Vaccination Strategies: Mixing Paths Versus Matching Tracks
Abstract
:1. Introduction
2. Unmet Needs and Emerging Challenges: Patients with Dysregulated Immune Responses
3. Mechanistic Insights into Immunization Strategies
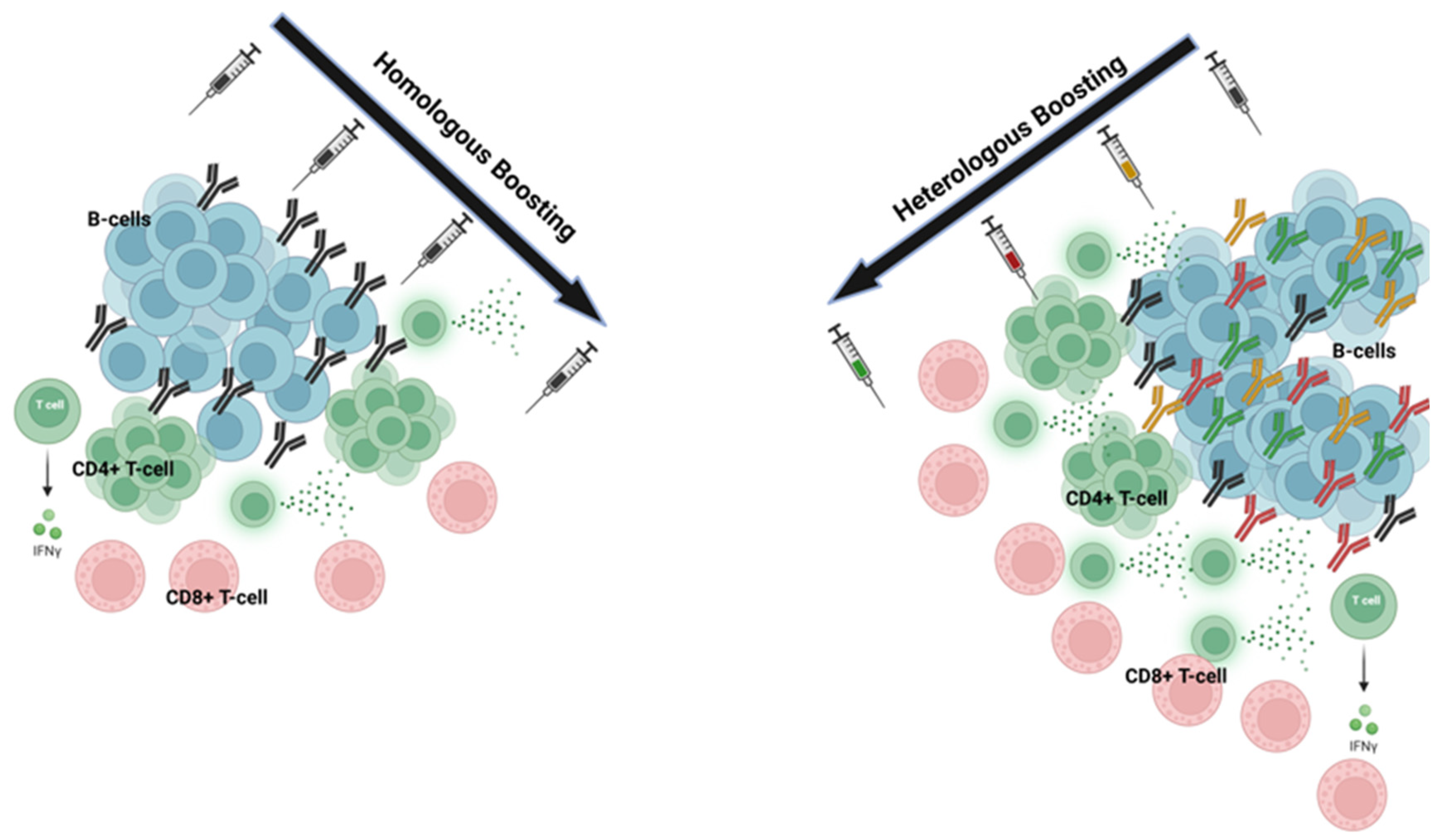
3.1. Homologous Prime-Boost Mechanisms
3.2. Heterologous Prime-Boost Mechanisms
3.3. Heterologous Immunization Outcomes
4. Role of Amino-Acid-Sequence Differences in Vaccines
5. Pre-COVID-19 Evidence and Insights Generated
6. The COVID-19 Case Study
6.1. Comparative Long-Term Efficacy of Heterologous Versus Homologous Regimens
6.2. Protection Against Emerging Variants
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deming, M.E.; Lyke, K.E. A ’mix and match’ approach to SARS-CoV-2 vaccination. Nat. Med. 2021, 27, 1510–1511. [Google Scholar] [CrossRef]
- Garg, I.; Sheikh, A.B.; Pal, S.; Shekhar, R. Mix-and-Match COVID-19 Vaccinations (Heterologous Boost): A Review. Infect. Dis. Rep. 2022, 14, 537–546. [Google Scholar] [CrossRef]
- Bhatnagar, N.; Kim, K.H.; Subbiah, J.; Muhammad-Worsham, S.; Park, B.R.; Liu, R.; Grovenstein, P.; Wang, B.Z.; Kang, S.M. Heterologous Prime-Boost Vaccination with Inactivated Influenza Viruses Induces More Effective Cross-Protection than Homologous Repeat Vaccination. Vaccines 2023, 11, 1209. [Google Scholar] [CrossRef] [PubMed]
- Pardo, I.; Maezato, A.M.; Callado, G.Y.; Gutfreund, M.C.; Hsieh, M.K.; Lin, V.; Kobayashi, T.; Salinas, J.L.; Subramanian, A.; Edmond, M.B.; et al. Effectiveness of heterologous and homologous COVID-19 vaccination among immunocompromised individuals: A systematic literature review and meta-analysis. Antimicrob. Steward. Healthc. Epidemiol. 2024, 4, e152. [Google Scholar] [CrossRef] [PubMed]
- Adnan, N.; Haq, M.A.; Akter, S.; Sajal, S.; Islam, M.F.; Mou, T.J.; Jamiruddin, M.R.; Jubyda, F.T.; Islam, M.S.; Tuli, J.F.; et al. Antibody Response after Homologous and Heterologous Prime-Boost COVID-19 Vaccination in a Bangladeshi Residential University Cohort. Vaccines 2024, 12, 482. [Google Scholar] [CrossRef] [PubMed]
- Garza-Silva, A.; Rivera-Salinas, D.; Rivera-Cavazos, A.; Fernández-Chau, I.F.; Cepeda-Medina, A.B.; Morales-Rodríguez, D.P.; Barco-Flores, I.A.; Sanz-Sánchez, M.; Acciardi, C.; Paez-Bo, G.; et al. Effectiveness of different booster vaccine combinations against SARS-CoV-2 during a six-month follow-up in Mexico and Argentina. Front. Immunol. 2024, 15, 1403784. [Google Scholar] [CrossRef]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Ramshaw, I.A.; Ramsay, A.J. The prime-boost strategy: Exciting prospects for improved vaccination. Immunol. Today 2000, 21, 163–165. [Google Scholar] [CrossRef]
- Lu, S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009, 21, 346–351. [Google Scholar] [CrossRef]
- Ledford, H. Could mixing COVID vaccines boost immune response? Nature 2021, 590, 375–376. [Google Scholar] [CrossRef]
- Nam, M.; Yun, S.G.; Kim, S.W.; Kim, C.G.; Cha, J.H.; Lee, C.; Kang, S.; Park, S.G.; Kim, S.B.; Lee, K.B.; et al. Humoral and Cellular Immune Responses to Vector, Mix-and-Match, or mRNA Vaccines against SARS-CoV-2 and the Relationship between the Two Immune Responses. Microbiol. Spectr. 2022, 10, e0249521. [Google Scholar] [CrossRef] [PubMed]
- Pascuale, C.A.; Varese, A.; Ojeda, D.S.; Pasinovich, M.E.; Lopez, L.; Rossi, A.H.; Rodriguez, P.E.; Miglietta, E.A.; Mazzitelli, I.; Di Diego Garcia, F.; et al. Immunogenicity and reactogenicity of heterologous immunization against SARS CoV-2 using Sputnik V, ChAdOx1-S, BBIBP-CorV, Ad5-nCoV, and mRNA-1273. Cell Rep. Med. 2022, 3, 100706. [Google Scholar] [CrossRef]
- Lim, S.Y.; Kim, J.Y.; Jung, J.; Yun, S.C.; Kim, S.H. Waning of humoral immunity depending on the types of COVID-19 vaccine. Infect. Dis. 2023, 55, 216–220. [Google Scholar] [CrossRef]
- Baglivo, F.; Magrì, M.; De Angelis, L.; Aprile, V.; Minelli, M.; Stifini, R.; Lopalco, P.; Rizzo, C.; Fedele, A. Performance comparison between heterologous and homologous COVID19 vaccine schedules on Omicron variant incidence: A real-world retrospective cohort study in Southern Italy. Vaccine 2023, 41, 5687–5695. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.; Greenland, M.; de Whalley, P.C.S.; Aley, P.K.; Plested, E.L.; Singh, N.; Koleva, S.; Tonner, S.; Macaulay, G.C.; Read, R.C.; et al. Reactogenicity, immunogenicity and breakthrough infections following heterologous or fractional second dose COVID-19 vaccination in adolescents (Com-COV3): A randomised controlled trial. J. Infect. 2023, 87, 230–241. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous COVID-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Happe, M.; Hofstetter, A.R.; Wang, J.; Yamshchikov, G.V.; Holman, L.A.; Novik, L.; Strom, L.; Kiweewa, F.; Wakabi, S.; Millard, M.; et al. Heterologous cAd3-Ebola and MVA-EbolaZ vaccines are safe and immunogenic in US and Uganda phase 1/1b trials. NPJ Vaccines 2024, 9, 67. [Google Scholar] [CrossRef]
- Brown, S.A.; Surman, S.L.; Sealy, R.; Jones, B.G.; Slobod, K.S.; Branum, K.; Lockey, T.D.; Howlett, N.; Freiden, P.; Flynn, P.; et al. Heterologous Prime-Boost HIV-1 Vaccination Regimens in Pre-Clinical and Clinical Trials. Viruses 2010, 2, 435–467. [Google Scholar] [CrossRef]
- EMA and ECDC Recommendations on Heterologous Vaccination Courses Against COVID-19: ‘Mix-and-Match’ Approach Can Be Used for Both Initial Courses and Boosters [Online]. Available online: https://www.ema.europa.eu/en/news/ema-ecdc-recommendations-heterologous-vaccination-courses-against-covid-19-mix-match-approach-can-be (accessed on 21 February 2025).
- Hung, P.V.; Nguyen, T.D.; Ha, L.T.; Toi, P.L.; Tram, T.H. Common Adverse Events from Mixing COVID-19 Vaccine Booster in Hanoi, Vietnam. Vaccines 2023, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Cheung, P.P. Effectiveness of heterologous and homologous COVID-19 vaccine regimens: Living systematic review with network meta-analysis. Bmj 2022, 377, e069989. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, K.; Prasad, N.; Dascomb, K.; Irving, S.A.; Yang, D.H.; Gaglani, M.; Klein, N.P.; DeSilva, M.B.; Ong, T.C.; Grannis, S.J.; et al. Effectiveness of Homologous and Heterologous COVID-19 Booster Doses Following 1 Ad.26.COV2.S (Janssen [Johnson & Johnson]) Vaccine Dose Against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults—VISION Network, 10 States, December 2021–March 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Collier, A.Y.; Yu, J.; Liu, J.; Chandrashekar, A.; McMahan, K.; Jacob-Dolan, C.; He, X.; Roy, V.; Hauser, B.M.; et al. Durability of Heterologous and Homologous COVID-19 Vaccine Boosts. JAMA Netw. Open 2022, 5, e2226335. [Google Scholar] [CrossRef]
- Klemis, V.; Schmidt, T.; Schub, D.; Mihm, J.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Hielscher, F.; Guckelmus, C.; Urschel, R.; et al. Comparative immunogenicity and reactogenicity of heterologous ChAdOx1-nCoV-19-priming and BNT162b2 or mRNA-1273-boosting with homologous COVID-19 vaccine regimens. Nat. Commun. 2022, 13, 4710. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, R.; Samieefar, N.; Masoumi, N.; Mohseni, S.; Rezaei, N. COVID-19 vaccines mix-and-match: The concept, the efficacy and the doubts. J. Med. Virol. 2022, 94, 1294–1299. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: “Mix and match” primary vaccines are safe and effective, study finds. Bmj 2021, 375, n3030. [Google Scholar] [CrossRef]
- Palanica, A.; Jeon, J. Initial Mix-and-Match COVID-19 Vaccination Perceptions, Concerns, and Side Effects across Canadians. Vaccines 2022, 10, 93. [Google Scholar] [CrossRef]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; García Morales, M.T.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Stuart, A.S.V.; Shaw, R.H.; Liu, X.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): A single-blind, randomised, phase 2, non-inferiority trial. Lancet 2022, 399, 36–49. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Stockinger, R.; Sprenger-Mähr, H.; Lhotta, K.; Zitt, E. Humoral and Cellular Immune Response After a 3-Dose Heterologous SARS-CoV-2 Vaccination Using the mRNA-BNT162b2 and Viral Vector Ad26COVS1 Vaccine in Hemodialysis Patients. Front. Immunol. 2022, 13, 907615. [Google Scholar] [CrossRef] [PubMed]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, Cd015477. [Google Scholar] [CrossRef] [PubMed]
- Jantarabenjakul, W.; Sodsai, P.; Chantasrisawad, N.; Jitsatja, A.; Ninwattana, S.; Thippamom, N.; Ruenjaiman, V.; Tan, C.W.; Pradit, R.; Sophonphan, J.; et al. Dynamics of Neutralizing Antibody and T-Cell Responses to SARS-CoV-2 and Variants of Concern after Primary Immunization with CoronaVac and Booster with BNT162b2 or ChAdOx1 in Health Care Workers. Vaccines 2022, 10, 639. [Google Scholar] [CrossRef]
- Mubarak, A.; Almutairi, S.; Al-Dhabbah, A.D.; Aldabas, S.Y.; Bhat, R.; Alqoufail, M.M.; Abdel-Maksoud, M.A.; Almanaa, T.N.; Farrag, M.A.; Alturaiki, W. Durability of SARS-CoV-2 Specific IgG Antibody Responses Following Two Doses of Match and Mixed COVID-19 Vaccines Regimens in Saudi Population. Infect. Drug Resist. 2022, 15, 3791–3800. [Google Scholar] [CrossRef]
- Vitiello, L.; Gatta, L.; Ilari, S.; Bonassi, S.; Cristina, M.; Ciatti, F.; Fini, M.; Proietti, S.; Russo, P.; Tomino, C.; et al. Long Lasting Cellular Immune Response Induced by mRNA Vaccination: Implication for Prevention Strategies. Front. Immunol. 2022, 13, 836495. [Google Scholar] [CrossRef]
- Hyun, H.; Jang, A.Y.; Park, H.; Heo, J.Y.; Seo, Y.B.; Nham, E.; Yoon, J.G.; Seong, H.; Noh, J.Y.; Cheong, H.J.; et al. Humoral and cellular immunogenicity of homologous and heterologous booster vaccination in Ad26.COV2.S-primed individuals: Comparison by breakthrough infection. Front. Immunol. 2023, 14, 1131229. [Google Scholar] [CrossRef]
- Mallah, S.I.; Alawadhi, A.; Jawad, J.; Wasif, P.; Alsaffar, B.; Alalawi, E.; Mohamed, A.M.; Butler, A.E.; Alalawi, B.; Qayed, D.; et al. Safety and efficacy of COVID-19 prime-boost vaccinations: Homologous BBIBP-CorV versus heterologous BNT162b2 boosters in BBIBP-CorV-primed individuals. Vaccine 2023, 41, 1925–1933. [Google Scholar] [CrossRef]
- Qu, M.M.; Song, B.; Yang, B.P.; Wang, Z.; Yu, M.; Zhang, Y.; Zhang, C.; Song, J.W.; Fan, X.; Xu, R.; et al. Effect of SARS-CoV-2 Breakthrough Infection on HIV Reservoirs and T-Cell Immune Recovery in 3-Dose Vaccinated People Living with HIV. Viruses 2023, 15, 2427. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, F.; Zhang, H.; Li, D.; Zhang, S.; Zhang, M.; Li, J.; Xie, J.; Zhang, L.; Yang, X.; et al. Waning neutralizing antibodies through 180 days after homologous and heterologous boosters of inactivated COVID-19 vaccine. Front. Public Health 2025, 13, 1478627. [Google Scholar] [CrossRef]
- Awadalla, M.; AlRawi, H.Z.; Henawi, R.A.; Barnawi, F.; Alkadi, H.; Alyami, A.; Alsughayir, A.; Alsaif, A.S.; Mubarak, A.; Alturaiki, W.; et al. Humoral and cellular immune durability of different COVID-19 vaccine platforms following homologous/heterologous boosters: One-year post vaccination. Front. Immunol. 2025, 16, 1526444. [Google Scholar] [CrossRef]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Erol, Ç.; Kuloğlu, Z.E.; Kayaaslan, B.; Esken, G.; Altunsoy, A.; Barlas, T.; Çınar, G.; Hasanoğlu, İ.; Oruç, E.; İncir, S.; et al. BNT162b2 or CoronaVac as the Third Dose against Omicron: Neutralizing Antibody Responses among Transplant Recipients Who Had Received Two Doses of CoronaVac. Viruses 2023, 15, 1534. [Google Scholar] [CrossRef]
- Gaete-Argel, A.; Saavedra-Alarcón, V.; Sauré, D.; Alonso-Palomares, L.; Acevedo, M.L.; Alarcón, M.; Bueno, S.M.; Kalergis, A.M.; Soto-Rifo, R.; Valiente-Echeverría, F.; et al. Impact of homologous and heterologous boosters in neutralizing antibodies titers against SARS-CoV-2 Omicron in solid-organ transplant recipients. Front. Immunol. 2023, 14, 1135478. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Lee, J.; Huh, K.H.; Joo, D.J.; Lee, J.G.; Kim, H.Y.; Lee, M.; Jung, I.; Kim, M.Y.; Kim, S.; et al. Comparison of humoral immunogenicity in solid organ transplant recipients after third-dose mRNA vaccine with homologous or heterologous schedules: An observational study. J. Clin. Virol. 2023, 159, 105374. [Google Scholar] [CrossRef]
- Narongkiatikhun, P.; Noppakun, K.; Chaiwarith, R.; Winichakoon, P.; Vongsanim, S.; Suteeka, Y.; Pongsuwan, K.; Kusirisin, P.; Wongsarikan, N.; Fanhchaksai, K.; et al. Correction: Narongkiatikhun et al. Immunogenicity and Safety of Homologous and Heterologous Prime-Boost of CoronaVac(®) and ChAdOx1 nCoV-19 among Hemodialysis Patients: An Observational Prospective Cohort Study. Vaccines 2023, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Sharifi Aliabadi, L.; Karami, M.; Barkhordar, M.; Hashemi Nazari, S.S.; Kavousi, A.; Ahmadvand, M.; Vaezi, M. Homologous versus Heterologous prime-boost COVID-19 Vaccination in autologous hematopoietic stem cell transplantation recipients: A blinded randomized controlled trial. Front. Immunol. 2023, 14, 1237916. [Google Scholar] [CrossRef]
- Sim, W.; Kang, H.; Jung, J.; Lee, J.; Ko, G.Y.; Park, H.S.; Choi, J.; Park, K.; Oh, E.J. Comparison of humoral and cellular immune responses between ChAd-BNT heterologous vaccination and BNT-BNT homologous vaccination following the third BNT dose: A prospective cohort study. Front. Immunol. 2023, 14, 1120556. [Google Scholar] [CrossRef]
- Thompson, E.A.; Ngecu, W.; Stoddart, L.; Johnston, T.S.; Chang, A.; Cascino, K.; Alejo, J.L.; Abedon, A.T.; Samaha, H.; Rouphael, N.; et al. Heterologous versus homologous boosting elicits qualitatively distinct, BA.5-cross-reactive T cells in transplant recipients. JCI Insight 2023, 8, e168470. [Google Scholar] [CrossRef]
| Platform | Advantages | Disadvantages | Examples in Homologous and Heterologous Strategies | References |
|---|---|---|---|---|
| mRNA (e.g., Pfizer, Moderna) | High efficacy, strong immune response, rapid adaptability to variants | Cold storage requirements, potential for myocarditis in young males | Homologous: Pfizer–Pfizer, Moderna–Moderna; Heterologous: Pfizer–AstraZeneca | Pardo et al., 2024 [4]; Adnan et al., 2024 [5] |
| Viral Vector (e.g., AstraZeneca, J&J) | Long-lasting immunity, no need for ultra-cold storage | Rare risk of blood clots, lower efficacy against some variants | Homologous: AstraZeneca–AstraZeneca; Heterologous: AstraZeneca–Pfizer, AstraZeneca–Moderna | Hung et al., 2023; [21] Garza-Silva et al., 2024 [6] |
| Protein Subunit (e.g., Novavax) | Established platform, fewer side effects | Requires adjuvant, slower production | Homologous: Novavax–Novavax; Heterologous: Novavax–mRNA | Kelly et al., 2023 [16] |
| Inactivated Virus (e.g., Sinopharm, Sinovac) | Well-studied platform, good safety profile | Lower efficacy, may require additional boosters | Homologous: Sinopharm–Sinopharm; Heterologous: Sinopharm–Pfizer, Sinovac–Moderna | Au et al., 2022 [22] |
| Vaccine Combination | Study (First Author, Year) | Population (n) | Immunogenicity | Efficacy | Safety | Improved over Homologous? |
|---|---|---|---|---|---|---|
| Pfizer + Moderna | Adnan et al., 2024 [5] | Bangladeshi university cohort (606) | Higher antibody levels with heterologous boosting | mRNA vaccines showed the highest immunogenicity | Safe, but waning immunity noted |  |
| AstraZeneca + Moderna | Hung et al., 2023 [21] | Hanoi, Vietnam (719) | Comparable immunogenicity to homologous AstraZeneca | Safe, with mild adverse events in 45.8% of participants | No major safety concerns |  |
| Gam-COVID-Vac + Pfizer | Garza-Silva et al., 2024 [6] | Mexico and Argentina (491) | High antibody titers maintained after six months | Effective, with protection comparable to homologous schemes | Safe, though moderate adverse events increased after booster |  |
| BNT162b2 + NVX-CoV2373 | Kelly et al., 2023 [16] | UK adolescents (148) | NVX induced stronger T-cell response and comparable antibody levels | NVX reduced breakthrough infection risk by 89% compared to BNT-30 | Safe, with lower reactogenicity in BNT-10 recipients |  |
| MOD-MOD-BNT | Baglivo et al., 2023 [15] | Southern Italy (469,069) | Heterologous boosting provided highest protection | Most effective in reducing Omicron infection | Safe, with lower infection risk than homologous |  |
| mRNA-1273 + Ad26.COV2.S | Atmar et al., 2022 [17] | US adults (458) | Heterologous boosting increased neutralizing antibody titers 6-73x | Higher T-cell responses compared to homologous boosting | Safe, though reactogenicity similar to primary series |  |
| Adenovirus Vector + mRNA | Au et al., 2022 [22] | Global meta-analysis (193,955,736) | 94% effectiveness against non-Delta/Omicron infections | Effective against hospitalization with OR 0.06 (95% CI: 0.02–0.21) | Safe, with effectiveness comparable to homologous three-dose regimens |  |
| BNT162b2 + Ad26.COV2.S | Tan et al., 2022 [24] | US cohort study (68) | Heterologous Ad26 boosting provided more durable antibody and T-cell responses | Effective against Omicron with sustained immunity over 16 weeks | Safe, with similar reactogenicity to homologous boosting |  |
| ChAdOx1 + BNT162b2 | Klemis et al., 2022 [25] | German healthcare workers (66) | Higher spike-specific CD8 T-cell responses than homologous regimens | Equivalent to or better than homologous BNT schedule | Safe, with pronounced reactogenicity in ChAdOx-primed individuals |  |
| ChAdOx1 + mRNA-1273 | Klemis et al., 2022 [25] | German healthcare workers (101) | Strongest T-cell response among all regimens studied | Higher antibody levels than homologous ChAdOx or BNT | Safe, but most reactogenic combination tested |  |
| Ad26.COV2.S + mRNA-1273 | Natarajan et al., 2022 [23] | US adults (25,244) | Heterologous boosting significantly improved neutralizing antibody response | 78% against hospitalization vs. 67% with homologous | Safe, but higher reactogenicity than homologous boosting |  |
| Heterologous Mix (Pfizer + Moderna, AstraZeneca + Pfizer) | Palanica et al., 2022 [28] | Canadian survey (1002) | N/A | Higher side effects reported with Moderna second dose | Safe, but concerns over long-term effects noted by participants |  |
| Pfizer + AstraZeneca (heterologous boost) | Awadalla et al., 2025 [41] | Saudi Arabia (484) | Higher IgG antibody levels one year post-vaccination; enhanced T-cell response (CD8+ IFN-γ production) | Superior ACE2-binding inhibition against Omicron variants; effective across all dose regimens | Safe; heterologous regimens showed longer-lasting immune durability |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livieratos, A.; Gogos, C.; Thomas, I.; Akinosoglou, K. Vaccination Strategies: Mixing Paths Versus Matching Tracks. Vaccines 2025, 13, 308. https://doi.org/10.3390/vaccines13030308
Livieratos A, Gogos C, Thomas I, Akinosoglou K. Vaccination Strategies: Mixing Paths Versus Matching Tracks. Vaccines. 2025; 13(3):308. https://doi.org/10.3390/vaccines13030308
Chicago/Turabian StyleLivieratos, Achilleas, Charalambos Gogos, Iason Thomas, and Karolina Akinosoglou. 2025. "Vaccination Strategies: Mixing Paths Versus Matching Tracks" Vaccines 13, no. 3: 308. https://doi.org/10.3390/vaccines13030308
APA StyleLivieratos, A., Gogos, C., Thomas, I., & Akinosoglou, K. (2025). Vaccination Strategies: Mixing Paths Versus Matching Tracks. Vaccines, 13(3), 308. https://doi.org/10.3390/vaccines13030308






