Effect of Current-Season-Only Versus Continuous Two-Season Influenza Vaccination on Mortality in Older Adults: A Propensity-Score-Matched Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
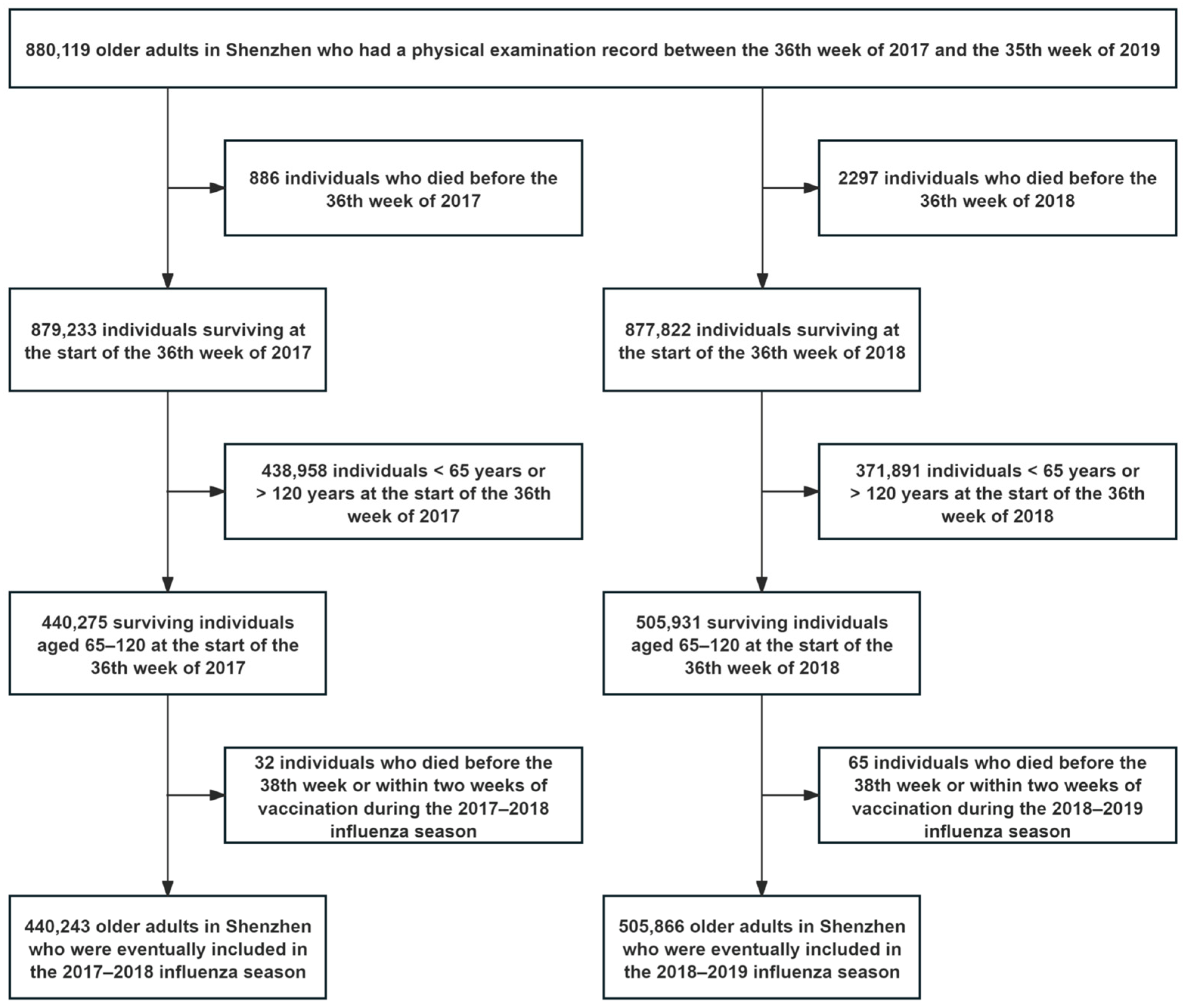
2.2. Study Population
- •
- Inclusion criteria: older adults who survived and were at least 65 years old at the start of the 36th week in 2017 and 2018 were included in this study;
- •
- Exclusion criteria: (1) for individuals who died during the 2017–2018 and 2018–2019 influenza seasons without the date of death or the underlying cause of death, when the number was low (<1%), they were excluded, and (2) for individuals who received an influenza vaccine during the 2017–2018 and 2018–2019 influenza seasons lacking information on the vaccination date, when the number was low (<1%), they were excluded.
2.3. Determination of Control and Exposure Groups
2.4. Outcomes
2.5. Statistical Analyses
2.6. Ethics Statement
3. Results
3.1. Propensity Score Matching
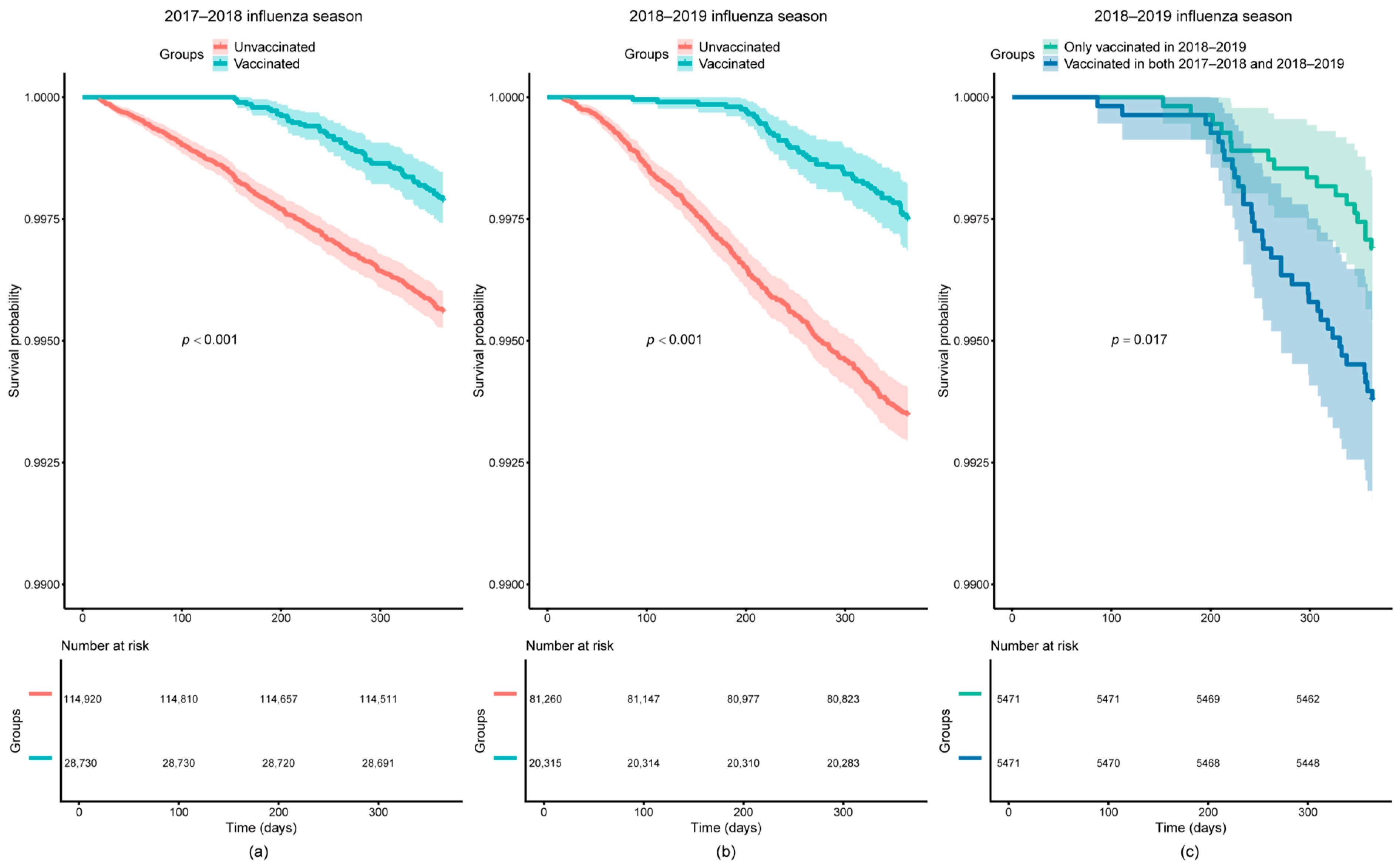
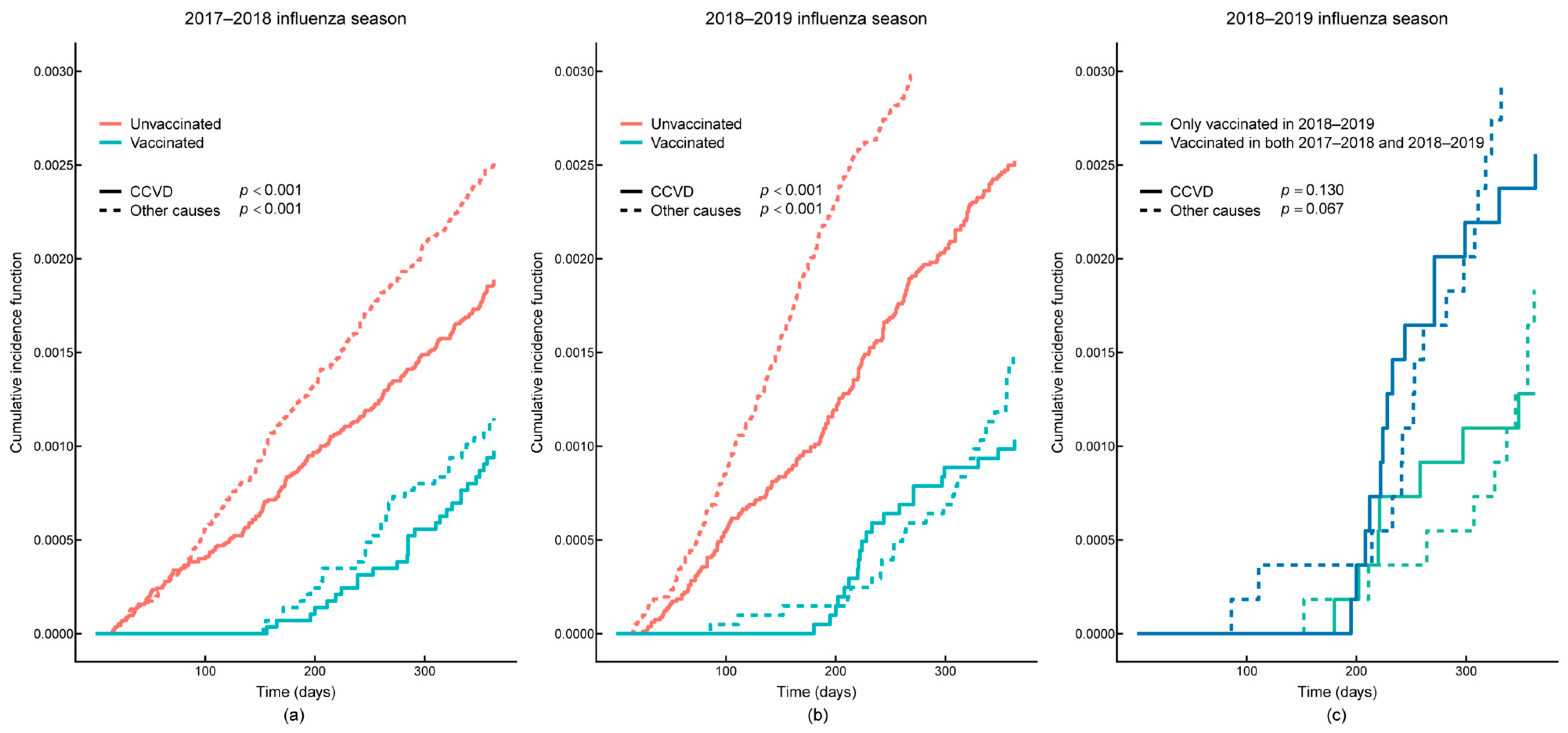
3.2. Survival Analysis
4. Discussion
4.1. Interpretation of Results
4.2. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| VE | Vaccine effectiveness |
| OR | Odds ratio |
| BMI | Body mass index |
| HGB | Hemoglobin |
| SCR | Serum creatinine |
| ICD-10 | The International Statistical Classification of Diseases and Related Health Problems 10th Revision |
| CCVD | Cardio-cerebral vascular diseases |
| SMD | Standardized mean difference |
| HR | Hazard ratio |
| CI | Confidence interval |
References
- World Health Organization. Influenza (Seasonal). Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 31 December 2024).
- Chen, J.; Deng, J.C.; Zemans, R.L.; Bahmed, K.; Kosmider, B.; Zhang, M.; Peters-Golden, M.; Goldstein, D.R. Age-induced prostaglandin E2 impairs mitochondrial fitness and increases mortality to influenza infection. Nat. Commun. 2022, 13, 6759. [Google Scholar] [CrossRef]
- Wang, H.; Fu, C.; Li, K.; Lu, J.; Chen, Y.; Lu, E.; Xiao, X.; Di, B.; Liu, H.; Yang, Z.; et al. Influenza associated mortality in Southern China, 2010–2012. Vaccine 2014, 32, 973–978. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccines Against Influenza: WHO Position Paper—May 2022. Available online: https://www.who.int/publications/i/item/who-wer9719 (accessed on 31 December 2024).
- Doyon-Plourde, P.; Przepiorkowski, J.; Young, K.; Zhao, L.; Sinilaite, A. Intraseasonal waning immunity of seasonal influenza vaccine—A systematic review and meta-analysis. Vaccine 2023, 41, 4462–4471. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Zhao, X.; Cook, A.R.; Parry, C.M.; Wilder-Smith, A.; MC, I.C. Do antibody responses to the influenza vaccine persist year-round in the elderly? A systematic review and meta-analysis. Vaccine 2017, 35, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Rivetti, D.; Rivetti, A.; Rudin, M.; Di Pietrantonj, C.; Demicheli, V. Efficacy and effectiveness of influenza vaccines in elderly people: A systematic review. Lancet 2005, 366, 1165–1174. [Google Scholar] [CrossRef]
- Nichol, K.L. Influenza vaccination in the elderly: Impact on hospitalisation and mortality. Drugs Aging 2005, 22, 495–515. [Google Scholar] [CrossRef]
- Darvishian, M.; Gefenaite, G.; Turner, R.M.; Pechlivanoglou, P.; Van der Hoek, W.; Van den Heuvel, E.R.; Hak, E. After adjusting for bias in meta-analysis seasonal influenza vaccine remains effective in community-dwelling elderly. J. Clin. Epidemiol. 2014, 67, 734–744. [Google Scholar] [CrossRef]
- Kopel, E.; Klempfner, R.; Goldenberg, I. Influenza vaccine and survival in acute heart failure. Eur. J. Heart Fail. 2014, 16, 264–270. [Google Scholar] [CrossRef]
- Gögenur, M.; Fransgård, T.; Krause, T.G.; Thygesen, L.C.; Gögenur, I. Association of postoperative influenza vaccine on overall mortality in patients undergoing curative surgery for solid tumors. Int. J. Cancer 2021, 148, 1821–1827. [Google Scholar] [CrossRef]
- Jones-Gray, E.; Robinson, E.J.; Kucharski, A.J.; Fox, A.; Sullivan, S.G. Does repeated influenza vaccination attenuate effectiveness? A systematic review and meta-analysis. Lancet Respir. Med. 2023, 11, 27–44. [Google Scholar] [CrossRef]
- Belongia, E.A.; Skowronski, D.M.; McLean, H.Q.; Chambers, C.; Sundaram, M.E.; De Serres, G. Repeated annual influenza vaccination and vaccine effectiveness: Review of evidence. Expert Rev. Vaccines 2017, 16, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Okoli, G.N.; Racovitan, F.; Abdulwahid, T.; Hyder, S.K.; Lansbury, L.; Righolt, C.H.; Mahmud, S.M.; Nguyen-Van-Tam, J.S. Decline in Seasonal Influenza Vaccine Effectiveness With Vaccination Program Maturation: A Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2021, 8, ofab069. [Google Scholar] [CrossRef] [PubMed]
- Keitel, W.A.; Voronca, D.C.; Atmar, R.L.; Paust, S.; Hill, H.; Wolff, M.C.; Bellamy, A.R. Effect of recent seasonal influenza vaccination on serum antibody responses to candidate pandemic influenza A/H5N1 vaccines: A meta-analysis. Vaccine 2019, 37, 5535–5543. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prevention and Control of Influenza Pandemics and Annual Epidemics. Available online: https://iris.who.int/handle/10665/78320 (accessed on 31 December 2024).
- Directorate-General for Health and Food Safety. COUNCIL RECOMMENDATION of 22 December 2009 on seasonal influenza vaccination. Off. J. Eur. Union 2009, 348, 71–72. [Google Scholar]
- Fan, J.; Cong, S.; Wang, N.; Bao, H.; Wang, B.; Feng, Y.; Lv, X.; Zhang, Y.; Zha, Z.; Yu, L.; et al. Influenza vaccination rate and its association with chronic diseases in China: Results of a national cross-sectional study. Vaccine 2020, 38, 2503–2511. [Google Scholar] [CrossRef]
- Uthoff, S.A.K.; Zinkevich, A.; Franiel, D.; Below, M.; Splieth, H.; Iwen, J.; Biedermann, M.; Heinemeier, D.; Ansmann, L. A complex intervention on vaccination uptake among older adults (≥ 60 years) in Germany—A study protocol with a mixed methods design. BMC Prim. Care 2023, 24, 148. [Google Scholar] [CrossRef]
- Liao, C.Y.; Mott, D.A.; Ford Ii, J.H.; Look, K.A.; Hayney, M.S. Influenza vaccination rates and location for receiving the influenza vaccination among older adult Medicare beneficiaries. J. Am. Pharm. Assoc. 2021, 61, 432–441.e432. [Google Scholar] [CrossRef]
- Itamochi, T.; Mieno, M.; Hatakeyama, S. Influenza vaccination coverage in Chiburijima Island, Japan: Impact of diversification of vaccination place. J. Gen. Fam. Med. 2020, 21, 178–184. [Google Scholar] [CrossRef]
- Iha, Y.; Kinjo, T.; Parrott, G.; Higa, F.; Mori, H.; Fujita, J. Comparative epidemiology of influenza A and B viral infection in a subtropical region: A 7-year surveillance in Okinawa, Japan. BMC Infect. Dis. 2016, 16, 650. [Google Scholar] [CrossRef]
- Ma, P.; Tang, X.; Zhang, L.; Wang, X.; Wang, W.; Zhang, X.; Wang, S.; Zhou, N. Influenza A and B outbreaks differed in their associations with climate conditions in Shenzhen, China. Int. J. Biometeorol. 2022, 66, 163–173. [Google Scholar] [CrossRef]
- Pyrkov, T.V.; Avchaciov, K.; Tarkhov, A.E.; Menshikov, L.I.; Gudkov, A.V.; Fedichev, P.O. Longitudinal analysis of blood markers reveals progressive loss of resilience and predicts human lifespan limit. Nat. Commun. 2021, 12, 2765. [Google Scholar] [CrossRef] [PubMed]
- Heymans, M.W.; Twisk, J.W.R. Handling missing data in clinical research. J. Clin. Epidemiol. 2022, 151, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, U.; Head, S.J.; Angelini, G.D.; Blackstone, E.H. Statistical primer: Propensity score matching and its alternatives. Eur. J. Cardiothorac. Surg. 2018, 53, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Ogundimu, E.O.; Altman, D.G.; Collins, G.S. Adequate sample size for developing prediction models is not simply related to events per variable. J. Clin. Epidemiol. 2016, 76, 175–182. [Google Scholar] [CrossRef]
- Nagashima, K.; Sato, Y. Information criteria for Firth’s penalized partial likelihood approach in Cox regression models. Stat. Med. 2017, 36, 3422–3436. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Kwong, J.C.; Chung, H.; Jung, J.K.H.; Buchan, S.A.; Campigotto, A.; Campitelli, M.A.; Crowcroft, N.S.; Gubbay, J.B.; Karnauchow, T.; Katz, K.; et al. The impact of repeated vaccination using 10-year vaccination history on protection against influenza in older adults: A test-negative design study across the 2010/11 to 2015/16 influenza seasons in Ontario, Canada. Eurosurveillance 2020, 25, 21–33. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, F.; Suo, L.; Li, W.; Qian, J.; Wang, T.; Lv, M.; Wu, J.; Yang, W.; Guo, M.; et al. Effectiveness of influenza vaccines in preventing acute cardiovascular events within 1 year in Beijing, China. npj Vaccines 2024, 9, 177. [Google Scholar] [CrossRef]
- Song, J.Y.; Noh, J.Y.; Lee, J.S.; Wie, S.H.; Kim, Y.K.; Lee, J.; Jeong, H.W.; Kim, S.W.; Lee, S.H.; Park, K.H.; et al. Effectiveness of repeated influenza vaccination among the elderly population with high annual vaccine uptake rates during the three consecutive A/H3N2 epidemics. Vaccine 2020, 38, 318–322. [Google Scholar] [CrossRef]
- Saito, N.; Komori, K.; Suzuki, M.; Morimoto, K.; Kishikawa, T.; Yasaka, T.; Ariyoshi, K. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: A test-negative case-control study in Japan. Vaccine 2017, 35, 687–693. [Google Scholar] [CrossRef]
- Richards, K.A.; Shannon, I.; Treanor, J.J.; Yang, H.; Nayak, J.L.; Sant, A.J. Evidence That Blunted CD4 T-Cell Responses Underlie Deficient Protective Antibody Responses to Influenza Vaccines in Repeatedly Vaccinated Human Subjects. J. Infect. Dis. 2020, 222, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, M.; Holmes, T.H.; Maecker, H.T.; Albrecht, R.A.; Dekker, C.L.; He, X.-S.; Greenberg, H.B. Diminished B-Cell Response After Repeat Influenza Vaccination. J. Infect. Dis. 2019, 219, 1586–1595. [Google Scholar] [CrossRef]
- Petrie, J.G.; Ohmit, S.E.; Johnson, E.; Truscon, R.; Monto, A.S. Persistence of Antibodies to Influenza Hemagglutinin and Neuraminidase Following One or Two Years of Influenza Vaccination. J. Infect. Dis. 2015, 212, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Sherman, A.C.; Lai, L.; Bower, M.; Natrajan, M.S.; Huerta, C.; Karmali, V.; Kleinhenz, J.; Xu, Y.; Rouphael, N.; Mulligan, M.J. The Effects of Imprinting and Repeated Seasonal Influenza Vaccination on Adaptive Immunity after Influenza Vaccination. Vaccines 2020, 8, 663. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.G.; Khvorov, A.; Carolan, L.; Dowson, L.; Hadiprodjo, A.J.; Sánchez-Ovando, S.; Liu, Y.; Leung, V.K.Y.; Hodgson, D.; Blyth, C.C.; et al. Antibody responses against influenza A decline with successive years of annual influenza vaccination. npj Vaccines 2025, 10, 11. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Chambers, C.; De Serres, G.; Sabaiduc, S.; Winter, A.L.; Dickinson, J.A.; Gubbay, J.B.; Fonseca, K.; Drews, S.J.; Charest, H.; et al. Serial Vaccination and the Antigenic Distance Hypothesis: Effects on Influenza Vaccine Effectiveness During A(H3N2) Epidemics in Canada, 2010-2011 to 2014-2015. J. Infect. Dis. 2017, 215, 1059–1099. [Google Scholar] [CrossRef]
- World Health Organization. Recommended Composition of Influenza Virus Vaccines for Use in the 2017–2018 Northern Hemisphere Influenza Season. Available online: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2017-2018-northern-hemisphere-influenza-season (accessed on 31 December 2024).
- World Health Organization. Recommended Composition of Influenza Virus Vaccines for Use in the 2018–2019 Northern Hemisphere Influenza Season. Available online: https://www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2018-2019-northern-hemisphere-influenza-season (accessed on 31 December 2024).
- Wang, X.; Wu, W.; Liu, H.; Sun, Y.; Fang, S. Epidemiological characteristics of influenza in Shenzhen, Guangdong province, 2014−2021. Dis. Surveill. 2023, 38, 776–780. [Google Scholar] [CrossRef]
- Lim, W.W.; Cowling, B.J.; Nakafero, G.; Feng, S.; Nguyen-Van-Tam, J.S.; Bolt, H. The impact of repeated vaccination on relative influenza vaccine effectiveness among vaccinated adults in the United Kingdom. Epidemiol. Infect. 2022, 150, e198. [Google Scholar] [CrossRef]
- Sinilaite, A.; Young, K.; Papenburg, J. Summary of the National Advisory Committee on Immunization (NACI) Statement-Recommendation on Repeated Seasonal Influenza Vaccination. Can. Commun. Dis. Rep. 2023, 49, 99–102. [Google Scholar] [CrossRef]
- Nichols, M.K.; Andrew, M.K.; Ye, L.; Hatchette, T.F.; Ambrose, A.; Boivin, G.; Bowie, W.; Dos Santos, G.; Elsherif, M.; Green, K.; et al. The Impact of Prior Season Vaccination on Subsequent Influenza Vaccine Effectiveness to Prevent Influenza-related Hospitalizations Over 4 Influenza Seasons in Canada. Clin. Infect. Dis. 2019, 69, 970–979. [Google Scholar] [CrossRef]
- Kitamura, S.; Matsushita, M.; Komatsu, N.; Yagi, Y.; Takeuchi, S.; Seo, H. Impact of repeated yearly vaccination on immune responses to influenza vaccine in an elderly population. Am. J. Infect. Control 2020, 48, 1422–1425. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.C.; Macartney, K.K.; Waterer, G.W.; Kotsimbos, T.; Kelly, P.M.; Blyth, C.C.; Influenza Complications Alert Network (FluCAN) Investigators. Repeated Vaccination Does Not Appear to Impact Upon Influenza Vaccine Effectiveness Against Hospitalization With Confirmed Influenza. Clin. Infect. Dis. 2017, 64, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Tong, F.; Tang, L.; Li, P.; Li, B.; Ye, L.; Zhou, J. Repeated vaccination does not appear to significantly weaken the protective effect of influenza vaccine in the elderly: A test-negative case-control study in China. Vaccine 2024, 42, 125986. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.A.; Chang, S.C.; Huang, Y.C.; Chiu, C.H.; Lin, T.Y. Antibody Responses to Trivalent Inactivated Influenza Vaccine in Health Care Personnel Previously Vaccinated and Vaccinated for The First Time. Sci. Rep. 2017, 7, 40027. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Shu, L.; Pang, Y.; Guo, Y.; Guo, Y.; Zong, K.; Chen, C.; Zheng, X.; Zhang, J.; Liu, M.; et al. Repeated influenza vaccination induces similar immune protection as first-time vaccination but with differing immune responses. Influenza Other Respir. Viruses 2023, 17, e13060. [Google Scholar] [CrossRef]
- Chan, T.C.; Fan-Ngai Hung, I.; Ka-Hay Luk, J.; Chu, L.W.; Hon-Wai Chan, F. Effectiveness of influenza vaccination in institutionalized older adults: A systematic review. J. Am. Med. Dir. Assoc. 2014, 15, 226.e221–226.e226. [Google Scholar] [CrossRef]
- Beyer, W.E.; McElhaney, J.; Smith, D.J.; Monto, A.S.; Nguyen-Van-Tam, J.S.; Osterhaus, A.D. Cochrane re-arranged: Support for policies to vaccinate elderly people against influenza. Vaccine 2013, 31, 6030–6033. [Google Scholar] [CrossRef]
- Behrouzi, B.; Araujo Campoverde, M.V.; Liang, K.; Talbot, H.K.; Bogoch, I.I.; McGeer, A.; Fröbert, O.; Loeb, M.; Vardeny, O.; Solomon, S.D.; et al. Influenza Vaccination to Reduce Cardiovascular Morbidity and Mortality in Patients With COVID-19: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1777–1794. [Google Scholar] [CrossRef]
- Modin, D.; Claggett, B.; Jørgensen, M.E.; Køber, L.; Benfield, T.; Schou, M.; Jensen, J.S.; Solomon, S.D.; Trebbien, R.; Fralick, M.; et al. Flu Vaccine and Mortality in Hypertension: A Nationwide Cohort Study. J. Am. Heart Assoc. 2022, 11, e021715. [Google Scholar] [CrossRef]
- Modin, D.; Claggett, B.; Køber, L.; Schou, M.; Jensen, J.U.S.; Solomon, S.D.; Vardeny, O.; Knop, F.K.; Nielsen, S.D.; Fralick, M.; et al. Influenza Vaccination Is Associated With Reduced Cardiovascular Mortality in Adults With Diabetes: A Nationwide Cohort Study. Diabetes Care 2020, 43, 2226–2233. [Google Scholar] [CrossRef] [PubMed]
- Bacurau, A.G.M.; Ferraz, R.O.; Donalisio, M.R.; Francisco, P. Mortality due to cerebrovascular disease among the elderly and vaccination against influenza: São Paulo State, Brazil, 1980–2012. Cad Saude Publica 2019, 35, e00145117. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, X.; Liu, G.; Lv, M.; Lu, M.; Wu, J.; Huang, Y. Effectiveness of influenza vaccination on in-hospital death and recurrent hospitalization in older adults with cardiovascular diseases. Int. J. Infect. Dis. 2022, 122, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Zahhar, J.A.; Salamatullah, H.K.; Almutairi, M.B.; Faidah, D.E.; Afif, L.M.; Banjar, T.A.; Alansari, N.; Betar, M.; Alghamdi, S.; Makkawi, S. Influenza vaccine effect on risk of stroke occurrence: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1324677. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Fajardo, A.; Oviedo-Orta, E. Influenza vaccination promotes stable atherosclerotic plaques in apoE knockout mice. Atherosclerosis 2011, 217, 97–105. [Google Scholar] [CrossRef]
- Fountoulaki, K.; Tsiodras, S.; Polyzogopoulou, E.; Olympios, C.; Parissis, J. Beneficial Effects of Vaccination on Cardiovascular Events: Myocardial Infarction, Stroke, Heart Failure. Cardiology 2018, 141, 98–106. [Google Scholar] [CrossRef]
- Ciszewski, A. Cardioprotective effect of influenza and pneumococcal vaccination in patients with cardiovascular diseases. Vaccine 2018, 36, 202–206. [Google Scholar] [CrossRef]
| Variable | Group | Before Propensity Score Matching | After Propensity Score Matching | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Vaccinated in Both 2017–2018 and 2018–2019 | Only Vaccinated in 2018–2019 | SMD | Overall | Vaccinated in Both 2017–2018 and 2018–2019 | Only Vaccinated in 2018–2019 | SMD | ||
| Sample size | 20,315 | 12,710 | 7605 | 10,942 | 5471 | 5471 | |||
| Death, n (%) | No | 20,264 (99.75) | 12,676 (99.73) | 7588 (99.78) | 0.009 | 10,891 (99.53) | 5437 (99.38) | 5454 (99.69) | 0.046 |
| Yes | 51 (0.25) | 34 (0.27) | 17 (0.22) | 51 (0.47) | 34 (0.62) | 17 (0.31) | |||
| Gender, n (%) | Male | 9555 (47.03) | 6075 (47.80) | 3480 (45.76) | 0.041 | 5090 (46.52) | 2571 (46.99) | 2519 (46.04) | 0.019 |
| Female | 10,760 (52.97) | 6635 (52.20) | 4125 (54.24) | 5852 (53.48) | 2900 (53.01) | 2952 (53.96) | |||
| Age, mean (SD) | 72.39 (5.82) | 72.61 (5.83) | 72.02 (5.77) | 0.102 | 72.04 (5.68) | 72.13 (5.68) | 71.95 (5.68) | 0.030 | |
| Ethnicity, n (%) | Han | 20,264 (99.75) | 12,680 (99.76) | 7584 (99.72) | 0.008 | 10,912 (99.73) | 5455 (99.71) | 5457 (99.74) | 0.007 |
| Minority | 51 (0.25) | 30 (0.24) | 21 (0.28) | 30 (0.27) | 16 (0.29) | 14 (0.26) | |||
| Marital status, n (%) | Unmarried | 10 (0.05) | 6 (0.05) | 4 (0.05) | 0.028 | 2 (0.02) | 1 (0.02) | 1 (0.02) | 0.026 |
| Married | 19,288 (94.94) | 12,092 (95.14) | 7196 (94.62) | 10,353 (94.62) | 5192 (94.90) | 5161 (94.33) | |||
| Divorce | 74 (0.36) | 49 (0.39) | 25 (0.33) | 32 (0.29) | 16 (0.29) | 16 (0.29) | |||
| Widowhood | 943 (4.64) | 563 (4.43) | 380 (5.00) | 555 (5.07) | 262 (4.79) | 293 (5.36) | |||
| Education level, n (%) | Junior high school and below | 10,668 (52.51) | 6368 (50.10) | 4300 (56.54) | 0.130 | 5730 (52.37) | 2834 (51.80) | 2896 (52.93) | 0.024 |
| High school | 5808 (28.59) | 3845 (30.25) | 1963 (25.81) | 3070 (28.06) | 1561 (28.53) | 1509 (27.58) | |||
| University and above | 3839 (18.90) | 2497 (19.65) | 1342 (17.65) | 2142 (19.58) | 1076 (19.67) | 1066 (19.48) | |||
| Insurance type, n (%) | Insurance payment | 12,668 (62.36) | 8847 (69.61) | 3821 (50.24) | 0.403 | 6298 (57.56) | 3208 (58.64) | 3090 (56.48) | 0.044 |
| Self-funded payment | 7647 (37.64) | 3863 (30.39) | 3784 (49.76) | 4644 (42.44) | 2263 (41.36) | 2381 (43.52) | |||
| Occupational type, n (%) | No occupation | 7719 (38.00) | 4527 (35.62) | 3192 (41.97) | 0.137 | 4286 (39.17) | 2118 (38.71) | 2168 (39.63) | 0.019 |
| production technology | 8839 (43.51) | 5672 (44.63) | 3167 (41.64) | 4641 (42.41) | 2337 (42.72) | 2304 (42.11) | |||
| Management | 3757 (18.49) | 2511 (19.76) | 1246 (16.38) | 2015 (18.42) | 1016 (18.57) | 999 (18.26) | |||
| Dietary habits, n (%) | Balanced | 18,814 (92.61) | 11,808 (92.90) | 7006 (92.12) | 0.031 | 10,111 (92.41) | 5063 (92.54) | 5048 (92.27) | 0.021 |
| Mainly meat | 272 (1.34) | 166 (1.31) | 106 (1.39) | 166 (1.52) | 81 (1.48) | 85 (1.55) | |||
| Mainly vegetarian | 1118 (5.50) | 667 (5.25) | 451 (5.93) | 605 (5.53) | 294 (5.37) | 311 (5.68) | |||
| Salt, oil, and sugar addiction | 111 (0.55) | 69 (0.54) | 42 (0.55) | 60 (0.55) | 33 (0.60) | 27 (0.49) | |||
| Frequency of exercise, n (%) | Never | 3200 (15.75) | 1931 (15.19) | 1269 (16.69) | 0.041 | 1816 (16.60) | 907 (16.58) | 909 (16.61) | 0.028 |
| Occasionally | 911 (4.48) | 569 (4.48) | 342 (4.50) | 491 (4.49) | 260 (4.75) | 231 (4.22) | |||
| More than once a week | 1556 (7.66) | 977 (7.69) | 579 (7.61) | 841 (7.69) | 427 (7.80) | 414 (7.57) | |||
| Every day | 14,648 (72.10) | 9233 (72.64) | 5415 (71.20) | 7794 (71.23) | 3877 (70.86) | 3917 (71.60) | |||
| Smoking, n (%) | Smoke | 1632 (8.03) | 987 (7.77) | 645 (8.48) | 0.038 | 850 (7.77) | 421 (7.70) | 429 (7.84) | 0.007 |
| Quit smoking | 2064 (10.16) | 1334 (10.50) | 730 (9.60) | 1076 (9.83) | 542 (9.91) | 534 (9.76) | |||
| Never smoke | 16,619 (81.81) | 10,389 (81.74) | 6230 (81.92) | 9016 (82.40) | 4508 (82.40) | 4508 (82.40) | |||
| Alcohol consumption, n (%) | Never | 17,083 (84.09) | 10,687 (84.08) | 6396 (84.10) | 0.027 | 9236 (84.41) | 4624 (84.52) | 4612 (84.30) | 0.016 |
| Occasionally | 1930 (9.50) | 1233 (9.70) | 697 (9.17) | 955 (8.73) | 482 (8.81) | 473 (8.65) | |||
| Often or every day | 1302 (6.41) | 790 (6.22) | 512 (6.73) | 751 (6.86) | 365 (6.67) | 386 (7.06) | |||
| BMI, n (%) | Normal | 12,184 (59.98) | 7566 (59.53) | 4618 (60.72) | 0.039 | 6671 (60.97) | 3329 (60.85) | 3342 (61.09) | 0.023 |
| Thin | 701 (3.45) | 418 (3.29) | 283 (3.72) | 401 (3.66) | 194 (3.55) | 207 (3.78) | |||
| Overweight | 6614 (32.56) | 4213 (33.15) | 2401 (31.57) | 3429 (31.34) | 1735 (31.71) | 1694 (30.96) | |||
| Obesity | 816 (4.02) | 513 (4.04) | 303 (3.98) | 441 (4.03) | 213 (3.89) | 228 (4.17) | |||
| HGB, n (%) | Non anemic | 19,414 (95.56) | 12,135 (95.48) | 7279 (95.71) | 0.012 | 10,461 (95.60) | 5220 (95.41) | 5241 (95.80) | 0.019 |
| Anemia | 901 (4.44) | 575 (4.52) | 326 (4.29) | 481 (4.40) | 251 (4.59) | 230 (4.20) | |||
| SCR, n (%) | Normal | 19,261 (94.81) | 12,039 (94.72) | 7222 (94.96) | 0.011 | 10,401 (95.06) | 5196 (94.97) | 5205 (95.14) | 0.017 |
| Low | 615 (3.03) | 392 (3.08) | 223 (2.93) | 318 (2.91) | 157 (2.87) | 161 (2.94) | |||
| High | 439 (2.16) | 279 (2.20) | 160 (2.10) | 223 (2.04) | 118 (2.16) | 105 (1.92) | |||
| Hypertension, n (%) | No | 9952 (48.99) | 6001 (47.21) | 3951 (51.95) | 0.095 | 5498 (50.25) | 2711 (49.55) | 2787 (50.94) | 0.028 |
| Yes | 10,363 (51.01) | 6709 (52.79) | 3654 (48.05) | 5444 (49.75) | 2760 (50.45) | 2684 (49.06) | |||
| Diabetes, n (%) | No | 16,045 (78.98) | 9899 (77.88) | 6146 (80.82) | 0.072 | 8742 (79.89) | 4374 (79.95) | 4368 (79.84) | 0.003 |
| Yes | 4270 (21.02) | 2811 (22.12) | 1459 (19.18) | 2200 (20.11) | 1097 (20.05) | 1103 (20.16) | |||
| Immunization program, n (%) | No | 2424 (11.93) | 180 (1.42) | 2244 (29.51) | 0.843 | 370 (3.38) | 180 (3.29) | 190 (3.47) | 0.010 |
| Yes | 17,891 (88.07) | 12,530 (98.58) | 5361 (70.49) | 10,572 (96.62) | 5291 (96.71) | 5281 (96.53) | |||
| Vaccine type, n (%) | IIV3 | 18,152 (89.35) | 11,512 (90.57) | 6640 (87.31) | 0.105 | 9186 (83.95) | 4598 (84.04) | 4588 (83.86) | 0.005 |
| IIV4 | 2163 (10.65) | 1198 (9.43) | 965 (12.69) | 1756 (16.05) | 873 (15.96) | 883 (16.14) | |||
| Vaccine manufacturer, n (%) | HUALAN BIO | 2829 (13.93) | 1625 (12.79) | 1204 (15.83) | 0.126 | 2095 (19.15) | 1035 (18.92) | 1060 (19.37) | 0.048 |
| Changchun Institute of Biological Products | 17,128 (84.31) | 10,917 (85.89) | 6211 (81.67) | 8548 (78.12) | 4307 (78.72) | 4241 (77.52) | |||
| Sanofi Pasteur | 358 (1.76) | 168 (1.32) | 190 (2.50) | 299 (2.73) | 129 (2.36) | 170 (3.11) | |||
| Influenza Season and Exposure Group | Study Design or Analytical Approach | All-Cause Mortality | CCVD Mortality | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| 2017–2018, vaccinated * | Main analysis | 0.61 (0.47, 0.80) | <0.001 | 0.68 (0.46, 1.02) | 0.060 |
| PSM with caliper values | 0.61 (0.47, 0.80) | <0.001 | 0.68 (0.46, 1.01) | 0.059 | |
| Nested case–control design | 0.63 (0.48, 0.83) | 0.001 | 0.69 (0.46, 1.05) | 0.087 | |
| Poisson’s regression | 0.61 (0.47, 0.80) | <0.001 | 0.68 (0.46, 1.02) | 0.059 | |
| 2018–2019, vaccinated * | Main analysis | 0.45 (0.33, 0.60) | <0.001 | 0.54 (0.34, 0.84) | 0.006 |
| PSM with caliper values | 0.45 (0.33, 0.60) | <0.001 | 0.54 (0.34, 0.84) | 0.006 | |
| Nested case–control design | 0.48 (0.36, 0.65) | <0.001 | 0.58 (0.37, 0.92) | 0.020 | |
| Poisson’s regression | 0.45 (0.34, 0.60) | <0.001 | 0.53 (0.34, 0.84) | 0.006 | |
| 2018–2019, only vaccinated in 2018–2019 *,# | Main analysis | 0.56 (0.31, 0.99) | 0.049 | 0.54 (0.21, 1.39) | 0.200 |
| PSM with caliper values | 0.57 (0.31, 0.99) | 0.049 | 0.54 (0.21, 1.38) | 0.190 | |
| Nested case–control design | 0.43 (0.19, 0.96) | 0.039 | 0.50 (0.13, 1.87) | 0.299 | |
| Poisson’s regression | 0.55 (0.31, 1.00) | 0.049 | 0.59 (0.24, 1.48) | 0.261 | |
| Variable | Group | 2017–2018 Influenza Season | 2018–2019 Influenza Season | ||
|---|---|---|---|---|---|
| HR (95%CI) | Interaction p-Value | HR (95%CI) | Interaction p-Value | ||
| Gender | Female | 0.43 (0.27, 0.68) | 0.132 | 0.27 (0.16, 0.46) | 0.029 |
| Male | 0.62 (0.45, 0.87) | 0.52 (0.36, 0.73) | |||
| Age (years) | 65–75 | 0.76 (0.48, 1.21) | 0.092 | 0.70 (0.45, 1.11) | 0.008 |
| >75 | 0.47 (0.34, 0.65) | 0.31 (0.21, 0.46) | |||
| Education level | Junior high school and below | 0.51 (0.36, 0.71) | 0.040 | 0.47 (0.33, 0.66) | 0.224 |
| High school | 0.40 (0.22, 0.72) | 0.24 (0.12, 0.50) | |||
| University and above | 1.26 (0.64, 2.50) | 0.46 (0.21, 1.01) | |||
| Insurance type | Insurance payment | 0.34 (0.24, 0.50) | <0.001 | 0.28 (0.20, 0.41) | <0.001 |
| Self-funded payment | 1.23 (0.81, 1.87) | 1.16 (0.70, 1.93) | |||
| Occupational type | Management | 0.46 (0.25, 0.86) | 0.826 | 0.45 (0.23, 0.86) | 0.764 |
| Production technology | 0.54 (0.30, 0.97) | 0.45 (0.26, 0.77) | |||
| No occupation | 0.57 (0.40, 0.80) | 0.38 (0.24, 0.55) | |||
| BMI | Normal | 0.51 (0.36, 0.72) | 0.646 | 0.36 (0.24, 0.54) | 0.695 |
| Thin | 0.64 (0.25, 1.65) | 0.58 (0.17, 1.95) | |||
| Overweight | 0.51 (0.30, 0.85) | 0.43 (0.25, 0.73) | |||
| Obesity | 0.91 (0.33, 2.47) | 0.63 (0.24, 1.61) | |||
| HGB | Non anemic | 0.54 (0.40, 0.73) | 0.639 | 0.43 (0.32, 0.59) | 0.459 |
| Anemia | 0.62 (0.36, 1.09) | 0.35 (0.15, 0.80) | |||
| Hypertension | No | 0.59 (0.43, 0.82) | 0.357 | 0.52 (0.35, 0.77) | 0.237 |
| Yes | 0.45 (0.28, 0.72) | 0.31 (0.20, 0.49) | |||
| Diabetes | No | 0.49 (0.37, 0.66) | 0.045 | 0.44 (0.33, 0.60) | 0.249 |
| Yes | 1.11 (0.56, 2.18) | 0.21 (0.08, 0.58) | |||
| Influenza Season | Cause of Death | Number of Events | ICD-10 | HR (95% CI) | p-Value |
|---|---|---|---|---|---|
| 2017–2018 | CCVD | 246 | I00–I99 | 0.68 (0.46, 1.02) | 0.060 |
| Ischemic heart disease | 118 | I20–I25 | 0.61 (0.33, 1.13) | 0.120 | |
| Cerebrovascular disease | 82 | I60–I69 | 0.65 (0.33, 1.29) | 0.220 | |
| Other circulatory system diseases | 46 | I00–I15, I26–I52, I70–I99 | 0.95 (0.43, 2.10) | 0.890 | |
| 2018–2019 | CCVD | 226 | I00–I99 | 0.54 (0.34, 0.84) | 0.006 |
| Ischemic heart disease | 100 | I20–I25 | 0.66 (0.36, 1.23) | 0.190 | |
| Cerebrovascular disease | 82 | I60–I69 | 0.49 (0.13, 0.80) | 0.014 | |
| Other circulatory system diseases | 44 | I00–I15, I26–I52, I70–I99 | 0.7 (0.26, 1.85) | 0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Wang, Y.; Wei, Y.; Hu, W.; Zhou, J.; Nama, N.; Ma, Y.; Liu, G.; Hao, Y. Effect of Current-Season-Only Versus Continuous Two-Season Influenza Vaccination on Mortality in Older Adults: A Propensity-Score-Matched Retrospective Cohort Study. Vaccines 2025, 13, 164. https://doi.org/10.3390/vaccines13020164
Sun H, Wang Y, Wei Y, Hu W, Zhou J, Nama N, Ma Y, Liu G, Hao Y. Effect of Current-Season-Only Versus Continuous Two-Season Influenza Vaccination on Mortality in Older Adults: A Propensity-Score-Matched Retrospective Cohort Study. Vaccines. 2025; 13(2):164. https://doi.org/10.3390/vaccines13020164
Chicago/Turabian StyleSun, Huimin, Yijing Wang, Yongyue Wei, Weihua Hu, Junwen Zhou, Nuosu Nama, Yujie Ma, Gang Liu, and Yuantao Hao. 2025. "Effect of Current-Season-Only Versus Continuous Two-Season Influenza Vaccination on Mortality in Older Adults: A Propensity-Score-Matched Retrospective Cohort Study" Vaccines 13, no. 2: 164. https://doi.org/10.3390/vaccines13020164
APA StyleSun, H., Wang, Y., Wei, Y., Hu, W., Zhou, J., Nama, N., Ma, Y., Liu, G., & Hao, Y. (2025). Effect of Current-Season-Only Versus Continuous Two-Season Influenza Vaccination on Mortality in Older Adults: A Propensity-Score-Matched Retrospective Cohort Study. Vaccines, 13(2), 164. https://doi.org/10.3390/vaccines13020164






