Synthetic rEg.P29 Peptides Induce Protective Immune Responses Against Echinococcus granulosus in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antigen and Vaccines
2.2. Experimental Protocol
2.3. Lymphocyte Preparation and Culture
2.4. ELISA and ELISPOT Assay
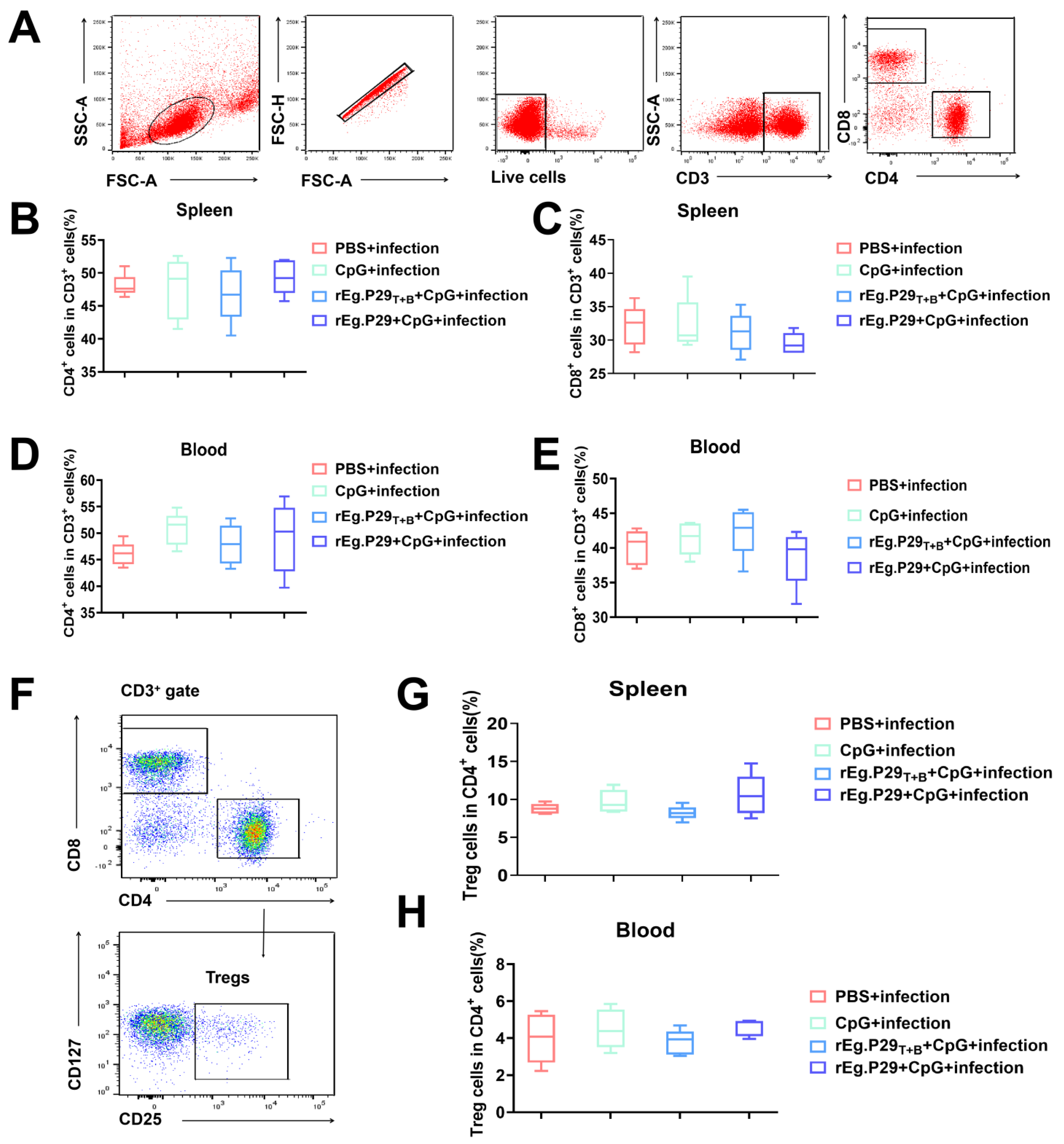
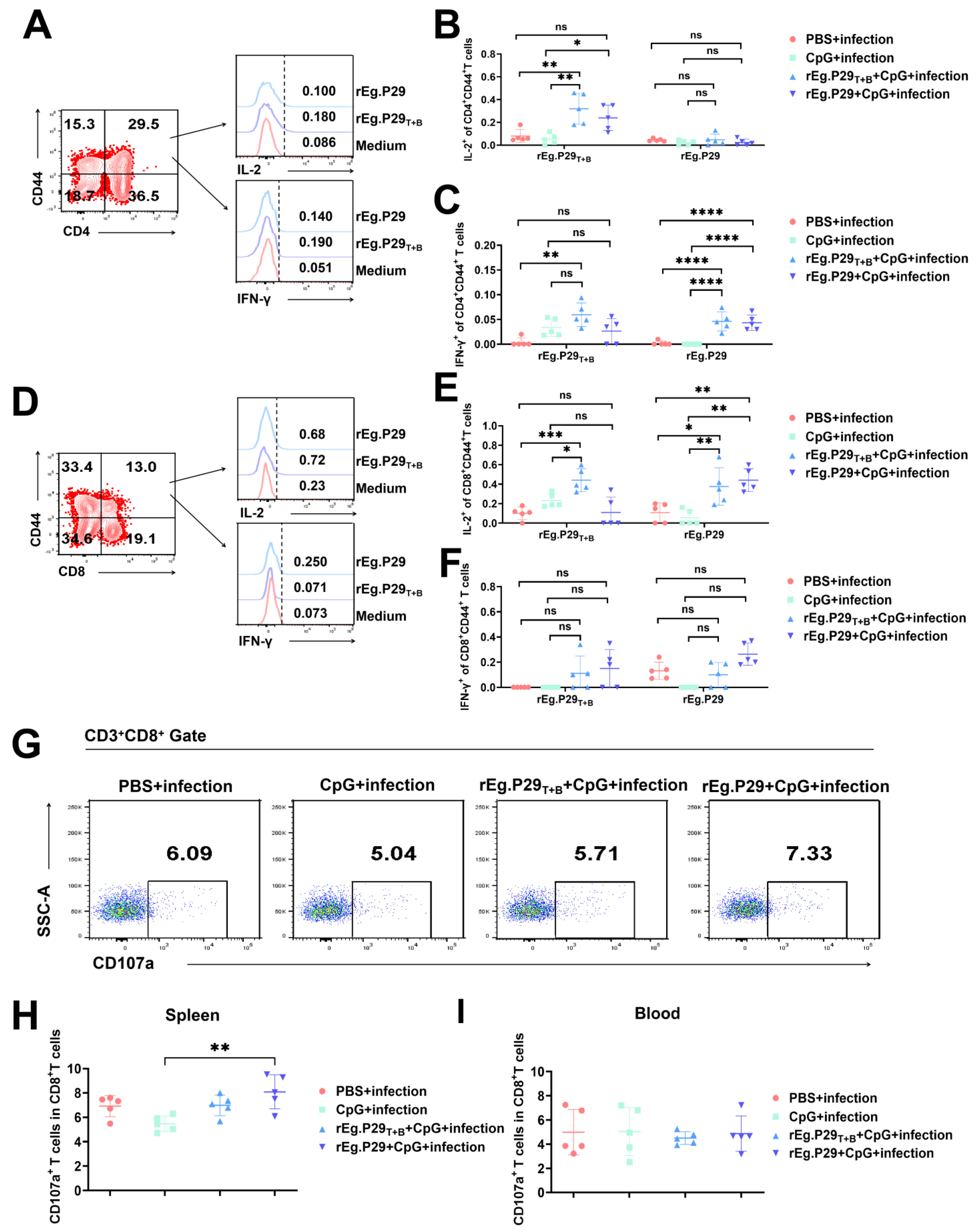
2.5. Flow Cytometry and Intracellular Cytokine Staining
2.6. Detection of Specific Antibody Response with ELISA
2.7. Statistical Analysis
3. Results
3.1. Evaluation of Potential Protective Effects of Designed Vaccines
3.2. Evaluation of Vaccine-Induced T-Cell Immune Response
3.3. Evaluation of Vaccine-Induced Humoral Immunoreaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| E. granulosus | Echinococcus granulosus |
| Th1 | T helper type 1 |
| ELISA | Enzyme-linked immunosorbent assay |
| ELISPOT | Enzyme-linked immune spot assay |
| SFCs | Spot-forming cells |
| PFA | paraformaldehyde |
| HRP | horse radish peroxidase |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| FCM | Flow cytometry |
| Tregs | Regulatory T cells |
| CTLs | Cytotoxic T Lymphocyte |
References
- Sanchez, L.; Mayta, H.; Jara, L.M.; Verastegui, M.; Gilman, R.H.; Gomez-Puerta, L.A.; Gavidia, C.M. Echinococcus granulosus sensu stricto and E. canadensis are distributed in livestock of highly endemic area in the Peruvian highlands. Acta Trop. 2022, 225, 106178. [Google Scholar] [CrossRef] [PubMed]
- Huzaifa, M.; Sharman, T. Ecchinococcus (Archived); StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Shahnazi, M.; Habibvand, M.; Johkool, M.G.; Hajialilo, E.; Sharifdini, M.; Javadi, A.; Saraei, M. Molecular Characterization of Echinococcus granulosus Sensu Stricto Isolated from the Livestock of Qazvin, Iran. Infect. Disord. Drug Targets 2021, 21, e161752283. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, A.B.; Sanei, B.; Yousefi, M.; Sharafi, S.M.; Safarnezhad, F.; Jafari, R.; Darani, H.Y. Albendazole and Treatment of Hydatid Cyst: Review of the Literature. Infect. Disord. Drug Targets 2019, 19, 101–104. [Google Scholar] [CrossRef]
- Chaabane-Banaoues, R.; Oudni-M’Rad, M.; M’Rad, S.; Mezhoud, H.; Babba, H. Environmental Contamination by Echinococcus granulosus sensu lato Eggs in Relation to Slaughterhouses in Urban and Rural Areas in Tunisia. Korean J. Parasitol. 2016, 54, 113–118. [Google Scholar] [CrossRef]
- Wen, H.; Vuitton, L.; Tuxun, T.; Li, J.; Vuitton, D.A.; Zhang, W.; McManus, D.P. Echinococcosis: Advances in the 21st Century. Clin. Microbiol. Rev. 2019, 32, 10–1128. [Google Scholar] [CrossRef]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef]
- Wen-Jun, Z.; Xiu-Min, H.; Ya-Min, G. Progress in researches of benzimidazole in treatment of echinococcosis. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2017, 29, 530–533. [Google Scholar]
- Brehm, K.; Koziol, U. On the importance of targeting parasite stem cells in anti-echinococcosis drug development. Parasite 2014, 21, 72. [Google Scholar] [CrossRef]
- Alkan, S.S.; Decker, T.; von Gabain, A. Vaccines—The key paradigm for the 21st century’s health care strategy: 5th Semmering vaccine symposium, Baden/Vienna. Editorial. Vaccine 2012, 30, 4299–4300. [Google Scholar] [CrossRef]
- Ottenhoff, T. Correlates of vaccine adjuvanticity, vaccine activity, protective immunity and disease in human infectious disease and cancer. Semin. Immunol. 2018, 39, 1–3. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, S.; Wang, Q.; Wang, C.; Zhu, M.; Ma, Z.; Zhao, W. Mechanisms underlying immune tolerance caused by recombinant Echinococcus granulosus antigens Eg mMDH and Eg10 in dendritic cells. PLoS ONE 2018, 13, e204868. [Google Scholar] [CrossRef] [PubMed]
- Amni, F.; Hajizadeh, M.; Elmi, T.; Hatam, N.K.; Shafaei, S.; Javadi, M.A.; Rafiei, S.R. Different manifestation of Echinococcus granulosus immunogenic antigens in the liver and lungs of intermediate host. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, H.; Shen, Y.; Wang, Y.; Wu, W.; Liu, H.; Yuan, Z.; Xu, Y.; Hu, Y.; Cao, J. Impairment of dendritic cell function and induction of CD4(+)CD25(+)Foxp3(+) T cells by excretory-secretory products: A potential mechanism of immune evasion adopted by Echinococcus granulosus. BMC Immunol. 2015, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Pan, C.; Cheng, P.; Wang, J.; Zhao, G.; Wu, X. Peptide-Based Vaccines for Tuberculosis. Front. Immunol. 2022, 13, 830497. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef]
- Oscherwitz, J. The promise and challenge of epitope-focused vaccines. Hum. Vaccin. Immunother. 2016, 12, 2113–2116. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Y.; Li, Z.; Li, Z.; Bo, Y.; Ma, R.; Zhao, W. Cloning, expression, and protective immunity in mice of a gene encoding the diagnostic antigen P-29 of Echinococcus granulosus. Acta Biochim. Biophys. Sin. 2009, 41, 79–85. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Gao, F.; Zhao, J.; Zhu, M.; He, X.; Niu, N.; Zhao, W. Immunoprotection of recombinant Eg.P29 against Echinococcus granulosus in sheep. Vet. Res. Commun. 2016, 40, 73–79. [Google Scholar] [CrossRef]
- Lv, Y.; Zhu, Y.; Chang, L.; Yang, J.; Zhao, Y.; Zhao, J.; Wang, Y.; Zhu, M.; Wu, C.; Zhao, W. Identification of a dominant murine T-cell epitope in recombinant protein P29 from Echinococcus granulosus. Acta Biochim. Biophys. Sin. 2022, 54, 482–493. [Google Scholar] [CrossRef]
- Lv, Y.; Li, S.; Zhang, T.; Zhu, Y.; Tao, J.; Yang, J.; Chang, L.; Wu, C.; Zhao, W. Identification of B-cell dominant epitopes in the recombinant protein P29 from Echinococcus granulosus. Immun. Inflamm. Dis. 2022, 10, e611. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chang, L.; Yang, J.; Wen, J.; Zhao, Y.; Zhu, M.; Wu, C.; Zhao, W. Immunogenicity of peptide-based vaccine composed of epitopes from Echinococcus granulosus rEg.P29. FASEB J. 2023, 37, e22819. [Google Scholar] [CrossRef]
- Woollard, D.J.; Heath, D.D.; Lightowlers, M.W. Assessment of protective immune responses against hydatid disease in sheep by immunization with synthetic peptide antigens. Parasitology 2000, 121 Pt 2, 145–153. [Google Scholar] [CrossRef]
- Woollard, D.J.; Gauci, C.G.; Heath, D.D.; Lightowlers, M.W. Protection against hydatid disease induced with the EG95 vaccine is associated with conformational epitopes. Vaccine 2000, 19, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Deplazes, P.; Casulli, A. Reinventing the Wheel of Echinococcus granulosus sensu lato Transmission to Humans. Trends Parasitol. 2020, 36, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Woolsey, I.D.; Miller, A.L. Echinococcus granulosus sensu lato and Echinococcus multilocularis: A review. Res. Vet. Sci. 2021, 135, 517–522. [Google Scholar] [CrossRef]
- Bosco, A.; Alves, L.C.; Cociancic, P.; Amadesi, A.; Pepe, P.; Morgoglione, M.E.; Maurelli, M.P.; Ferrer-Miranda, E.; Santoro, K.R.; Nascimento, R.R.; et al. Epidemiology and spatial distribution of Echinococcus granulosus in sheep and goats slaughtered in a hyperendemic European Mediterranean area. Parasit. Vectors 2021, 14, 421. [Google Scholar] [CrossRef]
- Rigano, R.; Buttari, B.; De Falco, E.; Profumo, E.; Ortona, E.; Margutti, P.; Scotta, C.; Teggi, A. Siracusano A: Echinococcus granulosus-specific T-cell lines derived from patients at various clinical stages of cystic echinococcosis. Parasite Immunol. 2004, 26, 45–52. [Google Scholar] [CrossRef]
- Mezioug, D.; Touil-Boukoffa, C. Cytokine profile in human hydatidosis: Possible role in the immunosurveillance of patients infected with Echinococcus granulosus. Parasite 2009, 16, 57–64. [Google Scholar] [CrossRef]
- Amri, M.; Mezioug, D.; Touil-Boukoffa, C. Involvement of IL-10 and IL-4 in evasion strategies of Echinococcus granulosus to host immune response. Eur. Cytokine Netw. 2009, 20, 63–68. [Google Scholar] [CrossRef]
- Zhang, X.F.; Gong, W.C.; Cao, S.K.; Xu, M.; Cao, J.P.; Shen, Y.J. Dynamic changes of myeloid-derived suppressor cells and regulatory T cells in livers of mice infected with Echinococcus granulosus. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2019, 31, 622–627. [Google Scholar] [PubMed]
- Foster, N.; Elsheikha, H.M. The immune response to parasitic helminths of veterinary importance and its potential manipulation for future vaccine control strategies. Parasitol. Res. 2012, 110, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Ismael, A.B.; Dimier-Poisson, I.; Lebrun, M.; Dubremetz, J.F.; Bout, D.; Mevelec, M.N. Mic1-3 knockout of Toxoplasma gondii is a successful vaccine against chronic and congenital toxoplasmosis in mice. J. Infect. Dis. 2006, 194, 1176–1183. [Google Scholar] [CrossRef]
- Fang, R.; Nie, H.; Wang, Z.; Tu, P.; Zhou, D.; Wang, L.; He, L.; Zhou, Y.; Zhao, J. Protective immune response in BALB/c mice induced by a suicidal DNA vaccine of the MIC3 gene of Toxoplasma gondii. Vet. Parasitol. 2009, 164, 134–140. [Google Scholar] [CrossRef]
- Flippe, L.; Bezie, S.; Anegon, I.; Guillonneau, C. Future prospects for CD8(+) regulatory T cells in immune tolerance. Immunol. Rev. 2019, 292, 209–224. [Google Scholar] [CrossRef]
- Betts, M.R.; Brenchley, J.M.; Price, D.A.; De Rosa, S.C.; Douek, D.C.; Roederer, M.; Koup, R.A. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 2003, 281, 65–78. [Google Scholar] [CrossRef]
- Lees, J.R. CD8+ T cells: The past and future of immune regulation. Cell Immunol. 2020, 357, 104212. [Google Scholar] [CrossRef]
- Heath, D.D.; Koolaard, J. Serological monitoring of protection of sheep against Echinococcus granulosus induced by the EG95 vaccine. Parasite Immunol. 2012, 34, 40–44. [Google Scholar] [CrossRef]
- Xu, T.; Liu, L.; Shi, C.; Liu, W.; Wang, M.; Tian, L.; Zheng, Y.; Wang, H.; Zheng, W.; He, H.; et al. A recombinant rabies virus expressing Echinococcus granulosus EG95 induces protective immunity in mice. Transbound. Emerg. Dis. 2022, 69, e254–e266. [Google Scholar] [CrossRef]
- Gauci, C.; Heath, D.; Chow, C.; Lightowlers, M.W. Hydatid disease: Vaccinology and development of the EG95 recombinant vaccine. Expert Rev. Vaccines 2005, 4, 103–112. [Google Scholar] [CrossRef]
- Grund, M.; Choi, S.J.; Powell, L.; Lukomski, S. Intranasal immunization with a Bucl8-based vaccine ameliorates bacterial burden and pathological inflammation, and promotes an IgG2a/b dominant response in an outbred mouse model of Burkholderia infection. Front. Immunol. 2023, 14, 1177650. [Google Scholar] [CrossRef]
- Collins, A.M. IgG subclass co-expression brings harmony to the quartet model of murine IgG function. Immunol. Cell Biol. 2016, 94, 949–954. [Google Scholar] [CrossRef]




| Species | Antigen | Fluorochrom | Clone | Supplier | Cat. No. |
|---|---|---|---|---|---|
| Mouse | CD19 | APC-Cy7 | ID3 | BD Pharmingen | 557655 |
| Mouse | CD138 | BV421 | 281-2 | BD Pharmingen | 562610 |
| Mouse | IgG | APC | Poly4053 | Biolegend | 405308 |
| Mouse | IgD | BV786 | C10-1 | BD Pharmingen | 563618 |
| Mouse | IgA | BV605 | 11-26c.2α | BD Pharmingen | 743295 |
| Mouse | CD3 | PE-CF594 | 145-2C11 | BD Pharmingen | 562286 |
| Mouse | CD4 | APC-Cy7 | GK1.5 | BD Pharmingen | 552051 |
| Mouse | CD8 | Pacific Blue | 53-6.7 | BD Pharmingen | 558106 |
| Mouse | IFN-γ | FITC | XMG1.3 | BD Pharmingen | 554411 |
| Mouse | IL-2 | PE | JES6-5H4 | BD Pharmingen | 554428 |
| Mouse | CD44 | APC | IM7 | BD Pharmingen | 561862 |
| Mouse | CD25 | PE | PC61 | BD Pharmingen | 553866 |
| Mouse | CD127 | FITC | A7R34 | Biolegend | 135007 |
| Mouse | CD107a | APC | 1D4B | Biolegend | 121614 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Tang, J.; Li, T.; Zhao, Y.; Wu, C.; Zhao, W. Synthetic rEg.P29 Peptides Induce Protective Immune Responses Against Echinococcus granulosus in Mice. Vaccines 2025, 13, 266. https://doi.org/10.3390/vaccines13030266
Lv Y, Tang J, Li T, Zhao Y, Wu C, Zhao W. Synthetic rEg.P29 Peptides Induce Protective Immune Responses Against Echinococcus granulosus in Mice. Vaccines. 2025; 13(3):266. https://doi.org/10.3390/vaccines13030266
Chicago/Turabian StyleLv, Yongxue, Jing Tang, Tao Li, Yinqi Zhao, Changyou Wu, and Wei Zhao. 2025. "Synthetic rEg.P29 Peptides Induce Protective Immune Responses Against Echinococcus granulosus in Mice" Vaccines 13, no. 3: 266. https://doi.org/10.3390/vaccines13030266
APA StyleLv, Y., Tang, J., Li, T., Zhao, Y., Wu, C., & Zhao, W. (2025). Synthetic rEg.P29 Peptides Induce Protective Immune Responses Against Echinococcus granulosus in Mice. Vaccines, 13(3), 266. https://doi.org/10.3390/vaccines13030266




