Intestinal Microbiota and Vaccinations: A Systematic Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Exclusion Criteria
2.3. Assessment of the Quality of Studies
2.4. Management of Missing Data
2.5. Assessment of Bias Reporting
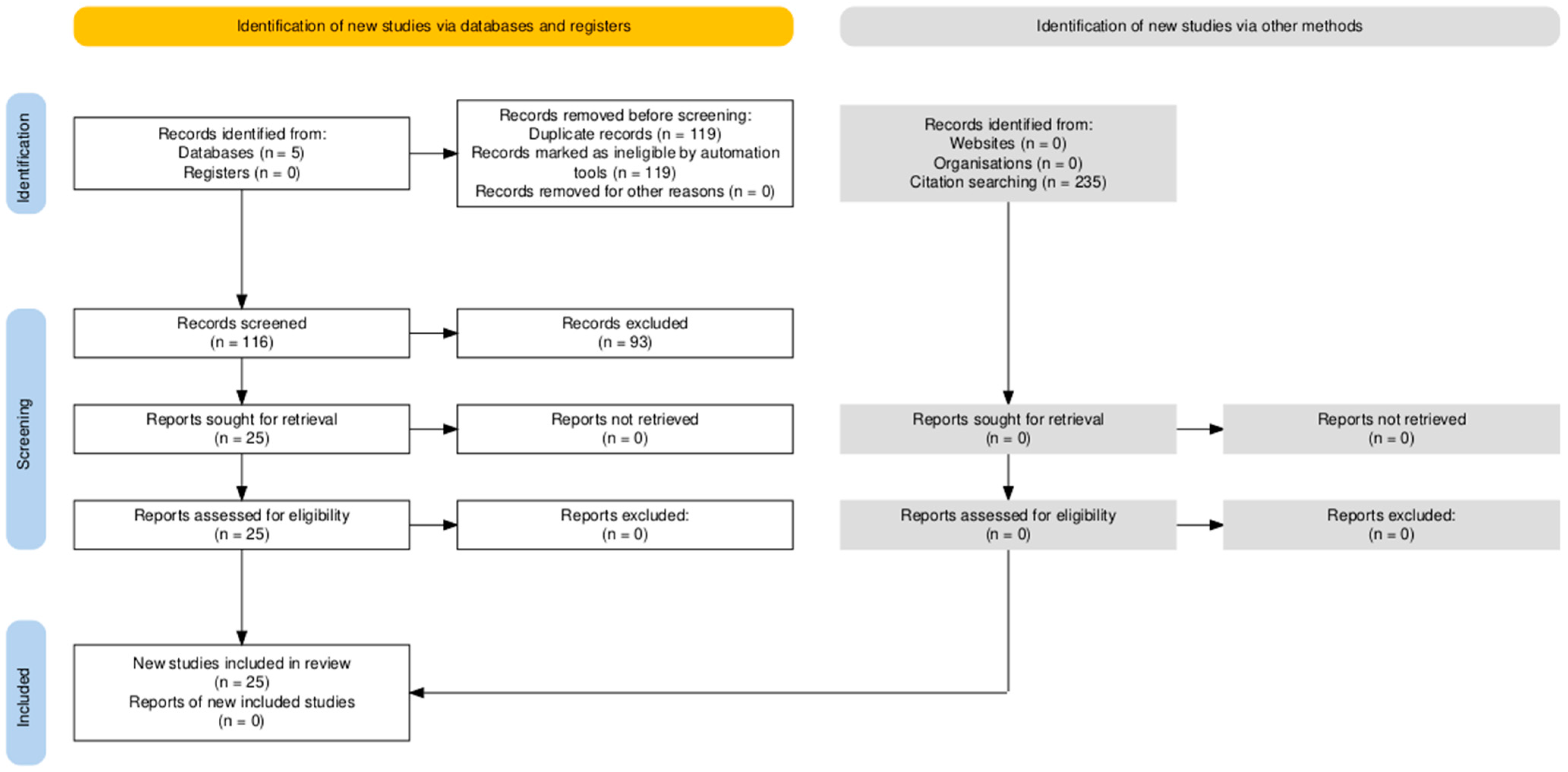
3. Results
3.1. Quality of the Study
3.2. Publication Bias
3.3. Clinical Trial Studies with COVID-19 Vaccines
3.4. Clinical Studies on Vibrio cholerae
3.5. Clinical Studies on Salmonella
3.6. Clinical Studies on Rotavirus
4. Discussion
4.1. COVID-19
4.2. Vibrio cholerae
4.3. Salmonella
4.4. Rotavirus
4.5. Limit of Our Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maurice, J.; Davey, S.; Banque Mondiale; Organisation Mondiale de la Santé. Unicef State of the World’s Vaccines and Immunization, 3rd ed.; World Health Organization: Geneve, Switzerland, 2009; ISBN 9789241563864. [Google Scholar]
- Zimmermann, P.; Ritz, N.; Perrett, K.P.; Messina, N.L.; van der Klis, F.R.M.; Curtis, N. Correlation of Vaccine Responses. Front. Immunol. 2021, 12, 646677. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Patriarca, P.A.; Wright, P.F.; John, T.J. Factors Affecting the Immunogenicity of Oral Poliovirus Vaccine in Developing Countries: Review. Rev. Infect. Dis. 1991, 13, 926–939. [Google Scholar] [CrossRef]
- Kirkpatrick, B.D.; Carmolli, M.P.; Taniuchi, M.; Haque, R.; Mychaleckyj, J.C.; Petri, W.A.; Walsh, M.C.; Ma, J.Z.; Diehl, S.A.; Alam, M.; et al. The “Performance of Rotavirus and Oral Polio Vaccines in Developing Countries” (PROVIDE) Study: Description of Methods of an Interventional Study Designed to Explore Complex Biologic Problems. Am. J. Trop. Med. Hyg. 2015, 92, 744–751. [Google Scholar] [CrossRef]
- Levine, M.M. Immunogenicity and Efficacy of Oral Vaccines in Developing Countries: Lessons from a Live Cholera Vaccine. BMC Biol. 2010, 8, 129. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The Influence of the Intestinal Microbiome on Vaccine Responses. Vaccine 2018, 36, 4433–4439. [Google Scholar] [CrossRef]
- Jordan, A.; Carding, S.R.; Hall, L.J. The Early-Life Gut Microbiome and Vaccine Efficacy. Lancet Microbe 2022, 3, e787–e794. [Google Scholar] [CrossRef]
- Lynn, D.J.; Benson, S.C.; Lynn, M.A.; Pulendran, B. Modulation of Immune Responses to Vaccination by the Microbiota: Implications and Potential Mechanisms. Nat. Rev. Immunol. 2021, 22, 33–46. [Google Scholar] [CrossRef]
- Fulde, M.; Hornef, M.W. Maturation of the Enteric Mucosal Innate Immune System during the Postnatal Period. Immunol. Rev. 2014, 260, 21–34. [Google Scholar] [CrossRef]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of Pathogens and Pathobionts by the Gut Microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef]
- Ijssennagger, N.; Belzer, C.; Hooiveld, G.J.; Dekker, J.; van Mil, S.W.C.; Müller, M.; Kleerebezem, M.; van der Meer, R. Gut Microbiota Facilitates Dietary Heme-Induced Epithelial Hyperproliferation by Opening the Mucus Barrier in Colon. Proc. Natl. Acad. Sci. USA 2015, 112, 10038–10043. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, C.; Bergentall, M.; Greiner, T.U.; Schaffner, F.; Östergren-Lundén, G.; Petersen, L.C.; Ruf, W.; Bäckhed, F. Tissue Factor and PAR1 Promote Microbiota-Induced Intestinal Vascular Remodelling. Nature 2012, 483, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial Endocrinology: The Interplay Between the Microbiota and the Endocrine System. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I.; et al. Antibiotics in Early Life Alter the Murine Colonic Microbiome and Adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-Chain Fatty Acids in Control of Body Weight and Insulin Sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Ramírez-Puebla, S.T.; Servín-Garcidueñas, L.E.; Jiménez-Marín, B.; Bolaños, L.M.; Rosenblueth, M.; Martínez, J.; Rogel, M.A.; Ormeño-Orrillo, E.; Martínez-Romero, E. Gut and Root Microbiota Commonalities. Appl. Environ. Microbiol. 2012, 79, 2–9. [Google Scholar] [CrossRef]
- Devlin, A.S.; Fischbach, M.A. A Biosynthetic Pathway for a Prominent Class of Microbiota-Derived Bile Acids. Nat. Chem. Biol. 2015, 11, 685–690. [Google Scholar] [CrossRef]
- Haiser, H.J.; Gootenberg, D.B.; Chatman, K.; Sirasani, G.; Balskus, E.P.; Turnbaugh, P.J. Predicting and Manipulating Cardiac Drug Inactivation by the Human Gut Bacterium Eggerthella Lenta. Science 2013, 341, 295–298. [Google Scholar] [CrossRef]
- Eckburg, P.B. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Reyes, A.; Haynes, M.; Hanson, N.; Angly, F.E.; Heath, A.C.; Rohwer, F.; Gordon, J.I. Viruses in the Faecal Microbiota of Monozygotic Twins and Their Mothers. Nature 2010, 466, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut Microbiota Composition Correlates with Diet and Health in the Elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Yallapragada, S.G.; Nash, C.B.; Robinson, D.T. Early-Life Exposure to Antibiotics, Alterations in the Intestinal Microbiome, and Risk of Metabolic Disease in Children and Adults. Pediatr. Ann. 2015, 44, e265–e269. [Google Scholar] [CrossRef]
- Johnson, C.C.; Ownby, D.R.; Alford, S.H.; Havstad, S.L.; Williams, L.K.; Zoratti, E.M.; Peterson, E.L.; Joseph, C.L.M. Antibiotic Exposure in Early Infancy and Risk for Childhood Atopy. J. Allergy Clin. Immunol. 2005, 115, 1218–1224. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-Level Analysis of Gut Microbiome Variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome During the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Wang, S.; Charbonnier, L.-M.; Noval Rivas, M.; Georgiev, P.; Li, N.; Gerber, G.; Bry, L.; Chatila, T.A. MyD88 Adaptor-Dependent Microbial Sensing by Regulatory T Cells Promotes Mucosal Tolerance and Enforces Commensalism. Immunity 2015, 43, 289–303. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2013, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Maurice, C.; Haiser, H.; Turnbaugh, P. Xenobiotics Shape the Physiology and Gene Expression of the Active Human Gut Microbiome. Cell 2013, 152, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.; Ahmed, A.M.S.; Subramanian, S.; Griffin, N.W.; Drewry, L.L.; Petri, W.A.; Haque, R.; Ahmed, T.; Gordon, J.I. Members of the Human Gut Microbiota Involved in Recovery from Vibrio Cholerae Infection. Nature 2014, 515, 423–426. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Fujimura, K.E.; Demoor, T.; Rauch, M.; Faruqi, A.A.; Jang, S.; Johnson, C.C.; Boushey, H.A.; Zoratti, E.; Ownby, D.; Lukacs, N.W.; et al. House Dust Exposure Mediates Gut Microbiome Lactobacillus Enrichment and Airway Immune Defense Against Allergens and Virus Infection. Proc. Natl. Acad. Sci. USA 2013, 111, 805–810. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions the COCHRANE COLLABORATION®; The Cochrane Collaboration and John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2008; pp. 1–649. ISBN 978-0-470-51845-8. [Google Scholar]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.; John, M.; Gavaghan, D.J.; McQuay, H.J. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Daddi, L.; Dorsett, Y.; Geng, T.; Bokoliya, S.; Yuan, H.; Wang, P.; Xu, W.; Zhou, Y. Baseline Gut Microbiome Signatures Correlate with Immunogenicity of SARS-CoV-2 MRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 11703. [Google Scholar] [CrossRef]
- Singer, J.; Tunbridge, M.; Perkins, G.B.; Salehi, T.; Ying, T.; Wu, H.; Coates, P.T.; Chadban, S.J. Rapamycin and Inulin for Third-Dose Vaccine Response Stimulation (RIVASTIM): Inulin—Study Protocol for a Pilot, Multicentre, Randomised, Double-Blinded, Controlled Trial of Dietary Inulin to Improve SARS-CoV-2 Vaccine Response in Kidney Transplant Recipients. BMJ Open 2022, 12, e062747. [Google Scholar]
- Cao, X.; Geng, Q.; Fan, D.; Wang, Q.; Wang, X.; Zhang, M.; Zhao, L.; Jiao, Y.; Deng, T.; Liu, H.; et al. M6A Methylation: A Process Reshaping the Tumour Immune Microenvironment and Regulating Immune Evasion. Mol. Cancer 2023, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT05195151 (accessed on 6 August 2024).
- Clinical Trails Gov. Available online: https://clinicaltrials.gov/study/NCT05623007 (accessed on 6 August 2024).
- Ng, S.C.; Peng, Y.; Zhang, L.; Mok, C.K.; Zhao, S.; Li, A.; Ching, J.Y.; Liu, Y.; Yan, S.; Chan, D.L.S.; et al. Gut Microbiota Composition Is Associated with SARS-CoV-2 Vaccine Immunogenicity and Adverse Events. Gut 2022, 71, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Mullish, B.H.; Danckert, N.P.; Liu, Z.; Olbei, M.L.; Saifuddin, A.; Torkizadeh, M.; Ibraheim, H.; Blanco, J.M.; Roberts, L.A.; et al. The Gut Microbiota and Metabolome Are Associated with Diminished COVID-19 Vaccine-Induced Antibody Responses in Immunosuppressed Inflammatory Bowel Disease Patients. eBioMedicine 2023, 88, 104430. [Google Scholar] [CrossRef] [PubMed]
- Yuki, Y.; Nojima, M.; Hosono, O.; Tanaka, H.; Kimura, Y.; Satoh, T.; Imoto, S.; Uematsu, S.; Kurokawa, S.; Kashima, K.; et al. Oral MucoRice-CTB Vaccine for Safety and Microbiota-Dependent Immunogenicity in Humans: A Phase 1 Randomised Trial. Lancet Microbe 2021, 2, e429–e440. [Google Scholar] [CrossRef]
- ICTRP Search Portal. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=KCT0002589 (accessed on 6 August 2024).
- Clinical Traials Gov. Available online: https://clinicaltrials.gov/study/NCT05657782 (accessed on 6 August 2024).
- Zhang, Y.; Brady, A.L.; Jones, C.R.; Song, Y.; Darton, T.C.; Blohmke, C.J.; Pollard, A.J.; Magder, L.S.; Fasano, A.; Sztein, M.B.; et al. Compositional and Functional Differences in the Human Gut Microbiome Correlate with Clinical Outcome Following Infection with Wild-Type Salmonella Enterica Serovar Typhi. mBio 2018, 9, e00686-18. [Google Scholar] [CrossRef]
- Eloe-Fadrosh, E.A.; McArthur, M.A.; Seekatz, A.M.; Drabek, E.F.; Rasko, D.A.; Sztein, M.B.; Fraser, C.M. Impact of Oral Typhoid Vaccination on the Human Gut Microbiota and Correlations with S. Typhi-Specific Immunological Responses. PLoS ONE 2013, 8, e62026. [Google Scholar] [CrossRef]
- Parker, E.P.K.; Bronowski, C.; Sindhu, K.N.C.; Babji, S.; Benny, B.; Carmona-Vicente, N.; Chasweka, N.; Chinyama, E.; Cunliffe, N.A.; Dube, Q.; et al. Impact of Maternal Antibodies and Microbiota Development on the Immunogenicity of Oral Rotavirus Vaccine in African, Indian, and European Infants. Nat. Commun. 2021, 12, 7288. [Google Scholar] [CrossRef]
- Robertson, R.C.; Church, J.; Edens, T.J.; Mutasa, K.; Geum, H.M.; Baharmand, I.; Gill, S.K.; Ntozini, R.; Chasekwa, B.; Carr, L.; et al. The Fecal Microbiome and Rotavirus Vaccine Immunogenicity in Rural Zimbabwean Infants. Vaccine 2021, 39, 5391–5400. [Google Scholar] [CrossRef]
- Fix, J.; Chandrashekhar, K.; Perez, J.; Bucardo, F.; Hudgens, M.G.; Yuan, L.; Twitchell, E.; Azcarate-Peril, M.A.; Vilchez, S.; Becker-Dreps, S. Association Between Gut Microbiome Composition and Rotavirus Vaccine Response Among Nicaraguan Infants. Am. J. Trop. Med. Hyg. 2019, 102, 213–219. [Google Scholar] [CrossRef]
- Harris, V.; Ali, A.; Fuentes, S.; Korpela, K.; Kazi, M.; Tate, J.; Parashar, U.; Wiersinga, W.J.; Giaquinto, C.; de Weerth, C.; et al. Rotavirus Vaccine Response Correlates with the Infant Gut Microbiota Composition in Pakistan. Gut Microbes 2017, 9, 93–101. [Google Scholar] [CrossRef]
- Sindhu, K.N.C.; Cunliffe, N.; Peak, M.; Turner, M.; Darby, A.; Grassly, N.; Gordon, M.; Dube, Q.; Babji, S.; Praharaj, I.; et al. Impact of Maternal Antibodies and Infant Gut Microbiota on the Immunogenicity of Rotavirus Vaccines in African, Indian and European Infants: Protocol for a Prospective Cohort Study. BMJ Open 2017, 7, e016577. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.C.; Armah, G.; Fuentes, S.; Korpela, K.E.; Parashar, U.; Victor, J.C.; Tate, J.; de Weerth, C.; Giaquinto, C.; Wiersinga, W.J.; et al. Significant Correlation between the Infant Gut Microbiome and Rotavirus Vaccine Response in Rural Ghana. J. Infect. Dis. 2016, 215, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials Gov. Available online: https://clinicaltrials.gov/study/NCT02538211 (accessed on 6 August 2024).
- ICTRP Search Portal. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12620000026921 (accessed on 6 August 2024).
- Parker, E.P.K.; Praharaj, I.; Zekavati, A.; Lazarus, R.P.; Giri, S.; Operario, D.J.; Liu, J.; Houpt, E.; Iturriza-Gómara, M.; Kampmann, B.; et al. Influence of the Intestinal Microbiota on the Immunogenicity of Oral Rotavirus Vaccine given to Infants in South India. Vaccine 2018, 36, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, R.P.; John, J.; Shanmugasundaram, E.; Rajan, A.K.; Thiagarajan, S.; Giri, S.; Babji, S.; Sarkar, R.; Kaliappan, P.S.; Venugopal, S.; et al. The Effect of Probiotics and Zinc Supplementation on the Immune Response to Oral Rotavirus Vaccine: A Randomized, Factorial Design, Placebo-Controlled Study among Indian Infants. Vaccine 2018, 36, 273–279. [Google Scholar] [CrossRef]
- Harris, V.C.; Haak, B.W.; Handley, S.A.; Jiang, B.; Velasquez, D.E.; Hykes, B.L.; Droit, L.; Berbers, G.A.; Kemper, E.M.; van Leeuwen, E.M.; et al. Effect of Antibiotic-Mediated Microbiome Modulation on Rotavirus Vaccine Immunogenicity: A Human, Randomized-Control Proof-of-Concept Trial. Cell Host Microbe 2018, 24, 197–207.e4. [Google Scholar] [CrossRef]
- Liu, Q.; Su, Q.; Zhang, F.; Tun, H.M.; Mak, J.W.Y.; Lui, G.C.-Y.; Ng, S.S.S.; Ching, J.Y.L.; Li, A.; Lu, W.; et al. Multi-Kingdom Gut Microbiota Analyses Define COVID-19 Severity and Post-Acute COVID-19 Syndrome. Nat. Commun. 2022, 13, 6806. [Google Scholar] [CrossRef]
- Mazzarelli, A.; Giancola, M.L.; Fontana, A.; Piselli, P.; Binda, E.; Trivieri, N.; Mencarelli, G.; Marchioni, L.; Vulcano, A.; De Giuli, C.; et al. Gut Microbiota Composition in COVID-19 Hospitalized Patients with Mild or Severe Symptoms. Front. Microbiol. 2022, 13, 1049215. [Google Scholar] [CrossRef]
- Jiao, J.; Shen, Y.; Wang, P.; Zuo, K.; Yang, X.; Chen, M.; Dong, Y.; Li, J. Characterization of the Intestinal Microbiome in Healthy Adults over SARS-CoV-2 Vaccination. Front. Biosci. (Landmark Ed.) 2022, 27, 280. [Google Scholar] [CrossRef]
- Rupawate, P.S.; Roylawar, P.; Khandagale, K.; Gawande, S.J.; Ade, A.B.; Jaiswal, D.K.; Borgave, S. Role of Gut Symbionts of Insect Pests: A Novel Target for Insect-Pest Control. Front. Microbiol. 2023, 14, 1146390. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, J.; Zhang, Z.; He, H.; Wang, C. A Study on Symbiotic Systems of Cicadas Provides New Insights into Distribution of Microbial Symbionts and Improves Fluorescence In Situ Hybridization Technique. Int. J. Mol. Sci. 2023, 24, 2434. [Google Scholar] [CrossRef]
- Xie, R.; Dong, C.; Wang, S.; Danso, B.; Dar, M.A.; Pandit, R.S.; Pawar, K.D.; Geng, A.; Zhu, D.; Li, X.; et al. Host-Specific Diversity of Culturable Bacteria in the Gut Systems of Fungus-Growing Termites and Their Potential Functions Towards Lignocellulose Bioconversion. Insects 2023, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Hinks, T.S.C.; Zhang, X.-W. MAIT Cell Activation and Functions. Front. Immunol. 2020, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, M.; Hinks, T. MAIT Cells and the Microbiome. Front. Immunol. 2023, 14, 1127588. [Google Scholar] [CrossRef]
- Gargano, F.; Guerrera, G.; Piras, E.; Serafini, B.; Di Paola, M.; Rizzetto, L.; Buscarinu, M.C.; Annibali, V.; Vuotto, C.; De Bardi, M.; et al. Proinflammatory Mucosal-Associated Invariant CD8+ T Cells React to Gut Flora Yeasts and Infiltrate Multiple Sclerosis Brain. Front. Immunol. 2022, 13, 890298. [Google Scholar] [CrossRef]
- Veneziani, I.; Alicata, C.; Moretta, L.; Maggi, E. The Latest Approach of Immunotherapy with Endosomal TLR Agonists Improving NK Cell Function: An Overview. Biomedicines 2022, 11, 64. [Google Scholar] [CrossRef]
- Shen, C.-F.; Yen, C.-L.; Fu, Y.-C.; Cheng, C.-M.; Shen, T.-C.; Chang, P.-D.; Cheng, K.-H.; Liu, C.-C.; Chang, Y.-T.; Chen, P.-L.; et al. Innate Immune Responses of Vaccinees Determine Early Neutralizing Antibody Production After ChAdOx1nCoV-19 Vaccination. Front. Immunol. 2022, 13, 807454. [Google Scholar] [CrossRef]
- Hägerbrand, K.; Westlund, J.; Yrlid, U.; Agace, W.; Johansson-Lindbom, B. MyD88 Signaling Regulates Steady-State Migration of Intestinal CD103+ Dendritic Cells Independently of TNF-α and the Gut Microbiota. J. Immunol. 2015, 195, 2888–2899. [Google Scholar] [CrossRef] [PubMed]
- Fryer, H.A.; Hartley, G.E.; Edwards, E.S.; O’Hehir, R.E.; van Zelm, M.C. Humoral Immunity and B-Cell Memory in Response to SARS-CoV-2 Infection and Vaccination. Biochem. Soc. Trans. 2022, 50, 1643–1658. [Google Scholar] [CrossRef]
- Detweiler, C.S. A New Way to Beat Intestinal Pathogens. Trends Microbiol. 2017, 25, 169–170. [Google Scholar] [CrossRef][Green Version]
- Stutz, M.R.; Dylla, N.P.; Pearson, S.D.; Lecompte-Osorio, P.; Nayak, R.; Khalid, M.; Adler, E.; Boissiere, J.; Lin, H.; Leiter, W.; et al. Immunomodulatory Fecal Metabolites Are Associated with Mortality in COVID-19 Patients with Respiratory Failure. Nat. Commun. 2022, 13, 6615. [Google Scholar] [CrossRef]
- Baradaran Ghavami, S.; Pourhamzeh, M.; Farmani, M.; Raftar, S.K.A.; Shahrokh, S.; Shpichka, A.; Asadzadeh Aghdaei, H.; Hakemi-Vala, M.; Hossein-khannazer, N.; Timashev, P.; et al. Cross-Talk between Immune System and Microbiota in COVID-19. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1281–1294. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Flores, T.D.; Pedraza-Brindis, E.J.; Cárdenas-Bedoya, J.; Ruíz-Carrillo, J.D.; Méndez-Clemente, A.S.; Martínez-Guzmán, M.A.; Iñiguez-Gutiérrez, L. Role of Micronutrients and Gut Microbiota-Derived Metabolites in COVID-19 Recovery. Int. J. Mol. Sci. 2022, 23, 12324. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Sanidad, K.Z.; Lucotti, S.; Lieber, C.M.; Cox, R.M.; Ananthanarayanan, A.; Basu, S.; Chen, J.; Shan, M.; Amir, M.; et al. Gut Microbiota-Derived Metabolites Confer Protection Against SARS-CoV-2 Infection. Gut Microbes 2022, 14, 2105609. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Lai, L.; Samaha, H.; Feng, Y.; Hu, M.; Hui, H.S.; Wali, B.; Ellis, M.; Davis-Gardner, M.E.; Huerta, C.; et al. Durability of Immune Responses to MRNA Booster Vaccination Against COVID-19. J. Clin. Invest. 2023, 133, e167955. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Yang, Y.L.; Tang, Y.X.; Qin, P.; Wang, G.; Xie, J.Y.; Chen, S.X.; Ding, C.; Huang, Y.W.; Zhu, S.J. Bile Acids Promote the Caveolae-Associated Entry of Swine Acute Diarrhea Syndrome Coronavirus in Porcine Intestinal Enteroids. PLoS Pathog. 2022, 18, e1010620. [Google Scholar] [CrossRef]
- Wu, R.; Abdullah, M.; Määttänen, P.; Pilar, A.; Scruten, E.; Johnson-Henry, K.C.; Napper, S.; O’Brien, C.; Jones, N.; Sherman, P.M. Protein Kinase c δ Signaling Is Required for Dietary Prebiotic-Induced Strengthening of Intestinal Epithelial Barrier Function. Sci. Rep. 2017, 7, 40820. [Google Scholar] [CrossRef]
- Harris, J.B.; LaRocque, R.C.; Qadri, F.; Ryan, E.T.; Calderwood, S.B. Cholera. Lancet 2012, 379, 2466–2476. [Google Scholar] [CrossRef]
- Ali, M.; Lopez, A.L.; Ae You, Y.; Eun Kim, Y.; Sah, B.; Maskery, B.; Clemens, J. The Global Burden of Cholera. Bull. World Health Organ. 2012, 90, 209–218. [Google Scholar] [CrossRef]
- Faruque, S.M.; Albert, M.J.; Mekalanos, J.J. Epidemiology, Genetics, and Ecology of ToxigenicVibrio Cholerae. Microbiol. Mol. Biol. Rev. 1998, 62, 1301–1314. [Google Scholar] [CrossRef]
- Ali, M.; Nelson, A.R.; Lopez, A.L.; Sack, D.A. Updated Global Burden of Cholera in Endemic Countries. PLoS Negl. Trop. Dis. 2015, 9, e0003832. [Google Scholar] [CrossRef]
- Khan, A.I.; Chowdhury, F.; Harris, J.B.; LaRocque, R.C.; Faruque, G.; Ryan, E.T.; Calderwood, S.B.; Qadri, F. Comparison of Clinical Features and Immunological Parameters of Patients with Dehydrating Diarrhoea Infected with Inaba or Ogawa Serotypes of Vibrio Cholerae O1. Scand. J. Infect. Dis. 2009, 42, 48–56. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, Y.; Li, J.; Gao, H.; Li, X.; Duan, R.; Cheng, Q.; Kan, B.; Liang, W. Serotype Conversion Gene RfbT Is Directly Regulated by Histone-like Nucleoid Structuring Protein (H-NS) in v. Cholerae O1. Front. Microbiol. 2023, 14, 1111895. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Hong, J.; Choi, Y.; Han, J.; Kim, S.; Jo, G.; Yoon, J.-Y.; Chae, H.; Yoon, H.; Lee, C.; et al. Generation and Characterization of Monoclonal Antibodies to the Ogawa Lipopolysaccharide of Vibrio cholerae O1 from Phage-Displayed Human Synthetic Fab Library. J. Microbiol. Biotechnol. 2020, 30, 1760–1768. [Google Scholar] [CrossRef]
- Hisatsune, K.; Kondo, S.; Isshiki, Y.; Iguchi, T.; Haishima, Y. Occurrence of 2-O-Methyl-N-(3-Deoxy-L-Glycero-Tetronyl)-D-Perosamine (4-Amino-4,6-Dideoxy-D-Manno-Pyranose) in Lipopolysaccharide from Ogawa but Not from Inaba O Forms of O1 Vibrio Cholerae. Biochem. Biophys. Res. Commun. 1993, 190, 302–307. [Google Scholar] [CrossRef]
- Cousins, S. Crisis-Driven Cholera Resurgence Switches Focus to Oral Vaccine. Bull. World Health Organ. 2018, 96, 446–447. [Google Scholar]
- Islam, M.T.; Clemens, J.D.; Qadri, F. Cholera Control and Prevention in Bangladesh: An Evaluation of the Situation and Solutions. J. Infect. Dis. 2018, 218, S171–S172. [Google Scholar] [CrossRef]
- Qadri, F.; Ali, M.; Lynch, J.; Chowdhury, F.; Khan, A.I.; Wierzba, T.F.; Excler, J.-L.; Saha, A.; Islam, M.T.; Begum, Y.A.; et al. Efficacy of a Single-Dose Regimen of Inactivated Whole-Cell Oral Cholera Vaccine: Results from 2 Years of Follow-up of a Randomised Trial. Lancet Infect. Dis. 2018, 18, 666–674. [Google Scholar] [CrossRef]
- Qadri, F.; Wierzba, T.F.; Ali, M.; Chowdhury, F.; Khan, A.I.; Saha, A.; Khan, I.A.; Asaduzzaman, M.; Akter, A.; Khan, A.; et al. Efficacy of a Single-Dose, Inactivated Oral Cholera Vaccine in Bangladesh. N. Engl. J. Med. 2016, 374, 1723–1732. [Google Scholar] [CrossRef]
- Qadri, F.; Ali, M.; Chowdhury, F.; Khan, A.I.; Saha, A.; Khan, I.A.; Begum, Y.A.; Bhuiyan, T.R.; Chowdhury, M.I.; Uddin, M.J.; et al. Feasibility and Effectiveness of Oral Cholera Vaccine in an Urban Endemic Setting in Bangladesh: A Cluster Randomised Open-Label Trial. Lancet 2015, 386, 1362–1371. [Google Scholar] [CrossRef]
- Sur, D.; Kanungo, S.; Sah, B.; Manna, B.; Ali, M.; Paisley, A.M.; Niyogi, S.K.; Park, J.K.; Sarkar, B.; Puri, M.K.; et al. Efficacy of a Low-Cost, Inactivated Whole-Cell Oral Cholera Vaccine: Results from 3 Years of Follow-Up of a Randomized, Controlled Trial. PLoS Negl. Trop. Dis. 2011, 5, e1289. [Google Scholar] [CrossRef]
- Sinclair, D.; Abba, K.; Zaman, K.; Qadri, F.; Graves, P.M. Oral Vaccines for Preventing Cholera. Cochrane Database Syst. Rev. 2011, 2011, CD008603. [Google Scholar] [PubMed]
- Rivera-Chávez, F.; Mekalanos, J.J. Cholera Toxin Promotes Pathogen Acquisition of Host-Derived Nutrients. Nature 2019, 572, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Sassone-Corsi, M.; Raffatellu, M. No Vacancy: How Beneficial Microbes Cooperate with Immunity to Provide Colonization Resistance to Pathogens. J. Immunol. 2015, 194, 4081–4087. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Martinez-Guryn, K.; Hubert, N.; Frazier, K.; Urlass, S.; Musch, M.W.; Ojeda, P.; Pierre, J.F.; Miyoshi, J.; Sontag, T.J.; Cham, C.M.; et al. Small Intestine Microbiota Regulate Host Digestive and Absorptive Adaptive Responses to Dietary Lipids. Cell Host Microbe 2018, 23, 458–469.e5. [Google Scholar] [CrossRef]
- Midani, F.S.; Weil, A.A.; Chowdhury, F.; Begum, Y.A.; Khan, A.I.; Debela, M.D.; Durand, H.K.; Reese, A.T.; Nimmagadda, S.N.; Silverman, J.D.; et al. Human Gut Microbiota Predicts Susceptibility to Vibrio Cholerae Infection. J. Infect. Dis. 2018, 218, 645–653. [Google Scholar] [CrossRef]
- Alavi, S.; Mitchell, J.D.; Cho, J.Y.; Liu, R.; Macbeth, J.C.; Hsiao, A. Interpersonal Gut Microbiome Variation Drives Susceptibility and Resistance to Cholera Infection. Cell 2020, 181, 1533–1546.e13. [Google Scholar] [CrossRef]
- You, J.S.; Yong, J.H.; Kim, G.H.; Moon, S.; Nam, K.T.; Ryu, J.H.; Yoon, M.Y.; Yoon, S.S. Commensal-Derived Metabolites Govern Vibrio Cholerae Pathogenesis in Host Intestine. Microbiome 2019, 7, 132. [Google Scholar] [CrossRef]
- Qin, Z.; Yang, X.; Chen, G.; Park, C.; Liu, Z. Crosstalks between Gut Microbiota and Vibrio Cholerae. Front. Cell Infect. Microbiol. 2020, 10, 582554. [Google Scholar] [CrossRef]
- Bachmann, V.; Kostiuk, B.; Unterweger, D.; Diaz-Satizabal, L.; Ogg, S.; Pukatzki, S. Bile Salts Modulate the Mucin-Activated Type vi Secretion System of Pandemic Vibrio Cholerae. PLoS Negl. Trop. Dis. 2015, 9, e0004031. [Google Scholar] [CrossRef]
- Hung, D.T.; Mekalanos, J.J. Bile Acids Induce Cholera Toxin Expression in Vibrio Cholerae in a ToxT-Independent Manner. Proc. Natl. Acad. Sci. USA 2005, 102, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Caro, F.; Robins, W.; Mekalanos, J.J. Antagonism toward the Intestinal Microbiota and Its Effect OnVibrio Choleraevirulence. Science 2018, 359, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Parry, C.M.; Hien, T.T.; Dougan, G.; White, N.J.; Farrar, J.J. Typhoid Fever. N. Engl. J. Med. 2002, 347, 1770–1782. [Google Scholar] [CrossRef]
- World Health Organization. The Immunological Basis for Immunization Series: Module 20: Salmonella Enterica Serovar Typhi(Typhoid) Vaccines; World Health Organization: Geneva, Switzerland, 2011; pp. 1–44. [Google Scholar]
- Connor, B.A.; Schwartz, E. Typhoid and Paratyphoid Fever in Travellers. Lancet Infect. Dis. 2005, 5, 623–628. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Nguyen, H.T.; Heasley, K.T.; Saechao, C.H.; Gil, L.M.; Rogers, A.H.; Miller, B.K.; Rolston, M.; Lopez, C.D.; Litvak, Y.; et al. Endogenous Enterobacteriaceae Underlie Variation in Susceptibility to Salmonella Infection. Nat. Microbiol. 2019, 4, 1057–1064. [Google Scholar] [CrossRef]
- Rogers, A.W.L.; Tsolis, R.M.; Bäumler, A.J. Salmonella versus the Microbiome. Microbiol. Mol. Biol. Rev. 2021, 85, e00027-19. [Google Scholar] [CrossRef]
- Vasquez, K.S.; Shiver, A.L.; Huang, K.C. Cutting the Gordian Knot of the Microbiota. Mol. Cell 2018, 70, 765–767. [Google Scholar] [CrossRef]
- Salerno-Goncalves, R.; Pasetti, M.F.; Sztein, M.B. Characterization of CD8+ Effector T Cell Responses in Volunteers Immunized with Salmonella enterica Serovar TyphiStrain Ty21a Typhoid Vaccine. J. Immunol. 2002, 169, 2196–2203. [Google Scholar] [CrossRef]
- Salerno-Gonçalves, R.; Fernández-Viña, M.; Lewinsohn, D.; Sztein, M.B. Identification of a Human HLA-E-Restricted CD8+ T Cell Subset in Volunteers Immunized with Salmonella enterica Serovar TyphiStrain Ty21a Typhoid Vaccine. J. Immunol. 2004, 173, 5852–5862. [Google Scholar] [CrossRef]
- Sztein, M.B. Cell-Mediated Immunity and Antibody Responses Elicited by Attenuated Salmonella enterica Serovar TyphiStrains Used as Live Oral Vaccines in Humans. Clin. Infect. Dis. 2007, 45, S15–S19. [Google Scholar] [CrossRef]
- Salerno-Gonçalves, R.; Wahid, R.; Sztein, M.B. Ex Vivo Kinetics of Early and Long-Term Multifunctional Human Leukocyte Antigen E-Specific CD8+ Cells in Volunteers Immunized with the Ty21a Typhoid Vaccine. Clin. Vaccine Immunol. 2010, 17, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.; Pasetti, M.F.; Maciel, M.; Simon, J.K.; Tacket, C.O.; Levine, M.M.; Sztein, M.B. Oral Priming with Salmonella TyphiVaccine Strain CVD 909 Followed by Parenteral Boost with the S. Typhivi Capsular Polysaccharide Vaccine Induces CD27+IgD− S. Typhi-Specific IgA and IgG B Memory Cells in Humans. Clin. Immunol. 2011, 138, 187–200. [Google Scholar] [CrossRef] [PubMed]
- McArthur, M.A.; Sztein, M.B. Heterogeneity of Multifunctional IL-17A Producing S. Typhi-Specific CD8+ T Cells in Volunteers Following Ty21a Typhoid Immunization. PLoS ONE 2012, 7, e38408. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.A.; Franco-Paredes, C.; Del Rio, C.; Edupuganti, S. Rethinking Typhoid Fever Vaccines: Implications for Travelers and People Living in Highly Endemic Areas. J. Travel. Med. 2009, 16, 46–52. [Google Scholar] [CrossRef]
- Guzman, C.A.; Borsutzky, S.; Griot-Wenk, M.; Metcalfe, I.C.; Pearman, J.; Collioud, A.; Favre, D.; Dietrich, G. Vaccines Against Typhoid Fever. Vaccine 2006, 24, 3804–3811. [Google Scholar] [CrossRef]
- Ferreira, R.B.R.; Antunes, L.C.M.; Finlay, B.B. Should the Human Microbiome Be Considered When Developing Vaccines? PLoS Pathog. 2010, 6, e1001190. [Google Scholar] [CrossRef]
- Gill, N.; Finlay, B.B. The Gut Microbiota: Challenging Immunology. Nat. Rev. Immunol. 2011, 11, 636–637. [Google Scholar] [CrossRef]
- Sczesnak, A.; Segata, N.; Qin, X.; Gevers, D.; Petrosino, J.F.; Huttenhower, C.; Littman, D.R.; Ivanov, I.I. The Genome of Th17 Cell-Inducing Segmented Filamentous Bacteria Reveals Extensive Auxotrophy and Adaptations to the Intestinal Environment. Cell Host Microbe 2011, 10, 260–272. [Google Scholar] [CrossRef]
- Benyacoub, J.; Rochat, F.; Saudan, K.-Y.; Rochat, I.; Antille, N.; Cherbut, C.; von der Weid, T.; Schiffrin, E.J.; Blum, S. Feeding a Diet Containing a Fructooligosaccharide Mix Can Enhance Salmonella Vaccine Efficacy in Mice. J. Nutr. 2008, 138, 123–129. [Google Scholar] [CrossRef][Green Version]
- Fang, H.; Elina, T.; Heikki, A.; Seppo, S. Modulation of Humoral Immune Response through Probiotic Intake. FEMS Immunol. Med. Microbiol. 2000, 29, 47–52. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. Estimates of the Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoea in 195 Countries: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, G.M.; Pérez-Schael, I.; Velázquez, F.R.; Abate, H.; Breuer, T.; Clemens, S.C.; Cheuvart, B.; Espinoza, F.; Gillard, P.; Innis, B.L.; et al. Safety and Efficacy of an Attenuated Vaccine Against Severe Rotavirus Gastroenteritis. N. Engl. J. Med. 2006, 354, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T.; Matson, D.O.; Dennehy, P.; Van Damme, P.; Santosham, M.; Rodriguez, Z.; Dallas, M.J.; Heyse, J.F.; Goveia, M.G.; Black, S.B.; et al. Safety and Efficacy of a Pentavalent Human–Bovine (WC3) Reassortant Rotavirus Vaccine. N. Engl. J. Med. 2006, 354, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Zaman, K.; Anh, D.D.; Victor, J.C.; Shin, S.; Yunus, M.; Dallas, M.J.; Podder, G.; Thiem, V.D.; Mai, L.T.P.; Luby, S.P.; et al. Efficacy of Pentavalent Rotavirus Vaccine against Severe Rotavirus Gastroenteritis in Infants in Developing Countries in Asia: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2010, 376, 615–623. [Google Scholar] [CrossRef]
- Armah, G.E.; Sow, S.O.; Breiman, R.F.; Dallas, M.J.; Tapia, M.D.; Feikin, D.R.; Binka, F.N.; Steele, A.D.; Laserson, K.F.; Ansah, N.A.; et al. Efficacy of Pentavalent Rotavirus Vaccine against Severe Rotavirus Gastroenteritis in Infants in Developing Countries in Sub-Saharan Africa: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2010, 376, 606–614. [Google Scholar] [CrossRef]
- Clarke, E.; Desselberger, U. Correlates of Protection against Human Rotavirus Disease and the Factors Influencing Protection in Low-Income Settings. Mucosal Immunol. 2014, 8, 1–17. [Google Scholar] [CrossRef]
- Moon, S.-S.; Groome, M.; Velasquez, D.E.; Parashar, U.D.; Jones, S.; Koen, A.; van Niekerk, N.; Jiang, B.; Madhi, S.A. Prevaccination Rotavirus Serum IgG and IgA Are Associated with Lower Immunogenicity of Live, Oral Human Rotavirus Vaccine in South African Infants. Clin. Infect. Dis. 2015, 62, 157–165. [Google Scholar] [CrossRef]
- Chilengi, R.; Simuyandi, M.; Beach, L.B.; Mwila, K.; Becker-Dreps, S.; Emperador, D.M.; Velasquez, D.E.; Bosomprah, S.; Jiang, B. Association of Maternal Immunity with Rotavirus Vaccine Immunogenicity in Zambian Infants. PLoS ONE 2016, 11, e0150100. [Google Scholar] [CrossRef]
- Emperador, D.M.; Velasquez, D.E.; Estivariz, C.F.; Lopman, B.; Jiang, B.; Parashar, U.; Anand, A.; Zaman, K. Interference of Monovalent, Bivalent, and Trivalent Oral Poliovirus Vaccines on Monovalent Rotavirus Vaccine Immunogenicity in Rural Bangladesh. Clin. Infect. Dis. 2015, 62, 150–156. [Google Scholar] [CrossRef]
- Nordgren, J.; Sharma, S.; Bucardo, F.; Nasir, W.; Günaydın, G.; Ouermi, D.; Nitiema, L.W.; Becker-Dreps, S.; Simpore, J.; Hammarström, L.; et al. Both Lewis and Secretor Status Mediate Susceptibility to Rotavirus Infections in a Rotavirus Genotype–Dependent Manner. Clin. Infect. Dis. 2014, 59, 1567–1573. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [PubMed]
- de Jong, S.E.; Olin, A.; Pulendran, B. The Impact of the Microbiome on Immunity to Vaccination in Humans. Cell Host Microbe 2020, 28, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the Human Infant Intestinal Microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef]
- Bezirtzoglou, E. The Intestinal Microflora during the First Weeks of Life. Anaerobe 1997, 3, 173–177. [Google Scholar] [CrossRef]
- Arboleya, S.; Solís, G.; Fernández, N.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Facultative to Strict Anaerobes Ratio in the Preterm Infant Microbiota. Gut Microbes 2012, 3, 583–588. [Google Scholar] [CrossRef]
- Penders, J.; Vink, C.; Driessen, C.; London, N.; Thijs, C.; Stobberingh, E.E. Quantification of Bifidobacterium Spp., Escherichia Coli and Clostridium Difficile in Faecal Samples of Breast-Fed and Formula-Fed Infants by Real-Time PCR. FEMS Microbiol. Lett. 2005, 243, 141–147. [Google Scholar] [CrossRef]
- Guaraldi, F.; Salvatori, G. Effect of Breast and Formula Feeding on Gut Microbiota Shaping in Newborns. Front. Cell. Infect. Microbiol. 2012, 2, 94. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of Microbial Consortia in the Developing Infant Gut Microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4578–4585. [Google Scholar] [CrossRef]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within Worlds: Evolution of the Vertebrate Gut Microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Honda, K. Intestinal Commensal Microbes as Immune Modulators. Cell Host Microbe 2012, 12, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ Regulatory T-Cell Development by a Commensal Bacterium of the Intestinal Microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Collins, M.D.; Lawson, P.A.; Willems, A.; Cordoba, J.J.; Fernandez-Garayzabal, J.; Garcia, P.; Cai, J.; Hippe, H.; Farrow, J.A.E. The Phylogeny of the Genus Clostridium: Proposal of Five New Genera and Eleven New Species Combinations. Int. J. Syst. Bacteriol. 1994, 44, 812–826. [Google Scholar] [CrossRef]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar]
- Nava, G.M.; Stappenbeck, T.S. Diversity of the Autochthonous Colonic Microbiota. Gut Microbes 2011, 2, 99–104. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of Colonic Regulatory T Cells by Indigenous Clostridium Species. Science 2010, 331, 337–341. [Google Scholar] [CrossRef]
- Ivanov, I.I.; de Llanos Frutos, R.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific Microbiota Direct the Differentiation of IL-17-Producing T-Helper Cells in the Mucosa of the Small Intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef]
- Umesaki, Y.; Okada, Y.; Matsumoto, S.; Imaoka, A.; Setoyama, H. Segmented Filamentous Bacteria Are Indigenous Intestinal Bacteria That Activate Intraepithelial Lymphocytes and Induce MHC Class II Molecules and Fucosyl Asialo GM1 Glycolipids on the Small Intestinal Epithelial Cells in the Ex-Germ-Free Mouse. Microbiol. Immunol. 1995, 39, 555–562. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. MetaArXiv 2020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loddo, F.; Laganà, P.; Rizzo, C.E.; Calderone, S.M.; Romeo, B.; Venuto, R.; Maisano, D.; Fedele, F.; Squeri, R.; Nicita, A.; et al. Intestinal Microbiota and Vaccinations: A Systematic Review of the Literature. Vaccines 2025, 13, 306. https://doi.org/10.3390/vaccines13030306
Loddo F, Laganà P, Rizzo CE, Calderone SM, Romeo B, Venuto R, Maisano D, Fedele F, Squeri R, Nicita A, et al. Intestinal Microbiota and Vaccinations: A Systematic Review of the Literature. Vaccines. 2025; 13(3):306. https://doi.org/10.3390/vaccines13030306
Chicago/Turabian StyleLoddo, Francesco, Pasqualina Laganà, Caterina Elisabetta Rizzo, Serena Maria Calderone, Bruno Romeo, Roberto Venuto, Daniele Maisano, Francesco Fedele, Raffaele Squeri, Alessandro Nicita, and et al. 2025. "Intestinal Microbiota and Vaccinations: A Systematic Review of the Literature" Vaccines 13, no. 3: 306. https://doi.org/10.3390/vaccines13030306
APA StyleLoddo, F., Laganà, P., Rizzo, C. E., Calderone, S. M., Romeo, B., Venuto, R., Maisano, D., Fedele, F., Squeri, R., Nicita, A., Nirta, A., Genovese, G., Bartucciotto, L., & Genovese, C. (2025). Intestinal Microbiota and Vaccinations: A Systematic Review of the Literature. Vaccines, 13(3), 306. https://doi.org/10.3390/vaccines13030306







