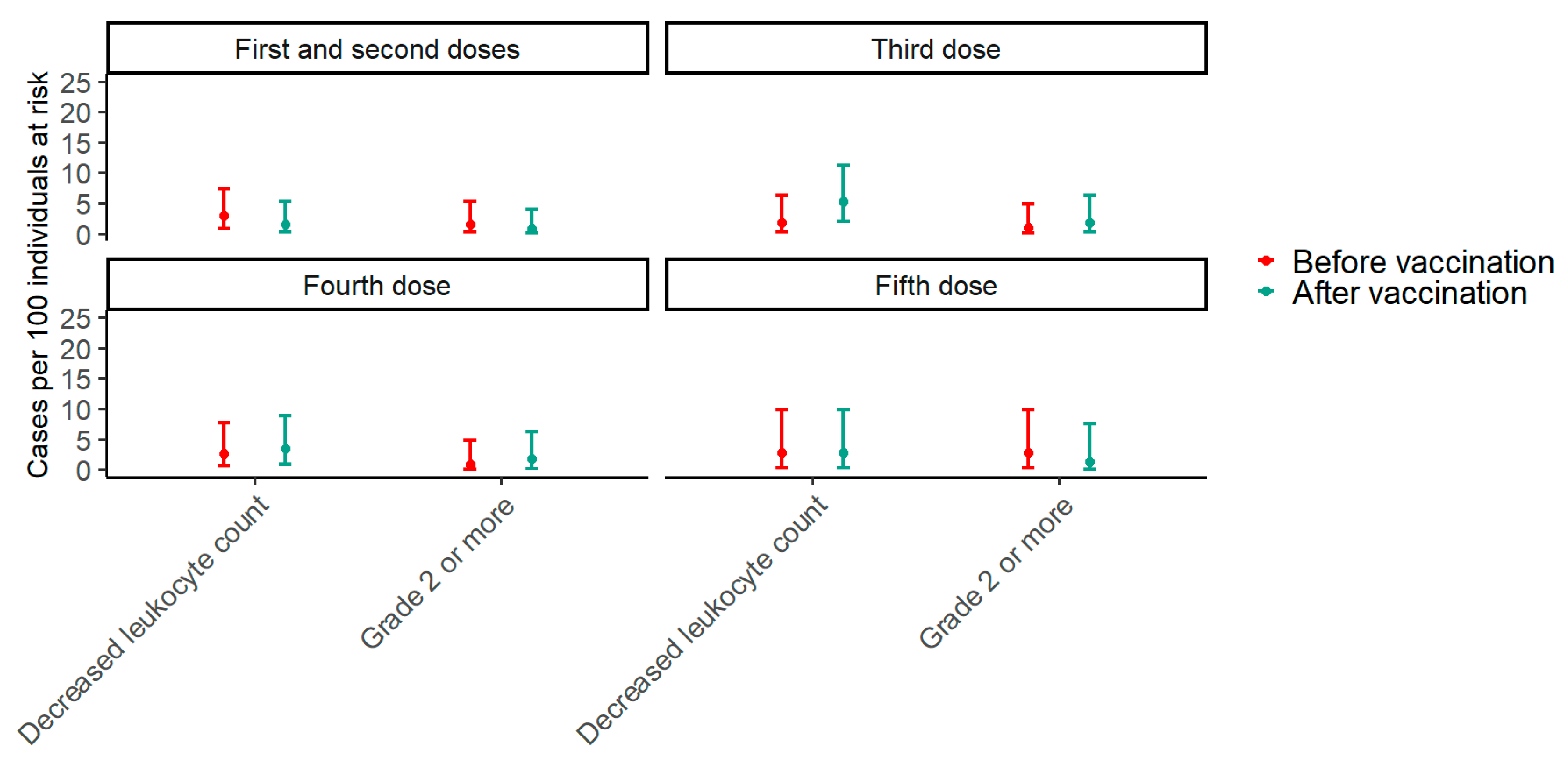
Paired data from before the first vaccine and after the second vaccine dose were available for 136 participants. In the period two months before the first vaccine dose, the prevalence of decreased leukocyte counts was 2.9% (95% CI: 0.8–7.4), while it was 1.4% (95% CI: 0.2–5.2) after the second vaccine dose (p = 0.617). Around the third vaccine dose, paired data were available for 113 participants. In the period two months before the third vaccine dose, the prevalence of decreased leukocyte counts was 0.9% (95% CI: 0.02–4.8) compared with 1.8% (95% CI: 0.2–6.2) after the third dose (p = 0.683). Paired data were available for 112 participants around the fourth vaccine dose. Two months before the fourth dose, the prevalence of decreased leukocyte counts was 0.9% (95% CI: 0.02–4.9), while it was 1.8% (95% CI: 0.2–6.3) after the fourth dose (p >0.999). Finally, around the fifth dose, paired data were available for 71 participants, and the prevalence of decreased leukocyte counts was 2.8% (95% CI: 0.3–9.8) before the fifth vaccine dose compared with 1.4% (95% CI: 0.03–7.6) after the fifth dose (p > 0.999) (Figure 1). To explore trends in the effect of vaccination on the prevalence of decreased leukocyte counts with an increasing number of vaccine doses, a GEE model including an interaction term between the timing of samples in relation to vaccination and the number of vaccine doses was fitted. The p-value for the interaction term was 0.376, indicating that the effect of vaccination on the prevalence of decreased leukocyte counts does not change significantly with the number of vaccine doses.
Figure 1.
The prevalence of decreased leukocyte counts two months before and after each SARS-CoV-2 vaccine dose. The plot shows estimates of prevalence with 95% confidence intervals of any decreased leukocyte count and a grade 2 or higher decrease in leukocyte count two months before and after each SARS-CoV-2 mRNA vaccine dose. For the first and second doses, the “before vaccination” period is the period two months before the first dose, and the “after vaccination” period is the period two months after the second dose. Red represents before vaccination, and blue represents after vaccination. Around the first and second doses, paired data were available for 136 participants; around the third dose, paired data were available for 113 participants; around the fourth dose, paired data were available for 112 participants; and around the fifth dose, paired data were available for 71 participants.
Of the 28 first events of decreased leukocyte counts, 20 (71%) were grade 1, 7 (25%) were grade 2, 1 (4%) was grade 3, and none were grade 4. Among the 28 individuals with episodes of decreased leukocyte counts, the highest grades observed throughout the study period were grade 1 in 12 (43%) individuals, grade 2 in 13 (46%) individuals, grade 3 in 2 (7%) cases, and grade 4 in 1 (4%) case.
3.3. Relative Incidence and Prevalence of Decreased Leukocyte Counts Before and After SARS-CoV-2 Infection
The risk of a decreased leukocyte count was higher 60 days after a PCR-confirmed SARS-CoV2 infection compared to the control periods (IRR: 7.1, 95% CI: 2.8–18.1, p < 0.001).
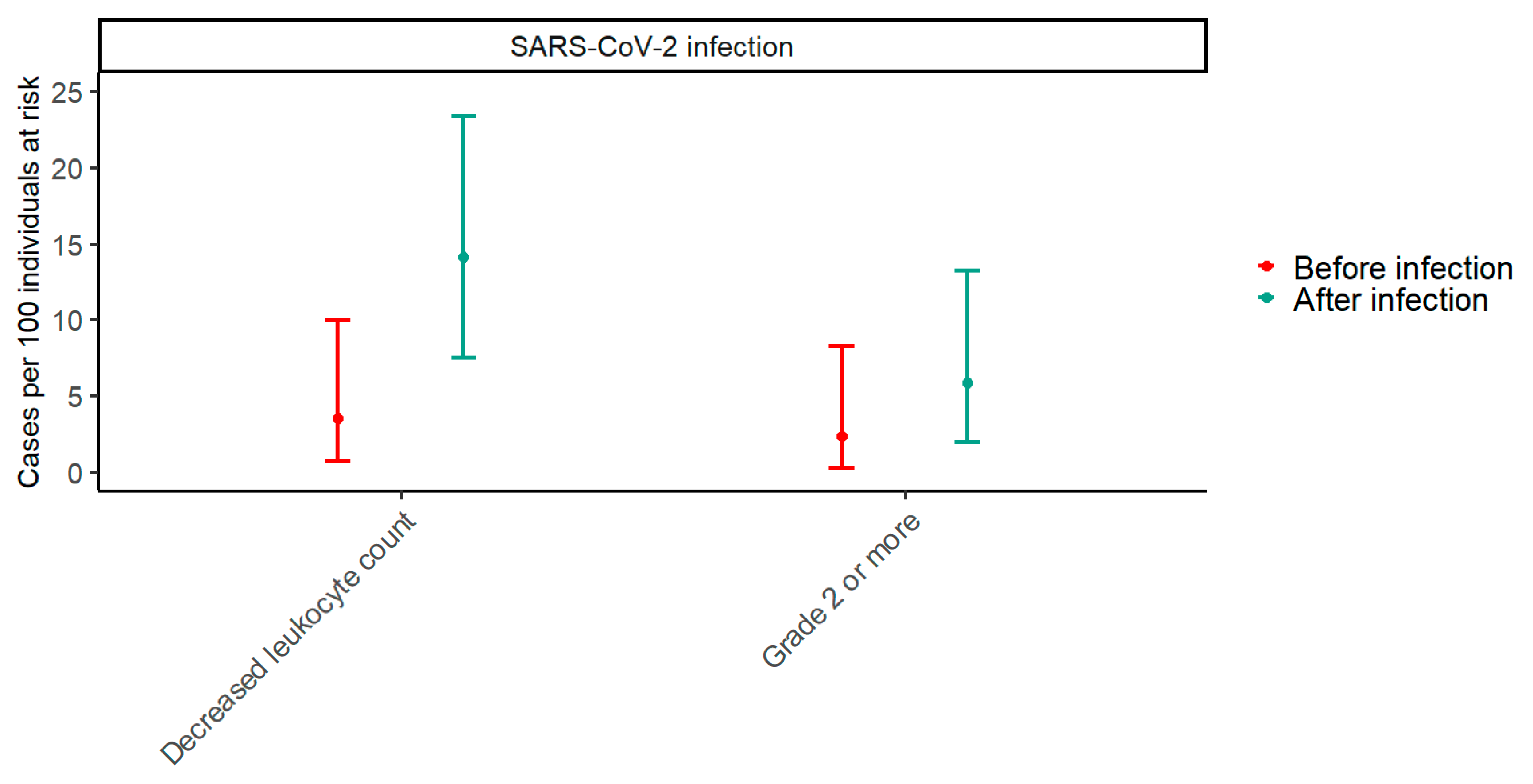
Paired data from before and after SARS-CoV-2 infection were available for 85 participants. In the period two months before SARS-CoV-2 infections, the prevalence of decreased leukocyte counts was 2.4% (95% CI: 0.3–8.2), while it was 5.9% (95% CI: 1.9–13.2) in the period two months after SARS-CoV-2 infections (p = 0.074) (Figure 2). To explore trends in the effect of SARS-CoV-2 on the prevalence of decreased leukocyte counts with an increasing number of vaccine doses, a GEE model including an interaction term between the timing of samples in relation to SARS-CoV-2 infection and the number of vaccine doses was fitted. The p-value for the interaction term was 0.310, indicating that the effect of SARS-CoV-2 infection on the prevalence of decreased leukocyte counts does not change significantly with the number of vaccine doses.
Figure 2.
The prevalence of decreased leukocyte counts two months before and after SARS-CoV-2 infection. The plot shows estimates of prevalence with 95% confidence intervals of any decreased leukocyte count and a grade 2 or higher decrease in leukocyte count two months before and after SARS-CoV-2 infection. Red represents before infection, and blue represents after infection. Paired data with samples from both before and after SARS-CoV-2 infection were available for 85 participants.
3.4. Geometric Mean of Leukocyte Count Before and After Vaccination
Two months prior to the first vaccine dose, the geometric mean leukocyte count was 7.3 × 109 cells/L (95% CI 6.9–7.6) (Table 2). From before to after the first vaccine dose, the geometric mean leukocyte count increased by 2.2% (95% CI: −2.1; +6.8, p = 0.319) (Table 3). Furthermore, the geometric mean leukocyte count decreased by 0.5% from before the first dose to after the second vaccine dose (95% CI: −3.9; +3.1, p = 0.799).

Table 2.
Geometric mean leukocyte counts and missing sample data before and after each vaccine dose.

Table 3.
Percentage of change in white blood cell count between each vaccine dose.
From two months before to two months after the third vaccine dose, the geometric mean leukocyte count increased by 2.9% (95% CI: −0.8; +6.7, p = 0.120), and from before to after the fourth vaccine dose, the geometric mean leukocyte count decreased by 1.8% (95% CI: −5.4; +1.9, p = 0.334). Finally, the geometric mean leukocyte count decreased by 0.8% from before to after the fifth vaccine dose (95% CI: −3.7; +5.4, p = 0.741) (Table 3).
For an overview of the geometric mean leukocyte count and missing data at each sampling time point, see Table 2.
4. Discussion
We found no evidence to support an association between SARS-CoV-2 mRNA vaccination and risk of decreased leukocyte count and no statistically significant differences in prevalence before and after each vaccine dose in SOT recipients. However, we found SARS-CoV-2 infection to be associated with increased risk of a decreased leukocyte count. Furthermore, we examined the leukocyte count before and after each dose of SARS-CoV-2 mRNA vaccine and found no statistically significant changes from before to after vaccination regardless of dose.
We found no statistically significant differences between the prevalence of decreased leukocyte counts before and after any doses of the SARS-CoV-2 mRNA vaccine. Similarly, the risk of a decreased leukocyte count was not higher after vaccination than during the control periods. To our knowledge, no previous studies have examined the prevalence, incidence, or risk of a decreased leukocyte count after SARS-CoV-2 mRNA vaccines in SOT recipients. Sing et al. found that among individuals with no history of prior hematological abnormalities who attended hospitals in Hong Kong, the second dose of BNT162b2 was associated with an IRR of 2.21 for a decreased leukocyte count [15]. Several factors may explain this discrepancy [38]. First, Sing et al. defined a decreased leukocyte count as a leukocyte count of less than 4.0 × 109 cell/L, a higher cutoff than in our study, potentially leading to a higher incidence of decreased leukocyte counts in their study. Second, Sing et al. only analyzed the first and second doses of BNT162b2 and analyzed each dose separately, while we investigated up to the fifth dose of monovalent or bivalent BNT162b2 vaccines without differentiating between doses. Finally, Sing et al. excluded participants receiving immunosuppressive treatment, while all participants in our study were receiving maintenance immunosuppressive treatment. Therefore, both beneficial and potentially harmful immunological responses to vaccines are expected to differ between the populations.
Notably, we observed an increased risk of a decreased leukocyte count in SOT recipients in the period after infection with SARS-CoV-2 compared to the control periods (IRR 7.1). This corroborates previous reports of leukopenia in kidney transplant recipients upon hospitalization with SARS-CoV-2 infection [25], and reports of leukopenia in individuals from Wuhan and New York upon hospitalization with SARS-CoV-2 infections in the early period of the pandemic [39,40]. Most cases of decreased leukocyte counts in our data were grade 1 and 2 adverse events. Grade 1 adverse events, as defined by CTCAE, are mild and/or asymptomatic and do not require clinical intervention. While the clinical implications may be minor, a grade 1 decreased leukocyte count has previously been associated with adverse outcomes such as blood stream infections [24,26] and grade 2 has been associated with invasive aspergillosis [25] and should not be ignored. Several mechanisms, such as de novo development of autoantibodies against leukocytes, migration of leukocytes from the blood to peripheral tissues, viral infiltration and suppression of the bone marrow, and the use of antiviral drugs, may result in a decreased leukocyte count after SARS-CoV-2 infection. Although we cannot determine the precise mechanism behind the association between SARS-CoV-2 infection and leukocyte count based on data in the present study, the lack of association between SARS-CoV-2 vaccination and a decreased leukocyte count suggests that the explanation is not to be found in exposure to the SARS-CoV-2 spike antigen which mRNA in BNT162b2 vaccines encode.
We examined leukocyte counts before and after up to five doses of SARS-CoV-2 mRNA vaccine and found no statistically significant or clinically relevant changes in the leukocyte count from before to after vaccination. To our knowledge, there are no previous reports on the leukocyte count before and after SARS-CoV-2 mRNA vaccination in SOT recipients. In line with our results, a study including 36 individuals who received either one dose of BNT162b2 or one dose of AZD1222 SARS-CoV-2 vaccine found that BNT162b2 did not induce a change in leukocyte counts. In contrast, the adenovector-based AZD1222 vaccine induced a decrease in leukocyte counts [41].
The strengths of our study include leukocyte counts in a well-described and large cohort of SOT recipients, with data from DDV and MiBa, which are national databases providing detailed and complete information on SARS-CoV-2 vaccines and infections, respectively. Furthermore, we have data on the leukocyte count for up to five vaccine doses, providing information relevant in a real-world setting, where most SOT recipients have received more than two vaccine doses. Another strength is the use of SCCS analyses, a statistical method originally developed to investigate rare adverse events after vaccines.
This study also had some limitations. One limitation in this study was the lack of an unvaccinated SOT recipient control group to allow for a comparison of the development in leukocyte count over time in the absence of SARS-CoV-2 mRNA vaccines. Furthermore, the lack of a vaccinated healthy control group hinders any conclusion regarding whether the development in leukocyte count and the incidence of leukopenia after SARS-CoV-2 mRNA vaccines are comparable in SOT recipients and healthy individuals. Finally, this study was limited by missing data and a risk that our sampling may be unbalanced, as the leukocyte count samples were not pre-scheduled and more ill patients may be prone to having additional samples on clinical indication.
Future studies to understand the mechanism behind the impact of SARS-CoV-2 infections on the leukocyte count in SOT recipients and its clinical implications are warranted. Additionally, studies on the leukocyte count after SARS-CoV-2 vaccination in SOT recipients with pre-scheduled blood sampling before and after vaccination would be of value. Finally, it remains important to continue studying potential adverse effects to vaccines in all populations to provide the best foundation for decision making by policymakers, clinicians, and patients.
5. Conclusions
In conclusion, we did not find SARS-CoV-2 mRNA vaccination to be associated with risk of a decreased leukocyte count or to affect the prevalence of decreased leukocyte counts. In contrast, SARS-CoV-2 infection was associated with a higher risk of a decreased leukocyte count, and the prevalence of decreased leukocyte counts was higher after SARS-CoV-2 infection than before infection. Furthermore, we did not find SARS-CoV-2 mRNA vaccination to impact leukocyte counts.
Author Contributions
Conceptualization, S.D.N., S.R.H. and C.R.P.; Methodology, S.R.H., S.D.N. and C.A.S.; Formal Analysis, S.R.H., Investigation, M.K., S.R.H., C.R.P., D.L.M., J.A.L., R.B.H., L.D.H., M.M.P.-H., M.P, S.S.S., A.R., P.G., K.K.I., H.B. and S.D.N.; Data Curation, S.R.H.; Writing—Original Draft Preparation, M.K. and S.R.H.; Writing—Review and Editing, M.K., S.R.H., C.R.P., D.L.M., J.A.L., R.B.H., L.D.H., M.M.P.-H., M.P., S.S.S., A.R., P.G., K.K.I., H.B., C.A.S. and S.D.N.; Visualization, S.R.H.; Supervision, S.D.N.; Funding Acquisition, S.D.N., P.G., H.B. and K.K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Foundation of Rigshospitalet and grants from the Carlsberg Foundation (grant no. CF20-0045), the Novo Nordisk Foundation (grant no. NFF205A0063505 and grant no. NNF20SA0064201), and the Svend Andersen Research Foundation (grant no. SARF2021). The funding sources had no role in the study design, data analysis, data interpretation, writing of the manuscript, or in the decision to submit the paper for publication.
Institutional Review Board Statement
This study was conducted following the Declaration of Helsinki. The study was approved by the Regional Scientific Ethics Committee of the Capital Region of Denmark (H-20079890).
Informed Consent Statement
Informed consent was obtained from all participants involved in this study.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
M.K., S.R.H., C.R.P., J.A.L., R.B.H., L.D.H., M.M.P-H., M.P., S.S.S., A.R., P.G., K.K.I., H.B. and C.A.S. declare no conflicts of interest. D.L.M. has received lecture fees from Takeda not related to this work. S.D.N. has received unrestricted research grants from the Novo Nordisk Foundation, Svend Andersen Fonden, Kirsten og Freddy Johansens Fond, and the Independent Research Fund Denmark and reports advisory board activity for Gilead Sciences, Takeda, and GlaxoSmithKline/ViiV Healthcare, all unrelated to this manuscript.
References
- Hadi, Y.B.; Naqvi, S.F.; Kupec, J.T.; Sofka, S.; Sarwari, A. Outcomes of COVID-19 in Solid Organ Transplant Recipients: A Propensity-matched Analysis of a Large Research Network. Transplantation 2021, 105, 1365–1371. [Google Scholar] [CrossRef]
- Ao, G.; Wang, Y.; Qi, X.; Nasr, B.; Bao, M.; Gao, M.; Sun, Y.; Xie, D. The association between severe or death COVID-19 and solid organ transplantation: A systematic review and meta-analysis. Transplant. Rev. 2021, 35, 100628. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, D.; Yotis, D.M.; Ferreira, V.H.; Shalhoub, S.; Belga, S.; Tyagi, V.; Ierullo, M.; Kulasingam, V.; Hébert, M.-J.; West, L.; et al. Immunogenicity, Safety, and Breakthrough Severe Acute Respiratory Syndrome Coronavirus 2 Infections After Coronavirus Disease 2019 Vaccination in Organ Transplant Recipients: A Prospective Multicenter Canadian Study. Open Forum Infect. Dis. 2023, 10, ofad200. [Google Scholar] [CrossRef] [PubMed]
- Solera, J.T.; Árbol, B.G.; Mittal, A.; Hall, V.; Marinelli, T.; Bahinskaya, I.; Selzner, N.; McDonald, M.; Schiff, J.; Sidhu, A.; et al. Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023. Am. J. Transplant. 2024, 24, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Huh, K.; Kang, M.; Kim, Y.-E.; Choi, Y.; An, S.J.; Seong, J.; Go, M.J.; Kang, J.-M.; Jung, J. Risk of Severe COVID-19 and Protective Effectiveness of Vaccination Among Solid Organ Transplant Recipients. J. Infect. Dis. 2023, 229, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.T.B.; Boyarsky, B.J.; Motter, J.D.M.; Greenberg, R.S.B.; Teles, A.T.B.; Ruddy, J.A.B.; Krach, M.R.; Jain, V.S.B.; Werbel, W.A.; Avery, R.K.; et al. Safety and Reactogenicity of 2 Doses of SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transplantation 2021, 105, 2170–2174. [Google Scholar] [CrossRef]
- Massa, F.; Cremoni, M.; Gérard, A.; Grabsi, H.; Rogier, L.; Blois, M.; Couzin, C.; Ben Hassen, N.; Rouleau, M.; Barbosa, S.; et al. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine 2021, 73, 103679. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; Chen, P.; Söderdahl, G.; Österborg, A.; Smith, C.I.E.; Wullimann, D.; et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine 2021, 74, 103705. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ierullo, M.; Ku, T.; Marinelli, T.; Majchrzak-Kita, B.; Yousuf, A.; Kulasingam, V.; Humar, A.; Kumar, D. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 3980–3989. [Google Scholar] [CrossRef]
- Hirama, T.; Akiba, M.; Shundo, Y.; Watanabe, T.; Watanabe, Y.; Oishi, H.; Niikawa, H.; Okada, Y. Efficacy and safety of mRNA SARS-CoV-2 vaccines in lung transplant recipients. J. Infect. Chemother. 2022, 28, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Shehab, M.; Alrashed, F.; Abdullah, I.; Alfadhli, A.; Ali, H.; Abu-Farha, M.; Channanath, A.M.; Abubaker, J.A.; Al-Mulla, F. Impact of BNT162b2 mRNA Vaccination on the Development of Short and Long-Term Vaccine-Related Adverse Events in Inflammatory Bowel Disease: A Multi-Center Prospective Study. Front. Med. 2022, 9, 881027. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.N.; Zhang, X.; Dai, X.; Watkins, R.; Adler, J.; Dubinsky, M.C.; Kastl, A.; Bousvaros, A.; A Strople, J.; Cross, R.K.; et al. Impact of SARS-CoV-2 Vaccination on Inflammatory Bowel Disease Activity and Development of Vaccine-Related Adverse Events: Results From PREVENT-COVID. Inflamm. Bowel Dis. 2021, 28, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Rahav, G.; Lustig, Y.; Lavee, J.; Benjamini, O.; Magen, H.; Hod, T.; Shem-Tov, N.; Shmueli, E.S.; Merkel, D.; Ben-Ari, Z.; et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: A prospective cohort study. eClinicalMedicine 2021, 41, 101158. [Google Scholar] [CrossRef]
- Lee, E.-J.; Cines, D.B.; Gernsheimer, T.; Kessler, C.; Michel, M.; Tarantino, M.D.; Semple, J.W.; Arnold, D.M.; Godeau, B.; Lambert, M.P.; et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021, 96, 534–537. [Google Scholar] [CrossRef]
- Sing, C.; Tang, C.T.L.; Chui, C.S.L.; Fan, M.; Lai, F.T.T.; Li, X.; Wan, E.Y.F.; Wong, C.K.H.; Chan, E.W.Y.; Hung, I.F.N.; et al. COVID-19 vaccines and risks of hematological abnormalities: Nested case–control and self-controlled case series study. Am. J. Hematol. 2022, 97, 470–480. [Google Scholar] [CrossRef]
- Tarawneh, O.; Tarawneh, H. Immune thrombocytopenia in a 22-year-old post COVID-19 vaccine. Am. J. Hematol. 2021, 96, E133–E134. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immunity Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Cummins, D.; Wilson, M.; Foulger, K.J.; Dawson, D.; Hogarth, A.M. Haematological changes associated with influenza vaccination in people aged over 65: Case report and prospective study. Clin. Lab. Haematol. 1998, 20, 285–287. [Google Scholar] [CrossRef]
- Brum, S.; Nolasco, F.; Sousa, J.; Ferreira, A.; Possante, M.; Pinto, J.; Barroso, E.; Santos, J. Leukopenia in Kidney Transplant Patients With the Association of Valganciclovir and Mycophenolate Mofetil. Transplant. Proc. 2008, 40, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Rerolle, J.-P.; Szelag, J.-C.; Le Meur, Y. Unexpected rate of severe leucopenia with the association of mycophenolate mofetil and valganciclovir in kidney transplant recipients. Nephrol. Dial. Transplant. 2006, 22, 671–672. [Google Scholar] [CrossRef]
- Akalin, E.; Azzi, Y.; Bartash, R.; Seethamraju, H.; Parides, M.; Hemmige, V.; Ross, M.; Forest, S.; Goldstein, Y.D.; Ajaimy, M.; et al. COVID-19 and Kidney Transplantation. N. Engl. J. Med. 2020, 382, 2475–2477. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, M.; Jaenigen, B.; Zschiedrich, S.; Pisarski, P.; Walz, G.; Schneider, J. Risk Factors and Management of Leukopenia After Kidney Transplantation: A Single-Center Experience. Transplant. Proc. 2021, 53, 1589–1598. [Google Scholar] [CrossRef]
- Heylen, L.; Maertens, J.; Naesens, M.; Van Wijngaerden, E.; Lagrou, K.; Bammens, B.; Claes, K.; Evenepoel, P.; Meijers, B.; Kuypers, D.; et al. Invasive Aspergillosis After Kidney Transplant: Case-Control Study. Clin. Infect. Dis. 2015, 60, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Møller, D.L.; Sørensen, S.S.; Perch, M.; Gustafsson, F.; Rezahosseini, O.; Knudsen, A.D.; Scheike, T.; Knudsen, J.D.; Lundgren, J.; Rasmussen, A.; et al. Bacterial and fungal bloodstream infections in solid organ transplant recipients: Results from a Danish cohort with nationwide follow-up. Clin. Microbiol. Infect. 2021, 28, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Zaccardi, F.; Watkinson, P.; Shankar-Hari, M.; Doidge, J.; et al. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. BMJ 2021, 374, n1931. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; E Abara, W.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect. Dis. 2022, 22, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Vaccination Mod Influenza Og COVID-19|Sundhedsstyrelsen. Available online: https://www.sst.dk/da/Vaccination (accessed on 30 September 2024).
- Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States|CDC. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (accessed on 30 September 2024).
- Hamm, S.R.; Møller, D.L.; Pérez-Alós, L.; Hansen, C.B.; Pries-Heje, M.M.; Heftdal, L.D.; Hasselbalch, R.B.; Fogh, K.; Madsen, J.R.; Armenteros, J.J.A.; et al. Decline in Antibody Concentration 6 Months After Two Doses of SARS-CoV-2 BNT162b2 Vaccine in Solid Organ Transplant Recipients and Healthy Controls. Front. Immunol. 2022, 13, 832501. [Google Scholar] [CrossRef]
- Hamm, S.R.; Loft, J.A.; Pérez-Alós, L.; Heftdal, L.D.; Hansen, C.B.; Møller, D.L.; Pries-Heje, M.M.; Hasselbalch, R.B.; Fogh, K.; Hald, A.; et al. The Impact of Time between Booster Doses on Humoral Immune Response in Solid Organ Transplant Recipients Vaccinated with BNT162b2 Vaccines. Viruses 2024, 16, 860. [Google Scholar] [CrossRef]
- Rezahosseini, O.; Hamm, S.R.; Heftdal, L.D.; Pérez-Alós, L.; Møller, D.L.; Perch, M.; Madsen, J.R.; Hald, A.; Hansen, C.B.; Armenteros, J.J.A.; et al. Humoral and T-cell response 12 months after the first BNT162b2 vaccination in solid organ transplant recipients and controls: Kinetics, associated factors, and role of SARS-CoV-2 infection. Front. Immunol. 2023, 13, 1075423. [Google Scholar] [CrossRef]
- Krause, T.G.; Jakobsen, S.; Haarh, M.; Mølbak, K. The Danish vaccination register. Eurosurveillance 2012, 17, 20155. [Google Scholar] [CrossRef]
- Voldstedlund, M.; Haarh, M.; Mølbak, K.; Representatives, t.M.B.o. The Danish Microbiology Database (MiBa) 2010 to 2013. Eurosurveillance 2014, 19. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE). 2021. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 29 October 2024).
- CRAN: Package LMMstar. Available online: https://cran.r-project.org/web/packages/LMMstar/index.html (accessed on 10 October 2024).
- Raval, A.D.; Kistler, K.D.; Tang, Y.; Vincenti, F. Burden of neutropenia and leukopenia among adult kidney transplant recipients: A systematic literature review of observational studies. Transpl. Infect. Dis. 2023, 25, e14000. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R., Jr.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of COVID-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Volzke, J.; Subin, B.; Müller, S.; Sombetzki, M.; Reisinger, E.C.; Müller-Hilke, B. Single-Dose SARS-CoV-2 Vaccinations with Either BNT162b2 or AZD1222 Induce Disparate Th1 Responses and IgA Production. BMC Med. 2022, 20, 29. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).