New High-Throughput Method for Aluminum Content Determination in Vaccine Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. MenB Vaccine and Aluminium Hydroxide Formulation
2.2. Microplate Readers
2.3. Protein Model Selection
2.4. High-Throughput—Automated Liquid Handling Platform
2.5. Performance Evaluation
2.5.1. Precision
2.5.2. Accuracy
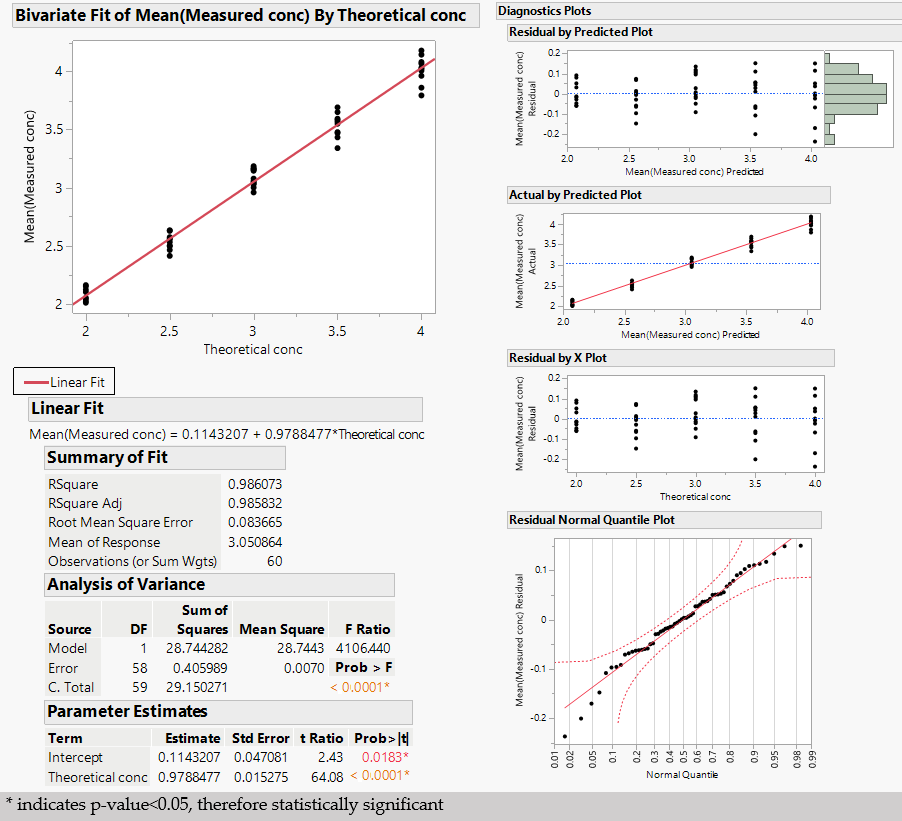
2.5.3. Linearity
2.5.4. Robustness
- -
- 5 samples having the 5 selected different concentrations of aluminum and containing 30% less of the DSs target concentration;
- -
- 5 samples having the 5 selected different concentrations of aluminum and containing 30% more of the DSs target concentration.
2.6. Performance Evaluation Design
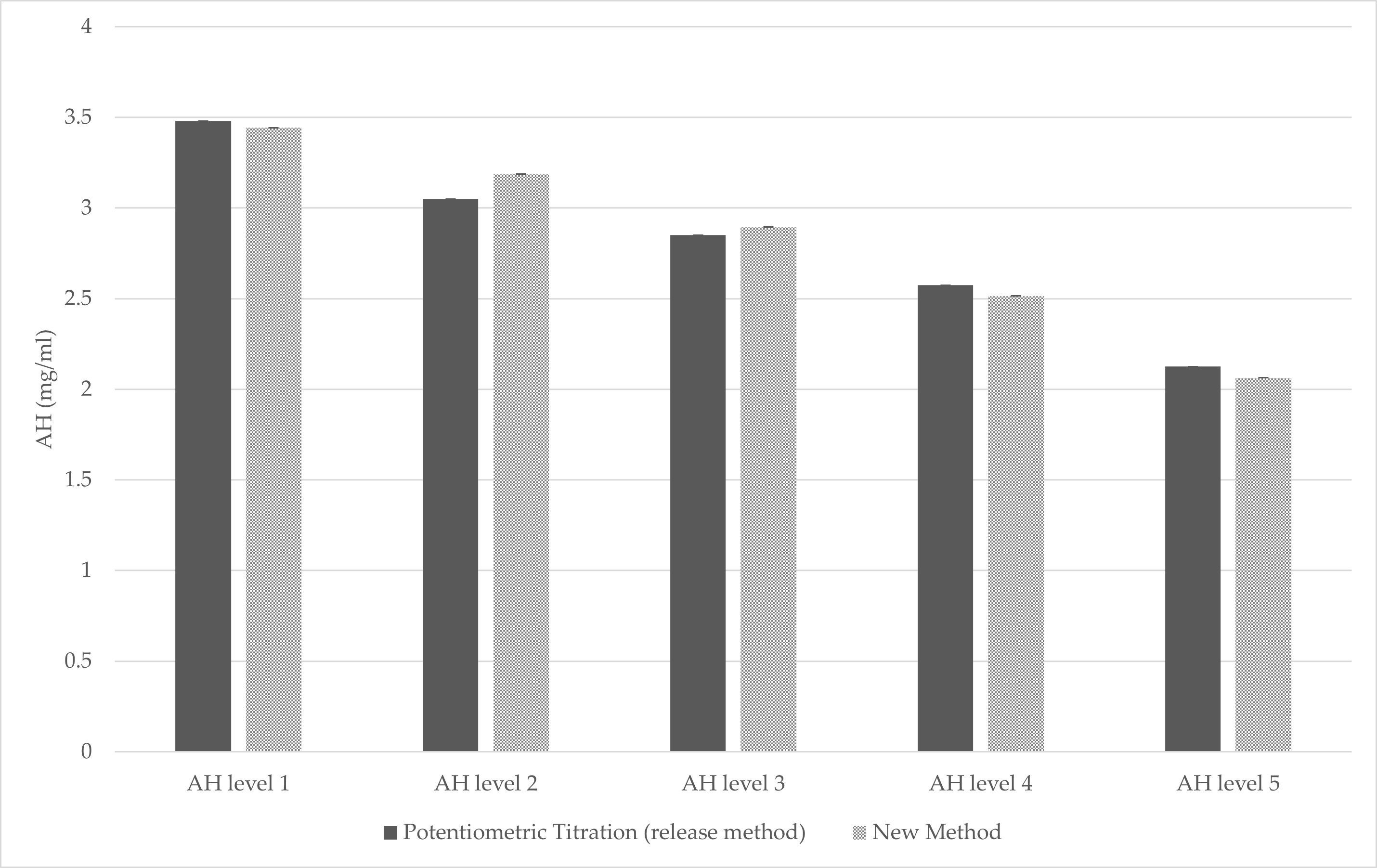
2.7. Bridging with an Orthogonal Method (The Titration Method)
3. Results and Discussions
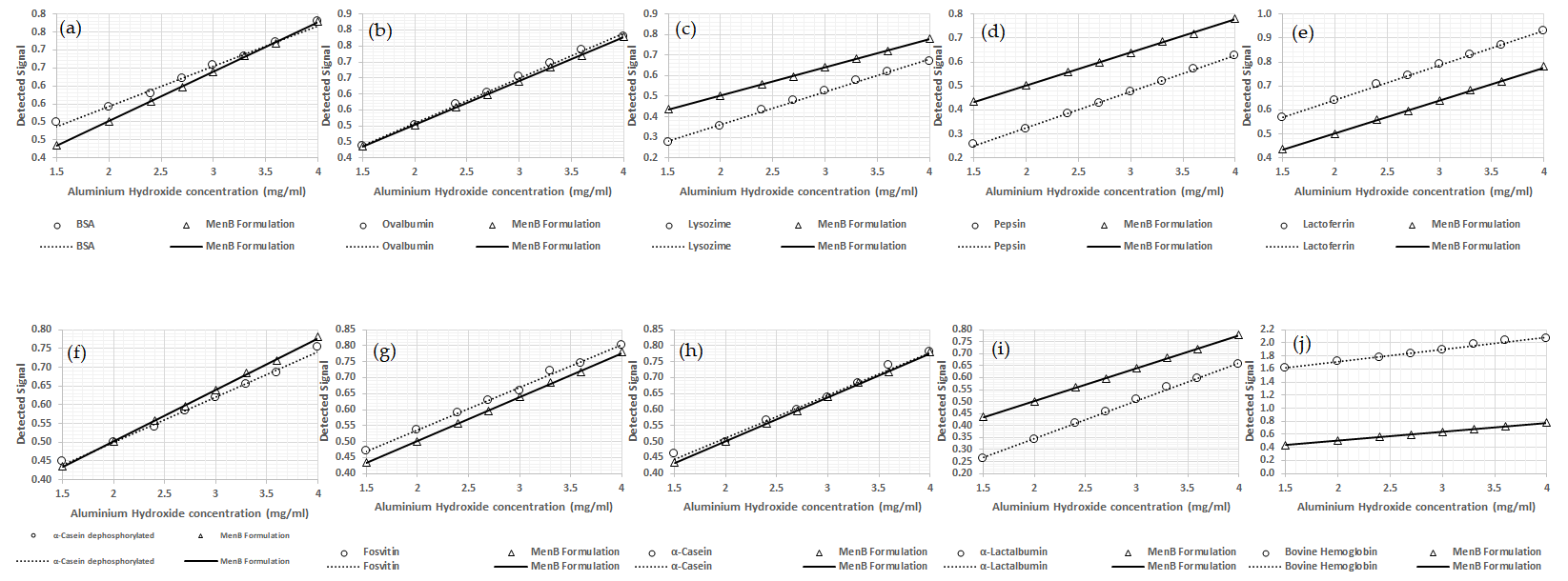
3.1. Protein Model Selection and Results
3.2. Performance Results

| Theoretical Conc. | Mean | Low PI95% | Up PI95% |
|---|---|---|---|
| 2.0 | 3.85 | −2.26 | 9.96 |
| 2.5 | 1.33 | −4.81 | 7.47 |
| 3.0 | 2.85 | −2.87 | 8.56 |
| 3.5 | 1.02 | −5.45 | 7.49 |
| 4.0 | 0.57 | −5.69 | 6.83 |
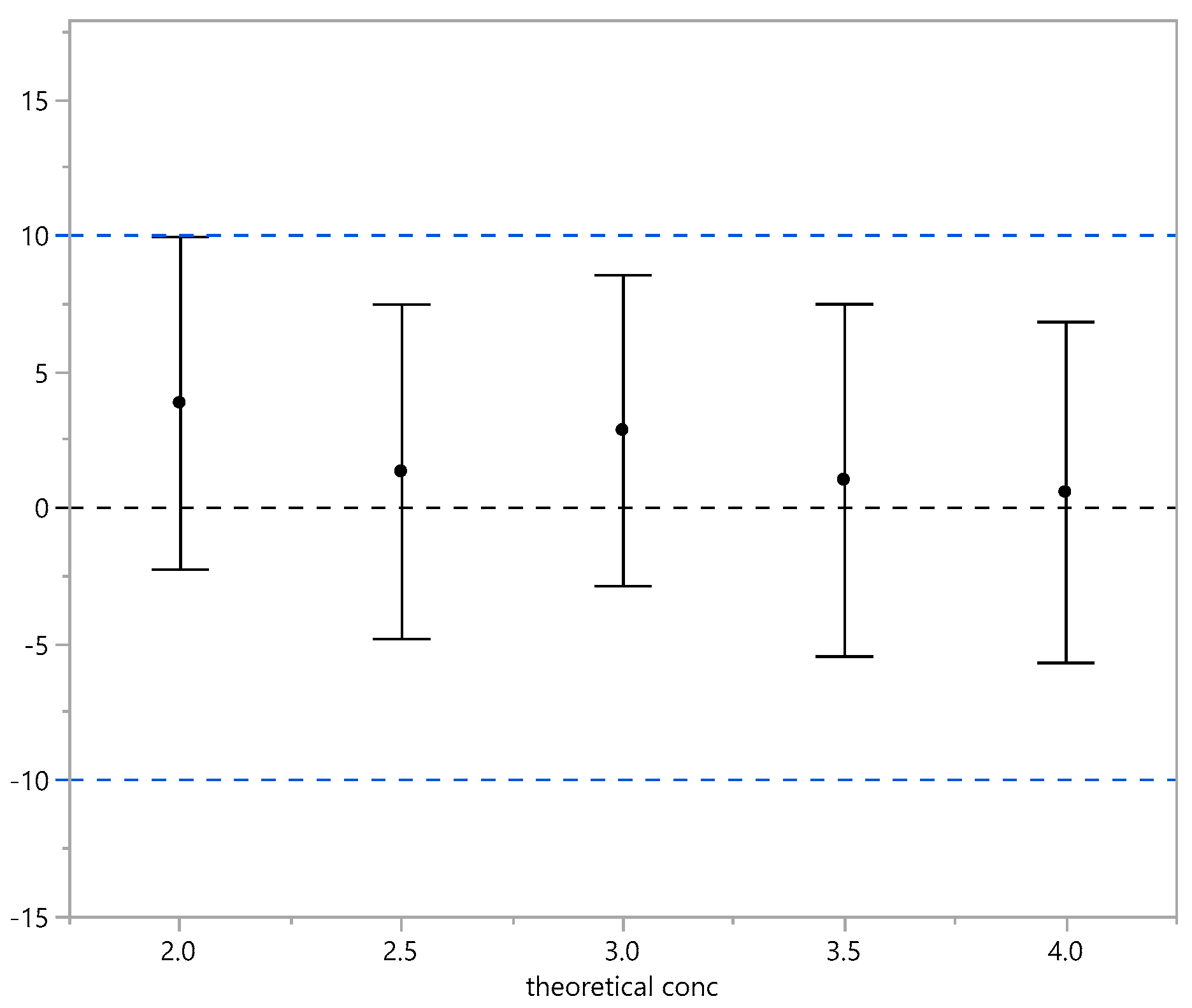
3.3. Bridging Results
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Operat. | Ovalb. Lot | Plate Lot | Session | Rep. | S1 2.0 mg/mL | S2 2.5 mg/mL | S3 3.0 mg/mL | S4 3.5 mg/mL | S5 4.0 mg/mL | Equation |
| Operator1 | 1 | 1 | 1 | 1 | 2.0 | 2.5 | 3.0 | 3.6 | 4.0 | y = 1.02x − 0.06 R2 0.9985 |
| Operator1 | 1 | 1 | 1 | 2 | 2.0 | 2.4 | 3.0 | 3.5 | 4.0 | |
| Operator1 | 2 | 2 | 2 | 1 | 2.1 | 2.5 | 3.2 | 3.6 | 4.0 | y = 0.99x + 0.11 R2 0.9908 |
| Operator1 | 2 | 2 | 2 | 2 | 2.1 | 2.5 | 3.2 | 3.5 | 4.1 | |
| Operator1 | 1 | 1 | 3 | 1 | 2.1 | 2.6 | 3.1 | 3.5 | 3.8 | y = 0.89x + 0.32 R2 0.9916 |
| Operator1 | 1 | 1 | 3 | 2 | 2.0 | 2.5 | 3.0 | 3.5 | 3.8 | |
| Operator1 | 2 | 2 | 4 | 1 | 2.1 | 2.6 | 3.0 | 3.6 | 4.1 | y = 1.02x + 0.02 R2 0.9992 |
| Operator1 | 2 | 2 | 4 | 2 | 2.0 | 2.6 | 3.1 | 3.6 | 4.1 | |
| Operator2 | 1 | 1 | 5 | 1 | 2.1 | 2.5 | 3.2 | 3.3 | 3.9 | y = 0.89x + 0.3 R2 0.9886 |
| Operator2 | 1 | 1 | 5 | 2 | 2.0 | 2.5 | 3.0 | 3.4 | 3.8 | |
| Operator2 | 2 | 2 | 6 | 1 | 2.2 | 2.4 | 3.0 | 3.5 | 3.9 | y = 1x − 0.03 R2 0.9968 |
| Operator2 | 2 | 2 | 6 | 2 | 1.8 | 2.4 | 3.0 | 3.5 | 4.0 | |
| Operator2 | 1 | 1 | 7 | 1 | 2.1 | 2.5 | 3.2 | 3.7 | 4.2 | y = 1.07x − 0.1 R2 0.9949 |
| Operator2 | 1 | 1 | 7 | 2 | 2.0 | 2.5 | 3.2 | 3.6 | 4.1 | |
| Operator2 | 2 | 2 | 8 | 1 | 2.0 | 2.5 | 3.1 | 3.4 | 4.1 | y = 0.98x + 0.09 R2 0.9971 |
| Operator2 | 2 | 2 | 8 | 2 | 2.1 | 2.6 | 3.0 | 3.5 | 4.0 | |
| Operator3 | 1 | 1 | 9 | 1 | 2.1 | 2.7 | 3.1 | 3.5 | 4.0 | y = 0.94x + 0.24 R2 0.9941 |
| Operator3 | 1 | 1 | 9 | 2 | 2.1 | 2.6 | 3.0 | 3.4 | 4.1 | |
| Operator3 | 2 | 2 | 10 | 1 | 2.1 | 2.5 | 3.2 | 3.6 | 4.1 | y = 0.99x + 0.13 R2 0.9981 |
| Operator3 | 2 | 2 | 10 | 2 | 2.2 | 2.6 | 3.0 | 3.6 | 4.1 | |
| Operator3 | 1 | 1 | 11 | 1 | 2.2 | 2.6 | 3.2 | 3.6 | 4.1 | y = 0.99x + 0.17 R2 0.9973 |
| Operator3 | 1 | 1 | 11 | 2 | 2.1 | 2.6 | 3.2 | 3.7 | 4.1 | |
| Operator3 | 2 | 2 | 12 | 1 | 2.1 | 2.5 | 3.1 | 3.7 | 3.9 | y = 1.02x + 0.03 R2 0.9958 |
| Operator3 | 2 | 2 | 12 | 2 | 2.1 | 2.5 | 3.2 | 3.5 | 4.3 |
| Operat. n. | Ovalb. Lot | Plate Lot | Session | Rep. | Level | S1 2.0 mg/mL | S2 2.5 mg/mL | S3 3 mg/mL | S4 3.5 mg/mL | S5 4.0 mg/mL |
| Operator1 | 1 | 1 | 1 | 1 | Low | 1.7 | 2.2 | 3.1 | 3.7 | 4.1 |
| Operator1 | 1 | 1 | 1 | 1 | High | 2.1 | 2.6 | 3.1 | 3.5 | 3.9 |
| Operator1 | 2 | 2 | 2 | 1 | Low | 1.9 | 2.4 | 3.1 | 3.4 | 3.8 |
| Operator1 | 2 | 2 | 2 | 1 | High | 2.2 | 2.6 | 3.1 | 3.5 | 4.0 |
| Operator1 | 1 | 1 | 3 | 1 | Low | 1.9 | 2.4 | 2.8 | 3.5 | 3.9 |
| Operator1 | 1 | 1 | 3 | 1 | High | 2.2 | 2.6 | 3.1 | 3.4 | 3.9 |
| Operator1 | 2 | 2 | 4 | 1 | Low | 2.0 | 2.6 | 3.0 | 3.5 | 3.8 |
| Operator1 | 2 | 2 | 4 | 1 | High | 2.1 | 2.6 | 3.1 | 3.5 | 4.0 |
| Operator2 | 1 | 1 | 5 | 1 | Low | 1.8 | 2.2 | 3.0 | 3.3 | 3.9 |
| Operator2 | 1 | 1 | 5 | 1 | High | 2.1 | 2.5 | 3.0 | 3.4 | 3.8 |
| Operator2 | 2 | 2 | 6 | 1 | Low | 1.8 | 2.5 | 2.9 | 3.6 | 4.2 |
| Operator2 | 2 | 2 | 6 | 1 | High | 2.1 | 2.6 | 3.0 | 3.5 | 3.8 |
| Operator2 | 1 | 1 | 7 | 1 | Low | 1.8 | 2.5 | 2.9 | 3.6 | 4.2 |
| Operator2 | 1 | 1 | 7 | 1 | High | 2.1 | 2.6 | 3.1 | 3.6 | 4.1 |
| Operator2 | 2 | 2 | 8 | 1 | Low | 1.9 | 2.5 | 3.0 | 3.6 | 4.0 |
| Operator2 | 2 | 2 | 8 | 1 | High | 2.1 | 2.6 | 3.1 | 3.6 | 4.0 |
| Operator3 | 1 | 1 | 9 | 1 | Low | 2.0 | 2.4 | 3.1 | 3.4 | 4.0 |
| Operator3 | 1 | 1 | 9 | 1 | High | 2.2 | 2.7 | 3.2 | 3.5 | 3.9 |
| Operator3 | 2 | 2 | 10 | 1 | Low | 2.0 | 2.5 | 2.8 | 3.7 | 3.8 |
| Operator3 | 2 | 2 | 10 | 1 | High | 2.2 | 2.8 | 3.3 | 3.3 | 4.1 |
| Operator3 | 1 | 1 | 11 | 1 | Low | 2.0 | 2.5 | 3.1 | 3.5 | 3.9 |
| Operator3 | 1 | 1 | 11 | 1 | High | 2.3 | 2.7 | 3.3 | 3.4 | 4.1 |
| Operator3 | 2 | 2 | 12 | 1 | Low | 2.0 | 2.4 | 2.8 | 3.5 | 4.1 |
| Operator3 | 2 | 2 | 12 | 1 | High | 2.2 | 2.6 | 3.2 | 3.4 | 4.0 |
References
- De Gregorio, E.; Tritto, E.; Rappuoli, R. Alum adjuvanticity: Unraveling a century old mystery. Eur. J. Immunol. 2008, 38, 2068–2071. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, S.; Bufali, S.; Skibinski, D.A.; O’hagan, D.T.; Singh, M. Aluminium adjuvant dose guidelines in vaccine formulation for preclinical evaluations. J. Pharm. Sci. 2012, 101, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.C.; Shafran, K.L.; Perry, C.C. Potentiometric determination of the ‘formal’ hydrolysis ratio of aluminium species in aqueous solutions. Anal. Chim. Acta 2008, 607, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Tritto, E.; Mosca, F.; De Gregorio, E. Mechanism of action of licensed vaccine adjuvants. Vaccine 2009, 27, 3331–3334. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; MacLennan, C.A. Outer membrane vesicle vaccines. Semin. Immunol. 2020, 50, 101433. [Google Scholar] [CrossRef] [PubMed]
- Rappuoli, R.; Pizza, M.; Masignani, V.; Vadivelu, K. Meningococcal B vaccine (4CMenB): The journey from research to real world experience. Expert Rev. Vaccines 2018, 17, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Vernikos, G.; Medini, D. Bexsero(R) chronicle. Pathog. Glob. Health 2014, 108, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Agnolon, V.; Bruno, C.; Galletti, B.; Mori, E.; Ugozzoli, M.; Pergola, C.; O’hagan, D.T.; Baudner, B.C. Multiplex immunoassay for in vitro characterization of acellular pertussis antigens in combination vaccines. Vaccine 2016, 34, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Laera, D.; Scarpellini, C.; Tavarini, S.; Baudner, B.; Marcelli, A.; Pergola, C.; Meppen, M.; O’Hagan, D.T. Maturation of Aluminium Adsorbed Antigens Contributes to the Creation of Homogeneous Vaccine Formulations. Vaccines 2023, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Lakos, A.; Torzsa, P.; Ferenci, T. Bexsero. a novel vaccine against meningococcus. Orv. Hetil. 2016, 157, 242–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, Z.; Yang, Y.; Yu, G.; Zhu, H.; Xia, X.; Chen, C.; Fu, D.; Li, M.; Cheng, G.; Xue, C.; et al. Engineering aluminum hydroxyphosphate nanoparticles with well-controlled surface property to enhance humoral immune responses as vaccine adjuvants. Biomaterials 2021, 275, 120960. [Google Scholar] [CrossRef]
- Giuliani, M.M.; Adu-Bobie, J.; Comanducci, M.; Aricò, B.; Savino, S.; Santini, L.; Brunelli, B.; Bambini, S.; Biolchi, A.; Capecchi, B.; et al. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 2006, 103, 10834–10839. [Google Scholar] [CrossRef] [PubMed]
- Hogenesch, H. Mechanism of immunopotentiation and safety of aluminium adjuvants. Front. Immunol. 2012, 3, 406. [Google Scholar]
- Aimanianda, V.; Haensler, J.; Lacroix-Desmazes, S.; Kaveri, S.V.; Bayry, J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol. Sci. 2009, 30, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Baylor, N.W.; Egan, W.; Richman, P. Aluminum salts in vaccines—US perspective. Vaccine 2002, 20, S18–S23. [Google Scholar] [CrossRef] [PubMed]
- Shirodkar, S.; Hutchinson, R.L.; Perry, D.L.; White, J.L.; Hem, S.L. Aluminum compounds used as adjuvants in vaccines. Pharm. Res. 1990, 7, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. NPJ Vaccines 2018, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mitkus, R.J.; King, D.B.; Hess, M.A.; Forshee, R.A.; Walderhaug, M.O. Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine 2011, 29, 9538–9543. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.S.; Jones, D.E.; Chou, C.H. Aluminum toxicokinetics regarding infant diet and vaccinations. Vaccine 2002, 20 (Suppl. S3), S13–S17. [Google Scholar] [CrossRef]

| Component | Concentration |
|---|---|
| AH | 3 mg/mL |
| NaCl | 6.25 mg/mL |
| Histidine pH 6.3 | 10 mM |
| 936-741 | 100 mcg/mL |
| 961c | 100 mcg/mL |
| 287-953 | 100 mcg/mL |
| OMV | 50 mcg/mL |
| Sucrose | 2.0% w/v |
| Protein | Product Code | IEP | Molecular Weight (kDa) | Amino Acid Residues | Number of Potentially Phosphorylated Residues |
|---|---|---|---|---|---|
| Ovalbumin | Sigma Cod.A7641 | 4.5 | 42.7 | 385 | Yes (1–2) |
| BSA | Sigma Cod.A9647 | 4.7 | 66.5 | 583 | No |
| Lysozyme (from chicken egg) | Sigma Cod.L6876 | 10.5–11.0 | 14.6 | 129 | No |
| Alpha-casein | Sigma Cod.C6780 | 4.2–4.8 | 22.0–25.0 | 195 | Yes (1–8) |
| Dephosphorylated alpha-casein | Sigma Cod.C8032 | 4.6 | 25 | 195 | No |
| Phosvitin | Sigma Cod.P1253 | 4 | 35 | 217 | Yes (~80) |
| Alpha-lactalbumin | Sigma Cod.L6010 | 4.2–4.5 | 14.2 | 123 | No |
| Bovine hemoglobin | Sigma Cod.H2500 | 6.8 | 64.5 | 574 | No |
| Pepsin | Sigma Cod.P7000 | 3.24 | 34.5 | 326 | No |
| Lactoferrin | Sigma Cod.L9507 | 8.7 | 80 | 700 | No |
| Solution Number | Solution Components | Component Concentration |
|---|---|---|
| 1. Stock solution Std Curve preparation | AH | AH 5 mg/mL; |
| NaCl; | NaCl 6.25 mg/mL; | |
| Histidine; | Histidine pH 6.5 10 mM; | |
| Sucrose; | Sucrose 2% | |
| Protein | Protein 350 mcg/mL | |
| 2. Diluent Solution Std Curve and control (Blank) preparation | NaCl; | NaCl 6.25 mg/mL; |
| Histidine | Histidine pH 6.5 10 mM; | |
| Sucrose | Sucrose 2% | |
| Protein | Protein 350 mcg/mL |
| DS Concentration (∆Target) | Formulation Samples | Al(OH)3 Concentration (mg/mL) |
|---|---|---|
| −30% | 1 LOW | 2.0 |
| −30% | 2 LOW | 2.5 |
| −30% | 3 LOW | 3.0 |
| −30% | 4 LOW | 3.5 |
| −30% | 5 LOW | 4.0 |
| +30% | 1 HIGH | 2.0 |
| +30% | 2 HIGH | 2.5 |
| +30% | 3 HIGH | 3.0 |
| +30% | 4 HIGH | 3.5 |
| +30% | 5 HIGH | 4.0 |
| Operator | Ovalb.Lot | Plate Lot | Session | Replicate | Samples | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1, 2, or 3 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 4 | 5 |
| 2 | 1 | 2 | 3 | 4 | 5 | ||||
| 2 | 2 | 2 | 1 | 1 | 2 | 3 | 4 | 5 | |
| 2 | 1 | 2 | 3 | 4 | 5 | ||||
| 1 | 1 | 3 | 1 | 1 | 2 | 3 | 4 | 5 | |
| 2 | 1 | 2 | 3 | 4 | 5 | ||||
| 2 | 2 | 4 | 1 | 1 | 2 | 3 | 4 | 5 | |
| 2 | 1 | 2 | 3 | 4 | 5 | ||||
| Protein | R2 | Equation |
|---|---|---|
| Ovalbumin | 0.998 | Y = 0.1406 + 0.2265 |
| BSA | 0.994 | Y = 0.1126x + 0.3182 |
| Lysozyme (from chicken egg) | 0.997 | Y = 0.1602x + 0.0400 |
| Alpha-casein | 0.991 | Y = 0.1338x + 0.2446 |
| Dephosphorylated alpha-casein | 0.995 | Y = 0.1211x + 0.2570 |
| Fosvitin | 0.996 | Y = 0.1328x + 0.2705 |
| Alpha-lactalbumin | 0.999 | Y = 0.1579x + 0.2940 |
| Bovine hemoglobin | 0.982 | Y = 0.1893x + 1.3284 |
| Pepsin | 0.999 | Y = 0.151x + 0.0221 |
| Lactoferrin | 0.999 | Y = 0.1436x + 0.3557 |
| MenB antigen reference sample | 0.999 | Y = 0.1374x + 0.2275 |
| Theoretical Alum Concentration | Mean | Random Effect | Variance Component | SD | CV% Repeatability | CV% Intermediate Precision |
|---|---|---|---|---|---|---|
| 2.0 mg/mL | 2.1 | operator | 0.0024871 | 0.049870833 | ||
| operator[session] | 0 | 0 | ||||
| Residual | 0.0048873 | 0.069909227 | 3.4% | |||
| Total | 0.0073744 | 0.085874327 | 4.1% | |||
| 2.5 mg/mL | 2.5 | operator | 0.0016754 | 0.04093165 | ||
| operator[session] | 0.0028267 | 0.053166719 | ||||
| Residual | 0.0008967 | 0.029944949 | 1.2% | |||
| Total | 0.0053988 | 0.073476527 | 2.9% | |||
| 3.0 mg/mL | 3.1 | operator | 0.0006915 | 0.026296388 | ||
| operator[session] | 0.0042188 | 0.06495229 | ||||
| Residual | 0.0017581 | 0.041929703 | 1.4% | |||
| Total | 0.0066683 | 0.08165966 | 2.6% | |||
| 3.5 mg/mL | 3.5 | operator | 0.0009079 | 0.030131379 | ||
| operator[session] | 0.0076644 | 0.087546559 | ||||
| Residual | 0.0028679 | 0.053552778 | 1.5% | |||
| Total | 0.0114402 | 0.106958871 | 3.0% | |||
| 4.0 mg/mL | 4.0 | operator | 0 | 0 | ||
| operator[session] | 0.0079019 | 0.088892632 | ||||
| Residual | 0.0080927 | 0.089959435 | 2.2% | |||
| Total | 0.0159947 | 0.126470155 | 3.1% |
| Theoretical Level | Mean (Conc) | Mean (RB%) | Low CI90% | Up CI90% |
|---|---|---|---|---|
| 2.0 | 2.077 | 3.85 | 2.47 | 5.23 |
| 2.5 | 2.533 | 1.33 | −0.06 | 2.72 |
| 3.0 | 3.085 | 2.85 | 1.55 | 4.14 |
| 3.5 | 3.536 | 1.02 | −0.44 | 2.48 |
| 4.0 | 4.023 | 0.57 | −0.84 | 1.99 |
| Sample/Level | Theoretical Alum Conc. (mg/mL) | Mean | IP—CV% |
|---|---|---|---|
| 1 LOW | 2.0 | 1.92 | 5.4 |
| 2 LOW | 2.5 | 2.43 | 4.4 |
| 3 LOW | 3.0 | 2.98 | 3.8 |
| 4 LOW | 3.5 | 3.52 | 3.2 |
| 5 LOW | 4.0 | 3.99 | 4.0 |
| 1 HIGH | 2.0 | 2.16 | 2.9 |
| 2 HIGH | 2.5 | 2.64 | 3.0 |
| 3 HIGH | 3.0 | 3.12 | 3.2 |
| 4 HIGH | 3.5 | 3.46 | 2.7 |
| 5 HIGH | 4.0 | 3.96 | 2.2 |
| Sample/Level | Theoretical Alum Conc. (mg/mL) | Mean | Mean (RB%) | Low CI90% | Up CI90% |
|---|---|---|---|---|---|
| 1 LOW | 2.0 | 1.92 | −4.2 | −6.88 | −1.51 |
| 2 LOW | 2.5 | 2.43 | −3.0 | −5.19 | −0.79 |
| 3 LOW | 3.0 | 2.98 | −0.6 | −2.52 | 1.42 |
| 4 LOW | 3.5 | 3.52 | 0.7 | −1.03 | 2.33 |
| 5 LOW | 4.0 | 3.99 | −0.3 | −2.39 | 1.77 |
| 1 HIGH | 2.0 | 2.16 | 8.0 | 6.37 | 9.56 |
| 2 HIGH | 2.5 | 2.64 | 5.5 | 3.89 | 7.15 |
| 3 HIGH | 3.0 | 3.12 | 4.0 | 2.34 | 5.75 |
| 4 HIGH | 3.5 | 3.46 | −1.1 | −2.42 | 0.32 |
| 5 HIGH | 4.0 | 3.96 | −1.0 | −2.10 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Meola, L.; Pasqui, D.; Tigli, C.; Luckham, S.; Colomba, S.; Paludi, M.; Denis, M.; Palmese, A.; Stranges, D.; Marcelli, A.; et al. New High-Throughput Method for Aluminum Content Determination in Vaccine Formulations. Vaccines 2025, 13, 105. https://doi.org/10.3390/vaccines13020105
Di Meola L, Pasqui D, Tigli C, Luckham S, Colomba S, Paludi M, Denis M, Palmese A, Stranges D, Marcelli A, et al. New High-Throughput Method for Aluminum Content Determination in Vaccine Formulations. Vaccines. 2025; 13(2):105. https://doi.org/10.3390/vaccines13020105
Chicago/Turabian StyleDi Meola, Lorenzo, Daniela Pasqui, Chiara Tigli, Stephen Luckham, Silvio Colomba, Marilena Paludi, Maxime Denis, Angelo Palmese, Daniela Stranges, Agnese Marcelli, and et al. 2025. "New High-Throughput Method for Aluminum Content Determination in Vaccine Formulations" Vaccines 13, no. 2: 105. https://doi.org/10.3390/vaccines13020105
APA StyleDi Meola, L., Pasqui, D., Tigli, C., Luckham, S., Colomba, S., Paludi, M., Denis, M., Palmese, A., Stranges, D., Marcelli, A., Moriconi, A., Meppen, M., & Pergola, C. (2025). New High-Throughput Method for Aluminum Content Determination in Vaccine Formulations. Vaccines, 13(2), 105. https://doi.org/10.3390/vaccines13020105






