Progress on Respiratory Syncytial Virus Vaccine Development and Evaluation Methods
Abstract
1. Introduction
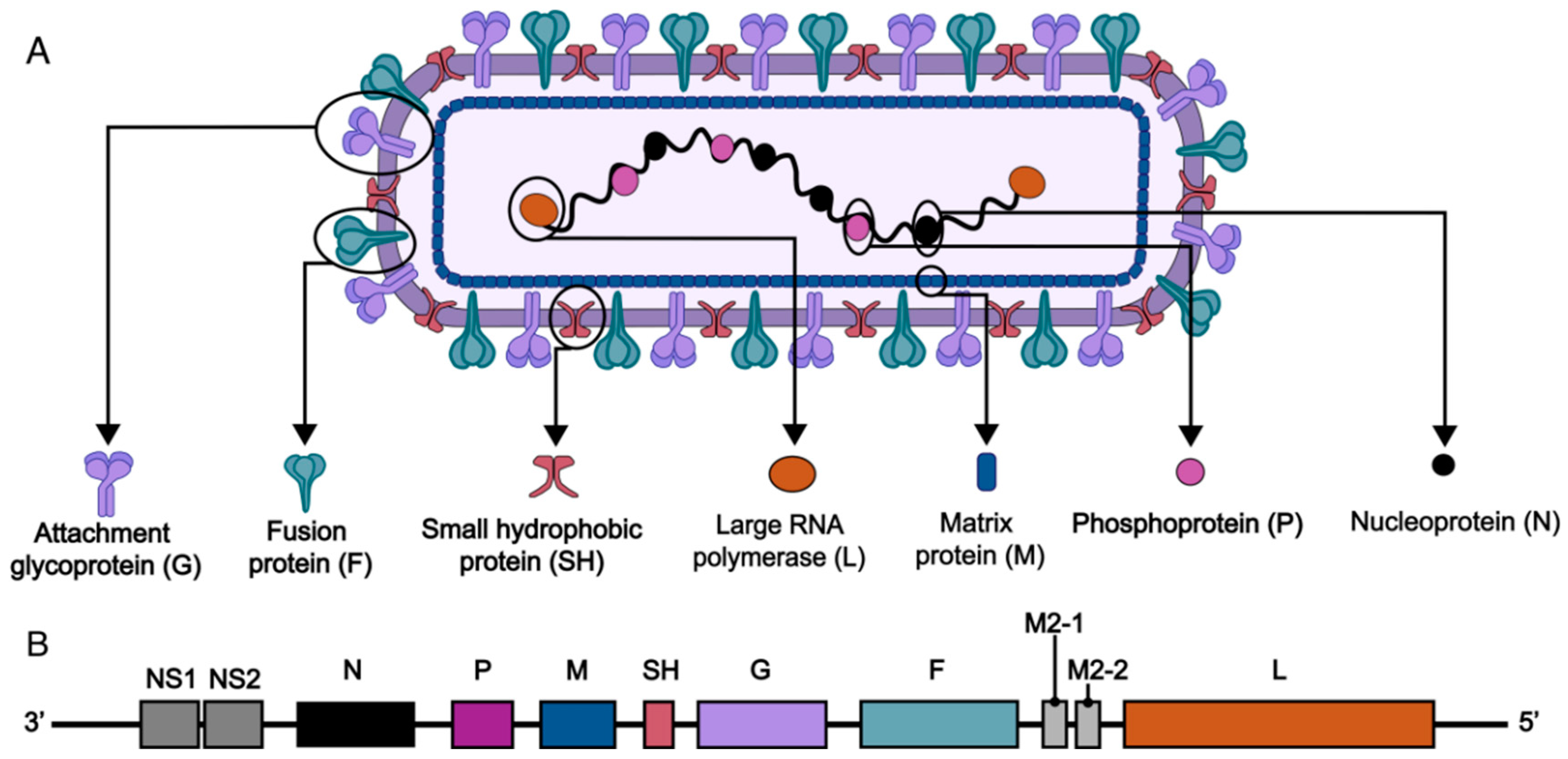
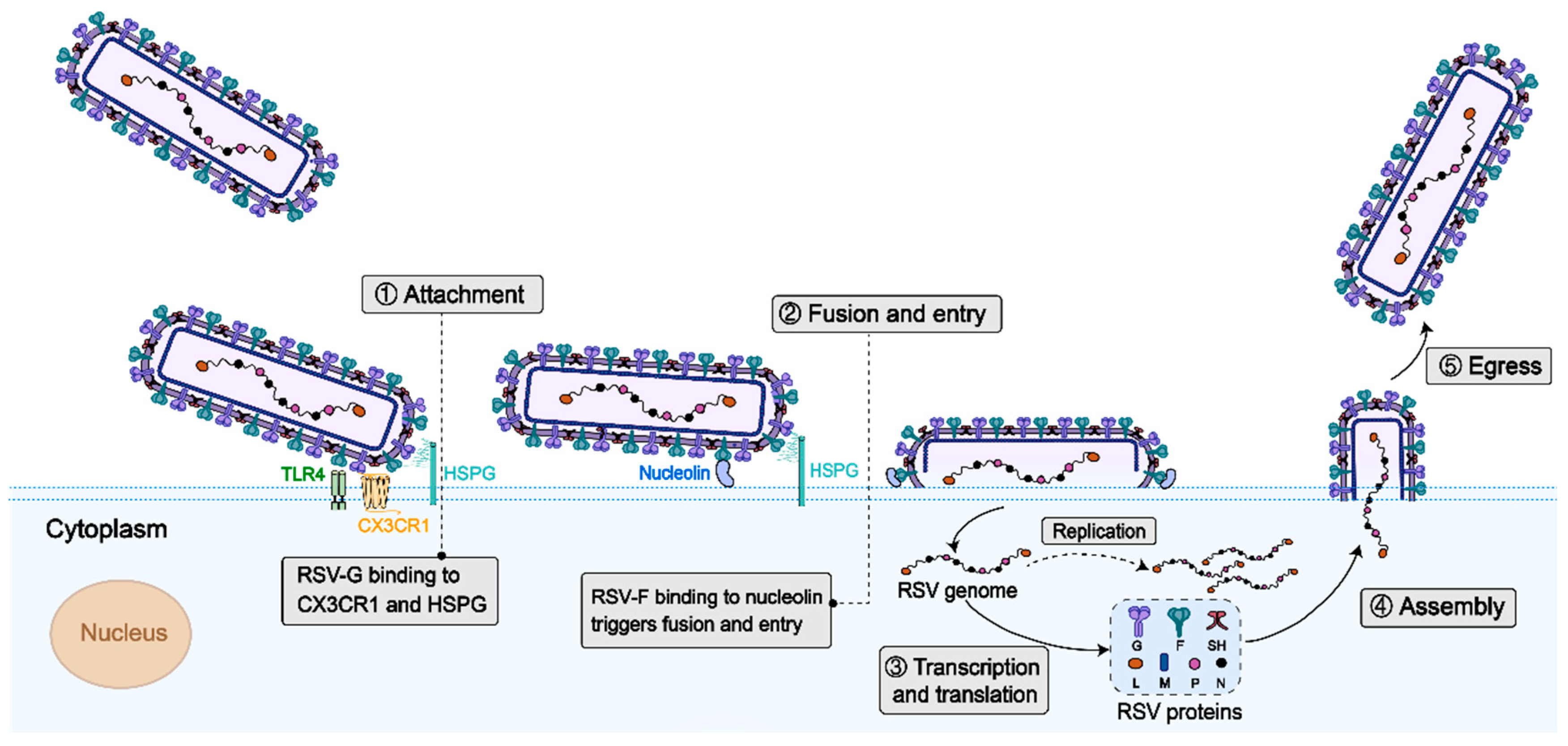
2. RSV Structure and Vaccine Targets
2.1. Virus Structure
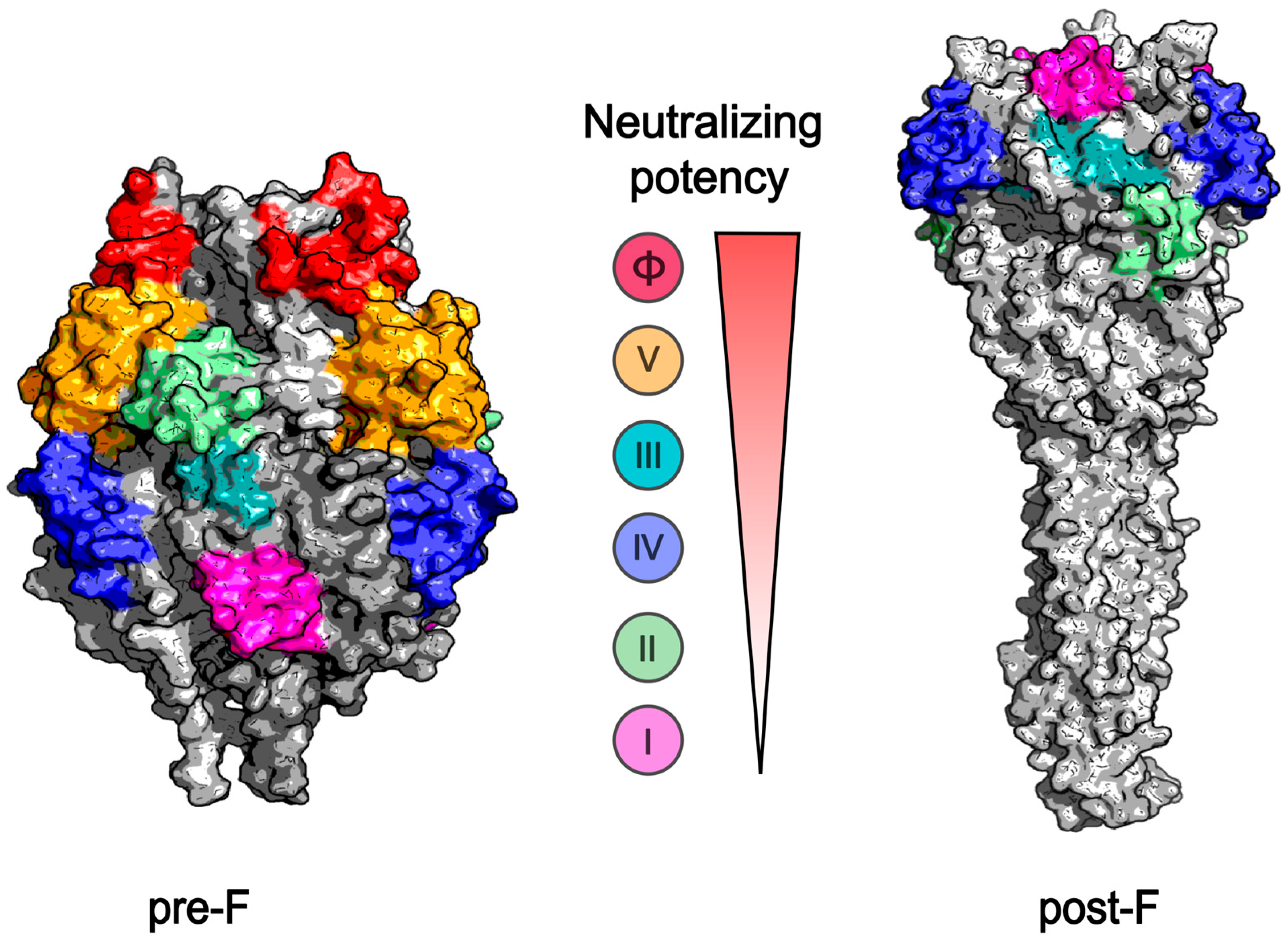
2.2. Target Immunogens of the RSV Vaccine
3. Progress in RSV Vaccine Research and Development
3.1. RSV Live Attenuated Vaccine
3.2. RSV Protein Subunit Vaccine
3.3. Vectored Vaccines
3.4. mRNA Vaccines
3.5. Nanoparticle Vaccines
| Platform | Examples | Target | Manufacturer | Status | References |
|---|---|---|---|---|---|
| Live attenuated vaccine | RSV/ΔNS2/Δ1313/I1314L | Deletion of NS2, deletion S1313 of L protein, I1314L of L protein of RSV | NA | Clinical phase II | [37] |
| RSV 276 | Deletion of M2-2 protein of RSV | NA | Clinical phase II | [38] | |
| Subunit vaccine | RSVpreF3 (Arexvy) | Pre-F | GSK | Approved by FDA | [18,33] |
| RSVpreF (Abrysvo) | Pre-F | Pfizer | Approved by FDA | [33,41] | |
| FG-Gb1 | F and G protein | NA | Preclinical | [42] | |
| Vectored vaccines | rBCG-N-hRSV | BCG vaccine expressing the nucleoprotein of RSV | IDT Biologika | Clinical phase I | [26] |
| SeVRSV | Sendai virus expressing F protein | NA | Clinical phase I | [43] | |
| rVSV-G-2A-F | VSV expressing both the G and F proteins | NA | Preclinical | [44] | |
| BLB201 | Type 5 (PIV5) that encodes the F protein | Blue Lake Biotechnology | Clinical phase I/IIa | [45] | |
| Ad26.RSV.preF | Adenovirus vector vaccine expressing pre-F | J&J/Jassen | Clinical phase IIb | [46] | |
| MVA-RSV | MVA expressing F, G, N, M2-1 protein of RSV A and G of RSV B | Bavarian Nordic | Clinical phase III (failed) | [48] | |
| mRNA vaccines | mRNA-1345 (mRESVIA) | Pre-F | Moderna | Approved by FDA | [34] |
| mRNA-1777 | Pre-F | Moderna | Clinical phase I | [52] | |
| LC2DM-LNP | Pre-F | NA | Preclinical | [54] | |
| Nanoparticle vaccines | ResVax | Pre-F | Novavax | Clinical phase III (failed) | [61] |
| pre-F-NP | Pre-F | NA | Preclinical | [60] |
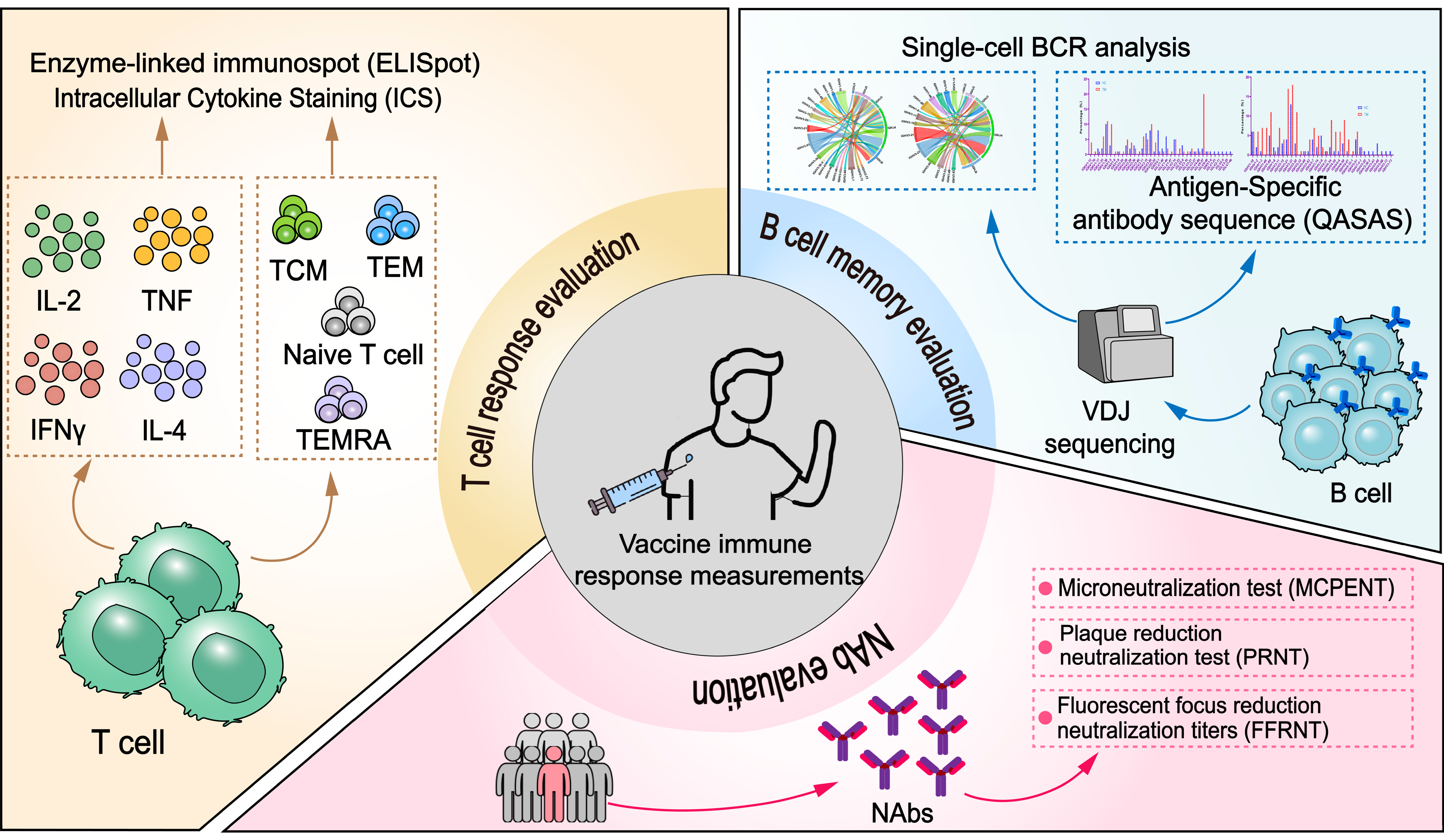
4. RSV Vaccine Testing and Evaluation
4.1. Immunological Surrogates for Vaccine Protection and Their Evaluation Methods
4.2. Immunological Evaluation of RSV and Its Correlation with Protective Immunity
4.2.1. Neutralizing Antibody Evaluation
4.2.2. T Cell Response Evaluation
4.2.3. B Cell Memory Evaluation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coultas, J.A.; Smyth, R.; Openshaw, P.J. Respiratory syncytial virus (RSV): A scourge from infancy to old age. Thorax 2019, 74, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Higdon, M.M.; Le, T.; O’Brien, K.L.; Murdoch, D.R.; Prosperi, C.; Baggett, H.C.; Brooks, W.A.; Feikin, D.R.; Hammitt, L.L.; Howie, S.R.C.; et al. Association of C-Reactive Protein With Bacterial and Respiratory Syncytial Virus-Associated Pneumonia Among Children Aged <5 Years in the PERCH Study. Clin. Infect. Dis. 2017, 64 (Suppl. 3), S378–S386. [Google Scholar] [CrossRef] [PubMed]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pircon, J.Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir. Viruses 2023, 17, e13031. [Google Scholar] [CrossRef]
- Kapikian, A.Z.; Mitchell, R.H.; Chanock, R.M.; Shvedoff, R.A.; Stewart, C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969, 89, 405–421. [Google Scholar] [CrossRef]
- Yin, W.; Li, H.Y.; Zheng, B.Y.; Deng, Y.Q.; Li, W.J.; Liu, Y.; Zeng, R.H. Lipopolysaccharide Inhibits FI-RSV Vaccine-enhanced Inflammation Through Regulating Th Responses. Curr. Med. Sci. 2019, 39, 363–370. [Google Scholar] [CrossRef]
- Killikelly, A.M.; Kanekiyo, M.; Graham, B.S. Pre-fusion F is absent on the surface of formalin-inactivated respiratory syncytial virus. Sci. Rep. 2016, 6, 34108. [Google Scholar] [CrossRef]
- Chatterjee, S.; Luthra, P.; Esaulova, E.; Agapov, E.; Yen, B.C.; Borek, D.M.; Edwards, M.R.; Mittal, A.; Jordan, D.S.; Ramanan, P.; et al. Structural basis for human respiratory syncytial virus NS1-mediated modulation of host responses. Nat. Microbiol. 2017, 2, 17101. [Google Scholar] [CrossRef]
- Grosfeld, H.; Hill, M.G.; Collins, P.L. RNA replication by respiratory syncytial virus (RSV) is directed by the N, P, and L proteins; transcription also occurs under these conditions but requires RSV superinfection for efficient synthesis of full-length mRNA. J. Virol. 1995, 69, 5677–5686. [Google Scholar] [CrossRef]
- Galloux, M.; Gabiane, G.; Sourimant, J.; Richard, C.A.; England, P.; Moudjou, M.; Aumont-Nicaise, M.; Fix, J.; Rameix-Welti, M.A.; Eleouet, J.F. Identification and characterization of the binding site of the respiratory syncytial virus phosphoprotein to RNA-free nucleoprotein. J. Virol. 2015, 89, 3484–3496. [Google Scholar] [CrossRef]
- Gan, S.W.; Tan, E.; Lin, X.; Yu, D.; Wang, J.; Tan, G.M.; Vararattanavech, A.; Yeo, C.Y.; Soon, C.H.; Soong, T.W.; et al. The small hydrophobic protein of the human respiratory syncytial virus forms pentameric ion channels. J. Biol. Chem. 2012, 287, 24671–24689. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Henke, D.M.; Avadhanula, V.; Shaw, C.A.; Tapia, L.I.; Piedra, P.A. Sequence variability of the respiratory syncytial virus (RSV) fusion gene among contemporary and historical genotypes of RSV/A and RSV/B. PLoS ONE 2017, 12, e0175792. [Google Scholar] [CrossRef]
- Zimmer, G.; Budz, L.; Herrler, G. Proteolytic activation of respiratory syncytial virus fusion protein: Cleavage at two furin consensus sequences. J. Biol. Chem. 2001, 276, 31642–31650. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Chen, M.; Joyce, M.G.; Sastry, M.; Stewart-Jones, G.B.; Yang, Y.; Zhang, B.; Chen, L.; Srivatsan, S.; Zheng, A.; et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013, 342, 592–598. [Google Scholar] [CrossRef]
- Krarup, A.; Truan, D.; Furmanova-Hollenstein, P.; Bogaert, L.; Bouchier, P.; Bisschop, I.J.M.; Widjojoatmodjo, M.N.; Zahn, R.; Schuitemaker, H.; McLellan, J.S.; et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat. Commun. 2015, 6, 8143. [Google Scholar] [CrossRef]
- Joyce, M.G.; Zhang, B.; Ou, L.; Chen, M.; Chuang, G.Y.; Druz, A.; Kong, W.P.; Lai, Y.T.; Rundlet, E.J.; Tsybovsky, Y.; et al. Iterative structure-based improvement of a fusion-glycoprotein vaccine against RSV. Nat. Struct. Mol. Biol. 2016, 23, 811–820. [Google Scholar] [CrossRef]
- Che, Y.; Gribenko, A.V.; Song, X.; Handke, L.D.; Efferen, K.S.; Tompkins, K.; Kodali, S.; Nunez, L.; Prasad, A.K.; Phelan, L.M.; et al. Rational design of a highly immunogenic prefusion-stabilized F glycoprotein antigen for a respiratory syncytial virus vaccine. Sci. Transl. Med. 2023, 15, eade6422. [Google Scholar] [CrossRef]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef]
- Walsh, E.E.; Eiras, D.; Woodside, J.; Jiang, Q.; Patton, M.; Marc, G.P.; Llapur, C.; Ramet, M.; Fukushima, Y.; Hussen, N.; et al. Efficacy, Immunogenicity, and Safety of the Bivalent RSV Prefusion F (RSVpreF) Vaccine in Older Adults Over 2 RSV Seasons. Clin. Infect. Dis. 2025, ciaf061. [Google Scholar] [CrossRef]
- Shaw, C.A.; Essink, B.; Harper, C.; Mithani, R.; Kapoor, A.; Dhar, R.; Wilson, L.; Guo, R.; Panozzo, C.A.; Wilson, E.; et al. Safety and Immunogenicity of an mRNA-Based RSV Vaccine Including a 12-Month Booster in a Phase 1 Clinical Trial in Healthy Older Adults. J. Infect. Dis. 2024, 230, e647–e656. [Google Scholar] [CrossRef]
- Fedechkin, S.O.; George, N.L.; Wolff, J.T.; Kauvar, L.M.; DuBois, R.M. Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies. Sci. Immunol. 2018, 3, eaar3534. [Google Scholar] [CrossRef] [PubMed]
- Tripp, R.A.; Jones, L.P.; Haynes, L.M.; Zheng, H.; Murphy, P.M.; Anderson, L.J. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2001, 2, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Jadhao, S.J.; Paden, C.R.; Tong, S. Functional Features of the Respiratory Syncytial Virus G Protein. Viruses 2021, 13, 1214. [Google Scholar] [CrossRef] [PubMed]
- Tripp, R.A.; Power, U.F.; Openshaw, P.J.M.; Kauvar, L.M. Respiratory Syncytial Virus: Targeting the G Protein Provides a New Approach for an Old Problem. J. Virol. 2018, 92, e01302-17. [Google Scholar] [CrossRef]
- Rainho-Tomko, J.N.; Pavot, V.; Kishko, M.; Swanson, K.; Edwards, D.; Yoon, H.; Lanza, L.; Alamares-Sapuay, J.; Osei-Bonsu, R.; Mundle, S.T.; et al. Immunogenicity and protective efficacy of RSV G central conserved domain vaccine with a prefusion nanoparticle. NPJ Vaccines 2022, 7, 74. [Google Scholar] [CrossRef]
- Abarca, K.; Rey-Jurado, E.; Munoz-Durango, N.; Vazquez, Y.; Soto, J.A.; Galvez, N.M.S.; Valdes-Ferrada, J.; Iturriaga, C.; Urzua, M.; Borzutzky, A.; et al. Safety and immunogenicity evaluation of recombinant BCG vaccine against respiratory syncytial virus in a randomized, double-blind, placebo-controlled phase I clinical trial. EClinicalMedicine 2020, 27, 100517. [Google Scholar] [CrossRef]
- Jordan, E.; Lawrence, S.J.; Meyer, T.P.H.; Schmidt, D.; Schultz, S.; Mueller, J.; Stroukova, D.; Koenen, B.; Gruenert, R.; Silbernagl, G.; et al. Broad Antibody and Cellular Immune Response From a Phase 2 Clinical Trial With a Novel Multivalent Poxvirus-Based Respiratory Syncytial Virus Vaccine. J. Infect. Dis. 2021, 223, 1062–1072. [Google Scholar] [CrossRef]
- Triantafilou, K.; Kar, S.; Vakakis, E.; Kotecha, S.; Triantafilou, M. Human respiratory syncytial virus viroporin SH: A viral recognition pathway used by the host to signal inflammasome activation. Thorax 2013, 68, 66–75. [Google Scholar] [CrossRef]
- Schepens, B.; Sedeyn, K.; Vande Ginste, L.; De Baets, S.; Schotsaert, M.; Roose, K.; Houspie, L.; Van Ranst, M.; Gilbert, B.; van Rooijen, N.; et al. Protection and mechanism of action of a novel human respiratory syncytial virus vaccine candidate based on the extracellular domain of small hydrophobic protein. EMBO Mol. Med. 2014, 6, 1436–1454. [Google Scholar] [CrossRef]
- Langley, J.M.; MacDonald, L.D.; Weir, G.M.; MacKinnon-Cameron, D.; Ye, L.; McNeil, S.; Schepens, B.; Saelens, X.; Stanford, M.M.; Halperin, S.A. A Respiratory Syncytial Virus Vaccine Based on the Small Hydrophobic Protein Ectodomain Presented With a Novel Lipid-Based Formulation Is Highly Immunogenic and Safe in Adults: A First-in-Humans Study. J. Infect. Dis. 2018, 218, 378–387. [Google Scholar] [CrossRef]
- Acosta, P.L.; Caballero, M.T.; Polack, F.P. Brief History and Characterization of Enhanced Respiratory Syncytial Virus Disease. Clin. Vaccine Immunol. 2015, 23, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Venkatesan, P. First RSV vaccine approvals. Lancet Microbe 2023, 4, e577. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves mRNA-based RSV vaccine. Nat. Rev. Drug Discov. 2024, 23, 487. [Google Scholar] [CrossRef] [PubMed]
- Karron, R.A.; Atwell, J.E.; McFarland, E.J.; Cunningham, C.K.; Muresan, P.; Perlowski, C.; Libous, J.; Spector, S.A.; Yogev, R.; Aziz, M.; et al. Live-attenuated Vaccines Prevent Respiratory Syncytial Virus-associated Illness in Young Children. Am. J. Respir. Crit. Care Med. 2021, 203, 594–603. [Google Scholar] [CrossRef]
- Yang, H.; Ye, C. Reverse genetics approaches for live-attenuated vaccine development of infectious bursal disease virus. Curr. Opin. Virol. 2020, 44, 139–144. [Google Scholar] [CrossRef]
- Karron, R.A.; Luongo, C.; Mateo, J.S.; Wanionek, K.; Collins, P.L.; Buchholz, U.J. Safety and Immunogenicity of the Respiratory Syncytial Virus Vaccine RSV/DeltaNS2/Delta1313/I1314L in RSV-Seronegative Children. J. Infect. Dis. 2020, 222, 82–91. [Google Scholar] [CrossRef]
- Cunningham, C.K.; Karron, R.A.; Muresan, P.; Kelly, M.S.; McFarland, E.J.; Perlowski, C.; Libous, J.; Oliva, J.; Jean-Philippe, P.; Moye, J.; et al. Evaluation of Recombinant Live-Attenuated Respiratory Syncytial Virus (RSV) Vaccines RSV/DeltaNS2/Delta1313/I1314L and RSV/276 in RSV-Seronegative Children. J. Infect. Dis. 2022, 226, 2069–2078. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, M.; Bian, Y.; Zheng, X.; Tong, R.; Sun, X. Advanced subunit vaccine delivery technologies: From vaccine cascade obstacles to design strategies. Acta Pharm. Sin. B 2023, 13, 3321–3338. [Google Scholar] [CrossRef]
- Syed, Y.Y. Respiratory Syncytial Virus Prefusion F Subunit Vaccine: First Approval of a Maternal Vaccine to Protect Infants. Paediatr. Drugs 2023, 25, 729–734. [Google Scholar] [CrossRef]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simoes, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Perez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Huang, J.; Li, X.; Xie, J.; Zhu, N. Nasal immunization with RSV F and G protein fragments conjugated to an M cell-targeting ligand induces an enhanced immune response and protection against RSV infection. Antivir. Res. 2018, 159, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Scaggs Huang, F.; Bernstein, D.I.; Slobod, K.S.; Portner, A.; Takimoto, T.; Russell, C.J.; Meagher, M.; Jones, B.G.; Sealy, R.E.; Coleclough, C.; et al. Safety and immunogenicity of an intranasal sendai virus-based vaccine for human parainfluenza virus type I and respiratory syncytial virus (SeVRSV) in adults. Hum. Vaccin. Immunother. 2021, 17, 554–559. [Google Scholar] [CrossRef]
- Brakel, K.A.; Binjawadagi, B.; French-Kim, K.; Watts, M.; Harder, O.; Ma, Y.; Li, J.; Niewiesk, S. Coexpression of respiratory syncytial virus (RSV) fusion (F) protein and attachment glycoprotein (G) in a vesicular stomatitis virus (VSV) vector system provides synergistic effects against RSV infection in a cotton rat model. Vaccine 2021, 39, 6817–6828. [Google Scholar] [CrossRef]
- Spearman, P.; Jin, H.; Knopp, K.; Xiao, P.; Gingerich, M.C.; Kidd, J.; Singh, K.; Tellier, M.; Radziewicz, H.; Wu, S.; et al. Intranasal parainfluenza virus type 5 (PIV5)-vectored RSV vaccine is safe and immunogenic in healthy adults in a phase 1 clinical study. Sci. Adv. 2023, 9, eadj7611. [Google Scholar] [CrossRef]
- Falsey, A.R.; Williams, K.; Gymnopoulou, E.; Bart, S.; Ervin, J.; Bastian, A.R.; Menten, J.; De Paepe, E.; Vandenberghe, S.; Chan, E.K.H.; et al. Efficacy and Safety of an Ad26.RSV.preF-RSV preF Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 609–620. [Google Scholar] [CrossRef]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral vectored vaccines: Design, development, preventive and therapeutic applications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef]
- Jordan, E.; Jenkins, V.; Silbernagl, G.; Chavez, M.P.V.; Schmidt, D.; Schnorfeil, F.; Schultz, S.; Chen, L.; Salgado, F.; Jacquet, J.M.; et al. A multivalent RSV vaccine based on the modified vaccinia Ankara vector shows moderate protection against disease caused by RSV in older adults in a phase 3 clinical study. Vaccine 2024, 42, 126427. [Google Scholar] [CrossRef]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in mRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Ramet, M.; et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- Aliprantis, A.O.; Shaw, C.A.; Griffin, P.; Farinola, N.; Railkar, R.A.; Cao, X.; Liu, W.; Sachs, J.R.; Swenson, C.J.; Lee, H.; et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum. Vaccin. Immunother. 2021, 17, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Espeseth, A.S.; Cejas, P.J.; Citron, M.P.; Wang, D.; DiStefano, D.J.; Callahan, C.; Donnell, G.O.; Galli, J.D.; Swoyer, R.; Touch, S.; et al. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 2020, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yin, Y.; Zhao, X.; Wang, C.; Zhu, X.; Zhan, L.; Chen, L.; Wang, S.; Lin, X.; Zhang, J.; et al. A truncated pre-F protein mRNA vaccine elicits an enhanced immune response and protection against respiratory syncytial virus. Nat. Commun. 2025, 16, 1386. [Google Scholar] [CrossRef]
- Grego, E.A.; Siddoway, A.C.; Uz, M.; Liu, L.; Christiansen, J.C.; Ross, K.A.; Kelly, S.M.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Polymeric Nanoparticle-Based Vaccine Adjuvants and Delivery Vehicles. Curr. Top. Microbiol. Immunol. 2021, 433, 29–76. [Google Scholar] [CrossRef]
- Liao, X.; Liu, Y.; Zheng, J.; Zhao, X.; Cui, L.; Hu, S.; Xia, T.; Si, S. Diverse Pathways of Engineered Nanoparticle-Induced NLRP3 Inflammasome Activation. Nanomaterials 2022, 12, 3908. [Google Scholar] [CrossRef]
- Lunov, O.; Syrovets, T.; Loos, C.; Nienhaus, G.U.; Mailander, V.; Landfester, K.; Rouis, M.; Simmet, T. Amino-functionalized polystyrene nanoparticles activate the NLRP3 inflammasome in human macrophages. ACS Nano 2011, 5, 9648–9657. [Google Scholar] [CrossRef]
- Kim, B.G.; Lee, P.H.; Lee, S.H.; Park, M.K.; Jang, A.S. Effect of TiO2 Nanoparticles on Inflammasome-Mediated Airway Inflammation and Responsiveness. Allergy Asthma Immunol. Res. 2017, 9, 257–264. [Google Scholar] [CrossRef]
- Sharma, S.; Mukkur, T.K.; Benson, H.A.; Chen, Y. Enhanced immune response against pertussis toxoid by IgA-loaded chitosan-dextran sulfate nanoparticles. J. Pharm. Sci. 2012, 101, 233–244. [Google Scholar] [CrossRef]
- Swanson, K.A.; Rainho-Tomko, J.N.; Williams, Z.P.; Lanza, L.; Peredelchuk, M.; Kishko, M.; Pavot, V.; Alamares-Sapuay, J.; Adhikarla, H.; Gupta, S.; et al. A respiratory syncytial virus (RSV) F protein nanoparticle vaccine focuses antibody responses to a conserved neutralization domain. Sci. Immunol. 2020, 5, aba6466. [Google Scholar] [CrossRef]
- Jares Baglivo, S.; Polack, F.P. The long road to protect infants against severe RSV lower respiratory tract illness. F1000Research 2019, 8, F1000. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.C.; Wittes, J. Correlates, surrogates, and vaccines. J. Infect. Dis. 2007, 196, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Benkeser, D.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Janes, H.E.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Comparing antibody assays as correlates of protection against COVID-19 in the COVE mRNA-1273 vaccine efficacy trial. Sci. Transl. Med. 2023, 15, eade9078. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Li, J.; Pan, H.; Wu, Y.; Zhu, F. Immunological surrogate endpoints of COVID-2019 vaccines: The evidence we have versus the evidence we need. Signal Transduct. Target. Ther. 2021, 6, 48. [Google Scholar] [CrossRef]
- Coudeville, L.; Bailleux, F.; Riche, B.; Megas, F.; Andre, P.; Ecochard, R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: Development and application of a bayesian random-effects model. BMC Med. Res. Methodol. 2010, 10, 18. [Google Scholar] [CrossRef]
- Saeland, E.; van der Fits, L.; Bolder, R.; Heemskerk-van der Meer, M.; Drijver, J.; van Polanen, Y.; Vaneman, C.; Tettero, L.; Serroyen, J.; Schuitemaker, H.; et al. Immunogenicity and protective efficacy of adenoviral and subunit RSV vaccines based on stabilized prefusion F protein in pre-clinical models. Vaccine 2022, 40, 934–944. [Google Scholar] [CrossRef]
- Stensballe, L.G.; Ravn, H.; Kristensen, K.; Agerskov, K.; Meakins, T.; Aaby, P.; Simoes, E.A. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J. Allergy Clin. Immunol. 2009, 123, 398–403. [Google Scholar] [CrossRef]
- Eick, A.; Karron, R.; Shaw, J.; Thumar, B.; Reid, R.; Santosham, M.; O’Brien, K.L. The role of neutralizing antibodies in protection of American Indian infants against respiratory syncytial virus disease. Pediatr. Infect. Dis. J. 2008, 27, 207–212. [Google Scholar] [CrossRef]
- Lamprecht, C.L.; Krause, H.E.; Mufson, M.A. Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J. Infect. Dis. 1976, 134, 211–217. [Google Scholar] [CrossRef]
- Glezen, W.P.; Paredes, A.; Allison, J.E.; Taber, L.H.; Frank, A.L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 1981, 98, 708–715. [Google Scholar] [CrossRef]
- Johnson, S.; Oliver, C.; Prince, G.A.; Hemming, V.G.; Pfarr, D.S.; Wang, S.C.; Dormitzer, M.; O’Grady, J.; Koenig, S.; Tamura, J.K.; et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997, 176, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Jozwik, A.; Habibi, M.S.; Paras, A.; Zhu, J.; Guvenel, A.; Dhariwal, J.; Almond, M.; Wong, E.H.C.; Sykes, A.; Maybeno, M.; et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 2015, 6, 10224. [Google Scholar] [CrossRef] [PubMed]
- Ruckwardt, T.J.; Morabito, K.M.; Graham, B.S. Immunological Lessons from Respiratory Syncytial Virus Vaccine Development. Immunity 2019, 51, 429–442. [Google Scholar] [CrossRef]
- Leroux-Roels, I.; Davis, M.G.; Steenackers, K.; Essink, B.; Vandermeulen, C.; Fogarty, C.; Andrews, C.P.; Kerwin, E.; David, M.P.; Fissette, L.; et al. Safety and Immunogenicity of a Respiratory Syncytial Virus Prefusion F (RSVPreF3) Candidate Vaccine in Older Adults: Phase 1/2 Randomized Clinical Trial. J. Infect. Dis. 2023, 227, 761–772. [Google Scholar] [CrossRef]
- McGuire, A.T.; Glenn, J.A.; Lippy, A.; Stamatatos, L. Diverse recombinant HIV-1 Envs fail to activate B cells expressing the germline B cell receptors of the broadly neutralizing anti-HIV-1 antibodies PG9 and 447-52D. J. Virol. 2014, 88, 2645–2657. [Google Scholar] [CrossRef]
- McGuire, A.T.; Hoot, S.; Dreyer, A.M.; Lippy, A.; Stuart, A.; Cohen, K.W.; Jardine, J.; Menis, S.; Scheid, J.F.; West, A.P.; et al. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J. Exp. Med. 2013, 210, 655–663. [Google Scholar] [CrossRef]
- Wheatley, A.K.; Whittle, J.R.; Lingwood, D.; Kanekiyo, M.; Yassine, H.M.; Ma, S.S.; Narpala, S.R.; Prabhakaran, M.S.; Matus-Nicodemos, R.A.; Bailer, R.T.; et al. H5N1 Vaccine-Elicited Memory B Cells Are Genetically Constrained by the IGHV Locus in the Recognition of a Neutralizing Epitope in the Hemagglutinin Stem. J. Immunol. 2015, 195, 602–610. [Google Scholar] [CrossRef]
- Wrammert, J.; Koutsonanos, D.; Li, G.M.; Edupuganti, S.; Sui, J.; Morrissey, M.; McCausland, M.; Skountzou, I.; Hornig, M.; Lipkin, W.I.; et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011, 208, 181–193. [Google Scholar] [CrossRef]
- Jorquera, P.A.; Tripp, R.A. Quantification of RSV Infectious Particles by Plaque Assay and Immunostaining Assay. Methods Mol. Biol. 2016, 1442, 33–40. [Google Scholar] [CrossRef]
- Fuentes, S.; Arenas, D.; Moore, M.M.; Golding, H.; Khurana, S. Development of bioluminescence imaging of respiratory syncytial virus (RSV) in virus-infected live mice and its use for evaluation of therapeutics and vaccines. Vaccine 2017, 35, 694–702. [Google Scholar] [CrossRef]
- Shambaugh, C.; Azshirvani, S.; Yu, L.; Pache, J.; Lambert, S.L.; Zuo, F.; Esser, M.T. Development of a High-Throughput Respiratory Syncytial Virus Fluorescent Focus-Based Microneutralization Assay. Clin. Vaccine Immunol. 2017, 24, e00225-17. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hosman, T.; Bastian, A.R.; Vandenberghe, S.; Chan, E.K.H.; Douoguih, M.; Heijnen, E.; Comeaux, C.A.; Callendret, B.; CYPRESS Investigators. Long-term efficacy and immunogenicity of Ad26.RSV.preF-RSV preF protein vaccine (CYPRESS): A randomised, double-blind, placebo-controlled, phase 2b study. Lancet Infect. Dis. 2024, 24, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Saeland, E.; van der Fits, L.; Bolder, R.; Heemskerk-van der Meer, M.; Drijver, J.; van Polanen, Y.; Vaneman, C.; Tettero, L.; Cox, F.; Serroyen, J.; et al. Combination Ad26.RSV.preF/preF protein vaccine induces superior protective immunity compared with individual vaccine components in preclinical models. NPJ Vaccines 2023, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Bouzya, B.; Rouxel, R.N.; Sacconnay, L.; Mascolo, R.; Nols, L.; Quique, S.; Francois, L.; Atas, A.; Warter, L.; Dezutter, N.; et al. Immunogenicity of an AS01-adjuvanted respiratory syncytial virus prefusion F (RSVPreF3) vaccine in animal models. NPJ Vaccines 2023, 8, 143. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; Wang, Y.; Lin, Z.; Li, W.; Cao, J.; Mei, X.; Wei, R.; Yang, J.; Zhai, X.; et al. Diverse BCR usage and T cell activation induced by different COVID-19 sequential vaccinations. mBio 2024, 15, e0142924. [Google Scholar] [CrossRef]
- Funakoshi, Y.; Yakushijin, K.; Ohji, G.; Matsutani, T.; Doi, K.; Sakai, H.; Sasaki, T.; Kusakabe, T.; Matsumoto, S.; Saito, Y.; et al. Analysis of B-cell receptor repertoire to evaluate immunogenicity of monovalent Omicron XBB.1.5 mRNA vaccines. EJHaem 2024, 5, 661–668. [Google Scholar] [CrossRef]
- Schneikart, G.; Tavarini, S.; Sammicheli, C.; Torricelli, G.; Guidotti, S.; Andreano, E.; Buricchi, F.; D’Oro, U.; Finco, O.; Bardelli, M. The respiratory syncytial virus fusion protein-specific B cell receptor repertoire reshaped by post-fusion subunit vaccination. Vaccine 2020, 38, 7916–7927. [Google Scholar] [CrossRef]
- Funakoshi, Y.; Yakushijin, K.; Ohji, G.; Matsutani, T.; Hojo, W.; Sakai, H.; Matsumoto, S.; Watanabe, M.; Kitao, A.; Saito, Y.; et al. Response to mRNA SARS-CoV-2 vaccination evaluated by B-cell receptor repertoire after tixagevimab/cilgavimab administration. Br. J. Haematol. 2023, 202, 504–516. [Google Scholar] [CrossRef]
| Methods | Evaluation Parameters or Indication | Features | Applications |
|---|---|---|---|
| Neutralizing antibody evaluation | |||
| MCPENT | Neutralization based on CPE | Based on CPE, needs professional personnel | Preclinical and clinical |
| PRNT/FRNT | Neutralization based on plaque or foci reduction | Involves staining or immunostaining | Preclinical and clinical |
| CFFRNT | Neutralizing antibodies based on fluorescence-expressing virus | High-throughput | Preclinical and clinical |
| T cell response evaluation | |||
| ELISpot | Cytokine-positive cells | Easily performed | Preclinical and clinical |
| ICS | Cytokine-positive cells and the phenotypes, memory phase of T cells | Needs professional personnel, phenotype analysis | Preclinical |
| AIM | Activation-induced markers | Needs professional personnel, phenotype analysis | Preclinical |
| Tetramer | Epitope-specific T cells | HLA-restricted | May not be applicable |
| B cell evaluation | |||
| BCR | Germline BCR (VH, VL germlines), BCR repertoire | Involves single-cell BCR technology | Preclinical |
| QASAS | CDR3 data | Involves single-cell BCR technology | Preclinical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Cao, H.; Li, G.; Zhou, K.; Fu, Z.; Zhong, J.; Wang, Z.; Yang, X. Progress on Respiratory Syncytial Virus Vaccine Development and Evaluation Methods. Vaccines 2025, 13, 304. https://doi.org/10.3390/vaccines13030304
Deng L, Cao H, Li G, Zhou K, Fu Z, Zhong J, Wang Z, Yang X. Progress on Respiratory Syncytial Virus Vaccine Development and Evaluation Methods. Vaccines. 2025; 13(3):304. https://doi.org/10.3390/vaccines13030304
Chicago/Turabian StyleDeng, Lie, Hongjie Cao, Guichang Li, Kaiwen Zhou, Zihan Fu, Jiaying Zhong, Zhongfang Wang, and Xiaoyun Yang. 2025. "Progress on Respiratory Syncytial Virus Vaccine Development and Evaluation Methods" Vaccines 13, no. 3: 304. https://doi.org/10.3390/vaccines13030304
APA StyleDeng, L., Cao, H., Li, G., Zhou, K., Fu, Z., Zhong, J., Wang, Z., & Yang, X. (2025). Progress on Respiratory Syncytial Virus Vaccine Development and Evaluation Methods. Vaccines, 13(3), 304. https://doi.org/10.3390/vaccines13030304






