Immunological Insights and Therapeutic Advances in COPD: Exploring Oral Bacterial Vaccines for Immune Modulation and Clinical Improvement
Abstract
1. Introduction
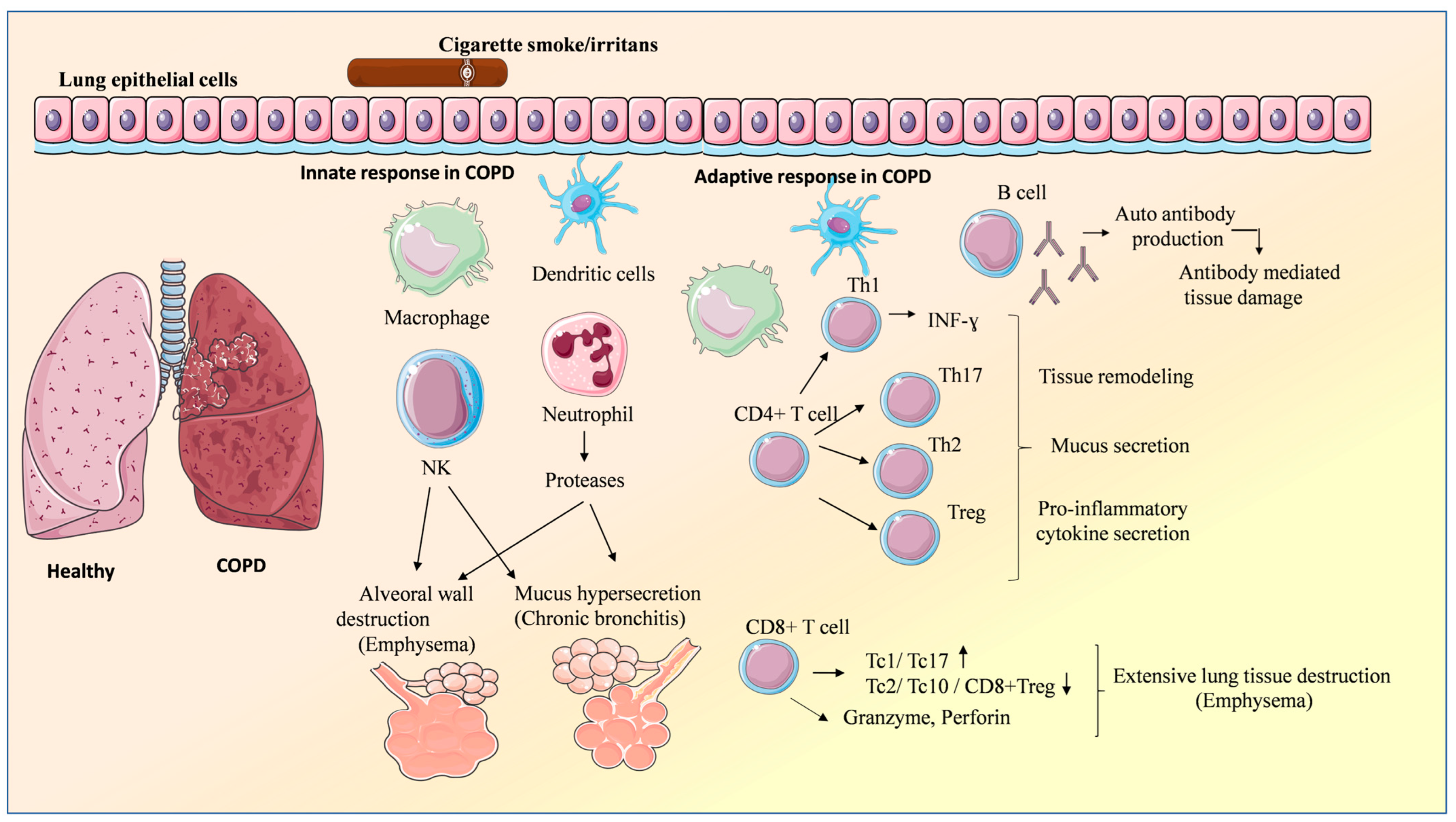
2. Characteristic of COPD
3. Role of Immune System in COPD
| Cells Type | Lungs | ||
|---|---|---|---|
| Cell Frequency | Cell Phenotype/Subpopulations | Correlation with COPD Severity | |
| Neutrophils | Increase | Proteases neutrophil elastase, cathepsin G, proteinase 3 | Positive Alveoral wall destruction—emphysema; mucus hypersecretion—chronic bronchitis COPD severity |
| Macrophages | Increase | M2 | Positive |
| Dendritic cells | Increase | - | Positive |
| NK cells | Increase | CD56 ↓ | Positive Alveoral wall destruction—emphysema; mucus hypersecretion—chronic bronchitis COPD severity |
| CD57 ↑ | |||
| (CD69 + CD25-, CD69 + CD25+, CD69-CD25+)↑ | |||
| T cytotoxic CD8 | Increase | Tc1cell/Tc17cell ↑ | Positive Proliferation, apoptosis of pro-inflammatory CD8+ cell, mucus hypersecretion |
| Tc2 cell/Tc10 cell/CD8+ Treg cell ↓ | |||
| IFN-γ TNF-α ↑ | |||
| IL-4 IL-13 ↑ | |||
| Tc17 cell IL-17A, IL-17F ↑ | |||
| Granzyme, Perforin ↑ | |||
| T helper Th17 | Increase | ↑ IL-17 → IL-1β, IL-6, G-CSF, GM-CSF, TNF-α, CXCL8 | Positive Tissue remodeling |
| Treg cells | ↑ rTreg, ↓ aTreg ↑Treg (FrIII)- | Positive Pro-inflammatory cytokine secretion | |
| B cells | Increase | IgG B cell | Positive Antibody mediated tissue damage |
3.1. Innate Immunity
3.1.1. Lung Epithelial Cells
3.1.2. Natural Killer (NK) Cells
3.1.3. Neutrophils
3.2. Adaptive Immunity
3.2.1. CD8+ T Cells
3.2.2. CD4+ T Cells
3.2.3. B-Cells
4. Standard and Alternative Methods of COPD Treatment
4.1. Inhibitors
4.2. Antibodies
| Inhibitors | ||
| Roflumilast | PDE4 inhibitor, reduces exacerbations and hospitalizations in severe COPD. | [113,114] |
| IC-87114 | PI3K inhibitor, reduces neutrophils recruitment, restores corticosteroid sensitivity | [95] |
| LY 294002 | PI3K inhibitor, inhibits the expression of intercellular adhesion molecule−1(ICAM-1) in COPD patients, mediating monocyte/macrophage adhesion and infiltrating | [96] |
| NVS-PI3K-2, -3, -5 | PI3K inhibitor, suppresses lung inflammation and bacterial colonization in COPD patients. | [50] |
| Elafin | Serine protease inhibitor, offers protection against emphysematous lung damage from neutrophil elastase, enhancing host defenses by activating pulmonary dendritic cell | [97] |
| MK-7123/navarixin | IL-8 receptor antagonist, improvement in forced expiratory volume in 1 | [100] |
| Antibodies | ||
| Abx | Blocking IL-8 results in a significant improvement in dyspnea, measured using the transitional dyspnea index | [99] |
| Itepekimab | Blocking IL-33 reduces inflammation of the airways | [103] |
| Astegolimab | Blocking IL-33 binding to the ST2 reduces exacerbations in patients with moderate to very severe COPD | [104] |
| Tozorakimab | Blocking IL-33 binding to the ST2 receptor causes a decrease in excess inflammation and epithelial remodeling in diseases caused by IL-33 | [105] |
| Mepolizumab | Blocking IL-5 reduces the frequency of moderate and severe exacerbations and extends the time between subsequent exacerbations | [106] |
| Benralizumab | Blocking the alpha subunit of the interleukin 5 receptor reduces the risk of moderate or severe COPD exacerbation within 30 or 90 days | [107] |
| Dupilumab | Blocking IL-4/13 receptor results in fewer exacerbations, better lung function and quality of life, and less severe respiratory symptoms | [108] |
| Belimumab | Blocking B-cell activating factor (BAFF) reduces autoantibody anti-GRP78 levels | [111] |
5. Potential of Oral Bacterial Vaccines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, R.-R.; Hao, K.; Yang, T. Air Pollution and Chronic Obstructive Pulmonary Disease. Chronic Dis. Transl. Med. 2020, 6, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Footitt, J.; Marczynski, M.; Radicioni, G.; Cross, M.T.; Finney, L.J.; Trujillo-Torralbo, M.-B.; Calderazzo, M.; Zhu, J.; Aniscenko, J.; et al. Airway Mucins Promote Immunopathology in Virus-Exacerbated Chronic Obstructive Pulmonary Disease. J. Clin. Investig. 2022, 132, e120901. [Google Scholar] [CrossRef]
- Tsiligianni, I.; Kocks, J.W.H. Daytime Symptoms of Chronic Obstructive Pulmonary Disease: A Systematic Review. NPJ Prim. Care Respir. Med. 2020, 30, 6. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Wen, F. Clinical Significance of Airway Mucus Hypersecretion in Chronic Obstructive Pulmonary Disease. J. Transl. Int. Med. 2015, 3, 89–92. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Raja, A.; Brown, B.D. Chronic Obstructive Pulmonary Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Li, X. Impact of Exercise Capacity Upon Respiratory Functions, Perception of Dyspnea, and Quality of Life in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstruct. Pulmon. Dis. 2021, 16, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Gea, J.G.; Pasto, M.; Carmona, M.A.; Orozco-Levi, M.; Palomeque, J.; Broquetas, J. Metabolic Characteristics of the Deltoid Muscle in Patients with Chronic Obstructive Pulmonary Disease. Eur. Respir. J. 2001, 17, 939–945. [Google Scholar] [CrossRef]
- Martínez-Gestoso, S.; García-Sanz, M.-T.; Carreira, J.-M.; Salgado, F.-J.; Calvo-Álvarez, U.; Doval-Oubiña, L.; Camba-Matos, S.; Peleteiro-Pedraza, L.; González-Pérez, M.-A.; Penela-Penela, P.; et al. Impact of Anxiety and Depression on the Prognosis of Copd Exacerbations. BMC Pulm. Med. 2022, 22, 169. [Google Scholar] [CrossRef]
- Love, M.E.; Proud, D. Respiratory Viral and Bacterial Exacerbations of COPD—The Role of the Airway Epithelium. Cells 2022, 11, 1416. [Google Scholar] [CrossRef]
- Taucher, E.; Mykoliuk, I.; Lindenmann, J.; Smolle-Juettner, F.-M. Implications of the Immune Landscape in COPD and Lung Cancer: Smoking Versus Other Causes. Front. Immunol. 2022, 13, 846605. [Google Scholar] [CrossRef] [PubMed]
- Mallia, P.; Message, S.D.; Gielen, V.; Contoli, M.; Gray, K.; Kebadze, T.; Aniscenko, J.; Laza-Stanca, V.; Edwards, M.R.; Slater, L.; et al. Experimental Rhinovirus Infection as a Human Model of Chronic Obstructive Pulmonary Disease Exacerbation. Am. J. Respir. Crit. Care Med. 2011, 183, 734–742. [Google Scholar] [CrossRef]
- Sajjan, U.S. Susceptibility to Viral Infections in Chronic Obstructive Pulmonary Disease: Role of Epithelial Cells. Curr. Opin. Pulm. Med. 2013, 19, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kuwal, A.; Joshi, V.; Dutt, N.; Singh, S.; Agarwal, K.C.; Purohit, G. A Prospective Study of Bacteriological Etiology in Hospitalized Acute Exacerbation of COPD Patients: Relationship with Lung Function and Respiratory Failure. Turk. Thorac. J. 2018, 19, 19–27. [Google Scholar] [CrossRef]
- Tong, X.; Cheng, A.; Xu, H.; Jin, J.; Yang, Y.; Zhu, S.; Li, Y. Aspergillus Fumigatus during COPD Exacerbation: A Pair-Matched Retrospective Study. BMC Pulm. Med. 2018, 18, 55. [Google Scholar] [CrossRef]
- Sethi, S.; Murphy, T.F. Infection in the Pathogenesis and Course of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- De Sanctis, J.B.; Moreno, D.; Larocca, N.; Garmendia, J.V. IgG Antibody Titers Against Ascaris Lumbricoides, Strongyloides Stercolaris, and Toxocara Canis in Venezuelan Patients with Asthma or COPD. Trop. Med. Infect. Dis. 2024, 9, 253. [Google Scholar] [CrossRef]
- Pouwels, S.D.; Heijink, I.H.; ten Hacken, N.H.T.; Vandenabeele, P.; Krysko, D.V.; Nawijn, M.C.; van Oosterhout, A.J.M. DAMPs Activating Innate and Adaptive Immune Responses in COPD. Mucosal Immunol. 2014, 7, 215–226. [Google Scholar] [CrossRef]
- Gopallawa, I.; Dehinwal, R.; Bhatia, V.; Gujar, V.; Chirmule, N. A Four-Part Guide to Lung Immunology: Invasion, Inflammation, Immunity, and Intervention. Front. Immunol. 2023, 14, 1119564. [Google Scholar] [CrossRef]
- Eckhardt, C.M.; Wu, H. Environmental Exposures and Lung Aging: Molecular Mechanisms and Implications for Improving Respiratory Health. Curr. Environ. Health Rep. 2021, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Kia’i, N.; Bajaj, T. Histology, Respiratory Epithelium. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Johnston, S.L.; Goldblatt, D.L.; Evans, S.E.; Tuvim, M.J.; Dickey, B.F. Airway Epithelial Innate Immunity. Front. Physiol. 2021, 12, 749077. [Google Scholar] [CrossRef] [PubMed]
- Aghapour, M.; Ubags, N.D.; Bruder, D.; Hiemstra, P.S.; Sidhaye, V.; Rezaee, F.; Heijink, I.H. Role of Air Pollutants in Airway Epithelial Barrier Dysfunction in Asthma and COPD. Eur. Respir. Rev. 2022, 31, 210112. [Google Scholar] [CrossRef]
- Burgoyne, R.A.; Fisher, A.J.; Borthwick, L.A. The Role of Epithelial Damage in the Pulmonary Immune Response. Cells 2021, 10, 2763. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wu, Y.; Xu, Y.; Zheng, J. SIgA in Various Pulmonary Diseases. Eur. J. Med. Res. 2023, 28, 299. [Google Scholar] [CrossRef]
- Osterburg, A.R.; Lach, L.; Panos, R.J.; Borchers, M.T. Unique Natural Killer Cell Subpopulations Are Associated with Exacerbation Risk in Chronic Obstructive Pulmonary Disease. Sci. Rep. 2020, 10, 1238. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Montes, J.F.; Prats, A.; Rodríguez, E.; Montero, M.A.; García-Valero, J.; Ferrer, J. Significant Increase of CD57+ Cells in Pulmonary Lymphoid Follicles of COPD Patients. Eur. Respir. J. 2011, 37, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Brajer-Luftmann, B.; Trafas, T.; Stelmach-Mardas, M.; Bendowska, W.; Piorunek, T.; Grabicki, M.; Kaczmarek, M. Natural Killer Cells as a Further Insight into the Course of Chronic Obstructive Pulmonary Disease. Biomedicines 2024, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Urbanowicz, R.A.; Tighe, P.J.; Todd, I.; Corne, J.M.; Fairclough, L.C. Differential Activation of Killer Cells in the Circulation and the Lung: A Study of Current Smoking Status and Chronic Obstructive Pulmonary Disease (COPD). PLoS ONE 2013, 8, e58556. [Google Scholar] [CrossRef]
- Hodge, G.; Mukaro, V.; Holmes, M.; Reynolds, P.N.; Hodge, S. Enhanced Cytotoxic Function of Natural Killer and Natural Killer T-like Cells Associated with Decreased CD94 (Kp43) in the Chronic Obstructive Pulmonary Disease Airway. Respirology 2013, 18, 369–376. [Google Scholar] [CrossRef]
- Pascual-Guardia, S.; Ataya, M.; Ramírez-Martínez, I.; Yélamos, J.; Chalela, R.; Bellido, S.; López-Botet, M.; Gea, J. Adaptive NKG2C+ Natural Killer Cells Are Related to Exacerbations and Nutritional Abnormalities in COPD Patients. Respir. Res. 2020, 21, 63. [Google Scholar] [CrossRef]
- Freeman, C.M.; Stolberg, V.R.; Crudgington, S.; Martinez, F.J.; Han, M.K.; Chensue, S.W.; Arenberg, D.A.; Meldrum, C.A.; McCloskey, L.; Curtis, J.L. Human CD56+ Cytotoxic Lung Lymphocytes Kill Autologous Lung Cells in Chronic Obstructive Pulmonary Disease. PLoS ONE 2014, 9, e103840. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Wang, M.; Zou, Q.; Zhao, S.; Sun, B.; Xu, L.; Jiang, Y. Increased Numbers of NK Cells, NKT-like Cells, and NK Inhibitory Receptors in Peripheral Blood of Patients with Chronic Obstructive Pulmonary Disease. Clin. Dev. Immunol. 2013, 2013, 721782. [Google Scholar] [CrossRef]
- Finch, D.K.; Stolberg, V.R.; Ferguson, J.; Alikaj, H.; Kady, M.R.; Richmond, B.W.; Polosukhin, V.V.; Blackwell, T.S.; McCloskey, L.; Curtis, J.L.; et al. Lung Dendritic Cells Drive Natural Killer Cytotoxicity in Chronic Obstructive Pulmonary Disease via IL-15Rα. Am. J. Respir. Crit. Care Med. 2018, 198, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, R.A.; Lamb, J.R.; Todd, I.; Corne, J.M.; Fairclough, L.C. Altered Effector Function of Peripheral Cytotoxic Cells in COPD. Respir. Res. 2009, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Jasper, A.E.; McIver, W.J.; Sapey, E.; Walton, G.M. Understanding the Role of Neutrophils in Chronic Inflammatory Airway Disease. F1000Research 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Tregay, N.; Begg, M.; Cahn, A.; Farahi, N.; Povey, K.; Madhavan, S.; Simmonds, R.; Gillett, D.; Solanki, C.; Wong, A.; et al. Use of Autologous 99mTechnetium-Labelled Neutrophils to Quantify Lung Neutrophil Clearance in COPD. Thorax 2019, 74, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Zhong, S.; Yu, C.; Zhao, H.; Huang, H.; Meng, X.; Lin, C.; Cai, S. Abnormal Neutrophil Polarization in Chronic Obstructive Pulmonary Disease and How Cigarette Smoke Extracts Attract Neutrophils. Ann. Transl. Med. 2022, 10, 472. [Google Scholar] [CrossRef]
- Sapey, E.; Stockley, J.A.; Greenwood, H.; Ahmad, A.; Bayley, D.; Lord, J.M.; Insall, R.H.; Stockley, R.A. Behavioral and Structural Differences in Migrating Peripheral Neutrophils from Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2011, 183, 1176–1186. [Google Scholar] [CrossRef]
- Kuckleburg, C.J.; Tilkens, S.M.; Santoso, S.; Newman, P.J. Proteinase 3 Contributes to Transendothelial Migration of NB1-Positive Neutrophils. J. Immunol. 2012, 188, 2419. [Google Scholar] [CrossRef] [PubMed]
- Sapey, E. Neutrophil Modulation in Alpha-1 Antitrypsin Deficiency. Chronic Obstr. Pulm. Dis. J. COPD Found. 2020, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Sinden, N.J.; Stockley, R.A. Proteinase 3 Activity in Sputum from Subjects with Alpha-1-Antitrypsin Deficiency and COPD. Eur. Respir. J. 2013, 41, 1042–1050. [Google Scholar] [CrossRef]
- Singh, G.; Acharya, S.; Shukla, S.; Jain, D. Muco-Obstructive Lung Disease: A Systematic Review. Cureus 2023, 15, e46866. [Google Scholar] [CrossRef]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Abdul Roda, M.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e15. [Google Scholar] [CrossRef]
- Grabcanovic-Musija, F.; Obermayer, A.; Stoiber, W.; Krautgartner, W.-D.; Steinbacher, P.; Winterberg, N.; Bathke, A.C.; Klappacher, M.; Studnicka, M. Neutrophil Extracellular Trap (NET) Formation Characterises Stable and Exacerbated COPD and Correlates with Airflow Limitation. Respir. Res. 2015, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Dicker, A.J.; Crichton, M.L.; Pumphrey, E.G.; Cassidy, A.J.; Suarez-Cuartin, G.; Sibila, O.; Furrie, E.; Fong, C.J.; Ibrahim, W.; Brady, G.; et al. Neutrophil Extracellular Traps Are Associated with Disease Severity and Microbiota Diversity in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2018, 141, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of Neutrophil Extracellular Trap Degradation Is Associated with Lupus Nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef]
- Venge, P.; Rak, S.; Steinholtz, L.; Håkansson, L.; Lindblad, G. Neutrophil Function in Chronic Bronchitis. Eur. Respir. J. 1991, 4, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Wrench, C.; Belchamber, K.B.R.; Bercusson, A.; Shah, A.; Barnes, P.J.; Armstrong-James, D.; Donnelly, L.E. Reduced Clearance of Fungal Spores by Chronic Obstructive Pulmonary Disease GM-CSF- and M-CSF-Derived Macrophages. Am. J. Respir. Cell Mol. Biol. 2018, 58, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Bewley, M.A.; Belchamber, K.B.R.; Chana, K.K.; Budd, R.C.; Donaldson, G.; Wedzicha, J.A.; Brightling, C.E.; Kilty, I.; Donnelly, L.E.; Barnes, P.J.; et al. Differential Effects of P38, MAPK, PI3K or Rho Kinase Inhibitors on Bacterial Phagocytosis and Efferocytosis by Macrophages in COPD. PLoS ONE 2016, 11, e0163139. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; Finney-Hayward, T.K.; Quint, J.K.; Thomas, C.M.R.; Tudhope, S.J.; Wedzicha, J.A.; Barnes, P.J.; Donnelly, L.E. Defective Macrophage Phagocytosis of Bacteria in COPD. Eur. Respir. J. 2010, 35, 1039–1047. [Google Scholar] [CrossRef]
- Eapen, M.S.; McAlinden, K.; Tan, D.; Weston, S.; Ward, C.; Muller, H.K.; Walters, E.H.; Sohal, S.S. Profiling Cellular and Inflammatory Changes in the Airway Wall of Mild to Moderate COPD. Respirology 2017, 22, 1125–1132. [Google Scholar] [CrossRef]
- Forsslund, H.; Mikko, M.; Karimi, R.; Grunewald, J.; Wheelock, Å.M.; Wahlström, J.; Sköld, C.M. Distribution of T-Cell Subsets in BAL Fluid of Patients with Mild to Moderate COPD Depends on Current Smoking Status and Not Airway Obstruction. Chest 2014, 145, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Tzanakis, N.; Chrysofakis, G.; Tsoumakidou, M.; Kyriakou, D.; Tsiligianni, J.; Bouros, D.; Siafakas, N.M. Induced Sputum CD8+ T-Lymphocyte Subpopulations in Chronic Obstructive Pulmonary Disease. Respir. Med. 2004, 98, 57–65. [Google Scholar] [CrossRef]
- Löfdahl, M.J.; Roos-Engstrand, E.; Pourazar, J.; Bucht, A.; Dahlen, B.; Elmberger, G.; Blomberg, A.; Sköld, C.M. Increased Intraepithelial T-Cells in Stable COPD. Respir. Med. 2008, 102, 1812–1818. [Google Scholar] [CrossRef][Green Version]
- Chen, C.; Shen, Y.; Ni, C.; Zhu, Y.; Huang, J. Imbalance of Circulating T-Lymphocyte Subpopulation in COPD and Its Relationship with CAT Performance. J. Clin. Lab. Anal. 2012, 26, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, G.; Zhang, M.-Q.; Xiong, X.-Z.; Liu, H.-J.; Xin, J.-B.; Zhang, J.-C.; Wu, J.-H.; Meng, Z.-J.; Sun, S.-W. Imbalance between Subsets of CD8+ Peripheral Blood T Cells in Patients with Chronic Obstructive Pulmonary Disease. PeerJ 2016, 4, e2301. [Google Scholar] [CrossRef]
- Mathai, R.T.K.; Bhat, S. Peripheral Blood T-Cell Populations in COPD, Asymptomatic Smokers and Healthy Non-Smokers in Indian Subpopulation- A Pilot Study. J. Clin. Diagn. Res. 2013, 7, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.M.; Martinez, C.H.; Todt, J.C.; Martinez, F.J.; Han, M.K.; Thompson, D.L.; McCloskey, L.; Curtis, J.L. Acute Exacerbations of Chronic Obstructive Pulmonary Disease Are Associated with Decreased CD4+ & CD8+ T Cells and Increased Growth & Differentiation Factor-15 (GDF-15) in Peripheral Blood. Respir. Res. 2015, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhou, M.; Chen, L.; Meng, Z.-J.; Xiong, X.-Z.; Liu, H.-J.; Xin, J.-B.; Zhang, J.-C. Cigarette Smoke Disturbs the Survival of CD8+ Tc/Tregs Partially through Muscarinic Receptors-Dependent Mechanisms in Chronic Obstructive Pulmonary Disease. PLoS ONE 2016, 11, e0147232. [Google Scholar] [CrossRef]
- Hodge, G.; Nairn, J.; Holmes, M.; Reynolds, P.N.; Hodge, S. Increased Intracellular T Helper 1 Proinflammatory Cytokine Production in Peripheral Blood, Bronchoalveolar Lavage and Intraepithelial T Cells of COPD Subjects. Clin. Exp. Immunol. 2007, 150, 22–29. [Google Scholar] [CrossRef]
- Xu, W.-H.; Hu, X.-L.; Liu, X.-F.; Bai, P.; Sun, Y.-C. Peripheral Tc17 and Tc17/Interferon-γ Cells Are Increased and Associated with Lung Function in Patients with Chronic Obstructive Pulmonary Disease. Chin. Med. J. 2016, 129, 909–916. [Google Scholar] [CrossRef]
- Barczyk, A.; Pierzchała, W.; Kon, O.M.; Cosio, B.; Adcock, I.M.; Barnes, P.J. Cytokine Production by Bronchoalveolar Lavage T Lymphocytes in Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2006, 117, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Nadigel, J.; Boulais, N.; Bourbeau, J.; Maltais, F.; Eidelman, D.H.; Hamid, Q. CD8 Positive T Cells Express IL-17 in Patients with Chronic Obstructive Pulmonary Disease. Respir. Res. 2011, 12, 43. [Google Scholar] [CrossRef]
- Shiratsuchi, N.; Asai, K.; Kanazawa, H.; Kyoh, S.; Tochino, Y.; Kodama, T.; Hirata, K. Measurement of Soluble Perforin, a Marker of CD8+ T Lymphocyte Activation in Epithelial Lining Fluid. Respir. Med. 2011, 105, 1885–1890. [Google Scholar] [CrossRef]
- Kim, W.-D.; Chi, H.-S.; Choe, K.-H.; Oh, Y.-M.; Lee, S.-D.; Kim, K.-R.; Yoo, K.-H.; Ngan, D.A.; Elliott, W.M.; Granville, D.J.; et al. A Possible Role for CD8+ and Non-CD8+ Cell Granzyme B in Early Small Airway Wall Remodelling in Centrilobular Emphysema. Respirology 2013, 18, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Cosio, M.G. Characterization of T Lymphocytes in Chronic Obstructive Pulmonary Disease. PLoS Med. 2004, 1, e20. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Ma, J.; Li, Y.; Xie, C. Role of CD4+ T and CD8+ T Lymphocytes-Mediated Cellular Immunity in Pathogenesis of Chronic Obstructive Pulmonary Disease. J. Immunol. Res. 2022, 2022, 1429213. [Google Scholar] [CrossRef]
- Tsoumakidou, M.; Tzanakis, N.; Chrysofakis, G.; Kyriakou, D.; Siafakas, N.M. Changes in Sputum T-Lymphocyte Subpopulations at the Onset of Severe Exacerbations of Chronic Obstructive Pulmonary Disease. Respir. Med. 2005, 99, 572–579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Y.; Xu, W. Peripheral Blood Th17 and Th17/Th2 Cells and Their Association with Lung Function and Biomarkers in Asthma-COPD Overlap Syndrome. Eur. Respir. J. 2017, 50, PA1006. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, L.; Xie, Y.; Xu, Y.; Jiao, W.; Wu, J.; Deng, X.; Fang, G.; Xue, Q.; Zheng, Y.; et al. Th1/Th17 Cytokine Profiles Are Associated with Disease Severity and Exacerbation Frequency in COPD Patients. Int. J. Chron Obstruct. Pulmon. Dis. 2020, 15, 1287–1299. [Google Scholar] [CrossRef]
- Geng, W.-R.; He, H.-Y.; Zhang, Q.; Tong, Z.-H. Th17 Cells Are Involved in Mouse Chronic Obstructive Pulmonary Disease Complicated with Invasive Pulmonary Aspergillosis. Chin. Med. J. 2021, 134, 555–563. [Google Scholar] [CrossRef]
- Liu, M.; Wu, K.; Lin, J.; Xie, Q.; Liu, Y.; Huang, Y.; Zeng, J.; Yang, Z.; Wang, Y.; Dong, S.; et al. Emerging Biological Functions of IL-17A: A New Target in Chronic Obstructive Pulmonary Disease? Front. Pharmacol. 2021, 12, 695957. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, H.; Li, Z.; Wang, J.; Zhang, F.; Cao, K.; Li, F.; Ding, J. ILC2s Induce Adaptive Th2-Type Immunity in Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Mediat. Inflamm. 2019, 2019, 3140183. [Google Scholar] [CrossRef]
- Carlier, F.M.; de Fays, C.; Pilette, C. Epithelial Barrier Dysfunction in Chronic Respiratory Diseases. Front. Physiol. 2021, 12, 691227. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ying, H.; Wang, S.; Gu, X.; Weng, Y.; Peng, W.; Xia, D.; Yu, W. Imbalance of Peripheral Blood Th17 and Treg Responses in Patients with Chronic Obstructive Pulmonary Disease. Clin. Respir. J. 2015, 9, 330–341. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L.; Chen, J.; Gu, Y.; Xu, J.; Ouyang, Y. Dendritic Cells and Th17/Treg Ratio Play Critical Roles in Pathogenic Process of Chronic Obstructive Pulmonary Disease. Biomed. Pharmacother. 2018, 108, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Sun, Y.; Hao, Y.; Zhuo, J.; Liu, X.; Bai, P.; Han, J.; Zheng, X.; Zeng, H. Imbalance between Subpopulations of Regulatory T Cells in COPD. Thorax 2013, 68, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.J.C.; Starkey, C.; Vestbo, J.; Singh, D. CD4-Regulatory Cells in COPD Patients. Chest 2007, 132, 156–163. [Google Scholar] [CrossRef]
- Polverino, F.; Seys, L.J.M.; Bracke, K.R.; Owen, C.A. B Cells in Chronic Obstructive Pulmonary Disease: Moving to Center Stage. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L687–L695. [Google Scholar] [CrossRef]
- Litsiou, E.; Semitekolou, M.; Galani, I.E.; Morianos, I.; Tsoutsa, A.; Kara, P.; Rontogianni, D.; Bellenis, I.; Konstantinou, M.; Potaris, K.; et al. CXCL13 Production in B Cells via Toll-like Receptor/Lymphotoxin Receptor Signaling Is Involved in Lymphoid Neogenesis in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Alturaiki, W.; Alturaiki, W.H. Elevated Plasma Levels of CXCL13 Chemokine in Saudi Patients With Asthma Exacerbation. Cureus 2022, 14, e21142. [Google Scholar] [CrossRef] [PubMed]
- Dubey, L.K.; Lebon, L.; Mosconi, I.; Yang, C.-Y.; Scandella, E.; Ludewig, B.; Luther, S.A.; Harris, N.L. Lymphotoxin-Dependent B Cell-FRC Crosstalk Promotes De Novo Follicle Formation and Antibody Production Following Intestinal Helminth Infection. Cell Rep. 2016, 15, 1527–1541. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Laucho-Contreras, M.; Rojas Quintero, J.; Divo, M.; Pinto-Plata, V.; Sholl, L.; de-Torres, J.P.; Celli, B.R.; Owen, C.A. Increased Expression of A Proliferation-Inducing Ligand (APRIL) in Lung Leukocytes and Alveolar Epithelial Cells in COPD Patients with Non Small Cell Lung Cancer: A Possible Link between COPD and Lung Cancer? Multidiscip. Respir. Med. 2016, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhiming, W.; Luman, W.; Tingting, Q.; Yiwei, C. Chemokines and Receptors in Intestinal B Lymphocytes. J. Leukoc. Biol. 2018, 103, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, J.; Xie, J.; Wang, J. The Effects of BAFF on T Lymphocytes in Chronic Obstructive Pulmonary Disease. Respir. Res. 2020, 21, 66. [Google Scholar] [CrossRef]
- Brusselle, G.G.; Demoor, T.; Bracke, K.R.; Brandsma, C.-A.; Timens, W. Lymphoid Follicles in (Very) Severe COPD: Beneficial or Harmful? Eur. Respir. J. 2009, 34, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Maher, T.M.; Tudor, V.A.; Saunders, P.; Gibbons, M.A.; Fletcher, S.V.; Denton, C.P.; Hoyles, R.K.; Parfrey, H.; Renzoni, E.A.; Kokosi, M.; et al. Rituximab versus Intravenous Cyclophosphamide in Patients with Connective Tissue Disease-Associated Interstitial Lung Disease in the UK (RECITAL): A Double-Blind, Double-Dummy, Randomised, Controlled, Phase 2b Trial. Lancet Respir. Med. 2023, 11, 45–54. [Google Scholar] [CrossRef]
- Pahal, P.; Hashmi, M.F.; Sharma, S. Chronic Obstructive Pulmonary Disease Compensatory Measures. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Shaykhiev, R.; Crystal, R.G. Innate Immunity and Chronic Obstructive Pulmonary Disease-A Mini-Review. Gerontology 2013, 59, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Li, B.; Yang, T. Pharmacological Therapy for Stable Chronic Obstructive Pulmonary Disease. Chronic Dis. Transl. Med. 2023, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, P.G.; Wolff, M.; Hohlfeld, J.M.; Krug, N.; Dransfield, M.T.; Sutherland, E.R.; Criner, G.J.; Kim, V.; Prasse, A.; Nivens, M.C.; et al. Safety and Efficacy of an Inhaled Epidermal Growth Factor Receptor Inhibitor (BIBW 2948 BS) in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2010, 181, 438–445. [Google Scholar] [CrossRef]
- Silva, B.S.A.; Ramos, D.; Bertolini, G.N.; Freire, A.P.C.F.; Leite, M.R.; Camillo, C.A.; Gobbo, L.A.; Ramos, E.M.C. Resistance Exercise Training Improves Mucociliary Clearance in Subjects with COPD: A Randomized Clinical Trial. Pulmonology 2019, 25, 340–347. [Google Scholar] [CrossRef]
- Priego-Jiménez, S.; Torres-Costoso, A.; Guzmán-Pavón, M.J.; Lorenzo-García, P.; Lucerón-Lucas-Torres, M.I.; Álvarez-Bueno, C. Efficacy of Different Types of Physical Activity Interventions on Exercise Capacity in Patients with Chronic Obstructive Pulmonary Disease (COPD): A Network Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14539. [Google Scholar] [CrossRef]
- Rossios, C.; To, Y.; Osoata, G.; Ito, M.; Barnes, P.J.; Ito, K. Corticosteroid Insensitivity Is Reversed by Formoterol via Phosphoinositide-3-Kinase Inhibition. Br. J. Pharmacol. 2012, 167, 775–786. [Google Scholar] [CrossRef]
- Liu, C.-W.; Lee, T.-L.; Chen, Y.-C.; Liang, C.-J.; Wang, S.-H.; Lue, J.-H.; Tsai, J.-S.; Lee, S.-W.; Chen, S.-H.; Yang, Y.-F.; et al. PM2.5-Induced Oxidative Stress Increases Intercellular Adhesion Molecule-1 Expression in Lung Epithelial Cells through the IL-6/AKT/STAT3/NF-κB-Dependent Pathway. Part. Fibre Toxicol. 2018, 15, 4. [Google Scholar] [CrossRef]
- Roghanian, A.; Williams, S.E.; Sheldrake, T.A.; Brown, T.I.; Oberheim, K.; Xing, Z.; Howie, S.E.M.; Sallenave, J.-M. The Antimicrobial/Elastase Inhibitor Elafin Regulates Lung Dendritic Cells and Adaptive Immunity. Am. J. Respir. Cell Mol. Biol. 2006, 34, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.I.; Fogarty, C.; Kelsen, S.; Long, W.; Ramsdell, J.; Allison, J.; Mahler, D.; Saadeh, C.; Siler, T.; Snell, P.; et al. The Safety and Efficacy of Infliximab in Moderate to Severe Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 175, 926–934. [Google Scholar] [CrossRef]
- Mahler, D.A.; Huang, S.; Tabrizi, M.; Bell, G.M. Efficacy and Safety of a Monoclonal Antibody Recognizing Interleukin-8 in COPD: A Pilot Study. Chest 2004, 126, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Rennard, S.I.; Dale, D.C.; Donohue, J.F.; Kanniess, F.; Magnussen, H.; Sutherland, E.R.; Watz, H.; Lu, S.; Stryszak, P.; Rosenberg, E.; et al. CXCR2 Antagonist MK-7123. A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2015, 191, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Tao, S.; Zhang, S.; Wang, J.; Zhang, F.; Li, F.; Ding, J. Type 2 Innate Lymphoid Cells Participate in IL-33-Stimulated Th2-Associated Immune Response in Chronic Obstructive Pulmonary Disease. Exp. Ther. Med. 2019, 18, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.Y.; Prakash, Y.S. Contributions of IL-33 in Non-Hematopoietic Lung Cells to Obstructive Lung Disease. Front. Immunol. 2020, 11, 1798. [Google Scholar] [CrossRef]
- Kosloski, M.P.; Kalliolias, G.D.; Xu, C.R.; Harel, S.; Lai, C.-H.; Zheng, W.; Davis, J.D.; Kamal, M.A. Pharmacokinetics and Pharmacodynamics of Itepekimab in Healthy Adults and Patients with Asthma: Phase I First-in-Human and First-in-Patient Trials. Clin. Transl. Sci. 2022, 15, 384–395. [Google Scholar] [CrossRef]
- Yousuf, A.J.; Mohammed, S.; Carr, L.; Yavari Ramsheh, M.; Micieli, C.; Mistry, V.; Haldar, K.; Wright, A.; Novotny, P.; Parker, S.; et al. Astegolimab, an Anti-ST2, in Chronic Obstructive Pulmonary Disease (COPD-ST2OP): A Phase 2a, Placebo-Controlled Trial. Lancet Respir. Med. 2022, 10, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Reid, F.; Singh, D.; Albayaty, M.; Moate, R.; Jimenez, E.; Sadiq, M.W.; Howe, D.; Gavala, M.; Killick, H.; Williams, A.; et al. A Randomized Phase I Study of the Anti-Interleukin-33 Antibody Tozorakimab in Healthy Adults and Patients With Chronic Obstructive Pulmonary Disease. Clin. Pharmacol. Ther. 2024, 115, 565–575. [Google Scholar] [CrossRef]
- Pavord, I.D.; Chanez, P.; Criner, G.J.; Kerstjens, H.A.M.; Korn, S.; Lugogo, N.; Martinot, J.-B.; Sagara, H.; Albers, F.C.; Bradford, E.S.; et al. Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2017, 377, 1613–1629. [Google Scholar] [CrossRef] [PubMed]
- Criner, G.J.; Celli, B.R.; Brightling, C.E.; Agusti, A.; Papi, A.; Singh, D.; Sin, D.D.; Vogelmeier, C.F.; Sciurba, F.C.; Bafadhel, M.; et al. Benralizumab for the Prevention of COPD Exacerbations. N. Engl. J. Med. 2019, 381, 1023–1034. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Rabe, K.F.; Hanania, N.A.; Vogelmeier, C.F.; Cole, J.; Bafadhel, M.; Christenson, S.A.; Papi, A.; Singh, D.; Laws, E.; et al. Dupilumab for COPD with Type 2 Inflammation Indicated by Eosinophil Counts. N. Engl. J. Med. 2023, 389, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Ora, J.; Cavalli, F.; Rogliani, P.; Matera, M.G. An Overview of the Safety and Efficacy of Monoclonal Antibodies for the Chronic Obstructive Pulmonary Disease. Biologics 2021, 15, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Brightling, C.E.; Rabe, K.F.; Han, M.K.; Christenson, S.A.; Drummond, M.B.; Papi, A.; Pavord, I.D.; Molfino, N.A.; Almqvist, G.; et al. Efficacy and Safety of Tezepelumab versus Placebo in Adults with Moderate to Very Severe Chronic Obstructive Pulmonary Disease (COURSE): A Randomised, Placebo-Controlled, Phase 2a Trial. Lancet Respir. Med. 2024, 13, 47–58. [Google Scholar] [CrossRef]
- Mkorombindo, T.; Wade, R.C.; Yuan, K.; Robison, S.W.; Christenson, A.E.; Wells, J.M.; Dransfield, M.T.; Duncan, S.R. A Pilot Trial of Belimumab for Atherosclerosis Associated Autoimmunity in COPD Patients. Eur. Respir. J. 2022, 60, 4085. [Google Scholar] [CrossRef]
- ElSaygh, J.; Zaher, A.; Nathani, P.; Omballi, M. A Review of Clinical Trials That Contributed to Chronic Obstructive Pulmonary Disease Treatment Protocols. Cureus 2021, 13, e14618. [Google Scholar] [CrossRef] [PubMed]
- Cilli, A.; Bal, H.; Gunen, H. Efficacy and Safety Profile of Roflumilast in a Real-World Experience. J. Thorac. Dis. 2019, 11, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Watz, H.; Baraldo, S.; Pedersen, F.; Biondini, D.; Bagul, N.; Hanauer, G.; Göhring, U.-M.; Purkayastha, D.; Román, J.; et al. Anti-Inflammatory Effects of Roflumilast in Chronic Obstructive Pulmonary Disease (ROBERT): A 16-Week, Randomised, Placebo-Controlled Trial. Lancet Respir. Med. 2018, 6, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Vaccines Work. Nat. Commun. 2018, 9, 1666. [CrossRef] [PubMed]
- Pavot, V.; Rochereau, N.; Genin, C.; Verrier, B.; Paul, S. New Insights in Mucosal Vaccine Development. Vaccine 2012, 30, 142–154. [Google Scholar] [CrossRef]
- Koatz, A.M.; Coe, N.A.; Cicerán, A.; Alter, A.J. Clinical and Immunological Benefits of OM-85 Bacterial Lysate in Patients with Allergic Rhinitis, Asthma, and COPD and Recurrent Respiratory Infections. Lung 2016, 194, 687–697. [Google Scholar] [CrossRef]
- Solèr, M.; Mütterlein, R.; Cozma, G. Swiss-German OM-85 Study Group Double-Blind Study of OM-85 in Patients with Chronic Bronchitis or Mild Chronic Obstructive Pulmonary Disease. Respiration 2007, 74, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, J.; Yuan, J.; Zeng, G.; Zhong, N.; Lin, C. Protective Effect of a Bacterial Extract against Acute Exacerbation in Patients with Chronic Bronchitis Accompanied by Chronic Obstructive Pulmonary Disease. Chin. Med. J. 2004, 117, 828–834. [Google Scholar]
- Choi, J.Y.; Park, Y.B.; An, T.J.; Yoo, K.H.; Rhee, C.K. Effect of Broncho-Vaxom (OM-85) on the Frequency of Chronic Obstructive Pulmonary Disease (COPD) Exacerbations. BMC Pulm. Med. 2023, 23, 378. [Google Scholar] [CrossRef]
- Collet, J.P.; Shapiro, P.; Ernst, P.; Renzi, T.; Ducruet, T.; Robinson, A. Effects of an Immunostimulating Agent on Acute Exacerbations and Hospitalizations in Patients with Chronic Obstructive Pulmonary Disease. The PARI-IS Study Steering Committee and Research Group. Prevention of Acute Respiratory Infection by an Immunostimulant. Am. J. Respir. Crit. Care Med. 1997, 156, 1719–1724. [Google Scholar] [CrossRef]
- Debbas, N.; Derenne, J.P. Preventive Effects of an Immunostimulating Product on Recurrent Infections of Chronic Bronchitis in the Elderly. Lung 1990, 168, 737–740. [Google Scholar] [CrossRef]
- Tang, H.; Fang, Z.; Saborío, G.P.; Xiu, Q. Efficacy and Safety of OM-85 in Patients with Chronic Bronchitis and/or Chronic Obstructive Pulmonary Disease. Lung 2015, 193, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Huang, J.; Wang, B.; Chen, G. Efficacy of Broncho-Vaxom on chronic obstructive pulmonary disease in elderly patients. Chin. J. Geriatr. 2019, 12, 717–721. [Google Scholar]
- Clinical Effectiveness of Broncho-Vaxom (BV) in Patients with Chronic Obstructive Pulmonary Disease|Cochrane Library. Available online: https://www.cochranelibrary.com/es/central/doi/10.1002/central/CN-00188938/full (accessed on 3 January 2025).
- Gut Microbiota Modulation on Intestinal Mucosal Adaptive Immunity-PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6794961/ (accessed on 3 December 2024).
- Hellfritzsch, M.; Scherließ, R. Mucosal Vaccination via the Respiratory Tract. Pharmaceutics 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Jiang, X.-G.; Guo, J.; Tian, Y.; Liu, C.-T. Effects of OM-85 BV in Patients with Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis. J. Clin. Pharmacol. 2015, 55, 1086–1092. [Google Scholar] [CrossRef]
- Braido, F.; Melioli, G.; Cazzola, M.; Fabbri, L.; Blasi, F.; Moretta, L.; Canonica, G.W. AIACE Study Group Sub-Lingual Administration of a Polyvalent Mechanical Bacterial Lysate (PMBL) in Patients with Moderate, Severe, or Very Severe Chronic Obstructive Pulmonary Disease (COPD) According to the GOLD Spirometric Classification: A Multicentre, Double-Blind, Randomised, Controlled, Phase IV Study (AIACE Study: Advanced Immunological Approach in COPD Exacerbation). Pulm. Pharmacol. Ther. 2015, 33, 75–80. [Google Scholar] [CrossRef]
- Cardinale, F.; Lombardi, E.; Rossi, O.; Bagnasco, D.; Bellocchi, A.; Menzella, F. Epithelial Dysfunction, Respiratory Infections and Asthma: The Importance of Immunomodulation. A Focus on OM-85. Expert Rev. Respir. Med. 2020, 14, 1019–1026. [Google Scholar] [CrossRef]
- Cao, C.; Wang, J.; Li, Y.; Li, Y.; Ma, L.; Abdelrahim, M.E.A.; Zhu, Y. Efficacy and Safety of OM-85 in Paediatric Recurrent Respiratory Tract Infections Which Could Have a Possible Protective Effect on COVID-19 Pandemic: A Meta-Analysis. Int. J. Clin. Pract. 2021, 75, e13981. [Google Scholar] [CrossRef]
- Hohensinner, P.; Salzmann, M.; Wojta, J.; Plasenzotti, R. Modulation of the Innate and Adaptive Immune System during Coronavirus Infection. Eur. Respir. J. 2021, 58, OA4310. [Google Scholar] [CrossRef]
- Fang, L.; Zhou, L.; Tamm, M.; Roth, M. OM-85 Broncho-Vaxom®, a Bacterial Lysate, Reduces SARS-CoV-2 Binding Proteins on Human Bronchial Epithelial Cells. Biomedicines 2021, 9, 1544. [Google Scholar] [CrossRef]
- Mestecky, J. The Common Mucosal Immune System and Current Strategies for Induction of Immune Responses in External Secretions. J. Clin. Immunol. 1987, 7, 265–276. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Function of Mucosa-Associated Lymphoid Tissue in Antibody Formation. Immunol. Investig. 2010, 39, 303–355. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.; Kaushik, R.S. Mucosal Immune System of Cattle: All Immune Responses Begin Here. Vet. Clin. N. Am. Food. Anim. Pract. 2019, 35, 431–451. [Google Scholar] [CrossRef]
- Meng, Q.; Li, P.; Li, Y.; Chen, J.; Wang, L.; He, L.; Xie, J.; Gao, X. Broncho-Vaxom Alleviates Persistent Allergic Rhinitis in Patients by Improving Th1/Th2 Cytokine Balance of Nasal Mucosa. Rhinology 2019, 57, 451–459. [Google Scholar] [CrossRef]
- Vela Ramirez, J.E.; Sharpe, L.A.; Peppas, N.A. Current State and Challenges in Developing Oral Vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Rossi, G.A.; Esposito, S.; Feleszko, W.; Melioli, G.; Olivieri, D. Immunomodulation Therapy–Clinical Relevance of Bacterial Lysates OM-85. Eur. Respir. Pulm. Dis. 2019, 5, 17. [Google Scholar] [CrossRef]
- Esposito, S.; Ballarini, S.; Argentiero, A.; Ruggiero, L.; Rossi, G.A.; Principi, N. Microbiota Profiles in Pre-School Children with Respiratory Infections: Modifications Induced by the Oral Bacterial Lysate OM-85. Front. Cell. Infect. Microbiol. 2022, 12, 789436. [Google Scholar] [CrossRef]
- Russo, C.; Colaianni, V.; Ielo, G.; Valle, M.S.; Spicuzza, L.; Malaguarnera, L. Impact of Lung Microbiota on COPD. Biomedicines 2022, 10, 1337. [Google Scholar] [CrossRef]
- Yin, J.; Xu, B.; Zeng, X.; Shen, K. Broncho-Vaxom in Pediatric Recurrent Respiratory Tract Infections: A Systematic Review and Meta-Analysis. Int. Immunopharmacol. 2018, 54, 198–209. [Google Scholar] [CrossRef] [PubMed]
| The Described Effect of Including Oral Vaccines in the Treatment of CODP | Clinical Trials Confirming Beneficial Effects |
|---|---|
| Decrease in the incidence of acute exacerbations | [117,118,119] |
| No decrease in severe exacerbations | [120,121] |
| Delay in moderate COPD exacerbations | [120] |
| Reducing the incidence of respiratory tract infections | [117,122] |
| Significant reduction in the total number of exacerbations | [117,122,123] |
| Decreased in the need for antibiotics | [119,122,123] |
| Improve quality of life | [124] |
| Decrease in the need of hospitalization | [121] |
| Improve quality of life | [124] |
| Improve lung function | [123,124,125] |
| Improve lung function | [117,121,124,125] |
| Reduce number of hospitalizations | [117,119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewicki, S.; Bałan, B.J.; Stelmasiak, M.; Radomska-Leśniewska, D.M.; Szymański, Ł.; Rios-Turek, N.; Bień-Kalinowska, J.; Szarpak, Ł.; Hajduk, B. Immunological Insights and Therapeutic Advances in COPD: Exploring Oral Bacterial Vaccines for Immune Modulation and Clinical Improvement. Vaccines 2025, 13, 107. https://doi.org/10.3390/vaccines13020107
Lewicki S, Bałan BJ, Stelmasiak M, Radomska-Leśniewska DM, Szymański Ł, Rios-Turek N, Bień-Kalinowska J, Szarpak Ł, Hajduk B. Immunological Insights and Therapeutic Advances in COPD: Exploring Oral Bacterial Vaccines for Immune Modulation and Clinical Improvement. Vaccines. 2025; 13(2):107. https://doi.org/10.3390/vaccines13020107
Chicago/Turabian StyleLewicki, Sławomir, Barbara Joanna Bałan, Marta Stelmasiak, Dorota Magdalena Radomska-Leśniewska, Łukasz Szymański, Natalia Rios-Turek, Justyna Bień-Kalinowska, Łukasz Szarpak, and Bogdan Hajduk. 2025. "Immunological Insights and Therapeutic Advances in COPD: Exploring Oral Bacterial Vaccines for Immune Modulation and Clinical Improvement" Vaccines 13, no. 2: 107. https://doi.org/10.3390/vaccines13020107
APA StyleLewicki, S., Bałan, B. J., Stelmasiak, M., Radomska-Leśniewska, D. M., Szymański, Ł., Rios-Turek, N., Bień-Kalinowska, J., Szarpak, Ł., & Hajduk, B. (2025). Immunological Insights and Therapeutic Advances in COPD: Exploring Oral Bacterial Vaccines for Immune Modulation and Clinical Improvement. Vaccines, 13(2), 107. https://doi.org/10.3390/vaccines13020107










