Recent Progress in Developing Extracellular Vesicles as Nanovehicles to Deliver Carbohydrate-Based Therapeutics and Vaccines
Abstract
:1. Introduction
1.1. EVs and Their Biogenesis
1.1.1. Biogenesis of Mammalian EVs
1.1.2. Biogenesis of Bacterial EVs
1.2. Glyco-Biomedicine
1.2.1. Biogenesis and Biological Significance of Glycan
1.2.2. Production of Glycoprotein Therapeutics and Glycoconjugate Vaccines
2. Preparation of EVs as Nanovehicles for Drug and Vaccine Delivery
2.1. EVs’ Source and Isolation
2.2. EVs’ Characterization
2.3. Glycoengineering of EVs and Their Payload
3. The Application of EVs in Disease Treatment
3.1. EVs in Neurological Diseases
3.1.1. Alzheimer’s Disease (AD)
3.1.2. Parkinson’s Disease (PD)
3.2. EVs in Cardiovascular Diseases
3.3. EVs in Cancer Treatment
3.4. Infectious Disease
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ApoEVs | apoptotic bodies from programmed cell death or apoptosis |
| BBB | blood–brain barrier |
| BEVs | bacterial extracellular vesicles |
| CAR | chimeric antigen receptor |
| CDC | cardiac-derived cells |
| CHO | Chinese hamster ovary |
| CMVs | cytoplasmic membrane vesicles |
| CPCs | cardiac progenitor cells |
| CVD | cardiovascular disease |
| DCs | Dendritic cells |
| DC-SIGN | dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin |
| DLS | dynamic light scattering |
| DOX | doxorubicin |
| ELVs | exosome-like vesicles |
| EOMVs | explosive outer-membrane vesicles |
| ESCRT | endosomal sorting complex required for transport |
| EVs | extracellular vesicles |
| GMMAs | Generalized Modules for Membrane Antigens |
| glycOMVs | glycoengineered OMVs |
| GTs | glycosyltransferases |
| ILVs | intraluminal vesicles |
| iPSC-EVs | induced pluripotent stem cells |
| LPSs | lipopolysaccharides |
| MSC | mesenchymal stem cell |
| NSCLC | non-small-cell lung cancer |
| NSC-exos | neuronal stem cell-derived exosomes |
| OIMVs | outer–inner membrane vesicles |
| OMVs | outer membrane vesicles |
| PAMPs | pathogen-associated molecular patterns |
| PEG | polyethylene glycol |
| SEC | size-exclusion chromatography |
| SPT | single-particle tracking analysis |
| TEM | transmission electron microscopy |
References
- Di Bella, M.A. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef]
- Fyfe, J.; Casari, I.; Manfredi, M.; Falasca, M. Role of Lipid Signalling in Extracellular Vesicles-Mediated Cell-to-Cell Communication. Cytokine Growth Factor Rev. 2023, 73, 20–26. [Google Scholar] [CrossRef]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane Vesicles, Current State-of-the-Art: Emerging Role of Extracellular Vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Ramis, J.M. Extracellular Vesicles in Cell Biology and Medicine. Sci. Rep. 2020, 10, 8667. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiu, Y.; Jiang, W.; Shen, J.; Yao, X.; He, X.; Li, L.; Fu, B.; Liu, X. Biological Features of Extracellular Vesicles and Challenges. Front. Cell Dev. Biol. 2022, 10, 816698. [Google Scholar] [CrossRef]
- Wen, C.; Seeger, R.C.; Fabbri, M.; Wang, L.; Wayne, A.S.; Jong, A.Y. Biological Roles and Potential Applications of Immune Cell-Derived Extracellular Vesicles. J. Extracell. Vesicles 2017, 6, 1400370. [Google Scholar] [CrossRef]
- van der Pol, E.; Böing, A.N.; Harrison, P.; Sturk, A.; Nieuwland, R. Classification, Functions, and Clinical Relevance of Extracellular Vesicles. Pharmacol. Rev. 2012, 64, 676–705. [Google Scholar] [CrossRef]
- Witwer, K.W.; Théry, C. Extracellular Vesicles or Exosomes? On Primacy, Precision, and Popularity Influencing a Choice of Nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Hallal, S.; Tűzesi, Á.; Grau, G.E.; Buckland, M.E.; Alexander, K.L. Understanding the Extracellular Vesicle Surface for Clinical Molecular Biology. J. Extracell. Vesicles 2022, 11, e12260. [Google Scholar] [CrossRef]
- Hong, P.; Yu, M.; Tian, W. Diverse RNAs in Adipose-Derived Extracellular Vesicles and Their Therapeutic Potential. Mol. Ther. Nucleic Acids 2021, 26, 665–677. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Araujo-Abad, S.; Saceda, M.; de Juan Romero, C. Biomedical Application of Small Extracellular Vesicles in Cancer Treatment. Adv. Drug Deliv. Rev. 2022, 182, 114117. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Kostyushev, D.; Kostyusheva, A.; Brezgin, S.; Smirnov, V.; Volchkova, E.; Lukashev, A.; Chulanov, V. Gene Editing by Extracellular Vesicles. Int. J. Mol. Sci. 2020, 21, 7362. [Google Scholar] [CrossRef]
- Cheng, L.; Hill, A.F. Therapeutically Harnessing Extracellular Vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef]
- Gregory, C.D.; Rimmer, M.P. Extracellular Vesicles Arising from Apoptosis: Forms, Functions, and Applications. J. Pathol. 2023, 260, 592–608. [Google Scholar] [CrossRef]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-Specific Regulation of Extracellular Vesicle Biogenesis and Cargo Selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef]
- Isaac, R.; Reis, F.C.G.; Ying, W.; Olefsky, J.M. Exosomes as Mediators of Intercellular Crosstalk in Metabolism. Cell Metab. 2021, 33, 1744–1762. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, M.; Ma, X.; Chen, H.; Gao, X. Harnessing Exosomes for the Development of Brain Drug Delivery Systems. Bioconjug. Chem. 2019, 30, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering Exosomes for Targeted Drug Delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Viñas, J.L.; Spence, M.; Gutsol, A.; Knoll, W.; Burger, D.; Zimpelmann, J.; Allan, D.S.; Burns, K.D. Receptor-Ligand Interaction Mediates Targeting of Endothelial Colony Forming Cell-Derived Exosomes to the Kidney after Ischemic Injury. Sci. Rep. 2018, 8, 16320. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Yue, S.; Stadel, D.; Zöller, M. Toward Tailored Exosomes: The Exosomal Tetraspanin Web Contributes to Target Cell Selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef]
- Kita, S.; Maeda, N.; Shimomura, I. Interorgan Communication by Exosomes, Adipose Tissue, and Adiponectin in Metabolic Syndrome. J. Clin. Investig. 2019, 129, 4041–4049. [Google Scholar] [CrossRef]
- Dang, S.-Y.; Leng, Y.; Wang, Z.-X.; Xiao, X.; Zhang, X.; Wen, T.; Gong, H.-Z.; Hong, A.; Ma, Y. Exosomal Transfer of Obesity Adipose Tissue for Decreased miR-141-3p Mediate Insulin Resistance of Hepatocytes. Int. J. Biol. Sci. 2019, 15, 351–368. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, X.; Hoo, R.L.C.; Ye, D.; Chan, C.Y.C.; Feng, T.; Wang, Y.; Lam, K.S.L.; Xu, A. Adipocyte-Secreted Exosomal microRNA-34a Inhibits M2 Macrophage Polarization to Promote Obesity-Induced Adipose Inflammation. J. Clin. Investig. 2019, 129, 834–849. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, L.; Mei, H.; Zhang, J.; Zhu, Y.; Han, X.; Zhu, D. Inflamed Macrophage Microvesicles Induce Insulin Resistance in Human Adipocytes. Nutr. Metab. 2015, 12, 21. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384.e12. [Google Scholar] [CrossRef]
- Castaño, C.; Kalko, S.; Novials, A.; Párrizas, M. Obesity-Associated Exosomal miRNAs Modulate Glucose and Lipid Metabolism in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, 12158–12163. [Google Scholar] [CrossRef]
- Párrizas, M.; Brugnara, L.; Esteban, Y.; González-Franquesa, A.; Canivell, S.; Murillo, S.; Gordillo-Bastidas, E.; Cussó, R.; Cadefau, J.A.; García-Roves, P.M.; et al. Circulating miR-192 and miR-193b Are Markers of Prediabetes and Are Modulated by an Exercise Intervention. J. Clin. Endocrinol. Metab. 2015, 100, E407–E415. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Danielson, K.M.; Benton, M.C.; Ziegler, O.; Shah, R.; Stubbs, R.S.; Das, S.; Macartney-Coxson, D. miRNA Signatures of Insulin Resistance in Obesity. Obesity 2017, 25, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, S.E.; Grijalva, A.; Xu, X.; Ables, E.; Nomani, A.; Ferrante, A.W. A Lipase-Independent Pathway of Lipid Release and Immune Modulation by Adipocytes. Science 2019, 363, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Sedgwick, A.E.; D’Souza-Schorey, C. The Biology of Extracellular Microvesicles. Traffic 2018, 19, 319–327. [Google Scholar] [CrossRef]
- Ståhl, A.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and Microvesicles in Normal Physiology, Pathophysiology, and Renal Diseases. Pediatr. Nephrol. 2019, 34, 11–30. [Google Scholar] [CrossRef]
- Prada, I.; Amin, L.; Furlan, R.; Legname, G.; Verderio, C.; Cojoc, D. A New Approach to Follow a Single Extracellular Vesicle-Cell Interaction Using Optical Tweezers. Biotechniques 2016, 60, 35–41. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Heusermann, W.; Hean, J.; Trojer, D.; Steib, E.; von Bueren, S.; Graff-Meyer, A.; Genoud, C.; Martin, K.; Pizzato, N.; Voshol, J.; et al. Exosomes Surf on Filopodia to Enter Cells at Endocytic Hot Spots, Traffic within Endosomes, and Are Targeted to the ER. J. Cell Biol. 2016, 213, 173–184. [Google Scholar] [CrossRef]
- Chen, L.; Brigstock, D.R. Integrins and Heparan Sulfate Proteoglycans on Hepatic Stellate Cells (HSC) Are Novel Receptors for HSC-Derived Exosomes. FEBS Lett. 2016, 590, 4263–4274. [Google Scholar] [CrossRef]
- French, K.C.; Antonyak, M.A.; Cerione, R.A. Extracellular Vesicle Docking at the Cellular Port: Extracellular Vesicle Binding and Uptake. Semin. Cell Dev. Biol. 2017, 67, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, M.J.; Temoche-Diaz, M.M.; Karfilis, K.V.; Ri, S.; Schekman, R. Y-Box Protein 1 Is Required to Sort microRNAs into Exosomes in Cells and in a Cell-Free Reaction. eLife 2016, 5, e19276. [Google Scholar] [CrossRef] [PubMed]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.L.G.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of Transfer of Functional microRNAs between Mouse Dendritic Cells via Exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Chairoungdua, A.; Smith, D.L.; Pochard, P.; Hull, M.; Caplan, M.J. Exosome Release of β-Catenin: A Novel Mechanism That Antagonizes Wnt Signaling. J. Cell Biol. 2010, 190, 1079–1091. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, D.; Jin, F.; Bian, Z.; Li, L.; Liang, H.; Li, M.; Shi, L.; Pan, C.; Zhu, D.; et al. Pyruvate Kinase Type M2 Promotes Tumour Cell Exosome Release via Phosphorylating Synaptosome-Associated Protein 23. Nat. Commun. 2017, 8, 14041. [Google Scholar] [CrossRef]
- Cashikar, A.G.; Shim, S.; Roth, R.; Maldazys, M.R.; Heuser, J.E.; Hanson, P.I. Structure of Cellular ESCRT-III Spirals and Their Relationship to HIV Budding. eLife 2014, 3, e02184. [Google Scholar] [CrossRef] [PubMed]
- Adell, M.A.Y.; Vogel, G.F.; Pakdel, M.; Müller, M.; Lindner, H.; Hess, M.W.; Teis, D. Coordinated Binding of Vps4 to ESCRT-III Drives Membrane Neck Constriction during MVB Vesicle Formation. J. Cell Biol. 2014, 205, 33–49. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. Discovery of Double-Stranded Genomic DNA in Circulating Exosomes. Cold Spring Harb. Symp. Quant. Biol. 2016, 81, 275–280. [Google Scholar] [CrossRef]
- Newton, W.C.; Kim, J.W.; Luo, J.Z.Q.; Luo, L. Stem Cell-Derived Exosomes: A Novel Vector for Tissue Repair and Diabetic Therapy. J. Mol. Endocrinol. 2017, 59, R155–R165. [Google Scholar] [CrossRef]
- Lee, Y.; El Andaloussi, S.; Wood, M.J.A. Exosomes and Microvesicles: Extracellular Vesicles for Genetic Information Transfer and Gene Therapy. Hum. Mol. Genet. 2012, 21, R125–R134. [Google Scholar] [CrossRef]
- Tong, M.; Chamley, L.W. Placental Extracellular Vesicles and Feto-Maternal Communication. Cold Spring Harb. Perspect. Med. 2015, 5, a023028. [Google Scholar] [CrossRef] [PubMed]
- Melki, I.; Tessandier, N.; Zufferey, A.; Boilard, E. Platelet Microvesicles in Health and Disease. Platelets 2017, 28, 214–221. [Google Scholar] [CrossRef]
- Blander, J.M. The Many Ways Tissue Phagocytes Respond to Dying Cells. Immunol. Rev. 2017, 277, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Poon, I.K.H. Methods for Monitoring the Progression of Cell Death, Cell Disassembly and Cell Clearance. Apoptosis 2019, 24, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Renò, F.; Burattini, S.; Rossi, S.; Luchetti, F.; Columbaro, M.; Santi, S.; Papa, S.; Falcieri, E. Phospholipid Rearrangement of Apoptotic Membrane Does Not Depend on Nuclear Activity. Histochem. Cell Biol. 1998, 110, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Poon, I.K.H.; Lucas, C.D.; Rossi, A.G.; Ravichandran, K.S. Apoptotic Cell Clearance: Basic Biology and Therapeutic Potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef]
- Nagata, S.; Suzuki, J.; Segawa, K.; Fujii, T. Exposure of Phosphatidylserine on the Cell Surface. Cell Death Differ. 2016, 23, 952–961. [Google Scholar] [CrossRef]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging Role of Extracellular Vesicles in Inflammatory Diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [CrossRef]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic Bodies from Endothelial Cells Enhance the Number and Initiate the Differentiation of Human Endothelial Progenitor Cells in Vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef]
- Holmgren, L.; Szeles, A.; Rajnavölgyi, E.; Folkman, J.; Klein, G.; Ernberg, I.; Falk, K.I. Horizontal Transfer of DNA by the Uptake of Apoptotic Bodies. Blood 1999, 93, 3956–3963. [Google Scholar] [CrossRef]
- Lemke, G. How Macrophages Deal with Death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Eken, C.; Martin, P.J.; Sadallah, S.; Treves, S.; Schaller, M.; Schifferli, J.A. Ectosomes Released by Polymorphonuclear Neutrophils Induce a MerTK-Dependent Anti-Inflammatory Pathway in Macrophages. J. Biol. Chem. 2010, 285, 39914–39921. [Google Scholar] [CrossRef]
- Graham, D.K.; DeRyckere, D.; Davies, K.D.; Earp, H.S. The TAM Family: Phosphatidylserine Sensing Receptor Tyrosine Kinases Gone Awry in Cancer. Nat. Rev. Cancer 2014, 14, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Berda-Haddad, Y.; Robert, S.; Salers, P.; Zekraoui, L.; Farnarier, C.; Dinarello, C.A.; Dignat-George, F.; Kaplanski, G. Sterile Inflammation of Endothelial Cell-Derived Apoptotic Bodies Is Mediated by Interleukin-1α. Proc. Natl. Acad. Sci. USA 2011, 108, 20684–20689. [Google Scholar] [CrossRef]
- Winau, F.; Weber, S.; Sad, S.; de Diego, J.; Hoops, S.L.; Breiden, B.; Sandhoff, K.; Brinkmann, V.; Kaufmann, S.H.E.; Schaible, U.E. Apoptotic Vesicles Crossprime CD8 T Cells and Protect against Tuberculosis. Immunity 2006, 24, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Horrevorts, S.K.; Stolk, D.A.; van de Ven, R.; Hulst, M.; van Het Hof, B.; Duinkerken, S.; Heineke, M.H.; Ma, W.; Dusoswa, S.A.; Nieuwland, R.; et al. Glycan-Modified Apoptotic Melanoma-Derived Extracellular Vesicles as Antigen Source for Anti-Tumor Vaccination. Cancers 2019, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Peng, M.; Vaquerano, J.E.; Zhou, Y.M.; Clinton, R.A.; Hyun, W.C.; Giedlin, M.A.; Leong, S.P. Induction of Th1 Response by Dendritic Cells Pulsed with Autologous Melanoma Apoptotic Bodies. Anticancer Res. 2000, 20, 1329–1336. [Google Scholar] [PubMed]
- Hus, I.; Roliński, J.; Tabarkiewicz, J.; Wojas, K.; Bojarska-Junak, A.; Greiner, J.; Giannopoulos, K.; Dmoszyńska, A.; Schmitt, M. Allogeneic Dendritic Cells Pulsed with Tumor Lysates or Apoptotic Bodies as Immunotherapy for Patients with Early-Stage B-Cell Chronic Lymphocytic Leukemia. Leukemia 2005, 19, 1621–1627. [Google Scholar] [CrossRef]
- Muhsin-Sharafaldine, M.-R.; Saunderson, S.C.; Dunn, A.C.; Faed, J.M.; Kleffmann, T.; McLellan, A.D. Procoagulant and Immunogenic Properties of Melanoma Exosomes, Microvesicles and Apoptotic Vesicles. Oncotarget 2016, 7, 56279–56294. [Google Scholar] [CrossRef]
- Tixeira, R.; Phan, T.K.; Caruso, S.; Shi, B.; Atkin-Smith, G.K.; Nedeva, C.; Chow, J.D.Y.; Puthalakath, H.; Hulett, M.D.; Herold, M.J.; et al. ROCK1 but Not LIMK1 or PAK2 Is a Key Regulator of Apoptotic Membrane Blebbing and Cell Disassembly. Cell Death Differ. 2020, 27, 102–116. [Google Scholar] [CrossRef]
- Brock, C.K.; Wallin, S.T.; Ruiz, O.E.; Samms, K.M.; Mandal, A.; Sumner, E.A.; Eisenhoffer, G.T. Stem Cell Proliferation Is Induced by Apoptotic Bodies from Dying Cells during Epithelial Tissue Maintenance. Nat. Commun. 2019, 10, 1044. [Google Scholar] [CrossRef]
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y.; et al. Circulating Apoptotic Bodies Maintain Mesenchymal Stem Cell Homeostasis and Ameliorate Osteopenia via Transferring Multiple Cellular Factors. Cell Res. 2018, 28, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Tyukavin, A.I.; Belostotskaya, G.B.; Golovanova, T.A.; Galagudza, M.M.; Zakharov, E.A.; Burkova, N.V.; Ivkin, D.Y.; Karpov, A.A. Stimulation of Proliferation and Differentiation of Rat Resident Myocardial Cells with Apoptotic Bodies of Cardiomyocytes. Bull. Exp. Biol. Med. 2015, 159, 138–141. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, X.; Lv, Y.; Zheng, C.; Dong, Y.; Dou, G.; Zhu, B.; Liu, A.; Wang, W.; Zhou, J.; et al. Apoptotic Bodies Derived from Mesenchymal Stem Cells Promote Cutaneous Wound Healing via Regulating the Functions of Macrophages. Stem Cell Res. Ther. 2020, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liang, M.; Wu, Y.; Ding, N.; Duan, L.; Yu, T.; Bai, Y.; Kang, F.; Dong, S.; Xu, J.; et al. Mature Osteoclast-Derived Apoptotic Bodies Promote Osteogenic Differentiation via RANKL-Mediated Reverse Signaling. J. Biol. Chem. 2019, 294, 11240–11247. [Google Scholar] [CrossRef] [PubMed]
- Bitto, N.J.; Zavan, L.; Johnston, E.L.; Stinear, T.P.; Hill, A.F.; Kaparakis-Liaskos, M. Considerations for the Analysis of Bacterial Membrane Vesicles: Methods of Vesicle Production and Quantification Can Influence Biological and Experimental Outcomes. Microbiol. Spectr. 2021, 9, e0127321. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Q.; Wang, S.; Weng, W.; Jing, Y.; Su, J. Bacterial Extracellular Vesicles as Bioactive Nanocarriers for Drug Delivery: Advances and Perspectives. Bioact. Mater. 2022, 14, 169–181. [Google Scholar] [CrossRef]
- Liu, G.; Ma, N.; Cheng, K.; Feng, Q.; Ma, X.; Yue, Y.; Li, Y.; Zhang, T.; Gao, X.; Liang, J.; et al. Bacteria-Derived Nanovesicles Enhance Tumour Vaccination by Trained Immunity. Nat. Nanotechnol. 2024, 19, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Ñahui Palomino, R.A.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota-Host Communications: Bacterial Extracellular Vesicles as a Common Language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef]
- Wang, S.; Gao, J.; Wang, Z. Outer Membrane Vesicles for Vaccination and Targeted Drug Delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1523. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Schild, S.; Kaparakis-Liaskos, M.; Eberl, L. Composition and Functions of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2023, 21, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-Membrane Vesicles from Gram-Negative Bacteria: Biogenesis and Functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Bos, J.; Cisneros, L.H.; Mazel, D. Real-Time Tracking of Bacterial Membrane Vesicles Reveals Enhanced Membrane Traffic upon Antibiotic Exposure. Sci. Adv. 2021, 7, eabd1033. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kulp, A.; Kuehn, M.J. Modulation of Bacterial Outer Membrane Vesicle Production by Envelope Structure and Content. BMC Microbiol. 2014, 14, 324. [Google Scholar] [CrossRef] [PubMed]
- Roier, S.; Zingl, F.G.; Cakar, F.; Durakovic, S.; Kohl, P.; Eichmann, T.O.; Klug, L.; Gadermaier, B.; Weinzerl, K.; Prassl, R.; et al. A Novel Mechanism for the Biogenesis of Outer Membrane Vesicles in Gram-Negative Bacteria. Nat. Commun. 2016, 7, 10515. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Toyofuku, M.; Nomura, N.; Obana, N. Autolysis-Mediated Membrane Vesicle Formation in Bacillus Subtilis. Environ. Microbiol. 2021, 23, 2632–2647. [Google Scholar] [CrossRef]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-Negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Pérez-Cruz, C.; Delgado, L.; López-Iglesias, C.; Mercade, E. Outer-Inner Membrane Vesicles Naturally Secreted by Gram-Negative Pathogenic Bacteria. PLoS ONE 2015, 10, e0116896. [Google Scholar] [CrossRef]
- Thoma, J.; Manioglu, S.; Kalbermatter, D.; Bosshart, P.D.; Fotiadis, D.; Müller, D.J. Protein-Enriched Outer Membrane Vesicles as a Native Platform for Outer Membrane Protein Studies. Commun. Biol. 2018, 1, 23. [Google Scholar] [CrossRef]
- Uddin, M.J.; Dawan, J.; Jeon, G.; Yu, T.; He, X.; Ahn, J. The Role of Bacterial Membrane Vesicles in the Dissemination of Antibiotic Resistance and as Promising Carriers for Therapeutic Agent Delivery. Microorganisms 2020, 8, 670. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the Wall: Extracellular Vesicles in Gram-Positive Bacteria, Mycobacteria and Fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Giv, N.; Basas, A.; Hicks, C.; El-Omar, E.; El-Assaad, F.; Hosseini-Beheshti, E. Bacterial Extracellular Vesicles and Their Novel Therapeutic Applications in Health and Cancer. Front. Cell Infect. Microbiol. 2022, 12, 962216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Thompson, C.D.; Weidenmaier, C.; Lee, J.C. Release of Staphylococcus Aureus Extracellular Vesicles and Their Application as a Vaccine Platform. Nat. Commun. 2018, 9, 1379. [Google Scholar] [CrossRef]
- Toyofuku, M.; Cárcamo-Oyarce, G.; Yamamoto, T.; Eisenstein, F.; Hsiao, C.-C.; Kurosawa, M.; Gademann, K.; Pilhofer, M.; Nomura, N.; Eberl, L. Prophage-Triggered Membrane Vesicle Formation through Peptidoglycan Damage in Bacillus Subtilis. Nat. Commun. 2017, 8, 481. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, Y.; Chen, D.; Wu, R.; Guo, L.; Lin, H. Proteomic and Metabolic Characterization of Membrane Vesicles Derived from Streptococcus mutans at Different pH Values. Appl. Microbiol. Biotechnol. 2020, 104, 9733–9748. [Google Scholar] [CrossRef]
- Kobayashi, H.; Uematsu, K.; Hirayama, H.; Horikoshi, K. Novel Toluene Elimination System in a Toluene-Tolerant Microorganism. J. Bacteriol. 2000, 182, 6451–6455. [Google Scholar] [CrossRef]
- Giacomucci, S.; Mathieu-Denoncourt, A.; Vincent, A.T.; Jannadi, H.; Duperthuy, M. Experimental Evolution of Vibrio Cholerae Identifies Hypervesiculation as a Way to Increase Motility in the Presence of Polymyxin B. Front. Microbiol. 2022, 13, 932165. [Google Scholar] [CrossRef]
- Manning, A.J.; Kuehn, M.J. Contribution of Bacterial Outer Membrane Vesicles to Innate Bacterial Defense. BMC Microbiol. 2011, 11, 258. [Google Scholar] [CrossRef]
- Balhuizen, M.D.; van Dijk, A.; Jansen, J.W.A.; van de Lest, C.H.A.; Veldhuizen, E.J.A.; Haagsman, H.P. Outer Membrane Vesicles Protect Gram-Negative Bacteria against Host Defense Peptides. mSphere 2021, 6, e00523-21. [Google Scholar] [CrossRef]
- Loeb, M.R. Bacteriophage T4-Mediated Release of Envelope Components from Escherichia coli. J. Virol. 1974, 13, 631–641. [Google Scholar] [CrossRef]
- Loeb, M.R.; Kilner, J. Release of a Special Fraction of the Outer Membrane from Both Growing and Phage T4-Infected Escherichia coli B. Biochim. Biophys. Acta 1978, 514, 117–127. [Google Scholar] [CrossRef] [PubMed]
- McBroom, A.J.; Kuehn, M.J. Release of Outer Membrane Vesicles by Gram-Negative Bacteria Is a Novel Envelope Stress Response. Mol. Microbiol. 2007, 63, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Schooling, S.R.; Beveridge, T.J. Membrane Vesicles: An Overlooked Component of the Matrices of Biofilms. J. Bacteriol. 2006, 188, 5945–5957. [Google Scholar] [CrossRef] [PubMed]
- Bitto, N.J.; Chapman, R.; Pidot, S.; Costin, A.; Lo, C.; Choi, J.; D’Cruze, T.; Reynolds, E.C.; Dashper, S.G.; Turnbull, L.; et al. Bacterial Membrane Vesicles Transport Their DNA Cargo into Host Cells. Sci. Rep. 2017, 7, 7072. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lin, H. Characterization and Function of Membrane Vesicles in Gram-Positive Bacteria. Appl. Microbiol. Biotechnol. 2021, 105, 1795–1801. [Google Scholar] [CrossRef]
- Wolf, J.M.; Rivera, J.; Casadevall, A. Serum Albumin Disrupts Cryptococcus neoformans and Bacillus anthracis Extracellular Vesicles. Cell Microbiol. 2012, 14, 762–773. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune Modulation by Bacterial Outer Membrane Vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.S. Gram-Negative Outer Membrane Vesicles in Vaccine Development. Discov. Med. 2011, 12, 7–15. [Google Scholar]
- Flynn, R.A.; Pedram, K.; Malaker, S.A.; Batista, P.J.; Smith, B.A.H.; Johnson, A.G.; George, B.M.; Majzoub, K.; Villalta, P.W.; Carette, J.E.; et al. Small RNAs Are Modified with N-Glycans and Displayed on the Surface of Living Cells. Cell 2021, 184, 3109–3124.e22. [Google Scholar] [CrossRef]
- Varki, A. Biological Roles of Glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Cummings, R.D. The Repertoire of Glycan Determinants in the Human Glycome. Mol. Biosyst. 2009, 5, 1087–1104. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global View of Human Protein Glycosylation Pathways and Functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qian, K. Protein O-GlcNAcylation: Emerging Mechanisms and Functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mao, Y.; Narimatsu, Y.; Ye, Z.; Tian, W.; Goth, C.K.; Lira-Navarrete, E.; Pedersen, N.B.; Benito-Vicente, A.; Martin, C.; et al. Site-Specific O-Glycosylation of Members of the Low-Density Lipoprotein Receptor Superfamily Enhances Ligand Interactions. J. Biol. Chem. 2019, 294, 8349. [Google Scholar] [CrossRef]
- Pedersen, N.B.; Wang, S.; Narimatsu, Y.; Yang, Z.; Halim, A.; Schjoldager, K.T.-B.G.; Madsen, T.D.; Seidah, N.G.; Bennett, E.P.; Levery, S.B.; et al. Low Density Lipoprotein Receptor Class A Repeats Are O-Glycosylated in Linker Regions. J. Biol. Chem. 2014, 289, 17312–17324. [Google Scholar] [CrossRef]
- Tomana, M.; Novak, J.; Julian, B.A.; Matousovic, K.; Konecny, K.; Mestecky, J. Circulating Immune Complexes in IgA Nephropathy Consist of IgA1 with Galactose-Deficient Hinge Region and Antiglycan Antibodies. J. Clin. Investig. 1999, 104, 73–81. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in Health and Disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Sørensen, A.L.; Reis, C.A.; Tarp, M.A.; Mandel, U.; Ramachandran, K.; Sankaranarayanan, V.; Schwientek, T.; Graham, R.; Taylor-Papadimitriou, J.; Hollingsworth, M.A.; et al. Chemoenzymatically Synthesized Multimeric Tn/STn MUC1 Glycopeptides Elicit Cancer-Specific Anti-MUC1 Antibody Responses and Override Tolerance. Glycobiology 2006, 16, 96–107. [Google Scholar] [CrossRef]
- Lin, Y.; Yin, H.; Zhou, C.; Zhou, L.; Zeng, Y.; Yao, H. Phase I Clinical Trial of MUC1-Targeted CAR-T Cells with PD-1-Knockout in the Treatment of Advanced Breast Cancer. J. Clin. Oncol. 2024, 42, 1089. [Google Scholar] [CrossRef]
- Schäffer, C.; Graninger, M.; Messner, P. Prokaryotic Glycosylation. Proteomics 2001, 1, 248–261. [Google Scholar] [CrossRef]
- Herget, S.; Toukach, P.V.; Ranzinger, R.; Hull, W.E.; Knirel, Y.A.; von der Lieth, C.-W. Statistical Analysis of the Bacterial Carbohydrate Structure Data Base (BCSDB): Characteristics and Diversity of Bacterial Carbohydrates in Comparison with Mammalian Glycans. BMC Struct. Biol. 2008, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P. Role of O-Antigen Variation in the Immune Response. Trends Microbiol. 1995, 3, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, I.; Vanderleyden, J. O-Antigen Structural Variation: Mechanisms and Possible Roles in Animal/Plant–Microbe Interactions. FEMS Microbiol. Rev. 2002, 26, 17–47. [Google Scholar] [CrossRef]
- Szymanski, C.M.; Yao, R.; Ewing, C.P.; Trust, T.J.; Guerry, P. Evidence for a System of General Protein Glycosylation in Campylobacter jejuni. Mol. Microbiol. 1999, 32, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Power, P.M.; Seib, K.L.; Jennings, M.P. Pilin Glycosylation in Neisseria Meningitidis Occurs by a Similar Pathway to Wzy-Dependent O-Antigen Biosynthesis in Escherichia coli. Biochem. Biophys. Res. Commun. 2006, 347, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.D.; Morrison, M.J.; Aas, F.E.; Børud, B.; Koomey, M.; Imperiali, B. Biochemical Characterization of the O-Linked Glycosylation Pathway in Neisseria gonorrhoeae Responsible for Biosynthesis of Protein Glycans Containing N,N′-Diacetylbacillosamine. Biochemistry 2011, 50, 4936–4948. [Google Scholar] [CrossRef]
- Nothaft, H.; Szymanski, C.M. Protein Glycosylation in Bacteria: Sweeter than Ever. Nat. Rev. Microbiol. 2010, 8, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Jervis, A.J.; Langdon, R.; Hitchen, P.; Lawson, A.J.; Wood, A.; Fothergill, J.L.; Morris, H.R.; Dell, A.; Wren, B.; Linton, D. Characterization of N-Linked Protein Glycosylation in Helicobacter pullorum. J. Bacteriol. 2010, 192, 5228–5236. [Google Scholar] [CrossRef]
- Egge-Jacobsen, W.; Salomonsson, E.N.; Aas, F.E.; Forslund, A.-L.; Winther-Larsen, H.C.; Maier, J.; Macellaro, A.; Kuoppa, K.; Oyston, P.C.F.; Titball, R.W.; et al. O-Linked Glycosylation of the PilA Pilin Protein of Francisella tularensis: Identification of the Endogenous Protein-Targeting Oligosaccharyltransferase and Characterization of the Native Oligosaccharide. J. Bacteriol. 2011, 193, 5487–5497. [Google Scholar] [CrossRef]
- Iwashkiw, J.A.; Seper, A.; Weber, B.S.; Scott, N.E.; Vinogradov, E.; Stratilo, C.; Reiz, B.; Cordwell, S.J.; Whittal, R.; Schild, S.; et al. Identification of a General O-Linked Protein Glycosylation System in Acinetobacter Baumannii and Its Role in Virulence and Biofilm Formation. PLoS Pathog. 2012, 8, e1002758. [Google Scholar] [CrossRef]
- Wehmeier, S.; Varghese, A.S.; Gurcha, S.S.; Tissot, B.; Panico, M.; Hitchen, P.; Morris, H.R.; Besra, G.S.; Dell, A.; Smith, M.C.M. Glycosylation of the Phosphate Binding Protein, PstS, in Streptomyces Coelicolor by a Pathway That Resembles Protein O-Mannosylation in Eukaryotes. Mol. Microbiol. 2009, 71, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Dobos, K.M.; Khoo, K.H.; Swiderek, K.M.; Brennan, P.J.; Belisle, J.T. Definition of the Full Extent of Glycosylation of the 45-Kilodalton Glycoprotein of Mycobacterium tuberculosis. J. Bacteriol. 1996, 178, 2498–2506. [Google Scholar] [CrossRef]
- Grass, S.; Buscher, A.Z.; Swords, W.E.; Apicella, M.A.; Barenkamp, S.J.; Ozchlewski, N.; St Geme, J.W. The Haemophilus Influenzae HMW1 Adhesin Is Glycosylated in a Process That Requires HMW1C and Phosphoglucomutase, an Enzyme Involved in Lipooligosaccharide Biosynthesis. Mol. Microbiol. 2003, 48, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Thibault, P.; Logan, S.M.; Kelly, J.F.; Brisson, J.R.; Ewing, C.P.; Trust, T.J.; Guerry, P. Identification of the Carbohydrate Moieties and Glycosylation motifs in Campylobacter jejuni Flagellin. J. Biol. Chem. 2001, 276, 34862–34870. [Google Scholar] [CrossRef] [PubMed]
- Schirm, M.; Schoenhofen, I.C.; Logan, S.M.; Waldron, K.C.; Thibault, P. Identification of Unusual Bacterial Glycosylation by Tandem Mass Spectrometry Analyses of Intact Proteins. Anal. Chem. 2005, 77, 7774–7782. [Google Scholar] [CrossRef] [PubMed]
- Von Behring, E. Nobel Prize in Physiology or Medicine 1901. Available online: https://www.nobelprize.org/prizes/medicine/1901/behring/lecture/ (accessed on 1 February 2025).
- Walsh, G.; Walsh, E. Biopharmaceutical Benchmarks 2022. Nat. Biotechnol. 2022, 40, 1722–1760. [Google Scholar] [CrossRef] [PubMed]
- Seeberger, P.H.; Overkleeft, H.S. Chemical Synthesis of Glycans and Glycoconjugates. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022; ISBN 978-1-62182-421-3. [Google Scholar]
- Kiss, B.; Gottschalk, U.; Pohlscheidt, M. (Eds.) New Bioprocessing Strategies: Development and Manufacturing of Recombinant Antibodies and Proteins; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2018; Volume 165, ISBN 978-3-319-97108-7. [Google Scholar]
- Jefferis, R. Isotype and Glycoform Selection for Antibody Therapeutics. Arch. Biochem. Biophys. 2012, 526, 159–166. [Google Scholar] [CrossRef]
- Umaña, P.; Jean-Mairet, J.; Moudry, R.; Amstutz, H.; Bailey, J.E. Engineered Glycoforms of an Antineuroblastoma IgG1 with Optimized Antibody-Dependent Cellular Cytotoxic Activity. Nat. Biotechnol. 1999, 17, 176–180. [Google Scholar] [CrossRef]
- Mastrangeli, R.; Palinsky, W.; Bierau, H. Glycoengineered Antibodies: Towards the next-Generation of Immunotherapeutics. Glycobiology 2019, 29, 199–210. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, S.; Halim, A.; Schulz, M.A.; Frodin, M.; Rahman, S.H.; Vester-Christensen, M.B.; Behrens, C.; Kristensen, C.; Vakhrushev, S.Y.; et al. Engineered CHO Cells for Production of Diverse, Homogeneous Glycoproteins. Nat. Biotechnol. 2015, 33, 842–844. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Narimatsu, Y.; Clausen, T.M.; Gomes, C.; Karlsson, R.; Steentoft, C.; Spliid, C.B.; Gustavsson, T.; Salanti, A.; Persson, A.; et al. The GAGOme: A Cell-Based Library of Displayed Glycosaminoglycans. Nat. Methods 2018, 15, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Jaroentomeechai, T.; Karlsson, R.; Goerdeler, F.; Teoh, F.K.Y.; Grønset, M.N.; de Wit, D.; Chen, Y.-H.; Furukawa, S.; Psomiadou, V.; Hurtado-Guerrero, R.; et al. Mammalian Cell-Based Production of Glycans, Glycopeptides and Glycomodules. Nat. Commun. 2024, 15, 9668. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, Y.; Joshi, H.J.; Nason, R.; Van Coillie, J.; Karlsson, R.; Sun, L.; Ye, Z.; Chen, Y.-H.; Schjoldager, K.T.; Steentoft, C.; et al. An Atlas of Human Glycosylation Pathways Enables Display of the Human Glycome by Gene Engineered Cells. Mol. Cell 2019, 75, 394–407.e5. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Klychko, E.; Thorne, T.; Misener, S.; Schultz, K.M.; Millay, M.; Ito, A.; Liu, T.; Kamide, C.; Agrawal, H.; et al. Exosomes from Human CD34(+) Stem Cells Mediate Their Proangiogenic Paracrine Activity. Circ. Res. 2011, 109, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Surman, M.; Hoja-Łukowicz, D.; Szwed, S.; Drożdż, A.; Stępień, E.; Przybyło, M. Human Melanoma-Derived Ectosomes Are Enriched with Specific Glycan Epitopes. Life Sci. 2018, 207, 395–411. [Google Scholar] [CrossRef]
- Guo, Y.; Tao, J.; Li, Y.; Feng, Y.; Ju, H.; Wang, Z.; Ding, L. Quantitative Localized Analysis Reveals Distinct Exosomal Protein-Specific Glycosignatures: Implications in Cancer Cell Subtyping, Exosome Biogenesis, and Function. J. Am. Chem. Soc. 2020, 142, 7404–7412. [Google Scholar] [CrossRef]
- Tian, W.; Zagami, C.; Chen, J.; Blomberg, A.L.; Guiu, L.S.; Skovbakke, S.L.; Goletz, S. Cell-Based Glycoengineering of Extracellular Vesicles through Precise Genome Editing. New Biotechnol. 2024, 83, 101–109. [Google Scholar] [CrossRef]
- Wacker, M.; Linton, D.; Hitchen, P.G.; Nita-Lazar, M.; Haslam, S.M.; North, S.J.; Panico, M.; Morris, H.R.; Dell, A.; Wren, B.W.; et al. N-Linked Glycosylation in Campylobacter jejuni and Its Functional Transfer into E. coli. Science 2002, 298, 1790–1793. [Google Scholar] [CrossRef]
- Du, T.; Buenbrazo, N.; Kell, L.; Rahmani, S.; Sim, L.; Withers, S.G.; DeFrees, S.; Wakarchuk, W. A Bacterial Expression Platform for Production of Therapeutic Proteins Containing Human-like O-Linked Glycans. Cell Chem. Biol. 2019, 26, 203–212.e5. [Google Scholar] [CrossRef]
- Natarajan, A.; Jaroentomeechai, T.; Cabrera-Sánchez, M.; Mohammed, J.C.; Cox, E.C.; Young, O.; Shajahan, A.; Vilkhovoy, M.; Vadhin, S.; Varner, J.D.; et al. Engineering Orthogonal Human O-Linked Glycoprotein Biosynthesis in Bacteria. Nat. Chem. Biol. 2020, 16, 1062–1070. [Google Scholar] [CrossRef]
- Jaroentomeechai, T.; Zheng, X.; Hershewe, J.; Stark, J.C.; Jewett, M.C.; DeLisa, M.P. A Pipeline for Studying and Engineering Single-Subunit Oligosaccharyltransferases. Methods Enzymol. 2017, 597, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Lizak, C.; Gerber, S.; Numao, S.; Aebi, M.; Locher, K.P. X-Ray Structure of a Bacterial Oligosaccharyltransferase. Nature 2011, 474, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zheng, X.; Shanker, S.; Jaroentomeechai, T.; Moeller, T.D.; Hulbert, S.W.; Koçer, I.; Byrne, J.; Cox, E.C.; Fu, Q.; et al. Shotgun Scanning Glycomutagenesis: A Simple and Efficient Strategy for Constructing and Characterizing Neoglycoproteins. Proc. Natl. Acad. Sci. USA 2021, 118, e2107440118. [Google Scholar] [CrossRef] [PubMed]
- Ollis, A.A.; Zhang, S.; Fisher, A.C.; DeLisa, M.P. Engineered Oligosaccharyltransferases with Greatly Relaxed Acceptor-Site Specificity. Nat. Chem. Biol. 2014, 10, 816–822. [Google Scholar] [CrossRef]
- Glasscock, C.J.; Yates, L.E.; Jaroentomeechai, T.; Wilson, J.D.; Merritt, J.H.; Lucks, J.B.; DeLisa, M.P. A Flow Cytometric Approach to Engineering Escherichia coli for Improved Eukaryotic Protein Glycosylation. Metab. Eng. 2018, 47, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Ollis, A.A.; Chai, Y.; Natarajan, A.; Perregaux, E.; Jaroentomeechai, T.; Guarino, C.; Smith, J.; Zhang, S.; DeLisa, M.P. Substitute Sweeteners: Diverse Bacterial Oligosaccharyltransferases with Unique N-Glycosylation Site Preferences. Sci. Rep. 2015, 5, 15237. [Google Scholar] [CrossRef]
- Robinson, M.-P.; Ke, N.; Lobstein, J.; Peterson, C.; Szkodny, A.; Mansell, T.J.; Tuckey, C.; Riggs, P.D.; Colussi, P.A.; Noren, C.J.; et al. Efficient Expression of Full-Length Antibodies in the Cytoplasm of Engineered Bacteria. Nat. Commun. 2015, 6, 8072. [Google Scholar] [CrossRef]
- Robinson, M.-P.; Jung, J.; Lopez-Barbosa, N.; Chang, M.; Li, M.; Jaroentomeechai, T.; Cox, E.C.; Zheng, X.; Berkmen, M.; DeLisa, M.P. Isolation of Full-Length IgG Antibodies from Combinatorial Libraries Expressed in the Cytoplasm of Escherichia coli. Nat. Commun. 2023, 14, 3514. [Google Scholar] [CrossRef]
- Mazor, Y.; Van Blarcom, T.; Mabry, R.; Iverson, B.L.; Georgiou, G. Isolation of Engineered, Full-Length Antibodies from Libraries Expressed in Escherichia coli. Nat. Biotechnol. 2007, 25, 563–565. [Google Scholar] [CrossRef]
- Chen, L.; Valentine, J.L.; Huang, C.-J.; Endicott, C.E.; Moeller, T.D.; Rasmussen, J.A.; Fletcher, J.R.; Boll, J.M.; Rosenthal, J.A.; Dobruchowska, J.; et al. Outer Membrane Vesicles Displaying Engineered Glycotopes Elicit Protective Antibodies. Proc. Natl. Acad. Sci. USA 2016, 113, E3609–E3618. [Google Scholar] [CrossRef]
- Feldman, M.F.; Wacker, M.; Hernandez, M.; Hitchen, P.G.; Marolda, C.L.; Kowarik, M.; Morris, H.R.; Dell, A.; Valvano, M.A.; Aebi, M. Engineering N-Linked Protein Glycosylation with Diverse O Antigen Lipopolysaccharide Structures in Escherichia coli. Proc. Natl. Acad. Sci. USA 2005, 102, 3016–3021. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.L.; Chen, L.; Perregaux, E.C.; Weyant, K.B.; Rosenthal, J.A.; Heiss, C.; Azadi, P.; Fisher, A.C.; Putnam, D.; Moe, G.R.; et al. Immunization with Outer Membrane Vesicles Displaying Designer Glycotopes Yields Class-Switched, Glycan-Specific Antibodies. Cell Chem. Biol. 2016, 23, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Prudden, A.R.; Capicciotti, C.J.; Bosman, G.P.; Yang, J.-Y.; Chapla, D.G.; Moremen, K.W.; Boons, G.-J. Streamlining the Chemoenzymatic Synthesis of Complex N-Glycans by a Stop and Go Strategy. Nature Chem. 2019, 11, 161–169. [Google Scholar] [CrossRef]
- Ma, S.; Gao, J.; Tian, Y.; Wen, L. Recent Progress in Chemoenzymatic Synthesis of Human Glycans. Org. Biomol. Chem. 2024, 22, 7767–7785. [Google Scholar] [CrossRef]
- Wei, F.; Zang, L.; Zhang, P.; Zhang, J.; Wen, L. Concise Chemoenzymatic Synthesis of N-Glycans. Chem 2024, 10, 2844–2860. [Google Scholar] [CrossRef]
- Jaroentomeechai, T.; Kwon, Y.H.; Liu, Y.; Young, O.; Bhawal, R.; Wilson, J.D.; Li, M.; Chapla, D.G.; Moremen, K.W.; Jewett, M.C.; et al. A Universal Glycoenzyme Biosynthesis Pipeline That Enables Efficient Cell-Free Remodeling of Glycans. Nat. Commun. 2022, 13, 6325. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, L.; Wei, N.; Yang, J.-Y.; Chapla, D.G.; Moremen, K.W.; Boons, G.-J. An Automated Platform for the Enzyme-Mediated Assembly of Complex Oligosaccharides. Nat. Chem. 2019, 11, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Jaroentomeechai, T.; Taw, M.N.; Li, M.; Aquino, A.; Agashe, N.; Chung, S.; Jewett, M.C.; DeLisa, M.P. Cell-Free Synthetic Glycobiology: Designing and Engineering Glycomolecules Outside of Living Cells. Front. Chem. 2020, 8, 645. [Google Scholar] [CrossRef]
- Jaroentomeechai, T.; Stark, J.C.; Natarajan, A.; Glasscock, C.J.; Yates, L.E.; Hsu, K.J.; Mrksich, M.; Jewett, M.C.; DeLisa, M.P. Single-Pot Glycoprotein Biosynthesis Using a Cell-Free Transcription-Translation System Enriched with Glycosylation Machinery. Nat. Commun. 2018, 9, 2686. [Google Scholar] [CrossRef]
- Stark, J.C.; Jaroentomeechai, T.; Moeller, T.D.; Hershewe, J.M.; Warfel, K.F.; Moricz, B.S.; Martini, A.M.; Dubner, R.S.; Hsu, K.J.; Stevenson, T.C.; et al. On-Demand Biomanufacturing of Protective Conjugate Vaccines. Sci. Adv. 2021, 7, eabe9444. [Google Scholar] [CrossRef]
- Zawada, J.F.; Yin, G.; Steiner, A.R.; Yang, J.; Naresh, A.; Roy, S.M.; Gold, D.S.; Heinsohn, H.G.; Murray, C.J. Microscale to Manufacturing Scale-up of Cell-free Cytokine Production—A New Approach for Shortening Protein Production Development Timelines. Biotechnol. Bioeng. 2011, 108, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Schoborg, J.A.; Hershewe, J.M.; Stark, J.C.; Kightlinger, W.; Kath, J.E.; Jaroentomeechai, T.; Natarajan, A.; DeLisa, M.P.; Jewett, M.C. A Cell-Free Platform for Rapid Synthesis and Testing of Active Oligosaccharyltransferases. Biotechnol. Bioeng. 2018, 115, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.C.; Vögeli, B.; Hassan, A.O.; Guerrero, L.; Kightlinger, W.; Yoesep, D.J.; Krüger, A.; DeWinter, M.; Diamond, M.S.; Karim, A.S.; et al. A Rapid Cell-Free Expression and Screening Platform for Antibody Discovery. Nat. Commun. 2023, 14, 3897. [Google Scholar] [CrossRef] [PubMed]
- Gurramkonda, C.; Rao, A.; Borhani, S.; Pilli, M.; Deldari, S.; Ge, X.; Pezeshk, N.; Han, T.; Tolosa, M.; Kostov, Y.; et al. Improving the Recombinant Human Erythropoietin Glycosylation Using Microsome Supplementation in CHO Cell-free System. Biotechnol. Bioeng. 2018, 115, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.W.; Majewska, N.I.; Chen, C.X.; Albanetti, T.E.; Jimenez, R.B.C.; Schmelzer, A.E.; Jewett, M.C.; Roy, V. Development of a CHO-Based Cell-Free Platform for Synthesis of Active Monoclonal Antibodies. ACS Synth. Biol. 2017, 6, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.; Vader, P.; Fuhrmann, G. Approaches to Surface Engineering of Extracellular Vesicles. Adv. Drug Deliv. Rev. 2021, 173, 416–426. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Stranford, D.M.; Simons, L.M.; Berman, K.E.; Cheng, L.; DiBiase, B.N.; Hung, M.E.; Lucks, J.B.; Hultquist, J.F.; Leonard, J.N. Bioengineering Multifunctional Extracellular Vesicles for Targeted Delivery of Biologics to T Cells. Nat. Biomed. Eng. 2024, 8, 397–414. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.-Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E.; et al. Current Methods for the Isolation of Extracellular Vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.K.; Yang, Y.-P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019, 27, 940–954.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Franklin, J.L.; Coffey, R.J. Comprehensive Isolation of Extracellular Vesicles and Nanoparticles. Nat. Protoc. 2023, 18, 1462–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Graves-Deal, R.; Trinh, V.Q.; Ramirez, M.A.; Sohn, Y.; Neininger, A.C.; Taneja, N.; McKinley, E.T.; et al. Supermeres Are Functional Extracellular Nanoparticles Replete with Disease Biomarkers and Therapeutic Targets. Nat. Cell Biol. 2021, 23, 1240–1254. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of Exosomes by Differential Centrifugation: Theoretical Analysis of a Commonly Used Protocol. Sci. Rep. 2015, 5, 17319. [Google Scholar] [CrossRef] [PubMed]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of Small Extracellular Vesicles Isolated from Plasma by Ultracentrifugation or Size-Exclusion Chromatography: Yield, Purity and Functional Potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Fernandez-Rhodes, M.; Law, A.; Peacock, B.; Lewis, M.P.; Davies, O.G. Comparison of Extracellular Vesicle Isolation Processes for Therapeutic Applications. J. Tissue Eng. 2023, 14, 20417314231174609. [Google Scholar] [CrossRef]
- Huang, T.; He, J. Characterization of Extracellular Vesicles by Size-Exclusion High-Performance Liquid Chromatography (HPLC). Methods Mol. Biol. 2017, 1660, 191–199. [Google Scholar] [CrossRef]
- Robinson, S.D.; Samuels, M.; Jones, W.; Stewart, N.; Eravci, M.; Mazarakis, N.K.; Gilbert, D.; Critchley, G.; Giamas, G. Confirming Size-Exclusion Chromatography as a Clinically Relevant Extracellular Vesicles Separation Method from 1 mL Plasma through a Comprehensive Comparison of Methods. BMC Methods 2024, 1, 7. [Google Scholar] [CrossRef]
- Kaddour, H.; Tranquille, M.; Okeoma, C.M. The Past, the Present, and the Future of the Size Exclusion Chromatography in Extracellular Vesicles Separation. Viruses 2021, 13, 2272. [Google Scholar] [CrossRef]
- Gámez-Valero, A.; Monguió-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.; Beyer, K.; Borràs, F.E. Size-Exclusion Chromatography-Based Isolation Minimally Alters Extracellular Vesicles’ Characteristics Compared to Precipitating Agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef] [PubMed]
- Mol, E.A.; Goumans, M.-J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher Functionality of Extracellular Vesicles Isolated Using Size-Exclusion Chromatography Compared to Ultracentrifugation. Nanomedicine 2017, 13, 2061–2065. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Morón-Font, M.; Gámez-Valero, A.; Carreras-Planella, L.; Borràs, F.E.; Franquesa, M. Extracellular-Vesicle Isolation from Different Biological Fluids by Size-Exclusion Chromatography. Curr. Protoc. Stem Cell Biol. 2019, 49, e82. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jalaludin, I.; Hwang, H.; Ko, M.; Adelipour, M.; Hwan, M.; Cho, N.; Kim, K.K.; Lubman, D.M.; Kim, J. Size-Exclusion Chromatography for the Characterization of Urinary Extracellular Vesicles. J. Chromatogr. B 2023, 1228, 123828. [Google Scholar] [CrossRef] [PubMed]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-Step Isolation of Extracellular Vesicles by Size-Exclusion Chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Malekian, F.; Baghban, N.; Kodam, S.P.; Ullah, M. Methodologies to Isolate and Purify Clinical Grade Extracellular Vesicles for Medical Applications. Cells 2022, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and Characterization of Extracellular Vesicles and Future Directions in Diagnosis and Therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1835. [Google Scholar] [CrossRef]
- Nordin, J.Z.; Lee, Y.; Vader, P.; Mäger, I.; Johansson, H.J.; Heusermann, W.; Wiklander, O.P.B.; Hällbrink, M.; Seow, Y.; Bultema, J.J.; et al. Ultrafiltration with Size-Exclusion Liquid Chromatography for High Yield Isolation of Extracellular Vesicles Preserving Intact Biophysical and Functional Properties. Nanomedicine 2015, 11, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.A.; Grauls, G.; Mariman, E.C.; Wouters, E.F.M.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R.; et al. Ultrafiltration Combined with Size Exclusion Chromatography Efficiently Isolates Extracellular Vesicles from Cell Culture Media for Compositional and Functional Studies. Sci. Rep. 2017, 7, 15297. [Google Scholar] [CrossRef]
- Andreu, Z.; Rivas, E.; Sanguino-Pascual, A.; Lamana, A.; Marazuela, M.; González-Alvaro, I.; Sánchez-Madrid, F.; de la Fuente, H.; Yáñez-Mó, M. Comparative Analysis of EV Isolation Procedures for miRNAs Detection in Serum Samples. J. Extracell. Vesicles 2016, 5, 31655. [Google Scholar] [CrossRef]
- Börger, V.; Staubach, S.; Dittrich, R.; Stambouli, O.; Giebel, B. Scaled Isolation of Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles. Curr. Protoc. Stem Cell Biol. 2020, 55, e128. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Sui, Z.; Shan, Y.; Hu, Y.; Chen, Y.; Zhang, L.; Zhang, Y. Effective Isolation of Exosomes with Polyethylene Glycol from Cell Culture Supernatant for In-Depth Proteome Profiling. Analyst 2016, 141, 4640–4646. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Pang, R.T.K.; Liu, W.; Li, Q.; Cheng, R.; Yeung, W.S.B. Polymer-Based Precipitation Preserves Biological Activities of Extracellular Vesicles from an Endometrial Cell Line. PLoS ONE 2017, 12, e0186534. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, B.; Fang, C.; Liang, X.; Xie, Y.; Sun, X.; Wang, W.; Zheng, L.; Wang, D. Extracellular Vesicles of Mesenchymal Stem Cells Are More Effectively Accessed through Polyethylene Glycol-Based Precipitation than by Ultracentrifugation. Stem Cells Int. 2022, 2022, 3577015. [Google Scholar] [CrossRef]
- Ludwig, A.-K.; De Miroschedji, K.; Doeppner, T.R.; Börger, V.; Ruesing, J.; Rebmann, V.; Durst, S.; Jansen, S.; Bremer, M.; Behrmann, E.; et al. Precipitation with Polyethylene Glycol Followed by Washing and Pelleting by Ultracentrifugation Enriches Extracellular Vesicles from Tissue Culture Supernatants in Small and Large Scales. J. Extracell. Vesicles 2018, 7, 1528109. [Google Scholar] [CrossRef] [PubMed]
- Djeungoue Petga, M.A.; Taylor, C.; Macpherson, A.; Dhadi, S.R.; Rollin, T.; Roy, J.W.; Ghosh, A.; Lewis, S.M.; Ouellette, R.J. A Simple Scalable Extracellular Vesicle Isolation Method Using Polyethylenimine Polymers for Use in Cellular Delivery. Extracell. Vesicle 2024, 3, 100033. [Google Scholar] [CrossRef]
- Multia, E.; Liangsupree, T.; Jussila, M.; Ruiz-Jimenez, J.; Kemell, M.; Riekkola, M.-L. Automated On-Line Isolation and Fractionation System for Nanosized Biomacromolecules from Human Plasma. Anal. Chem. 2020, 92, 13058–13065. [Google Scholar] [CrossRef] [PubMed]
- Ku, A.; Ravi, N.; Yang, M.; Evander, M.; Laurell, T.; Lilja, H.; Ceder, Y. A Urinary Extracellular Vesicle microRNA Biomarker Discovery Pipeline; from Automated Extracellular Vesicle Enrichment by Acoustic Trapping to microRNA Sequencing. PLoS ONE 2019, 14, e0217507. [Google Scholar] [CrossRef]
- Bajo-Santos, C.; Priedols, M.; Kaukis, P.; Paidere, G.; Gerulis-Bergmanis, R.; Mozolevskis, G.; Abols, A.; Rimsa, R. Extracellular Vesicles Isolation from Large Volume Samples Using a Polydimethylsiloxane-Free Microfluidic Device. Int. J. Mol. Sci. 2023, 24, 7971. [Google Scholar] [CrossRef]
- Wu, B.; Chen, X.; Wang, J.; Qing, X.; Wang, Z.; Ding, X.; Xie, Z.; Niu, L.; Guo, X.; Cai, T.; et al. Separation and Characterization of Extracellular Vesicles from Human Plasma by Asymmetrical Flow Field-Flow Fractionation. Anal. Chim. Acta 2020, 1127, 234–245. [Google Scholar] [CrossRef]
- Sitar, S.; Kejžar, A.; Pahovnik, D.; Kogej, K.; Tušek-Žnidarič, M.; Lenassi, M.; Žagar, E. Size Characterization and Quantification of Exosomes by Asymmetrical-Flow Field-Flow Fractionation. Anal. Chem. 2015, 87, 9225–9233. [Google Scholar] [CrossRef] [PubMed]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern Isolation and Separation Techniques for Extracellular Vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.Y. Nickel Affinity: A Sensible Approach for Extracellular Vesicles Isolation? EBioMedicine 2019, 44, 14–15. [Google Scholar] [CrossRef] [PubMed]
- Kosanović, M.; Milutinović, B.; Goč, S.; Mitić, N.; Janković, M. Ion-Exchange Chromatography Purification of Extracellular Vesicles. Biotechniques 2017, 63, 65–71. [Google Scholar] [CrossRef]
- Heath, N.; Grant, L.; De Oliveira, T.M.; Rowlinson, R.; Osteikoetxea, X.; Dekker, N.; Overman, R. Rapid Isolation and Enrichment of Extracellular Vesicle Preparations Using Anion Exchange Chromatography. Sci. Rep. 2018, 8, 5730. [Google Scholar] [CrossRef] [PubMed]
- Van Deun, J.; Jo, A.; Li, H.; Lin, H.-Y.; Weissleder, R.; Im, H.; Lee, H. Integrated Dual-Mode Chromatography to Enrich Extracellular Vesicles from Plasma. Adv. Biosyst. 2020, 4, e1900310. [Google Scholar] [CrossRef]
- Lusky, M. Good Manufacturing Practice Production of Adenoviral Vectors for Clinical Trials. Hum. Gene Ther. 2005, 16, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Morani, M.; Mai, T.D.; Krupova, Z.; Defrenaix, P.; Multia, E.; Riekkola, M.-L.; Taverna, M. Electrokinetic Characterization of Extracellular Vesicles with Capillary Electrophoresis: A New Tool for Their Identification and Quantification. Anal. Chim. Acta 2020, 1128, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-Y.; Sung, C.W.-H.; Chen, C.; Cheng, C.-M.; Lin, D.P.-C.; Huang, C.-T.; Hsu, M.-Y. Advances in Exosomes Technology. Clin. Chim. Acta 2019, 493, 14–19. [Google Scholar] [CrossRef]
- Shami-Shah, A.; Travis, B.G.; Walt, D.R. Advances in Extracellular Vesicle Isolation Methods: A Path towards Cell-Type Specific EV Isolation. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 447–460. [Google Scholar] [CrossRef]
- Gaillard, M.; Thuaire, A.; Nonglaton, G.; Agache, V.; Roupioz, Y.; Raillon, C. Biosensing Extracellular Vesicles: Contribution of Biomolecules in Affinity-Based Methods for Detection and Isolation. Analyst 2020, 145, 1997–2013. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in Extracellular Vesicle Formation and Function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental pH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, H.; Zhu, X.; Wu, P.; Chen, W.; Zou, P.; Li, Q.; Chen, Z. Serum Deprivation Elevates the Levels of Microvesicles with Different Size Distributions and Selectively Enriched Proteins in Human Myeloma Cells in Vitro. Acta Pharmacol. Sin. 2014, 35, 381–393. [Google Scholar] [CrossRef]
- Hahm, J.; Kim, J.; Park, J. Strategies to Enhance Extracellular Vesicle Production. Tissue Eng. Regen. Med. 2021, 18, 513–524. [Google Scholar] [CrossRef]
- Dorayappan, K.D.P.; Wanner, R.; Wallbillich, J.J.; Saini, U.; Zingarelli, R.; Suarez, A.A.; Cohn, D.E.; Selvendiran, K. Hypoxia-Induced Exosomes Contribute to a More Aggressive and Chemoresistant Ovarian Cancer Phenotype: A Novel Mechanism Linking STAT3/Rab Proteins. Oncogene 2018, 37, 3806–3821. [Google Scholar] [CrossRef]
- Saludas, L.; Garbayo, E.; Ruiz-Villalba, A.; Hernández, S.; Vader, P.; Prósper, F.; Blanco-Prieto, M.J. Isolation Methods of Large and Small Extracellular Vesicles Derived from Cardiovascular Progenitors: A Comparative Study. Eur. J. Pharm. Biopharm. 2022, 170, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Nomura, S.; Miyake, T.; Kagawa, H.; Kitada, C.; Taniguchi, H.; Komiyama, Y.; Fujimura, Y.; Ikeda, Y.; Fukuhara, S. High Shear Stress Can Initiate Both Platelet Aggregation and Shedding of Procoagulant Containing Microparticles. Blood 1996, 88, 3456–3464. [Google Scholar] [CrossRef]
- Ambattu, L.A.; Ramesan, S.; Dekiwadia, C.; Hanssen, E.; Li, H.; Yeo, L.Y. High Frequency Acoustic Cell Stimulation Promotes Exosome Generation Regulated by a Calcium-Dependent Mechanism. Commun. Biol. 2020, 3, 553. [Google Scholar] [CrossRef]
- Bagheri, H.S.; Mousavi, M.; Rezabakhsh, A.; Rezaie, J.; Rasta, S.H.; Nourazarian, A.; Avci, Ç.B.; Tajalli, H.; Talebi, M.; Oryan, A.; et al. Low-Level Laser Irradiation at a High Power Intensity Increased Human Endothelial Cell Exosome Secretion via Wnt Signaling. Lasers Med. Sci. 2018, 33, 1131–1145. [Google Scholar] [CrossRef]
- Syromiatnikova, V.; Prokopeva, A.; Gomzikova, M. Methods of the Large-Scale Production of Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 10522. [Google Scholar] [CrossRef] [PubMed]
- Gudbergsson, J.M.; Johnsen, K.B.; Skov, M.N.; Duroux, M. Systematic Review of Factors Influencing Extracellular Vesicle Yield from Cell Cultures. Cytotechnology 2016, 68, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques Used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef]
- Bryant, G.; Abeynayake, C.; Thomas, J.C. Improved Particle Size Distribution Measurements Using Multiangle Dynamic Light Scattering. 2. Refinements and Applications. Langmuir 1996, 12, 6224–6228. [Google Scholar] [CrossRef]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A Comparison of Atomic Force Microscopy (AFM) and Dynamic Light Scattering (DLS) Methods to Characterize Nanoparticle Size Distributions. J. Nanopart. Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Reimer, L. Transmission Electron Microscopy: Physics of Image Formation and Microanalysis; Springer: Berlin/Heidelberg, Germany, 2013; Volume 36, ISBN 3-662-13553-1. [Google Scholar]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and Characterization of Extracellular Vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Ahmad, N.; Nelayah, J.; Wilhelm, C.; Silva, A.; Gazeau, F.; Alloyeau, D. Monitoring the Dynamics of Cell-Derived Extracellular Vesicles at the Nanoscale by Liquid-Cell Transmission Electron Microscopy. Nanoscale 2018, 10, 1234–1244. [Google Scholar] [CrossRef]
- Nikishin, I.; Dulimov, R.; Skryabin, G.; Galetsky, S.; Tchevkina, E.; Bagrov, D. ScanEV—A Neural Network-Based Tool for the Automated Detection of Extracellular Vesicles in TEM Images. Micron 2021, 145, 103044. [Google Scholar] [CrossRef]
- Linares, R.; Tan, S.; Gounou, C.; Brisson, A.R. Imaging and Quantification of Extracellular Vesicles by Transmission Electron Microscopy. Methods Mol. Biol. 2017, 1545, 43–54. [Google Scholar] [CrossRef]
- Corona, M.L.; Hurbain, I.; Raposo, G.; van Niel, G. Characterization of Extracellular Vesicles by Transmission Electron Microscopy and Immunolabeling Electron Microscopy. Methods Mol. Biol. 2023, 2668, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Erdbrügger, U.; Rudy, C.K.; Etter, M.E.; Dryden, K.A.; Yeager, M.; Klibanov, A.L.; Lannigan, J. Imaging Flow Cytometry Elucidates Limitations of Microparticle Analysis by Conventional Flow Cytometry. Cytom. Part A 2014, 85, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Desgeorges, A.; Hollerweger, J.; Lassacher, T.; Rohde, E.; Helmbrecht, C.; Gimona, M. Differential Fluorescence Nanoparticle Tracking Analysis for Enumeration of the Extracellular Vesicle Content in Mixed Particulate Solutions. Methods 2020, 177, 67–73. [Google Scholar] [CrossRef]
- Bachurski, D.; Schuldner, M.; Nguyen, P.-H.; Malz, A.; Reiners, K.S.; Grenzi, P.C.; Babatz, F.; Schauss, A.C.; Hansen, H.P.; Hallek, M.; et al. Extracellular Vesicle Measurements with Nanoparticle Tracking Analysis—An Accuracy and Repeatability Comparison between NanoSight NS300 and ZetaView. J. Extracell. Vesicles 2019, 8, 1596016. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and Phenotyping of Cellular Vesicles Using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Saveyn, H.; De Baets, B.; Thas, O.; Hole, P.; Smith, J.; Van der Meeren, P. Accurate Particle Size Distribution Determination by Nanoparticle Tracking Analysis Based on 2-D Brownian Dynamics Simulation. J. Colloid Interface Sci. 2010, 352, 593–600. [Google Scholar] [CrossRef]
- van der Pol, E.; Coumans, F.A.W.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R. Particle Size Distribution of Exosomes and Microvesicles Determined by Transmission Electron Microscopy, Flow Cytometry, Nanoparticle Tracking Analysis, and Resistive Pulse Sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sun, L.; Henriquez, R.R.; Crooks, R.M. A Carbon Nanotube-Based Coulter Nanoparticle Counter. Acc. Chem. Res. 2004, 37, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.I. Impedance-Based Flow Cytometry for the Measurement of Microparticles. Semin. Thromb. Hemost. 2010, 36, 819–823. [Google Scholar] [CrossRef]
- Rosa-Fernandes, L.; Rocha, V.B.; Carregari, V.C.; Urbani, A.; Palmisano, G. A Perspective on Extracellular Vesicles Proteomics. Front. Chem. 2017, 5, 102. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Baeta, H.; Silva, B.C.; Moraes, M.C.S.; Bodo, C.; Beck, H.C.; Rodriguez, M.S.; Saraswat, M.; Pandey, A.; Matthiesen, R. Extra-Cellular Vesicles Carry Proteome of Cancer Hallmarks. Front. Biosci. 2020, 25, 398–436. [Google Scholar] [CrossRef]
- Charest, A. Experimental and Biological Insights from Proteomic Analyses of Extracellular Vesicle Cargos in Normalcy and Disease. Adv. Biosyst. 2020, 4, e2000069. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-S.; Faruque, H.A.; Kim, J.-H.; Kim, K.J.; Choi, J.E.; Kim, B.A.; Kim, B.; Kim, Y.J.; Woo, M.H.; Park, J.Y.; et al. CD5L as an Extracellular Vesicle-Derived Biomarker for Liquid Biopsy of Lung Cancer. Diagnostics 2021, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Cufaro, M.C.; Pieragostino, D.; Lanuti, P.; Rossi, C.; Cicalini, I.; Federici, L.; De Laurenzi, V.; Del Boccio, P. Extracellular Vesicles and Their Potential Use in Monitoring Cancer Progression and Therapy: The Contribution of Proteomics. J. Oncol. 2019, 2019, 1639854. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, F.; Carluccio, M.; Zuccarini, M.; Giuliani, P.; Ricci-Vitiani, L.; Pallini, R.; De Sanctis, P.; Di Pietro, R.; Ciccarelli, R.; Angelucci, S. Proteomic Characterization of Two Extracellular Vesicle Subtypes Isolated from Human Glioblastoma Stem Cell Secretome by Sequential Centrifugal Ultrafiltration. Biomedicines 2021, 9, 146. [Google Scholar] [CrossRef]
- Ganig, N.; Baenke, F.; Thepkaysone, M.-L.; Lin, K.; Rao, V.S.; Wong, F.C.; Polster, H.; Schneider, M.; Helm, D.; Pecqueux, M.; et al. Proteomic Analyses of Fibroblast- and Serum-Derived Exosomes Identify QSOX1 as a Marker for Non-Invasive Detection of Colorectal Cancer. Cancers 2021, 13, 1351. [Google Scholar] [CrossRef]
- Haney, M.J.; Zhao, Y.; Fallon, J.K.; Yue, W.; Li, S.M.; Lentz, E.E.; Erie, D.; Smith, P.C.; Batrakova, E.V. Extracellular Vesicles as Drug Delivery System for Treatment of Neurodegenerative Disorders: Optimization of the Cell Source. Adv. Nanobiomed. Res. 2021, 1, 2100064. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Didiot, M.-C.; Sapp, E.; Leszyk, J.; Shaffer, S.A.; Rockwell, H.E.; Gao, F.; Narain, N.R.; DiFiglia, M.; Kiebish, M.A.; et al. High-Resolution Proteomic and Lipidomic Analysis of Exosomes and Microvesicles from Different Cell Sources. J. Extracell. Vesicles 2016, 5, 32570. [Google Scholar] [CrossRef]
- Kitamura, Y.; Kojima, M.; Kurosawa, T.; Sasaki, R.; Ichihara, S.; Hiraku, Y.; Tomimoto, H.; Murata, M.; Oikawa, S. Proteomic Profiling of Exosomal Proteins for Blood-Based Biomarkers in Parkinson’s Disease. Neuroscience 2018, 392, 121–128. [Google Scholar] [CrossRef]
- Larssen, P.; Wik, L.; Czarnewski, P.; Eldh, M.; Löf, L.; Ronquist, K.G.; Dubois, L.; Freyhult, E.; Gallant, C.J.; Oelrich, J.; et al. Tracing Cellular Origin of Human Exosomes Using Multiplex Proximity Extension Assays. Mol. Cell Proteom. 2017, 16, 502–511. [Google Scholar] [CrossRef]
- Martin-Jaular, L.; Nevo, N.; Schessner, J.P.; Tkach, M.; Jouve, M.; Dingli, F.; Loew, D.; Witwer, K.W.; Ostrowski, M.; Borner, G.H.H.; et al. Unbiased Proteomic Profiling of Host Cell Extracellular Vesicle Composition and Dynamics upon HIV-1 Infection. EMBO J. 2021, 40, e105492. [Google Scholar] [CrossRef] [PubMed]
- Montecchi, T.; Shaba, E.; De Tommaso, D.; Di Giuseppe, F.; Angelucci, S.; Bini, L.; Landi, C.; Baldari, C.T.; Ulivieri, C. Differential Proteomic Analysis of Astrocytes and Astrocytes-Derived Extracellular Vesicles from Control and Rai Knockout Mice: Insights into the Mechanisms of Neuroprotection. Int. J. Mol. Sci. 2021, 22, 7933. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, S.; Hirano, M.; Isoyama, J.; Nagayama, S.; Tomonaga, T.; Adachi, J. Comprehensive Proteomic Profiling of Plasma and Serum Phosphatidylserine-Positive Extracellular Vesicles Reveals Tissue-Specific Proteins. iScience 2022, 25, 104012. [Google Scholar] [CrossRef]
- Nielsen, J.E.; Honoré, B.; Vestergård, K.; Maltesen, R.G.; Christiansen, G.; Bøge, A.U.; Kristensen, S.R.; Pedersen, S. Shotgun-Based Proteomics of Extracellular Vesicles in Alzheimer’s Disease Reveals Biomarkers Involved in Immunological and Coagulation Pathways. Sci. Rep. 2021, 11, 18518. [Google Scholar] [CrossRef] [PubMed]
- Pane, K.; Quintavalle, C.; Nuzzo, S.; Ingenito, F.; Roscigno, G.; Affinito, A.; Scognamiglio, I.; Pattanayak, B.; Gallo, E.; Accardo, A.; et al. Comparative Proteomic Profiling of Secreted Extracellular Vesicles from Breast Fibroadenoma and Malignant Lesions: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 3989. [Google Scholar] [CrossRef]
- Pecankova, K.; Pecherkova, P.; Gasova, Z.; Sovova, Z.; Riedel, T.; Jäger, E.; Cermak, J.; Majek, P. Proteome Changes of Plasma-Derived Extracellular Vesicles in Patients with Myelodysplastic Syndrome. PLoS ONE 2022, 17, e0262484. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Poh, Q.H.; Fatmous, M.; Fang, H.; Gurung, S.; Vollenhoven, B.; Salamonsen, L.A.; Greening, D.W. Proteomic Profiling of Human Uterine Extracellular Vesicles Reveal Dynamic Regulation of Key Players of Embryo Implantation and Fertility during Menstrual Cycle. Proteomics 2021, 21, e2000211. [Google Scholar] [CrossRef]
- Ruhen, O.; Qu, X.; Jamaluddin, M.F.B.; Salomon, C.; Gandhi, A.; Millward, M.; Nixon, B.; Dun, M.D.; Meehan, K. Dynamic Landscape of Extracellular Vesicle-Associated Proteins Is Related to Treatment Response of Patients with Metastatic Breast Cancer. Membranes 2021, 11, 880. [Google Scholar] [CrossRef]
- Shaba, E.; Landi, C.; Carleo, A.; Vantaggiato, L.; Paccagnini, E.; Gentile, M.; Bianchi, L.; Lupetti, P.; Bargagli, E.; Prasse, A.; et al. Proteome Characterization of BALF Extracellular Vesicles in Idiopathic Pulmonary Fibrosis: Unveiling Undercover Molecular Pathways. Int. J. Mol. Sci. 2021, 22, 5696. [Google Scholar] [CrossRef]
- Vagner, T.; Chin, A.; Mariscal, J.; Bannykh, S.; Engman, D.M.; Di Vizio, D. Protein Composition Reflects Extracellular Vesicle Heterogeneity. Proteomics 2019, 19, e1800167. [Google Scholar] [CrossRef]
- Turchinovich, A.; Drapkina, O.; Tonevitsky, A. Transcriptome of Extracellular Vesicles: State-of-the-Art. Front. Immunol. 2019, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, A.; Story, M.D. The Long and Short of It: The Emerging Roles of Non-Coding RNA in Small Extracellular Vesicles. Cancers 2020, 12, 1445. [Google Scholar] [CrossRef] [PubMed]
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 700. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Li, Q.; Lai, H.; Zhang, H.; Hu, Z.; Li, Y.; Huang, S. EV-Origin: Enumerating the Tissue-Cellular Origin of Circulating Extracellular Vesicles Using exLR Profile. Comput. Struct. Biotechnol. J. 2020, 18, 2851–2859. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Chiu, P.K.-F.; Wong, C.Y.-P.; Cheng, C.K.-L.; Teoh, J.Y.-C.; Ng, C.-F. Identification of piRNA Targets in Urinary Extracellular Vesicles for the Diagnosis of Prostate Cancer. Diagnostics 2021, 11, 1828. [Google Scholar] [CrossRef] [PubMed]
- Corrado, C.; Barreca, M.M.; Zichittella, C.; Alessandro, R.; Conigliaro, A. Molecular Mediators of RNA Loading into Extracellular Vesicles. Cells 2021, 10, 3355. [Google Scholar] [CrossRef]
- Magaña, S.M.; Peterson, T.E.; Evans, J.E.; Decker, P.A.; Simon, V.; Eckel-Passow, J.E.; Daniels, D.J.; Parney, I.F. Pediatric Brain Tumor Cell Lines Exhibit miRNA-Depleted, Y RNA-Enriched Extracellular Vesicles. J. Neuro-Oncol. 2022, 156, 269–279. [Google Scholar] [CrossRef]
- Iparraguirre, L.; Alberro, A.; Hansen, T.B.; Castillo-Triviño, T.; Muñoz-Culla, M.; Otaegui, D. Profiling of Plasma Extracellular Vesicle Transcriptome Reveals That circRNAs Are Prevalent and Differ between Multiple Sclerosis Patients and Healthy Controls. Biomedicines 2021, 9, 1850. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, H.Y.; Hur, J.Y.; Kim, H.J.; Kim, I.A.; Kim, W.S.; Lee, K.Y. Genomic Profiling of Extracellular Vesicle-Derived DNA from Bronchoalveolar Lavage Fluid of Patients with Lung Adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 104–116. [Google Scholar] [CrossRef]
- Maire, C.L.; Fuh, M.M.; Kaulich, K.; Fita, K.D.; Stevic, I.; Heiland, D.H.; Welsh, J.A.; Jones, J.C.; Görgens, A.; Ricklefs, T.; et al. Genome-Wide Methylation Profiling of Glioblastoma Cell-Derived Extracellular Vesicle DNA Allows Tumor Classification. Neuro-Oncology 2021, 23, 1087–1099. Available online: https://academic.oup.com/neuro-oncology/article/23/7/1087/6122789 (accessed on 16 February 2025). [CrossRef]
- Hur, J.Y.; Lee, K.Y. Characteristics and Clinical Application of Extracellular Vesicle-Derived DNA. Cancers 2021, 13, 3827. [Google Scholar] [CrossRef] [PubMed]
- Malkin, E.Z.; Bratman, S.V. Bioactive DNA from Extracellular Vesicles and Particles. Cell Death Dis. 2020, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Kanada, M.; Bachmann, M.H.; Hardy, J.W.; Frimannson, D.O.; Bronsart, L.; Wang, A.; Sylvester, M.D.; Schmidt, T.L.; Kaspar, R.L.; Butte, M.J.; et al. Differential Fates of Biomolecules Delivered to Target Cells via Extracellular Vesicles. Proc. Natl. Acad. Sci. USA 2015, 112, E1433–E1442. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Rustam, Y.H.; Masters, C.L.; Makalic, E.; McLean, C.A.; Hill, A.F.; Barnham, K.J.; Reid, G.E.; Vella, L.J. Characterization of Brain-Derived Extracellular Vesicle Lipids in Alzheimer’s Disease. J. Extracell. Vesicles 2021, 10, e12089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ma, Z.; Wang, X.; Liang, M.; Wang, W.; Su, F.; Yang, H.; Gao, Y.; Ren, Y. Lipidomic Biomarkers of Extracellular Vesicles for the Prediction of Preterm Birth in the Early Second Trimester. J. Proteome Res. 2020, 19, 4104–4113. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in Exosomes: Current Knowledge and the Way Forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Donoso-Quezada, J.; Ayala-Mar, S.; González-Valdez, J. The Role of Lipids in Exosome Biology and Intercellular Communication: Function, Analytics and Applications. Traffic 2021, 22, 204–220. [Google Scholar] [CrossRef]
- Kreimer, S.; Belov, A.M.; Ghiran, I.; Murthy, S.K.; Frank, D.A.; Ivanov, A.R. Mass-Spectrometry-Based Molecular Characterization of Extracellular Vesicles: Lipidomics and Proteomics. J. Proteome Res. 2015, 14, 2367–2384. Available online: https://pubs.acs.org/doi/10.1021/pr501279t (accessed on 16 February 2025). [CrossRef]
- Choi, D.-S.; Kim, D.-K.; Kim, Y.-K.; Gho, Y.S. Proteomics, Transcriptomics and Lipidomics of Exosomes and Ectosomes. Proteomics 2013, 13, 1554–1571. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/pmic.201200329 (accessed on 16 February 2025). [CrossRef]
- Clos-Garcia, M.; Loizaga-Iriarte, A.; Zuñiga-Garcia, P.; Sánchez-Mosquera, P.; Rosa Cortazar, A.; González, E.; Torrano, V.; Alonso, C.; Pérez-Cormenzana, M.; Ugalde-Olano, A.; et al. Metabolic Alterations in Urine Extracellular Vesicles Are Associated to Prostate Cancer Pathogenesis and Progression. J. Extracell. Vesicles 2018, 7, 1470442. Available online: https://isevjournals.onlinelibrary.wiley.com/doi/10.1080/20013078.2018.1470442 (accessed on 16 February 2025). [CrossRef]
- Zebrowska, A.; Skowronek, A.; Wojakowska, A.; Widlak, P.; Pietrowska, M. Metabolome of Exosomes: Focus on Vesicles Released by Cancer Cells and Present in Human Body Fluids. Int. J. Mol. Sci. 2019, 20, 3461. Available online: https://www.mdpi.com/1422-0067/20/14/3461 (accessed on 16 February 2025). [CrossRef] [PubMed]
- Lou, D.; Shi, K.; Li, H.P.; Zhu, Q.; Hu, L.; Luo, J.; Yang, R.; Liu, F. Quantitative Metabolic Analysis of Plasma Extracellular Vesicles for the Diagnosis of Severe Acute Pancreatitis. J. Nanobiotechnol. 2022, 20, 52. Available online: https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-022-01239-6 (accessed on 16 February 2025). [CrossRef]
- Harmati, M.; Bukva, M.; Böröczky, T.; Buzás, K.; Gyukity-Sebestyén, E. The Role of the Metabolite Cargo of Extracellular Vesicles in Tumor Progression. Cancer Metastasis Rev. 2021, 40, 1203–1221. [Google Scholar] [CrossRef] [PubMed]
- Palomo, L.; Casal, E.; Royo, F.; Cabrera, D.; van-Liempd, S.; Falcon-Perez, J.M. Considerations for Applying Metabolomics to the Analysis of Extracellular Vesicles. Front. Immunol. 2014, 5, 651. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Hiemori, K.; Kiyoi, K.; Tateno, H. Glycome Analysis of Extracellular Vesicles Derived from Human Induced Pluripotent Stem Cells Using Lectin Microarray. Sci. Rep. 2018, 8, 3997. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Royo, F.; Aizpurua-Olaizola, O.; Pazos, R.; Boons, G.-J.; Reichardt, N.-C.; Falcon-Perez, J.M. Glycosylation of Extracellular Vesicles: Current Knowledge, Tools and Clinical Perspectives. J. Extracell. Vesicles 2018, 7, 1442985. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Xu, G.; Li, Q.; Goonatilleke, E.; Lebrilla, C.B. Mass Spectrometry Approaches to Glycomic and Glycoproteomic Analyses. Chem. Rev. 2018, 118, 7886–7930. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.A.; Aguilar Díaz De León, J.S.; Busatto, S.; Wurtz, G.A.; Zubair, A.C.; Borges, C.R.; Wolfram, J. Glycan Node Analysis of Plasma-Derived Extracellular Vesicles. Cells 2020, 9, 1946. [Google Scholar] [CrossRef]
- Pendiuk Goncalves, J.; Walker, S.A.; Aguilar Díaz de León, J.S.; Yang, Y.; Davidovich, I.; Busatto, S.; Sarkaria, J.; Talmon, Y.; Borges, C.R.; Wolfram, J. Glycan Node Analysis Detects Varying Glycosaminoglycan Levels in Melanoma-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 8506. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Chen, W.; Lu, H.; Zhang, Y. Comprehensive Review of MS-Based Studies on N-Glycoproteome and N-Glycome of Extracellular Vesicles. Proteomics 2024, 24, e2300065. [Google Scholar] [CrossRef]
- Costa, J.; Gatermann, M.; Nimtz, M.; Kandzia, S.; Glatzel, M.; Conradt, H.S. N-Glycosylation of Extracellular Vesicles from HEK-293 and Glioma Cell Lines. Anal. Chem. 2018, 90, 7871–7879. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, J.Q.; Griffin, M.D. Getting to Know the Extracellular Vesicle Glycome. Mol. BioSyst. 2016, 12, 1071–1081. [Google Scholar] [CrossRef]
- Shimoda, A.; Miura, R.; Tateno, H.; Seo, N.; Shiku, H.; Sawada, S.-I.; Sasaki, Y.; Akiyoshi, K. Assessment of Surface Glycan Diversity on Extracellular Vesicles by Lectin Microarray and Glycoengineering Strategies for Drug Delivery Applications. Small Methods 2022, 6, e2100785. [Google Scholar] [CrossRef]
- Martins, Á.M.; Ramos, C.C.; Freitas, D.; Reis, C.A. Glycosylation of Cancer Extracellular Vesicles: Capture Strategies, Functional Roles and Potential Clinical Applications. Cells 2021, 10, 109. [Google Scholar] [CrossRef]
- Temchura, V.V.; Tenbusch, M.; Nchinda, G.; Nabi, G.; Tippler, B.; Zelenyuk, M.; Wildner, O.; Uberla, K.; Kuate, S. Enhancement of Immunostimulatory Properties of Exosomal Vaccines by Incorporation of Fusion-Competent G Protein of Vesicular Stomatitis Virus. Vaccine 2008, 26, 3662–3672. [Google Scholar] [CrossRef] [PubMed]
- Dusoswa, S.A.; Horrevorts, S.K.; Ambrosini, M.; Kalay, H.; Paauw, N.J.; Nieuwland, R.; Pegtel, M.D.; Würdinger, T.; Van Kooyk, Y.; Garcia-Vallejo, J.J. Glycan Modification of Glioblastoma-Derived Extracellular Vesicles Enhances Receptor-Mediated Targeting of Dendritic Cells. J. Extracell. Vesicles 2019, 8, 1648995. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Song, J.; Kang, Y.Y.; Mok, H. Mannose-Modified Serum Exosomes for the Elevated Uptake to Murine Dendritic Cells and Lymphatic Accumulation. Macromol. Biosci. 2019, 19, e1900042. [Google Scholar] [CrossRef]
- Rughetti, A.; Rahimi, H.; Belleudi, F.; Napoletano, C.; Battisti, F.; Zizzari, I.G.; Antonilli, M.; Bellati, F.; Wandall, H.H.; Benedetti Panici, P.; et al. Microvesicle Cargo of Tumor-Associated MUC1 to Dendritic Cells Allows Cross-Presentation and Specific Carbohydrate Processing. Cancer Immunol. Res. 2014, 2, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and Microglial Activation in Alzheimer Disease: Where Do We Go from Here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Cui, G.-H.; Guo, H.-D.; Li, H.; Zhai, Y.; Gong, Z.-B.; Wu, J.; Liu, J.-S.; Dong, Y.-R.; Hou, S.-X.; Liu, J.-R. RVG-Modified Exosomes Derived from Mesenchymal Stem Cells Rescue Memory Deficits by Regulating Inflammatory Responses in a Mouse Model of Alzheimer’s Disease. Immun. Ageing 2019, 16, 10. [Google Scholar] [CrossRef]
- Cuesta, C.M.; Guerri, C.; Ureña, J.; Pascual, M. Role of Microbiota-Derived Extracellular Vesicles in Gut-Brain Communication. Int. J. Mol. Sci. 2021, 22, 4235. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Hareendran, S.; Loh, Y.P. Function of Exosomes in Neurological Disorders and Brain Tumors. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 55–79. [Google Scholar] [CrossRef]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered Exosomes: Desirable Target-Tracking Characteristics for Cerebrovascular and Neurodegenerative Disease Therapies. Theranostics 2021, 11, 8926–8944. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.L.; Vassar, R. The Role of Amyloid Precursor Protein Processing by BACE1, the Beta-Secretase, in Alzheimer Disease Pathophysiology. J. Biol. Chem. 2008, 283, 29621–29625. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Jeong, E.S.; Khan, M.Z.; Shin, J.H.; Kim, J.D. Stem Cells-Derived Exosomes Alleviate Neurodegeneration and Alzheimer’s Pathogenesis by Ameliorating Neuroinflamation, and Regulating the Associated Molecular Pathways. Sci. Rep. 2023, 13, 15731. [Google Scholar] [CrossRef]
- Xie, X.; Song, Q.; Dai, C.; Cui, S.; Tang, R.; Li, S.; Chang, J.; Li, P.; Wang, J.; Li, J.; et al. Clinical Safety and Efficacy of Allogenic Human Adipose Mesenchymal Stromal Cells-Derived Exosomes in Patients with Mild to Moderate Alzheimer’s Disease: A Phase I/II Clinical Trial. Gen. Psychiatry 2023, 36, e101143. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10582850/ (accessed on 15 February 2025). [CrossRef] [PubMed]
- Meade, R.M.; Fairlie, D.P.; Mason, J.M. Alpha-Synuclein Structure and Parkinson’s Disease—Lessons and Emerging Principles. Mol. Neurodegener. 2019, 14, 29. [Google Scholar] [CrossRef]
- Cooper, J.M.; Wiklander, P.B.O.; Nordin, J.Z.; Al-Shawi, R.; Wood, M.J.; Vithlani, M.; Schapira, A.H.V.; Simons, J.P.; El-Andaloussi, S.; Alvarez-Erviti, L. Systemic Exosomal siRNA Delivery Reduced Alpha-Synuclein Aggregates in Brains of Transgenic Mice. Mov. Disord. 2014, 29, 1476–1485. [Google Scholar] [CrossRef]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.-E.; El-Baba, M.D.; Saxena, P.; Ausländer, S.; Tan, K.R.; Fussenegger, M. Designer Exosomes Produced by Implanted Cells Intracerebrally Deliver Therapeutic Cargo for Parkinson’s Disease Treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Cabrera-Pastor, A. Emerging Role of Extracellular Vesicles as Biomarkers in Neurodegenerative Diseases and Their Clinical and Therapeutic Potential in Central Nervous System Pathologies. Int. J. Mol. Sci. 2024, 25, 10068. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Maung-U, K.; Jagadeesh, G. Prevalence and Prevention of Cardiovascular Disease and Diabetes Mellitus. Pharmacol. Res. 2016, 113, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Braunwald, E. Clinical Benefit of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 435–447. [Google Scholar] [CrossRef]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Andersson, C.; Vasan, R.S. Epidemiology of Cardiovascular Disease in Young Individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef]
- Lassiter, G.; Melancon, C.; Rooney, T.; Murat, A.-M.; Kaye, J.S.; Kaye, A.M.; Kaye, R.J.; Cornett, E.M.; Kaye, A.D.; Shah, R.J.; et al. Ozanimod to Treat Relapsing Forms of Multiple Sclerosis: A Comprehensive Review of Disease, Drug Efficacy and Side Effects. Neurol. Int. 2020, 12, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Torregroza, C.; Huhn, R.; Hollmann, M.W.; Preckel, B. Perioperative Cardioprotection: Clinical Implications. Anesth. Analg. 2020, 131, 1751–1764. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F. Extracellular Vesicles, Tunneling Nanotubes, and Cellular Interplay: Synergies and Missing Links. Front. Mol. Biosci. 2017, 4, 50. [Google Scholar] [CrossRef]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Abdul Roda, M.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e15. [Google Scholar] [CrossRef]
- Sánchez-Alonso, S.; Alcaraz-Serna, A.; Sánchez-Madrid, F.; Alfranca, A. Extracellular Vesicle-Mediated Immune Regulation of Tissue Remodeling and Angiogenesis After Myocardial Infarction. Front. Immunol. 2018, 9, 2799. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Jin, Z.; Yu, Z.; Wu, X.; Chang, X.; Fan, Z.; Li, F.; Wang, X.; Li, Z.; Zhou, Q.; et al. Cathepsin L Promotes Angiogenesis by Regulating the CDP/Cux/VEGF-D Pathway in Human Gastric Cancer. Gastric Cancer 2020, 23, 974–987. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Afshari, J.T.; Esmaeili, S.-A.; Saburi, E.; Joneidi, Z.; Momtazi-Borojeni, A.A. Cardioprotective microRNAs: Lessons from Stem Cell-Derived Exosomal microRNAs to Treat Cardiovascular Disease. Atherosclerosis 2019, 285, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Wang, L.; Sun, X.-J.; Zhao, X.; Zhang, G.-W.; Li-Ling, J. Sevoflurane Preconditioning Promotes Activation of Resident CSCs by Transplanted BMSCs via miR-210 in a Rat Model for Myocardial Infarction. Oncotarget 2017, 8, 114637–114647. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Zhu, X.-Y.; Herrmann, S.; Hickson, L.; Tang, H.; Dietz, A.B.; van Wijnen, A.J.; Lerman, L.; Textor, S. Adipose-Derived Mesenchymal Stem Cells from Patients with Atherosclerotic Renovascular Disease Have Increased DNA Damage and Reduced Angiogenesis That Can Be Modified by Hypoxia. Stem Cell Res. Ther. 2016, 7, 128. [Google Scholar] [CrossRef]
- Bezerra, J.B.; Scheinberg, M.A.; Abuchan, R. [Severe agranulocytosis induced by gold salts: Reversal by peritoneal dialysis]. AMB Rev. Assoc. Medica Bras. 1978, 24, 377–378. [Google Scholar]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.-T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef]
- Chandy, M.; Rhee, J.-W.; Ozen, M.O.; Williams, D.R.; Pepic, L.; Liu, C.; Zhang, H.; Malisa, J.; Lau, E.; Demirci, U.; et al. Atlas of Exosomal microRNAs Secreted from Human iPSC-Derived Cardiac Cell Types. Circulation 2020, 142, 1794–1796. [Google Scholar] [CrossRef]
- Musunuru, K.; Sheikh, F.; Gupta, R.M.; Houser, S.R.; Maher, K.O.; Milan, D.J.; Terzic, A.; Wu, J.C.; American Heart Association Council on Functional Genomics and Translational Biology; Council on Cardiovascular Disease in the Young; et al. Induced Pluripotent Stem Cells for Cardiovascular Disease Modeling and Precision Medicine: A Scientific Statement from the American Heart Association. Circ. Genom. Precis. Med. 2018, 11, e000043. [Google Scholar] [CrossRef]
- Rustagi, Y.; Jaiswal, H.K.; Rawal, K.; Kundu, G.C.; Rani, V. Comparative Characterization of Cardiac Development Specific microRNAs: Fetal Regulators for Future. PLoS ONE 2015, 10, e0139359. [Google Scholar] [CrossRef]
- Adamiak, M.; Sahoo, S. Exosomes in Myocardial Repair: Advances and Challenges in the Development of Next-Generation Therapeutics. Mol. Ther. 2018, 26, 1635–1643. [Google Scholar] [CrossRef]
- Sahoo, S.; Losordo, D.W. Exosomes and Cardiac Repair after Myocardial Infarction. Circ. Res. 2014, 114, 333–344. [Google Scholar] [CrossRef]
- Jadli, A.S.; Parasor, A.; Gomes, K.P.; Shandilya, R.; Patel, V.B. Exosomes in Cardiovascular Diseases: Pathological Potential of Nano-Messenger. Front. Cardiovasc. Med. 2021, 8, 767488. [Google Scholar] [CrossRef]
- Menasché, P.; Renault, N.K.; Hagège, A.; Puscas, T.; Bellamy, V.; Humbert, C.; Le, L.; Blons, H.; Granier, C.; Benhamouda, N.; et al. First-in-Man Use of a Cardiovascular Cell-Derived Secretome in Heart Failure. Case Report. Ebiomedicine 2024, 103, 105145. Available online: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(24)00180-4/fulltext (accessed on 15 February 2025). [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.; Cheng, Q.; Bai, L.; Huang, S.; Gao, J. Extracellular Vesicles in Cardiovascular Diseases: Diagnosis and Therapy. Front. Cell Dev. Biol. 2022, 10, 875376. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Kim, Y.H. Cancer Prevention from the Perspective of Global Cancer Burden Patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef]
- Tolcher, A.W. Antibody Drug Conjugates: Lessons from 20 Years of Clinical Experience. Ann. Oncol. 2016, 27, 2168–2172. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Borthakur, G.; Estey, A.E.E. Therapy-Related Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Curr. Oncol. Rep. 2007, 9, 373–377. [Google Scholar] [CrossRef]
- Braunstein, S.; Nakamura, J.L. Radiotherapy-Induced Malignancies: Review of Clinical Features, Pathobiology, and Evolving Approaches for Mitigating Risk. Front. Oncol. 2013, 3, 73. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A Doxorubicin Delivery Platform Using Engineered Natural Membrane Vesicle Exosomes for Targeted Tumor Therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, Y.; Gong, C.; Wang, Z.; Xia, Q.; Gu, F.; Hu, C.; Zhang, L.; Guo, H.; Gao, S. A33 Antibody-Functionalized Exosomes for Targeted Delivery of Doxorubicin against Colorectal Cancer. Nanomedicine 2018, 14, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Duan, J.; Liu, R.; Du, Y.; Luo, Q.; Cui, Y.; Su, Z.; Xu, J.; Xie, Y.; Lu, W. Engineered Targeting tLyp-1 Exosomes as Gene Therapy Vectors for Efficient Delivery of siRNA into Lung Cancer Cells. Asian J. Pharm. Sci. 2020, 15, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-Mediated siRNA Delivery to Suppress Postoperative Breast Cancer Metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef]
- Gujrati, V.; Kim, S.; Kim, S.-H.; Min, J.J.; Choy, H.E.; Kim, S.C.; Jon, S. Bioengineered Bacterial Outer Membrane Vesicles as Cell-Specific Drug-Delivery Vehicles for Cancer Therapy. ACS Nano 2014, 8, 1525–1537. [Google Scholar] [CrossRef]
- Meksiriporn, B.; Spangler, J.B. Directed-Evolution Approach to Empower EGFR Targeting for Immunotherapy. Cell Rep. Methods 2024, 4, 100762. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Lei, C.; Liu, S.; Cui, Y.; Wang, C.; Qian, K.; Li, T.; Shen, Y.; Fan, X.; Lin, F.; et al. CAR Exosomes Derived from Effector CAR-T Cells Have Potent Antitumour Effects and Low Toxicity. Nat. Commun. 2019, 10, 4355. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, Q.; Hou, T.; Han, M.; Smbatyan, G.; Lang, J.E.; Epstein, A.L.; Lenz, H.-J.; Zhang, Y. Genetically Engineered Cell-Derived Nanoparticles for Targeted Breast Cancer Immunotherapy. Mol. Ther. 2020, 28, 536–547. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Cheng, K.; Zhang, K.; Wang, Y.; Zhang, Y.; Li, Y.; Liu, G.; Xu, J.; Xu, J.; et al. Bacterial Outer Membrane Vesicles Presenting Programmed Death 1 for Improved Cancer Immunotherapy via Immune Activation and Checkpoint Inhibition. ACS Nano 2020, 14, 16698–16711. [Google Scholar] [CrossRef]
- Holst, J.; Martin, D.; Arnold, R.; Huergo, C.C.; Oster, P.; O’Hallahan, J.; Rosenqvist, E. Properties and Clinical Performance of Vaccines Containing Outer Membrane Vesicles from Neisseria meningitidis. Vaccine 2009, 27 (Suppl. 2), B3–B12. [Google Scholar] [CrossRef] [PubMed]
- van de Waterbeemd, B.; Mommen, G.P.M.; Pennings, J.L.A.; Eppink, M.H.; Wijffels, R.H.; van der Pol, L.A.; de Jong, A.P.J.M. Quantitative Proteomics Reveals Distinct Differences in the Protein Content of Outer Membrane Vesicle Vaccines. J. Proteome Res. 2013, 12, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Grandi, A.; Fantappiè, L.; Irene, C.; Valensin, S.; Tomasi, M.; Stupia, S.; Corbellari, R.; Caproni, E.; Zanella, I.; Isaac, S.J.; et al. Vaccination with a FAT1-Derived B Cell Epitope Combined with Tumor-Specific B and T Cell Epitopes Elicits Additive Protection in Cancer Mouse Models. Front. Oncol. 2018, 8, 481. [Google Scholar] [CrossRef]
- Cheng, K.; Zhao, R.; Li, Y.; Qi, Y.; Wang, Y.; Zhang, Y.; Qin, H.; Qin, Y.; Chen, L.; Li, C.; et al. Bioengineered Bacteria-Derived Outer Membrane Vesicles as a Versatile Antigen Display Platform for Tumor Vaccination via Plug-and-Display Technology. Nat. Commun. 2021, 12, 2041. [Google Scholar] [CrossRef] [PubMed]
- Codiak BioSciences. A First-in-Human Study of CDK-002 (exoSTING) in Subjects with Advanced/Metastatic, Recurrent, Injectable Solid Tumors; NCI: Bethesda, MD, USA, 2020. Available online: https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/v?id=NCI-2020-12113 (accessed on 19 February 2025).
- Codiak BioSciences. Phase 1 Study of Macrophage Reprogramming Agent, exoASO-STAT6 (CDK-004), in Patients with Advanced Hepatocellular Carcinoma (HCC) and Patients with Liver Metastases from Either Primary Gastric Cancer or Colorectal Cancer (CRC); Clinicaltrials.gov: Bethesda, MD, USA, 2023.
- Surana, R.; LeBleu, V.S.; Lee, J.J.; Smaglo, B.G.; Zhao, D.; Lee, M.S.; Wolff, R.A.; Overman, M.J.; Mendt, M.C.; McAndrews, K.M.; et al. Phase I Study of Mesenchymal Stem Cell (MSC)-Derived Exosomes with KRASG12D siRNA in Patients with Metastatic Pancreatic Cancer Harboring a KRASG12D Mutation. J. Clin. Oncol. 2022, 40, TPS633. [Google Scholar] [CrossRef]
- Huang, B. Phase II Study of Tumor Cell-Derived Microparticles Used as Vectors of Chemotherapeutic Drugs to Treat Malignant Ascites and Pleural Effusion; Clinicaltrials.gov: Bethesda, MD, USA, 2013.
- Gustave Roussy, Cancer Campus, Grand Paris. Phase II Trial of a Vaccination with Tumor Antigen-Loaded Dendritic Cell-Derived Exosomes on Patients with Unresectable Non Small Cell Lung Cancer Responding to Induction Chemotherapy; Clinicaltrials.gov: Bethesda, MD, USA, 2018.
- Shattock, A.J.; Johnson, H.C.; Sim, S.Y.; Carter, A.; Lambach, P.; Hutubessy, R.C.W.; Thompson, K.M.; Badizadegan, K.; Lambert, B.; Ferrari, M.J.; et al. Contribution of Vaccination to Improved Survival and Health: Modelling 50 Years of the Expanded Programme on Immunization. Lancet 2024, 403, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Amanna, I.J.; Slifka, M.K. Successful Vaccines. In Current Topics in Microbiology and Immunology; Springer Science and Business Media LLC: Berlin, Germany, 2020; Volume 428, pp. 1–30. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; da Veiga, A.B.G.; Kaminski, V.d.L.; Valverde-Villegas, J.M.; de Freitas, A.W.Q.; Chies, J.A.B. Control and Prevention of Infectious Diseases from a One Health Perspective. Genet. Mol. Biol. 2021, 44, e20200256. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K. Live Attenuated Vaccines in Patients Receiving Immunosuppressive Agents. Pediatr. Nephrol. 2023, 38, 3889–3900. [Google Scholar] [CrossRef]
- Shinjoh, M.; Miyairi, I.; Hoshino, K.; Takahashi, T.; Nakayama, T. Effective and Safe Immunizations with Live-Attenuated Vaccines for Children after Living Donor Liver Transplantation. Vaccine 2008, 26, 6859–6863. [Google Scholar] [CrossRef]
- Avci, F.Y.; Kasper, D.L. How Bacterial Carbohydrates Influence the Adaptive Immune System. Annu. Rev. Immunol. 2010, 28, 107–130. [Google Scholar] [CrossRef]
- Coutinho, A.; Möller, G. B Cell Mitogenic Properties of Thymus-Independent Antigens. Nat. New Biol. 1973, 245, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Mond, J.J.; Lees, A.; Snapper, C.M. T Cell-Independent Antigens Type 2. Annu. Rev. Immunol. 1995, 13, 655–692. [Google Scholar] [CrossRef] [PubMed]
- Guttormsen, H.K.; Sharpe, A.H.; Chandraker, A.K.; Brigtsen, A.K.; Sayegh, M.H.; Kasper, D.L. Cognate Stimulatory B-Cell-T-Cell Interactions Are Critical for T-Cell Help Recruited by Glycoconjugate Vaccines. Infect. Immun. 1999, 67, 6375–6384. [Google Scholar] [CrossRef]
- Guttormsen, H.K.; Wetzler, L.M.; Finberg, R.W.; Kasper, D.L. Immunologic Memory Induced by a Glycoconjugate Vaccine in a Murine Adoptive Lymphocyte Transfer Model. Infect. Immun. 1998, 66, 2026–2032. [Google Scholar] [CrossRef] [PubMed]
- Schneerson, R.; Barrera, O.; Sutton, A.; Robbins, J.B. Preparation, Characterization, and Immunogenicity of Haemophilus Influenzae Type b Polysaccharide-Protein Conjugates. J. Exp. Med. 1980, 152, 361–376. [Google Scholar] [CrossRef]
- Geno, K.A.; Gilbert, G.L.; Song, J.Y.; Skovsted, I.C.; Klugman, K.P.; Jones, C.; Konradsen, H.B.; Nahm, M.H. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev. 2015, 28, 871–899. [Google Scholar] [CrossRef]
- Pace, D.; Pollard, A.J. Meningococcal A, C, Y and W-135 Polysaccharide-Protein Conjugate Vaccines. Arch. Dis. Child. 2007, 92, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Klugman, K.P.; Gilbertson, I.T.; Koornhof, H.J.; Robbins, J.B.; Schneerson, R.; Schulz, D.; Cadoz, M.; Armand, J. Protective Activity of Vi Capsular Polysaccharide Vaccine against Typhoid Fever. Lancet 1987, 2, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Acharya, I.L.; Lowe, C.U.; Thapa, R.; Gurubacharya, V.L.; Shrestha, M.B.; Cadoz, M.; Schulz, D.; Armand, J.; Bryla, D.A.; Trollfors, B. Prevention of Typhoid Fever in Nepal with the Vi Capsular Polysaccharide of Salmonella typhi. N. Engl. J. Med. 1987, 317, 1101–1104. [Google Scholar] [CrossRef]
- Tacket, C.O.; Levine, M.M.; Robbins, J.B. Persistence of Antibody Titres Three Years after Vaccination with Vi Polysaccharide Vaccine against Typhoid Fever. Vaccine 1988, 6, 307–308. [Google Scholar] [CrossRef]
- Wo, J.; Lv, Z.-Y.; Sun, J.-N.; Tang, H.; Qi, N.; Ye, B.-C. Engineering Probiotic-Derived Outer Membrane Vesicles as Functional Vaccine Carriers to Enhance Immunity against SARS-CoV-2. iScience 2023, 26, 105772. [Google Scholar] [CrossRef] [PubMed]
- Siegers, C.P.; Bartels, L.; Riemann, D. Effects of Fasting and Glutathione Depletors on the GSH-Dependent Enzyme System in the Gastrointestinal Mucosa of the Rat. Pharmacology 1989, 38, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Gnopo, Y.M.D.; Watkins, H.C.; Stevenson, T.C.; DeLisa, M.P.; Putnam, D. Designer Outer Membrane Vesicles as Immunomodulatory Systems—Reprogramming Bacteria for Vaccine Delivery. Adv. Drug Deliv. Rev. 2017, 114, 132–142. [Google Scholar] [CrossRef]
- Piccioli, D.; Bartolini, E.; Micoli, F. GMMA as a “plug and Play” Technology to Tackle Infectious Disease to Improve Global Health: Context and Perspectives for the Future. Expert Rev. Vaccines 2022, 21, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; MacLennan, C.A. Outer Membrane Vesicle Vaccines. Semin. Immunol. 2020, 50, 101433. [Google Scholar] [CrossRef]
- Donnelly, J.J.; Deck, R.R.; Liu, M.A. Immunogenicity of a Haemophilus Influenzae Polysaccharide-Neisseria meningitidis Outer Membrane Protein Complex Conjugate Vaccine. J. Immunol. 1990, 145, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Latz, E.; Franko, J.; Golenbock, D.T.; Schreiber, J.R. Haemophilus Influenzae Type B-Outer Membrane Protein Complex Glycoconjugate Vaccine Induces Cytokine Production by Engaging Human Toll-like Receptor 2 (TLR2) and Requires the Presence of TLR2 for Optimal Immunogenicity. J. Immunol. 2004, 172, 2431–2438. [Google Scholar] [CrossRef]
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Oldrini, D.; Pitirollo, O.; Gasperini, G.; et al. Generalized Modules for Membrane Antigens as Carrier for Polysaccharides: Impact of Sugar Length, Density, and Attachment Site on the Immune Response Elicited in Animal Models. Front. Immunol. 2021, 12, 719315. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, E.; Kis, Z.; Ozanne, J.; Di Benedetto, R.; Ricchetti, B.; Massai, L.; Carducci, M.; Oldrini, D.; Gasperini, G.; Aruta, M.G.; et al. GMMA as an Alternative Carrier for a Glycoconjugate Vaccine against Group A Streptococcus. Vaccines 2022, 10, 1034. [Google Scholar] [CrossRef]
- Gasperini, G.; Alfini, R.; Arato, V.; Mancini, F.; Aruta, M.G.; Kanvatirth, P.; Pickard, D.; Necchi, F.; Saul, A.; Rossi, O.; et al. Salmonella paratyphi A Outer Membrane Vesicles Displaying Vi Polysaccharide as a Multivalent Vaccine against Enteric Fever. Infect. Immun. 2021, 89, e00699-20. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Alfini, R.; Di Benedetto, R.; Necchi, F.; Schiavo, F.; Mancini, F.; Carducci, M.; Palmieri, E.; Balocchi, C.; Gasperini, G.; et al. GMMA Is a Versatile Platform to Design Effective Multivalent Combination Vaccines. Vaccines 2020, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Valguarnera, E.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles as a Platform for Vaccine Development. Methods Enzymol. 2017, 597, 285–310. [Google Scholar] [CrossRef]
- Price, N.L.; Goyette-Desjardins, G.; Nothaft, H.; Valguarnera, E.; Szymanski, C.M.; Segura, M.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles: A Novel Platform for Bacterial Vaccines. Sci. Rep. 2016, 6, 24931. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, T.C.; Cywes-Bentley, C.; Moeller, T.D.; Weyant, K.B.; Putnam, D.; Chang, Y.-F.; Jones, B.D.; Pier, G.B.; DeLisa, M.P. Immunization with Outer Membrane Vesicles Displaying Conserved Surface Polysaccharide Antigen Elicits Broadly Antimicrobial Antibodies. Proc. Natl. Acad. Sci. USA 2018, 115, E3106–E3115. [Google Scholar] [CrossRef] [PubMed]
- Low, K.E.; Howell, P.L. Gram-Negative Synthase-Dependent Exopolysaccharide Biosynthetic Machines. Curr. Opin. Struct. Biol. 2018, 53, 32–44. [Google Scholar] [CrossRef]
- Tian, H.; Li, B.; Xu, T.; Yu, H.; Chen, J.; Yu, H.; Li, S.; Zeng, L.; Huang, X.; Liu, Q. Outer Membrane Vesicles Derived from Salmonella Enterica Serotype Typhimurium Can Deliver Shigella Flexneri 2a O-Polysaccharide Antigen to Prevent Shigella flexneri 2a Infection in Mice. Appl. Environ. Microbiol. 2021, 87, e0096821. [Google Scholar] [CrossRef]
- Weyant, K.B.; Oloyede, A.; Pal, S.; Liao, J.; Jesus, M.R.-D.; Jaroentomeechai, T.; Moeller, T.D.; Hoang-Phou, S.; Gilmore, S.F.; Singh, R.; et al. A Modular Vaccine Platform Enabled by Decoration of Bacterial Outer Membrane Vesicles with Biotinylated Antigens. Nat. Commun. 2023, 14, 464. [Google Scholar] [CrossRef] [PubMed]
- Cheever, M.A.; Allison, J.P.; Ferris, A.S.; Finn, O.J.; Hastings, B.M.; Hecht, T.T.; Mellman, I.; Prindiville, S.A.; Viner, J.L.; Weiner, L.M.; et al. The Prioritization of Cancer Antigens: A National Cancer Institute Pilot Project for the Acceleration of Translational Research. Clin. Cancer Res. 2009, 15, 5323–5337. [Google Scholar] [CrossRef]
- Ihssen, J.; Kowarik, M.; Dilettoso, S.; Tanner, C.; Wacker, M.; Thöny-Meyer, L. Production of Glycoprotein Vaccines in Escherichia coli. Microb. Cell Factories 2010, 9, 61. [Google Scholar] [CrossRef]
- Stark, J.C.; Jaroentomeechai, T.; Warfel, K.F.; Hershewe, J.M.; DeLisa, M.P.; Jewett, M.C. Rapid Biosynthesis of Glycoprotein Therapeutics and Vaccines from Freeze-Dried Bacterial Cell Lysates. Nat. Protoc. 2023, 18, 2374–2398. [Google Scholar] [CrossRef]
- Warfel, K.F.; Williams, A.; Wong, D.A.; Sobol, S.E.; Desai, P.; Li, J.; Chang, Y.-F.; DeLisa, M.P.; Karim, A.S.; Jewett, M.C. A Low-Cost, Thermostable, Cell-Free Protein Synthesis Platform for On-Demand Production of Conjugate Vaccines. ACS Synth. Biol. 2023, 12, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Deghmane, A.-E.; Taha, M.-K. Product Review on the IMD Serogroup B Vaccine Bexsero®. Hum. Vaccines Immunother. 2022, 18, 2020043. [Google Scholar] [CrossRef] [PubMed]
- Sierra-González, V.G. Cuban Meningococcal Vaccine VA-MENGOC-BC:30 Years of Use and Future Potential. MEDICC Rev. 2019, 21, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Adamo, R.; Nakakana, U. Outer Membrane Vesicle Vaccine Platforms. BioDrugs 2024, 38, 47–59. [Google Scholar] [CrossRef]
- Gu, W.; Luozhong, S.; Cai, S.; Londhe, K.; Elkasri, N.; Hawkins, R.; Yuan, Z.; Su-Greene, K.; Yin, Y.; Cruz, M.; et al. Extracellular Vesicles Incorporating Retrovirus-like Capsids for the Enhanced Packaging and Systemic Delivery of mRNA into Neurons. Nat. Biomed. Eng. 2024, 8, 415–426. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Zhou, Y.; Arias, G.F.; Dittmer, D.P. Extracellular Vesicle Isolation by a Tangential-Flow Filtration-Based Large-Scale Purification Method. Methods Mol. Biol. 2023, 2668, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, C.; Lang, K.; Kuplennik, N.; Sosnik, A.; Steinfeld, R. High-Grade Extracellular Vesicles Preparation by Combined Size-Exclusion and Affinity Chromatography. Sci. Rep. 2021, 11, 10550. [Google Scholar] [CrossRef]
- Petersen, K.E.; Shiri, F.; White, T.; Bardi, G.T.; Sant, H.; Gale, B.K.; Hood, J.L. Exosome Isolation: Cyclical Electrical Field Flow Fractionation in Low-Ionic-Strength Fluids. Anal. Chem. 2018, 90, 12783–12790. [Google Scholar] [CrossRef]
- Bahadorani, M.; Nasiri, M.; Dellinger, K.; Aravamudhan, S.; Zadegan, R. Engineering Exosomes for Therapeutic Applications: Decoding Biogenesis, Content Modification, and Cargo Loading Strategies. Int. J. Nanomed. 2024, 19, 7137–7164. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A Review on Exosomes Application in Clinical Trials: Perspective, Questions, and Challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.P.; Zhao, E.L.; Soltani, M.; Frei, M.; Nelson, J.A.D.; Bundy, B.C. Streamlining the Preparation of “Endotoxin-Free” ClearColi Cell Extract with Autoinduction Media for Cell-Free Protein Synthesis of the Therapeutic Protein Crisantaspase. Synth. Syst. Biotechnol. 2019, 4, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular Vesicles: A Rising Star for Therapeutics and Drug Delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent Advances in Extracellular Vesicles for Therapeutic Cargo Delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef] [PubMed]

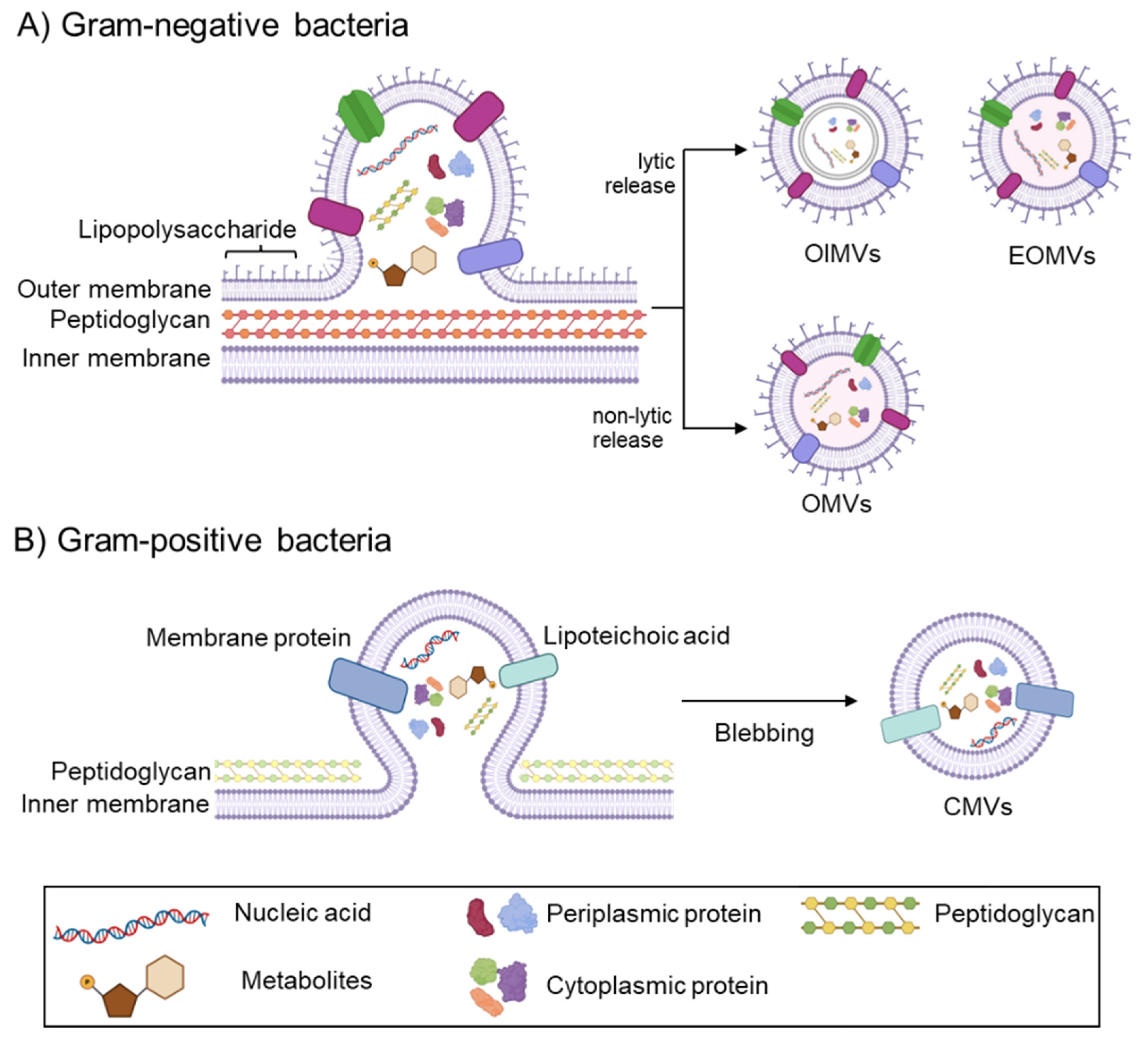
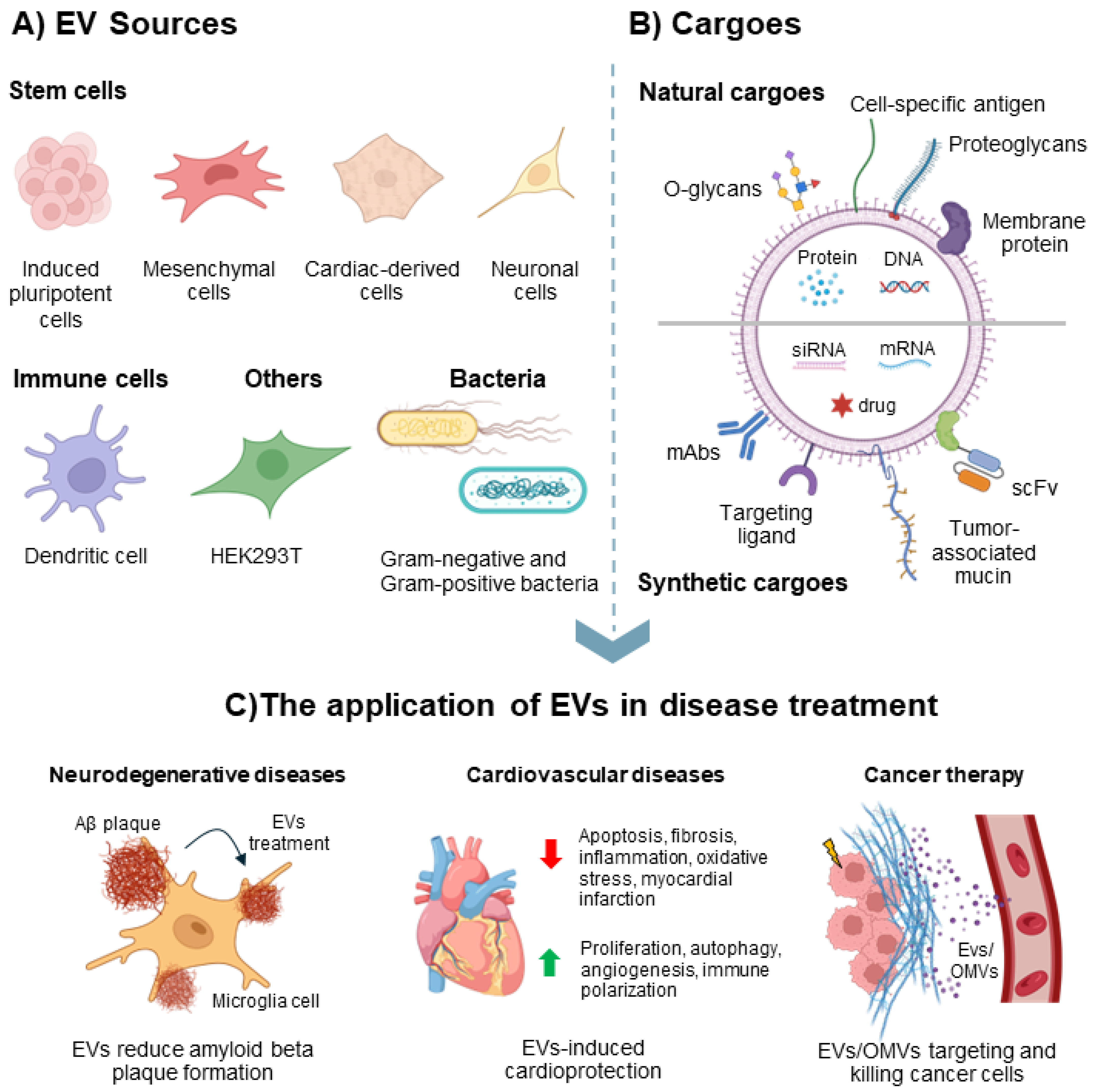
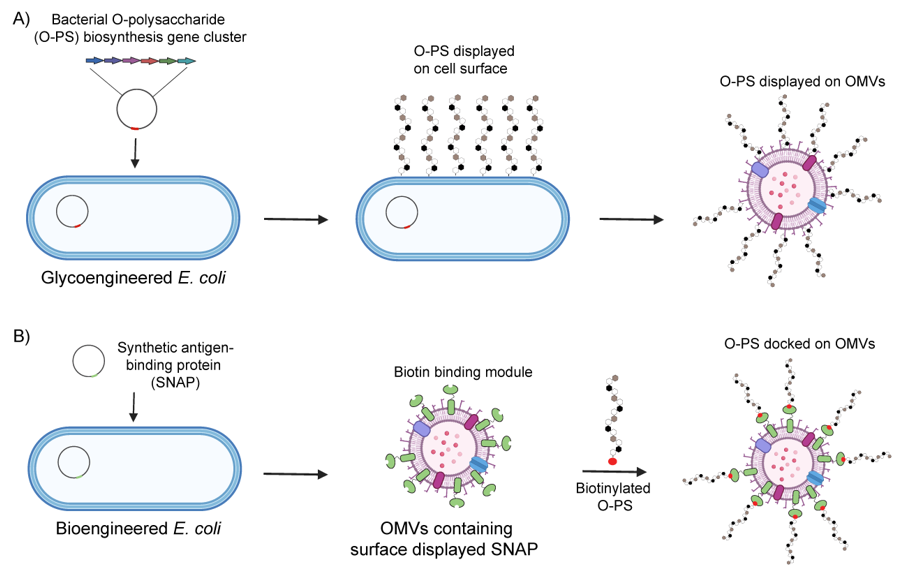
| Techniques | Description | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Traditional techniques | ||||
| Ultracentrifuge (UC) | Separation by size and density through sequential centrifugation |
|
| [184,185,186,187,188,189,190] |
| Size-exclusion chromatography (SEC) | Separation of EVs from contaminating proteins based on size differences |
|
| [191,192,193,194,195,196,197,198] |
| Ultrafiltration (UF) | Size-based separation of EV using membranes with specific pore sizes or properties |
|
| [197,199,200,201,202] |
| Precipitation | Use chemicals to induce precipitation, followed by low-speed centrifugation to isolate EVs pool |
|
| [203,204,205,206,207,208,209] |
| Emerging techniques | ||||
| Tangential flow filtration (TFF) | Ultrafiltration technique but with sample flows in parallel to the membrane |
|
| [210] |
| Asymmetrical flow field-flow fractionation (AsFlFFF) | A size-based separation technique based on diffusion coefficients for fractionating EVs |
|
| [183,211,212,213,214] |
| Charge-based techniques (exchange chromatography, electrophoresis, and dielectrophoresis) | Using surface charge on EVs for separation via electrostatic interaction or electrophoretic mobilities |
|
| [214,215,216,217,218,219,220] |
| Affinity-based techniques | Isolation technique using specific natural or engineered ligands on EV surface |
|
| [182,185,220,221,222,223,224] |
| Techniques | Description | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Traditional method | ||||
| Dynamic light scattering (DLS) | Analysis of light scattering from Brownian motion to determine particle size |
|
| [236,237,238] |
| Transmission electron microscopy (TEM) | Visualization of EVs using electron microscope |
|
| [236,239,240,241,242,243,244,245] |
| Nanoparticle Tracking Analysis (NTA) | Measure EVs’ size distributions and concentrations using real-time tracking of individual particles suspension (Brownian motion) |
|
| [235,246,247,248,249] |
| Flow cytometry | Microfluidic-based detection of EVs morphology and/or fluorescent signals |
|
| [235,241,250,251,252,253] |
| Omics approach | ||||
| Proteomics | Comprehensive mass spectrometry-based profiling of total proteome in particular EVs |
|
| [254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274] |
| Transcriptomics | Profiling total transcripts (mainly mRNA) using RT-PCR and NGS approach Providing insights into cellular state and serving as potential biomarkers during pathogenesis |
|
| [275,276,277,278,279,280,281,282] |
| Genomics | Sequencing of DNA within EVs |
|
| [283,284,285,286,287] |
| Lipidomics | Mass spectrometry-based approach to profile total lipid compositions and abundance within EVs |
|
| [288,289,290,291,292,293] |
| Metabolomics | Mass spectrometry-based or nuclear magnetic resonance (NMR)-based analysis to profile total small molecule metabolites within EVs |
|
| [294,295,296,297,298] |
| Glycomics | Mass spectrometry- and/or carbohydrate binding molecules-based profiling of glycans and glycoconjugates on the surface and within EVs |
|
| [299,300,301,302,303,304,305,306,307] |
| Vaccine | Pathogen | Company |
|---|---|---|
| Licensed vaccine | ||
| Bexsero (4CMenB) | Neisseria meningitidis serogroup B | GSK (Siena, Italy) |
| MenZB (NZ dOMV) | Neisseria meningitidis serogroup B | Novartis Vaccine and Diagnostics (Siena, Italy) b and National Institute of Public Health (Oslo, Norway) |
| VA-MENGO-BC | Neisseria meningitidis serogroup B | Finlay Institute (Havana, Cuba) |
| Norway MenBVAC | Neisseria meningitidis serogroup B | Norwegian Institute of Public Health (Oslo, Norway) a |
| PedvaxHib (PRP-OMPC) | Haemophilus influenzae type b | Merck Co. (Rahway, NJ, USA) |
| Procomvax/Comvax (PRP-OMPC and hepatitis B) | Haemophilus influenzae type b and hepatitis B | Merck Co. (Rahway, NJ, USA) a |
| Vaxelis (diphtheria and tetanus toxoids, acellular pertussis, inactivated poliovirus, PRP-OMPC and hepatitis B) | Diphtheria, tetanus, pertussis, poliomyelitis, H. influenzae type b and hepatitis B | Merck Co. (Rahway, NJ, USA) and Sanofi Pasteur (Lyon, France) |
| Phase I/II clinical trial | ||
| altSonflex1-2-3 | Shigella | GSK (Siena, Italy) |
| Avacc | COVID-19 | Intravacc (Bilthoven, Netherlands) |
| iNTS-GMMA | Invasive non-typhoidal Salmonella | GSK (Siena, Italy) |
| N/A | Neisseria gonorrhoea | GSK (Siena, Italy) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puagsopa, J.; Tongviseskul, N.; Jaroentomeechai, T.; Meksiriporn, B. Recent Progress in Developing Extracellular Vesicles as Nanovehicles to Deliver Carbohydrate-Based Therapeutics and Vaccines. Vaccines 2025, 13, 285. https://doi.org/10.3390/vaccines13030285
Puagsopa J, Tongviseskul N, Jaroentomeechai T, Meksiriporn B. Recent Progress in Developing Extracellular Vesicles as Nanovehicles to Deliver Carbohydrate-Based Therapeutics and Vaccines. Vaccines. 2025; 13(3):285. https://doi.org/10.3390/vaccines13030285
Chicago/Turabian StylePuagsopa, Japigorn, Niksa Tongviseskul, Thapakorn Jaroentomeechai, and Bunyarit Meksiriporn. 2025. "Recent Progress in Developing Extracellular Vesicles as Nanovehicles to Deliver Carbohydrate-Based Therapeutics and Vaccines" Vaccines 13, no. 3: 285. https://doi.org/10.3390/vaccines13030285
APA StylePuagsopa, J., Tongviseskul, N., Jaroentomeechai, T., & Meksiriporn, B. (2025). Recent Progress in Developing Extracellular Vesicles as Nanovehicles to Deliver Carbohydrate-Based Therapeutics and Vaccines. Vaccines, 13(3), 285. https://doi.org/10.3390/vaccines13030285





