The Sm14+GLA-SE Recombinant Vaccine Against Schistosoma mansoni and S. haematobium in Adults and School Children: Phase II Clinical Trials in West Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Vaccine Antigen and Adjuvant
2.2. Location and Study Cohorts
2.3. Clinical and Laboratory Safety Assessment
2.4. Investigation of Immune Responses
2.5. Investigation of Cell-Mediated Immunity
2.6. Vaccination of Adults—Phase IIa
2.6.1. Vaccination of Adults—Extended Phase IIa
2.6.2. Vaccination of Children—Phase IIb
2.6.3. Safety-Related Measures
3. Results
3.1. Adult Trial (Phase IIa)
3.1.1. Safety Outcome
3.1.2. Humoral Immunity
3.1.3. Cell-Mediated Immunity
3.2. Phase IIb
3.2.1. Safety Outcome
3.2.2. Humoral Immunity
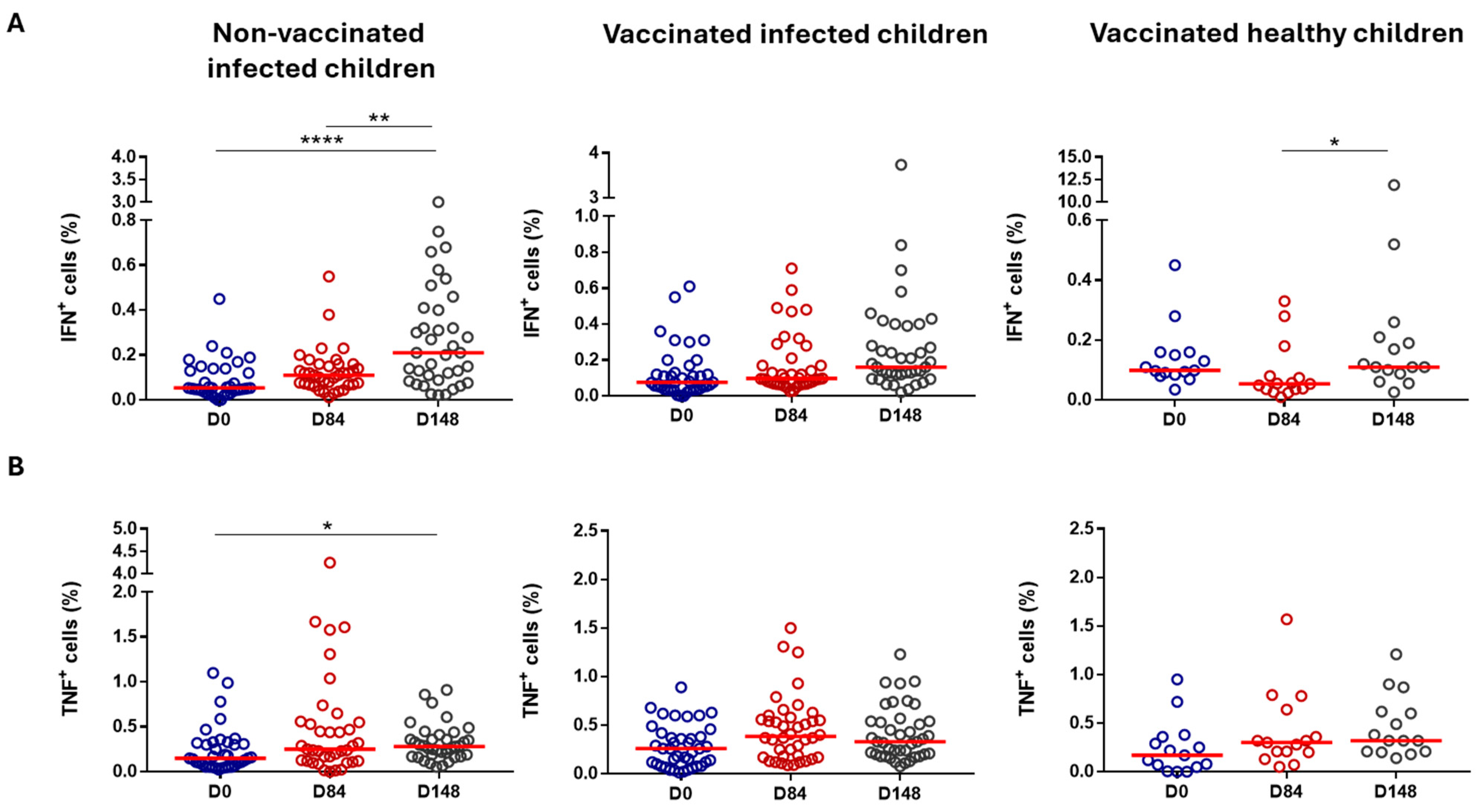
3.2.3. Cell-Mediated Immunity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Guideline on Control and Elimination of Human Schistosomiasis; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-004160-8. [Google Scholar]
- Wood, C.L.; Sokolow, S.H.; Jones, I.J.; Chamberlin, A.J.; Lafferty, K.D.; Kuris, A.M.; Jocque, M.; Hopkins, S.; Adams, G.; Buck, J.C.; et al. Precision Mapping of Snail Habitat Provides a Powerful Indicator of Human Schistosomiasis Transmission. Proc. Natl. Acad. Sci. USA 2019, 116, 23182–23191. [Google Scholar] [CrossRef]
- Crompton, D.W.T.; World Health Organization. WHO Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Anthelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers; Preventive Chemotherapy: Geneva, Switzerland, 2006. [Google Scholar]
- McManus, D.P.; Bergquist, R.; Cai, P.; Ranasinghe, S.; Tebeje, B.M.; You, H. Schistosomiasis—From Immunopathology to Vaccines. Semin. Immunopathol. 2020, 42, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Kura, K.; Truscott, J.E.; Toor, J.; Anderson, R.M. Modelling the Impact of a Schistosoma mansoni Vaccine and Mass Drug Administration to Achieve Morbidity Control and Transmission Elimination. PLoS Neglected Trop. Dis. 2019, 13, e0007349. [Google Scholar] [CrossRef] [PubMed]
- Tendler, M.; Almeida, M.S.; Pinto, R.M.; Noronha, D.; Katz, N. Schistosoma mansoni-New Zealand Rabbit Model: Resistance Induced by Infection Followed by Active Immunization with Protective Antigens. J. Parasitol. 1991, 77, 138–141. [Google Scholar] [CrossRef]
- Tendler, M.; Pinto, R.M.; Lima, A.d.O.; Savino, W.; Katz, N. Vaccination in Murine Schistosomiasis with Adult Worm-Derived Antigens: Variables Influencing Protection in Outbred Mice. Int. J. Parasitol. 1991, 21, 299–306. [Google Scholar] [CrossRef]
- Tendler, M.; Brito, C.A.; Vilar, M.M.; Serra-Freire, N.; Diogo, C.M.; Almeida, M.S.; Delbem, A.C.; Da Silva, J.F.; Savino, W.; Garratt, R.C.; et al. A Schistosoma mansoni Fatty Acid-Binding Protein, Sm14, Is the Potential Basis of a Dual-Purpose Anti-Helminth Vaccine. Proc. Natl. Acad. Sci. USA 1996, 93, 269–273. [Google Scholar] [CrossRef]
- Ramos, C.R.; Vilar, M.M.; Nascimento, A.L.; Ho, P.L.; Thaumaturgo, N.; Edelenyi, R.; Almeida, M.; Dias, W.O.; Diogo, C.M.; Tendler, M. R-Sm14-pRSETA Efficacy in Experimental Animals. Mem. Inst. Oswaldo Cruz 2001, 96, 131–135. [Google Scholar] [CrossRef]
- Ramos, C.R.R.; Figueredo, R.C.R.; Pertinhez, T.A.; Vilar, M.M.; do Nascimento, A.L.T.O.; Tendler, M.; Raw, I.; Spisni, A.; Ho, P.L. Gene Structure and M20T Polymorphism of the Schistosoma mansoni Sm14 Fatty Acid-Binding Protein. Molecular, Functioanl, and Immunoprotection Analysis. J. Biol. Chem. 2003, 278, 12745–12751. [Google Scholar] [CrossRef]
- Ramos, C.R.R.; Spisni, A.; Oyama, S.; Sforça, M.L.; Ramos, H.R.; Vilar, M.M.; Alves, A.C.; Figueredo, R.C.R.; Tendler, M.; Zanchin, N.I.T.; et al. Stability Improvement of the Fatty Acid Binding Protein Sm14 from S. Mansoni by Cys Replacement: Structural and Functional Characterization of a Vaccine Candidate. Biochim. Biophys. Acta 2009, 1794, 655–662. [Google Scholar] [CrossRef]
- Santini-Oliveira, M.; Coler, R.N.; Parra, J.; Veloso, V.; Jayashankar, L.; Pinto, P.M.; Ciol, M.A.; Bergquist, R.; Reed, S.G.; Tendler, M. Schistosomiasis Vaccine Candidate Sm14/GLA-SE: Phase 1 Safety and Immunogenicity Clinical Trial in Healthy, Male Adults. Vaccine 2016, 34, 586–594. [Google Scholar] [CrossRef]
- Santini-Oliveira, M.; Machado Pinto, P.; dos Santos, T.; Vilar, M.M.; Grinsztejn, B.; Veloso, V.; Paes-de-Almeida, E.C.; Amaral, M.A.Z.; Ramos, C.R.; Marroquin-Quelopana, M.; et al. Development of the Sm14/GLA-SE Schistosomiasis Vaccine Candidate: An Open, Non-Placebo-Controlled, Standardized-Dose Immunization Phase Ib Clinical Trial Targeting Healthy Young Women. Vaccines 2022, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
- Fundação Oswaldo Cruz. Safety and Immunogenicity Evaluation of the Vaccine Candidate Sm14 in Combination with the Adjuvant Glucopyranosyl Lipid A (GLA-SE) in Adults Living in Endemic Regions for S. mansoni and S. haematobium in Senegal. A Comparative, Randomized, Open-Label Trial. 2016; Recorded by the Senegalese Ethics Committee (CNERS) Under the Reference: SEN 16/26; and Its Extension Under the Reference: SEN 17/22. Available online: https://clinicaltrials.gov/study/NCT03041766 (accessed on 30 October 2024).
- Fundação Oswaldo Cruz. Safety and Immunogenicity Evaluation of the Vaccine Candidate Sm14 Against Schistosomiasis in Senegalese School Children Healthy or Infected with S. mansoni and/or S. haematobium. A Comparative, Randomized, Controlled, Open-Label Trial. 2019; Recorded by the Senegalese Ethics Committee Under the Reference: SEN 18/26. Available online: https://clinicaltrials.gov/study/NCT03799510 (accessed on 30 October 2024).
- International Centers for Tropical Disease Research Network. ICTDR Investigator Manual: Monitoring and Reporting Adverse Events; NIAID: Bethesda, MD, USA, 2003.
- Food and Drug Administration. FDA Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials; Center for Biologics Evaluation and Research: Rockville, MD, USA, 2007.
- Borkowf, C.B. Constructing Binomial Confidence Intervals with near Nominal Coverage by Adding a Single Imaginary Failure or Success. Stat. Med. 2006, 25, 3679–3695. [Google Scholar] [CrossRef] [PubMed]
- Queto, T.; Vasconcelos, Z.F.M.; Luz, R.A.; Anselmo, C.; Guiné, A.A.A.; e Silva, P.M.R.; Farache, J.; Cunha, J.M.T.; Bonomo, A.C.; Gaspar-Elsas, M.I.C.; et al. G-CSF Suppresses Allergic Pulmonary Inflammation, Downmodulating Cytokine, Chemokine and Eosinophil Production. Life Sci. 2011, 88, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Silva-Freitas, M.L.; Corrêa-Castro, G.; Cota, G.F.; Giacoia-Gripp, C.; Rabello, A.; Teixeira Dutra, J.; de Vasconcelos, Z.F.M.; Savino, W.; Da-Cruz, A.M.; Santos-Oliveira, J.R. Impaired Thymic Output Can Be Related to the Low Immune Reconstitution and T Cell Repertoire Disturbances in Relapsing Visceral Leishmaniasis Associated HIV/AIDS Patients. Front. Immunol. 2020, 11, 953. [Google Scholar] [CrossRef]
- Friedman, M. The Use of Ranks to Avoid the Assumption of Normality Implicit in the Analysis of Variance. J. Am. Stat. Assoc. 1937, 32, 675–701. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Siegel, S. Estatística não-paramétrica: Para as ciências do comportamento. In Estatística Não-Paramétrica: Para as Ciências do Comportamento; Mc Graw-Hill: São Paulo, Brazil, 1975; p. 350. [Google Scholar]
- Katz, N.; Chaves, A.; Pellegrino, J. A Simple Device for Quantitative Stool Thick-Smear Technique in Schistosomiasis Mansoni. Rev. Inst. Med. Trop. São Paulo 1972, 14, 397–400. [Google Scholar]
- Coler, R.N.; Duthie, M.S.; Hofmeyer, K.A.; Guderian, J.; Jayashankar, L.; Vergara, J.; Rolf, T.; Misquith, A.; Laurance, J.D.; Raman, V.S.; et al. From Mouse to Man: Safety, Immunogenicity and Efficacy of a Candidate Leishmaniasis Vaccine LEISH-F3+GLA-SE. Clin. Transl. Immunol. 2015, 4, e35. [Google Scholar] [CrossRef]
- Windish, H.P.; Duthie, M.S.; Misquith, A.; Ireton, G.; Lucas, E.; Laurance, J.D.; Bailor, R.H.; Coler, R.N.; Reed, S.G. Protection of Mice from Mycobacterium Tuberculosis by ID87/GLA-SE, a Novel Tuberculosis Subunit Vaccine Candidate. Vaccine 2011, 29, 7842–7848. [Google Scholar] [CrossRef]
- Duthie, M.S.; Coler, R.N.; Laurance, J.D.; Sampaio, L.H.; Oliveira, R.M.; Sousa, A.L.M.; Stefani, M.M.A.; Maeda, Y.; Matsuoka, M.; Makino, M.; et al. Protection against Mycobacterium Leprae Infection by the ID83/GLA-SE and ID93/GLA-SE Vaccines Developed for Tuberculosis. Infect. Immun. 2014, 82, 3979–3985. [Google Scholar] [CrossRef]
- Reed, S.G.; Carter, D.; Casper, C.; Duthie, M.S.; Fox, C.B. Correlates of GLA Family Adjuvants’ Activities. Semin. Immunol. 2018, 39, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.-A. 2—Vaccine Immunology. In Plotkin’s Vaccines, 7th ed.; Plotkin, S.A., Orenstein, W.A., Offit, P.A., Edwards, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 16–34.e7. ISBN 978-0-323-35761-6. [Google Scholar]
- Yamey, G.; McDade, K.K.; Anderson, R.M.; Bartsch, S.M.; Bottazzi, M.E.; Diemert, D.; Hotez, P.J.; Lee, B.Y.; McManus, D.; Molehin, A.J.; et al. Vaccine Value Profile for Schistosomiasis. Vaccine 2024, 126020. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, N.R.; Leonardo, L.R.; Mitchell, G.F. Vaccine-Linked Chemotherapy: Can Schistosomiasis Control Benefit from an Integrated Approach? Trends Parasitol. 2005, 21, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson, D.C.; Maayan, N.; Donegan, S.; Chaplin, M.; Garner, P. Public Health Deworming Programmes for Soil-transmitted Helminths in Children Living in Endemic Areas. Cochrane Database Syst. Rev. 2019, 2019, CD000371. [Google Scholar] [CrossRef]
- World Health Organization. Global Vaccine Action Plan 2011–2020; World Health Organization: Geneva, Switzerland, 2013; ISBN 978-92-4-150498-0. [Google Scholar]

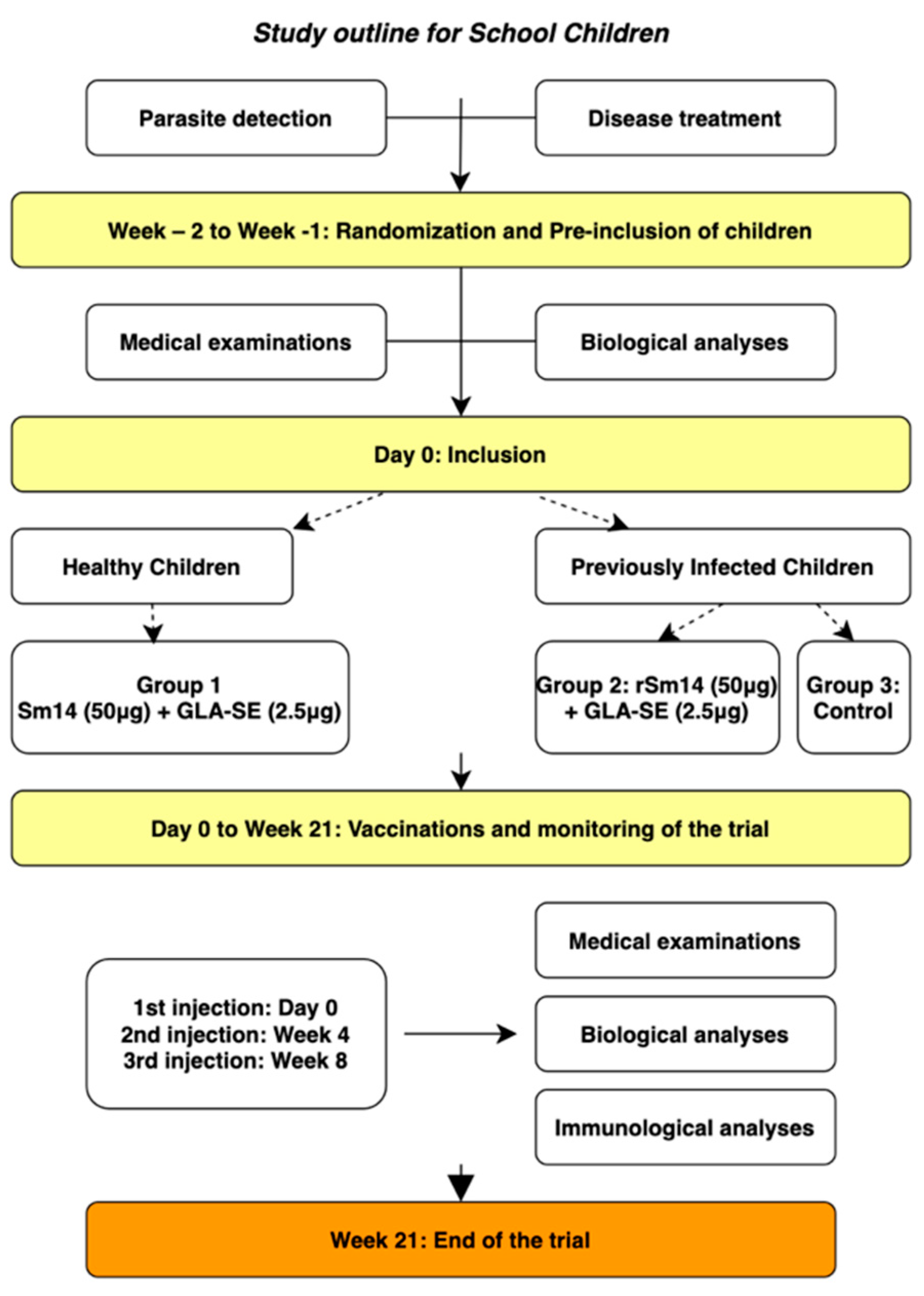




| Trial Characteristic | Healthy, Vaccinated Children (Group 1) | Previously Infected, Vaccinated Children (Group 2) | Previously Infected, Non-Vaccinated Children (Group 3) | |
|---|---|---|---|---|
| Sample size | 15 | 40 | 40 | |
| Age | ||||
| Years | Mean (SD) | 9.3 (1.2) | 9.4 (1.1) | 9.2 (1.1) |
| Median | (min, max) | 9 (8, 11) | 9.5 (8, 11) | 9.0 (8, 11) |
| Sex % | males (n) | 40.0 (6) | 50.0 (20) | 42.5 (17) |
| Infection type | ||||
| S. haematobium only | % (n) | 0 (0) | 50.0 (20) | 62.5 (25) |
| S. mansoni only | % (n) | 0 (0) | 35.0 (14) | 27.5 (11) |
| Mixed infection | % (n) | 0 (0) | 15.0 (6) | 10.0 (4) |
| Healthy group: first vaccine injection (D0) | |||||||
|---|---|---|---|---|---|---|---|
| Week 1 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | 1 | 2 | 3 | ||||
| Group 2 (Previously infected children + rSm14) | |||||||
| Group 3 (Unvaccinated, previously infected children) | |||||||
| Inclusion | Week 1 = 6 subjects | ||||||
| Vaccination | Week 1 = 6 subjects | ||||||
| Week 2 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | 3 | 3 | 3 | ||||
| Group 2 (Previously infected children + rSm14) | |||||||
| Group 3 (Unvaccinated, previously infected children) | |||||||
| Inclusion | Week 2 = 9 subjects | ||||||
| Vaccination | Week 2 = 9 subjects | ||||||
| Infected group: first vaccine injection (D0) | |||||||
| Week 3 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | |||||||
| Group 2 (Previously infected children + rSm14) | 1 | 2 | 3 | ||||
| Group 3 (Unvaccinated, previously infected children) | 1 | 2 | 3 | ||||
| Inclusions | Week 3 = 12 subjects | ||||||
| Vaccination | Week 3 = 6 subjects | ||||||
| Week 4 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | |||||||
| Group 2 (Previously infected children + rSm14) | 3 | 3 | 3 | ||||
| Group 3 (Unvaccinated, previously infected children) | 3 | 3 | 3 | ||||
| Inclusion | Week 4 = 18 subjects | ||||||
| Vaccination | Week 4 = 9 subjects | ||||||
| Week 5 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | |||||||
| Group 2 (Previously infected children + rSm14) | 3 | 3 | 3 | ||||
| Group 3 (Unvaccinated, previously infected children) | 3 | 3 | 3 | ||||
| Inclusion | Week 5 = 18 subjects | ||||||
| Vaccination | Week 5 = 9 subjects | ||||||
| Week 6 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | |||||||
| Group 2 (Previously infected children + rSm14) | 3 | 3 | 3 | ||||
| Group 3 (Unvaccinated, previously infected children) | 3 | 3 | 3 | ||||
| Inclusion | Week 6 = 18 subjects | ||||||
| Vaccination | Week 6 = 9 subjects | ||||||
| Week 7 | |||||||
| M | T | W | T | F | Sat | Sun | |
| Group 1 (Healthy children + rSm14) | |||||||
| Group 2 (Previously infected children + rSm14) | 3 | 3 | 1 | ||||
| Group 3 (Unvaccinated, previously infected children) | 3 | 3 | 1 | ||||
| Inclusion | Week 7 = 14 subjects | ||||||
| Vaccination | Week 7 = 7 subjects | ||||||
| Adverse Events | GLA-SE Content | AE After First Injection * (95% CI) | AE After Second Injection ** (95% CI) | AE After Third Injection *** (95% CI) |
|---|---|---|---|---|
| Serious | 2.5 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) |
| 5.0 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | |
| Pain at site of injection | 2.5 μg | 0.13 (0.02, 0.40) | 0.00 (0.00, 0.22) | 0.07 (0.002, 0.32) |
| 5.0 μg | 0.20 (0.04, 0.48) | 0.20 (0.04, 0.48) | 0.13 (0.02, 0.40) | |
| Heavy arm post injection | 2.5 μg | 0.00 (0.00, 0.22) | 0.20 (0.04, 0.48) | 0.07 (0.002, 0.32) |
| 5.0 μg | 0.07 (0.002, 0.32) | 0.27 (0.08, 0.55) | 0.20 (0.04, 0.48) | |
| Pruritus | 2.5 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.13 (0.02, 0.40) |
| 5.0 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | |
| Dizziness and/or headache | 2.5 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) |
| 5.0 μg | 0.00 (0.00, 0.22) | 0.07 (0.002, 0.32) | 0.00 (0.00, 0.22) | |
| Acute gastroenteritis | 2.5 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.07 (0.002, 0.32) |
| 5.0 μg | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) | 0.00 (0.00, 0.22) |
| Adverse Event (AE) | Group | No. a | AE After First Injection b Event (95% CI) | No. a | AE After Second Injection c Event (95% CI) | No. a | AE After Third Injection d Event (95% CI) |
|---|---|---|---|---|---|---|---|
| Serious (Grades 3–4) | Healthy | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | |
| Pain at site of infection | Healthy | 0/15 | 0.00 (0.00, 0.22) | 2/15 | 0.13 (0.02, 0.40) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 2/40 | 0.05 (0.01, 0.17) | 3/40 | 0.08 (0.02, 0.20) | 3/40 | 0.08 (0.02, 0.20) | |
| Heavy arm post inject. | Healthy | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | 1/40 | 0.02 (0.001, 0.13) | |
| Pruritus | Healthy | 1/15 | 0.00 (0.00, 0.22) | 1/15 | 0.00 (0.00, 0.22) | 1/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | 1/40 | 0.02 (0.001, 0.13) | |
| Swelling | Healthy | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | 1/40 | 0.02 (0.001, 0.13) | |
| Fever | Healthy | 1/15 | 0.07 (0.002, 0.32) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 0/40 | 0.00 (0.00, 0.09) | 1/40 | 0.02 (0.001, 0.13) | 1/40 | 0.02 (0.001, 0.13) | |
| Headache | Healthy | 1/15 | 0.07 (0.002, 0.32) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 1/40 | 0.02 (0.001, 0.13) | 2/40 | 0.05 (0.01, 0.17) | 0/40 | 0.00 (0.00, 0.09) | |
| Abdominal pain | Healthy | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 1/40 | 0.02 (0.001, 0.13) | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) | |
| Vomiting | Healthy | 0/15 | 0.00 (0.00, 0.22) | 1/15 | 0.07 (0.002, 0.32) | 0/15 | 0.00 (0.00, 0.22) |
| Pre-infected | 1/40 | 0.02 (0.001, 0.13) | 0/40 | 0.00 (0.00, 0.09) | 0/40 | 0.00 (0.00, 0.09) |
| Blood Collection (Day) | Group | ||||
|---|---|---|---|---|---|
| IgG Response | Healthy, Vaccinated a Group 1 | Previously Infected, Vaccinated b Group 2 | Previously Infected, Non-Vaccinated c Group 3 | p-Value d | |
| 0 | Mean (SD) | 56 (176) | 321 (627) | 324 (751) | |
| Median (min, max) | 0 (0, 688) | 2 (0, 2306) | 0 (0, 3370) | ||
| Responder % (n) | 6.7% (1) | 25.0% (10) | 20.0% (8) | 0.40 | |
| 84 | Mean (SD) | 3533 (1778) | 2555 (1672) | 262 (591) | |
| Median (min, max) | 3118 (1763, 9180) | 2211 (22, 7326) | 0 (0, 2332) | ||
| Responder % (n) | 100 (15) | 92.5 (37) | 20.0 (8) | <0.001 | |
| 148 | Mean (SD) | 2078 (1210) | 1626 (1177) | 231 (536) | |
| Median (min, max) | 1620 (920, 5624) | 1605 (1, 4998) | 0 (0, 2072) | ||
| Responder % (n) | 100 (15) | 90.0 (36) | 20.0 (8) | <0.001 | |
| 400+ e | Mean (SD) | 1322 (973) | 1205 (1014) | 317 (604) | |
| Median (min, max) | 1203 (61, 3476) | 1110 (0, 3336) | 1 (0, 2454) | ||
| Responder % (n) | 92.9 (13) | 73.7 (28) | 33.3 (13) | <0.001 | |
| Cell Population | Healthy, Vaccinated Children (Group 1) | Previously Infected, Vaccinated Children (Group 2) | Previously Infected, Non-Vaccinated Children (Group 3) | |||
|---|---|---|---|---|---|---|
| T cells (CD3+) | Not significant | ↓ Day 84 (%) | p = 0.0036 | ↑ Day 148 (%) | p = 0.0349 | |
| CD4+ T cells | Not significant | Not significant | Not significant | |||
| CD8+ T cells | ↓ Day 84 (AN) | p = 0.0105 | Not significant | Not significant | ||
| B cells | ↑ Day 84 (%) | p = 0.0105 | ↓ Day 84 (%) | p = 0.0003 | ↓ Day 148 (%) | p = 0.0017 |
| Monocytes | Not significant | ↑ Day 84/Day 148 (%/AN) | p = 0.0029/0.0063 p = 0.0110/0.0417 | ↑ Day 148 (%) | p = 0.0250 | |
| CD4 T-Cell Subpopulation | Healthy, Vaccinated Children (Group 1) | Previously Infected, Vaccinated Children (Group 2) | Previously Infected, Non-Vaccinated Children (Group 3) | ||
|---|---|---|---|---|---|
| Naïve T cells | ↑ Day 84 | p = 0.0016 | Not significant | ↑ Day 148 | p = 0.0250 |
| ↑ Day 148 | p < 0.0001 | ||||
| Effector T cells | ↓ Day 84 | p = 0.0030 | Not significant | Not significant | |
| ↓ Day 148 | p = 0.0030 | ||||
| EMT cells | ↓ Day 84 | p < 0.0001 | Not significant | Not significant | |
| ↓ Day 148 | p = 0.0105 | ||||
| CMT cells | Not significant | Not significant | Not significant | ||
| CD8 T-Cell Subpopulation | Healthy, Vaccinated Children (Group 1) | Previously Infected, Vaccinated Children (Group 2) | Previously Infected, Non-Vaccinated Children (Group 3) | ||
|---|---|---|---|---|---|
| Naïve T cells | ↑ Day 84 | p = 0.0001 | Not significant | Not significant | |
| ↑ Day 148 | p = 0.0411 | ||||
| Effector T cells | ↓ Day 148 | p = 0.0185 | Not significant | Not significant | |
| EMT cells | ↓ Day 84 | p = 0.0008 | ↓ Day 84 | p = 0.0052 | Not significant |
| CMT cells | Not significant | ↓ Day 84 | p = 0.0024 | Not significant | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, A.T.; Diop, D.; Diop, M.; Schacht, A.-M.; Mbengue, A.; Diagne, R.; Guisse, M.; Dompnier, J.-P.; Messias, C.; Coler, R.N.; et al. The Sm14+GLA-SE Recombinant Vaccine Against Schistosoma mansoni and S. haematobium in Adults and School Children: Phase II Clinical Trials in West Africa. Vaccines 2025, 13, 316. https://doi.org/10.3390/vaccines13030316
Ly AT, Diop D, Diop M, Schacht A-M, Mbengue A, Diagne R, Guisse M, Dompnier J-P, Messias C, Coler RN, et al. The Sm14+GLA-SE Recombinant Vaccine Against Schistosoma mansoni and S. haematobium in Adults and School Children: Phase II Clinical Trials in West Africa. Vaccines. 2025; 13(3):316. https://doi.org/10.3390/vaccines13030316
Chicago/Turabian StyleLy, Amadou Tidjani, Doudou Diop, Modou Diop, Anne-Marie Schacht, Abdoulaye Mbengue, Rokhaya Diagne, Marieme Guisse, Jean-Pierre Dompnier, Carolina Messias, Rhea N. Coler, and et al. 2025. "The Sm14+GLA-SE Recombinant Vaccine Against Schistosoma mansoni and S. haematobium in Adults and School Children: Phase II Clinical Trials in West Africa" Vaccines 13, no. 3: 316. https://doi.org/10.3390/vaccines13030316
APA StyleLy, A. T., Diop, D., Diop, M., Schacht, A.-M., Mbengue, A., Diagne, R., Guisse, M., Dompnier, J.-P., Messias, C., Coler, R. N., Ramos, C. R., Tendeng, J.-N., Ndiaye, S., Marroquin-Quelopana, M., de Carvalho Parra, J., dos Santos, T., Sirianni dos Santos Almeida, M., Mendes-da-Cruz, D. A., Reed, S., ... Tendler, M. (2025). The Sm14+GLA-SE Recombinant Vaccine Against Schistosoma mansoni and S. haematobium in Adults and School Children: Phase II Clinical Trials in West Africa. Vaccines, 13(3), 316. https://doi.org/10.3390/vaccines13030316






