An mRNA Vaccine for Herpes Zoster and Its Efficacy Evaluation in Naïve/Primed Murine Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Plasmid Templates
2.2. Plasmid Linearization and In Vitro Transcription (IVT)
2.3. Lipid Nanoparticle (LNP) Encapsulation
2.4. Characterization and Quality Control
2.5. Cell Transfection
2.6. Flow Cytometric Analysis
2.7. Western Blot
2.8. Animal Studies
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Intracellular Cytokine Staining (ICS)
2.11. Enzyme-Linked Immunosorbent Spot Assay (ELISpot)
2.12. Mouse Inflammatory Cytokine Cytometric Bead Array (CBA) and Th1/Th2 Cytokine CBA
2.13. RNA Optimization by AI Automation Screening Platform
2.14. Lyophilization
2.15. AlphaFold Prediction
2.16. Statistical Analysis
3. Results
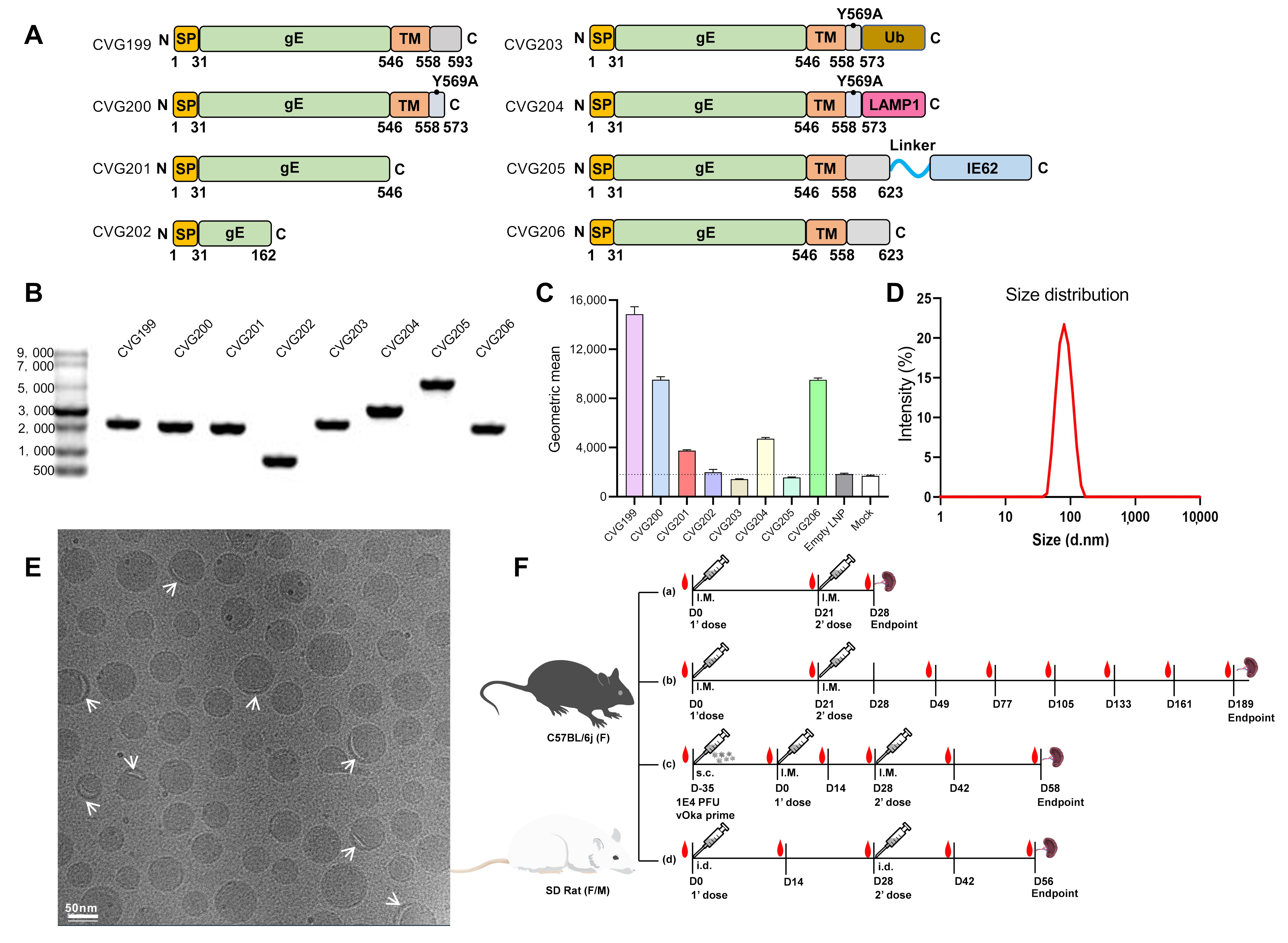
3.1. Rational Design and Characterization of Various VZV mRNA Constructs
3.2. mRNA Vaccine Preparations: In Vitro Transcription (IVT), Lipid Nanoparticle (LNP) Encapsulation, Physicochemical Characterization, and In Vitro Expression
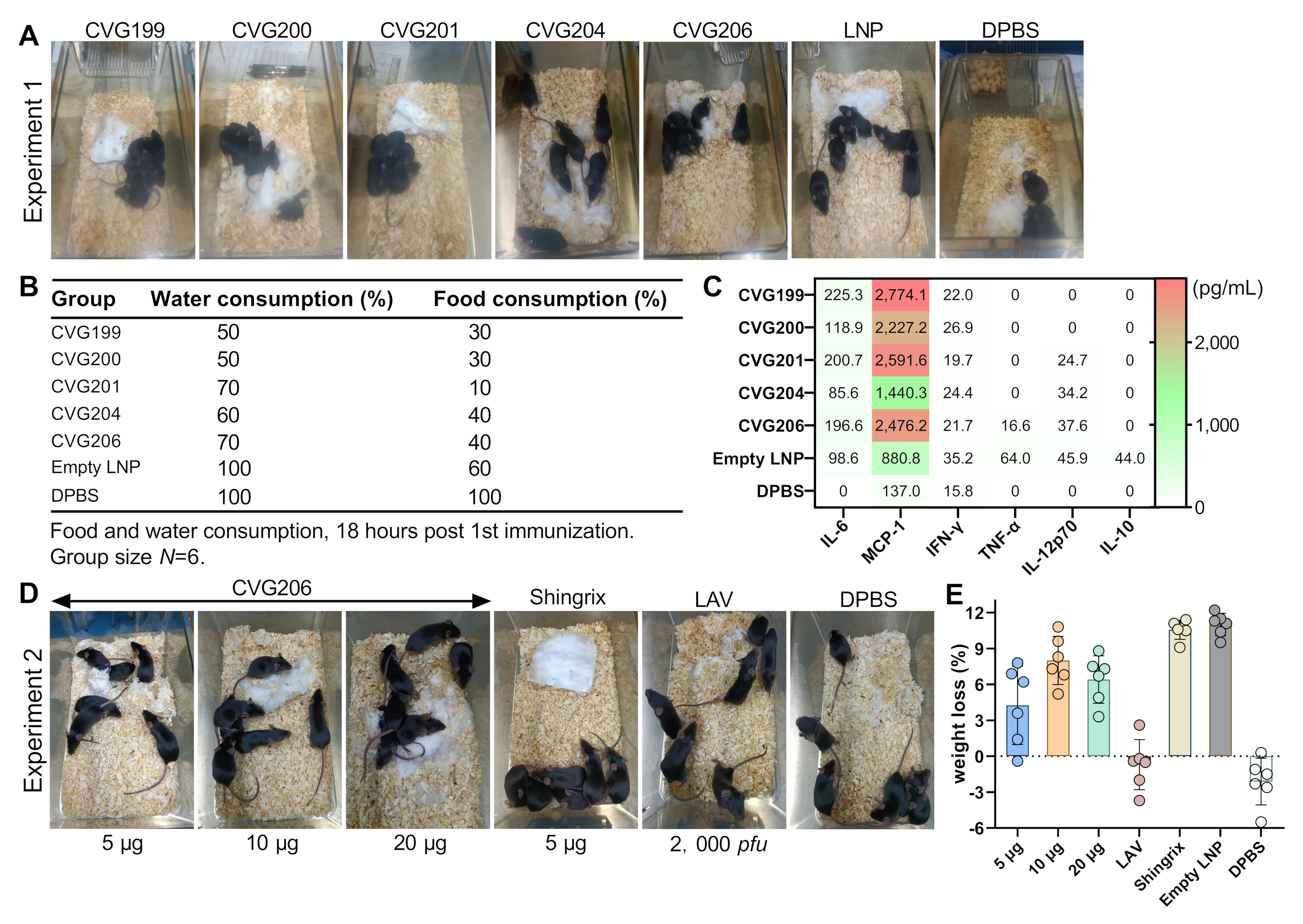
3.3. Behavioral Observation and Inflammatory Detection in Mice 18 h Post-Immunization
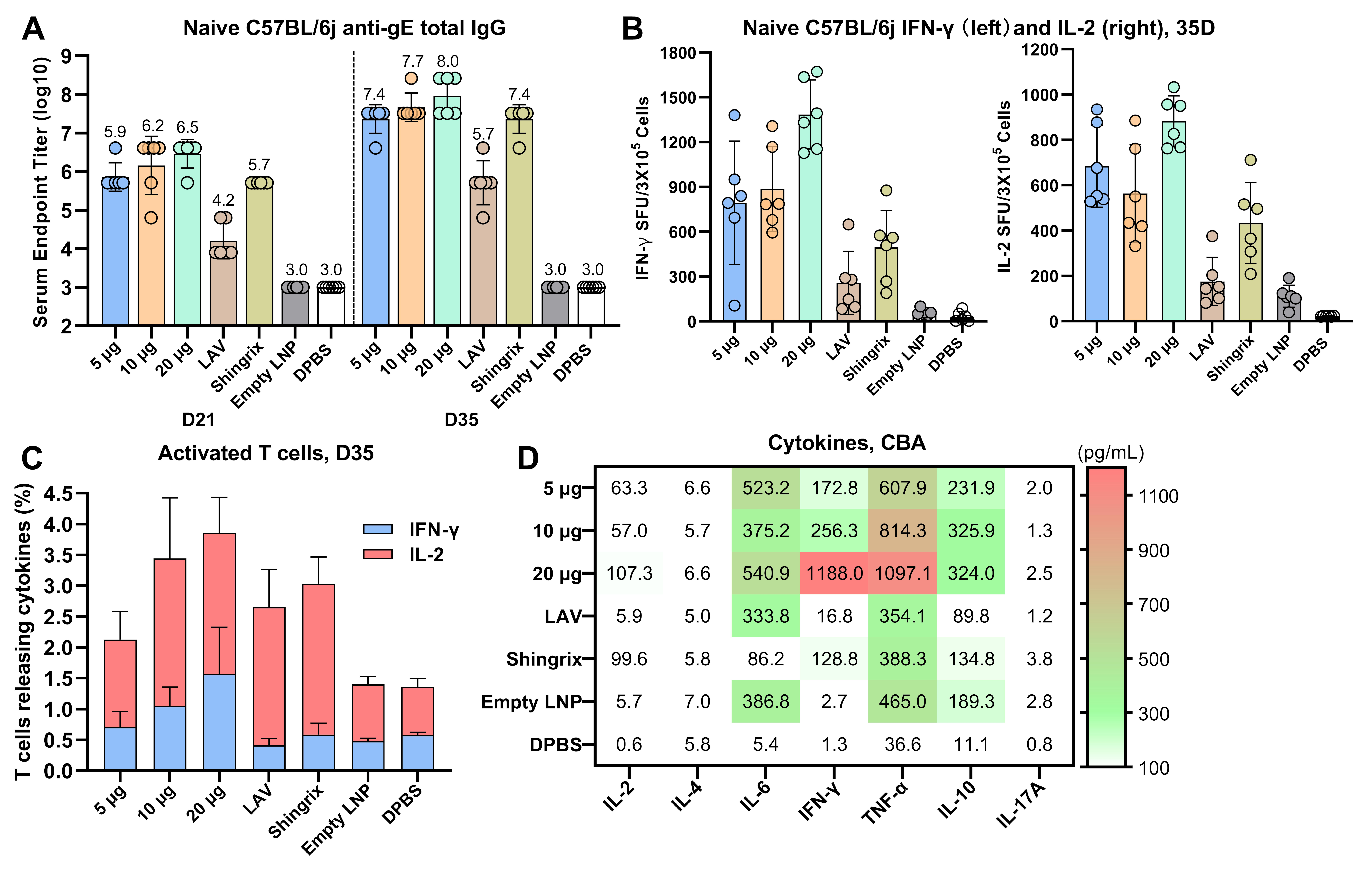
3.4. Robust Immune Responses Induced by VZV mRNAs in the Naïve Mouse Model
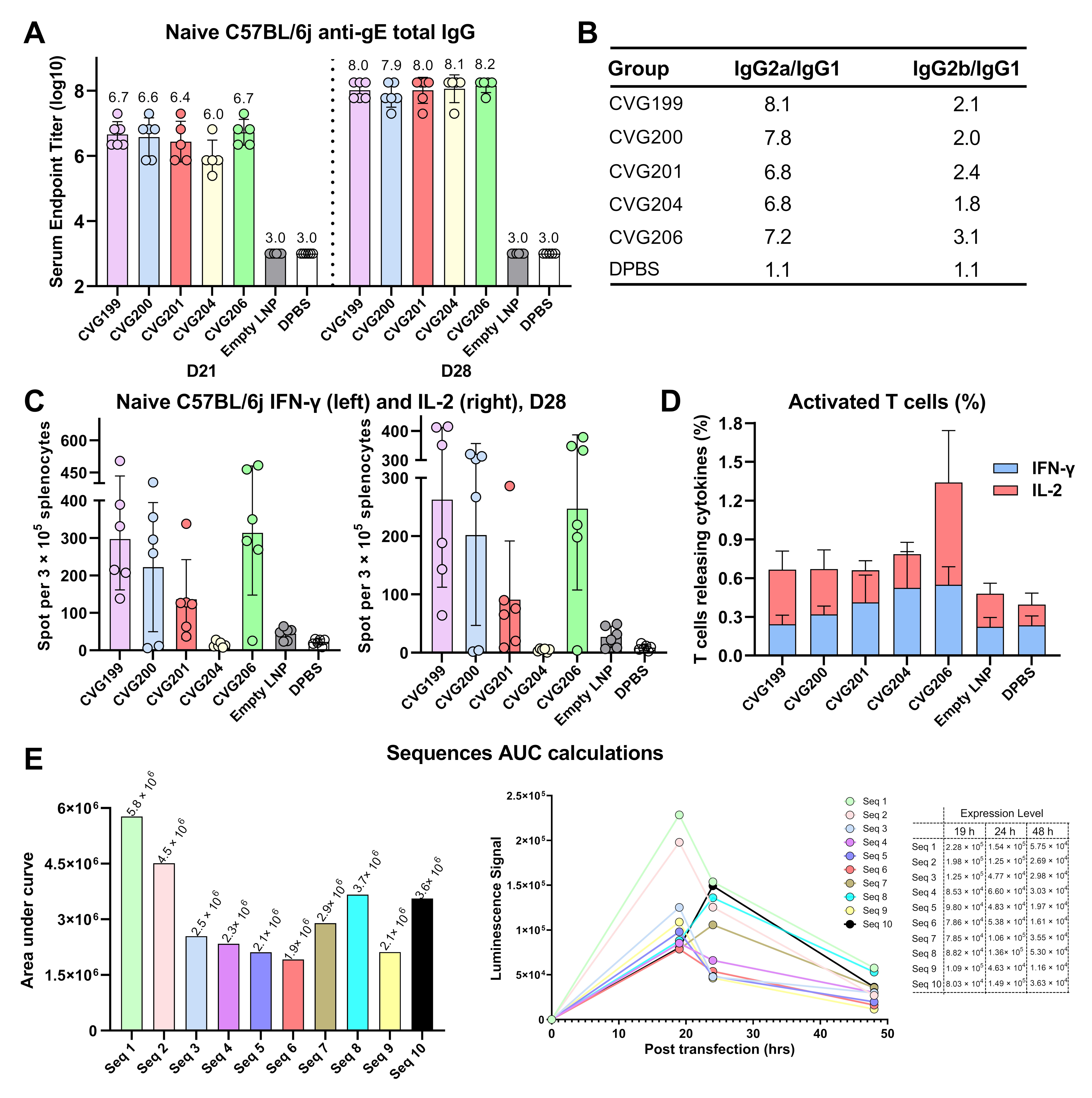
3.5. mRNA Sequence Optimization of CVG206 on Protein Accumulated Expression by Automated Screening Platform
3.6. 3D Structure Predictions and Immunogenicity Distribution Analysis of Full-Length and Derived Truncations of gE Protein

3.7. Dose Dependence of Humoral and Cellular Immune Responses to the CVG206 Vaccine in Mouse Model
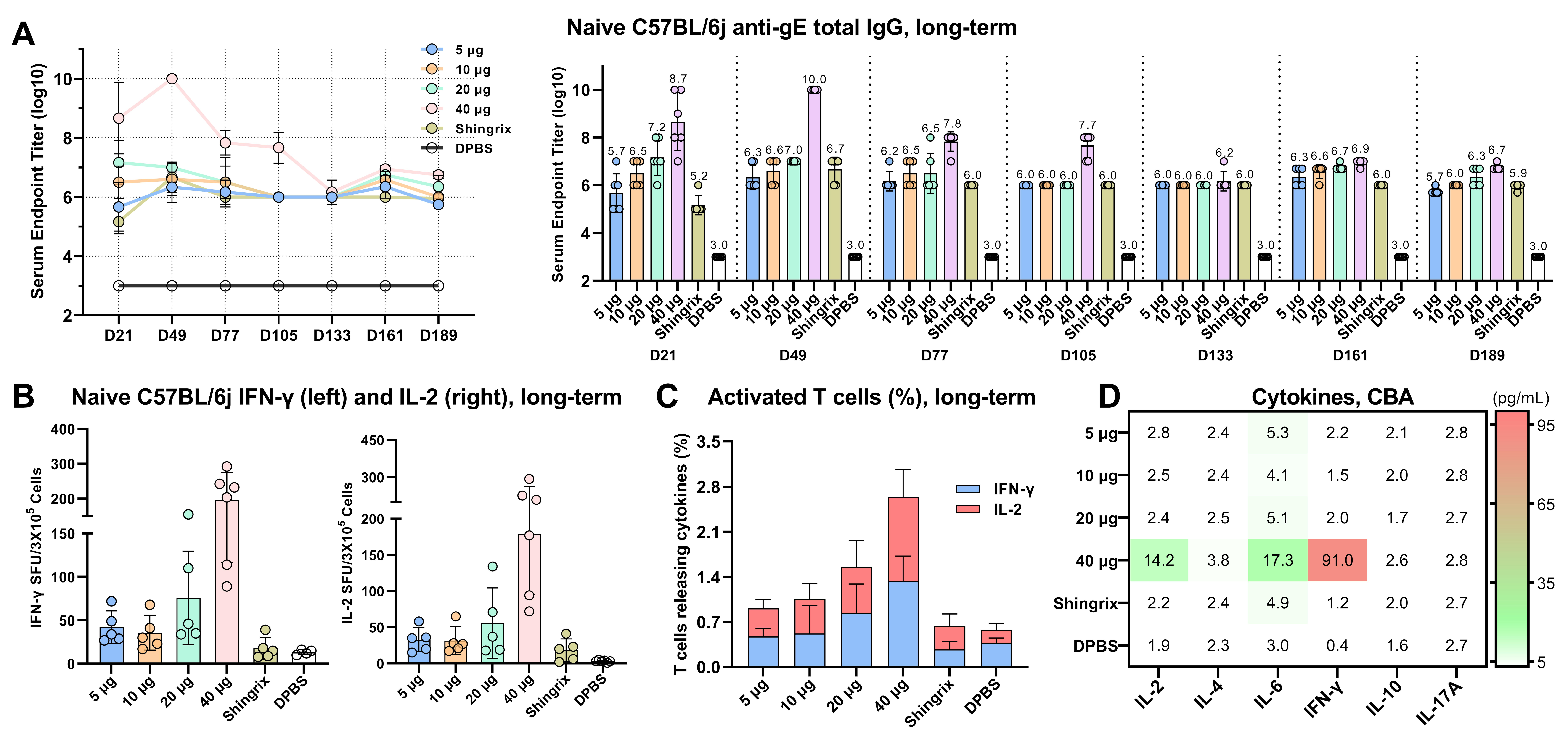
3.8. Long-Term Immunogenicity Evaluation of CVG206
3.9. Immunogenicity Induced by CVG206 in a VZV-Primed Animal Model

| Patch | Temp. | Month | Encapsulation (%) | Integrity (%) | Size (nm) | Zeta Pot. (mV) | Particle Distribution | mRNA Polymer (%) |
|---|---|---|---|---|---|---|---|---|
| 202401001 | 2–8 °C | 0 | 92.6 | 84.3 | 78 | 2.0 | Dv (10):42 Dv (50): 61 Dv (90):100 | 9.5 |
| 2–8 °C | 1 | 90.6 | 83.6 | 78 | 0.5 | Dv (10):45 Dv (50): 64 Dv (90):102 | 10.6 | |
| 2–8 °C | 2 | 91.0 | 83.2 | 77 | 1.0 | Dv (10):40 Dv (50): 58 Dv (90):98 | 9.1 | |
| 2–8 °C | 3 | 92.4 | 81.8 | 76 | 2.0 | Dv (10):40 Dv (50): 59 Dv (90):96 | 9.8 | |
| 2–8 °C | 6 | 89.6 | 82.6 | 75 | 2.0 | Dv (10):44 Dv (50): 63 Dv (90):96 | 11.9 | |
| 2–8 °C | 9 | 89.9 | 81.7 | 75 | 2.0 | Dv (10):40 Dv (50): 58 Dv (90):94 | 12.7 | |
| 2–8 °C | criterion | ≥80.0 | ≥65.0 | 50~150 | −30~30 | ≤15 | ||
| Patch | Temp | Month | Moisture (%) | mRNA Content (µg) | Insoluble Particles | Morphology | Translation Activity | mRNA–Lipid Adducts (%) |
| 202401001 | 2–8 °C | 0 | 0.5 | 56 | ≥10 µm: 792 ≥25 µm: 7 | White, loose | High | 0.6 |
| 2–8 °C | 1 | 1.0 | 51 | ≥10 µm: 1914 ≥25 µm: 13 | White, loose | High | 0.6 | |
| 2–8 °C | 2 | 1.0 | 48 | ≥10 µm: 2144 ≥25 µm: 11 | White, loose | High | 0.6 | |
| 2–8 °C | 3 | 1.1 | 49 | ≥10 µm: 1750 ≥25 µm: 14 | White, loose | High | 0.6 | |
| 2–8 °C | 6 | 0.9 | 49 | ≥10 µm: 2133 ≥25 µm: 19 | White, loose | High | 0.7 | |
| 2–8 °C | 9 | 0.9 | 56 | ≥10 µm: 2447 ≥25 µm: 19 | White, loose | High | 1.8 | |
| 2–8 °C | criterion | ≤3 | 40~60/Vial | ≥10 µm: 6000 ≥25 µm: 600 | White, loose | ≥50% | ≤10 |
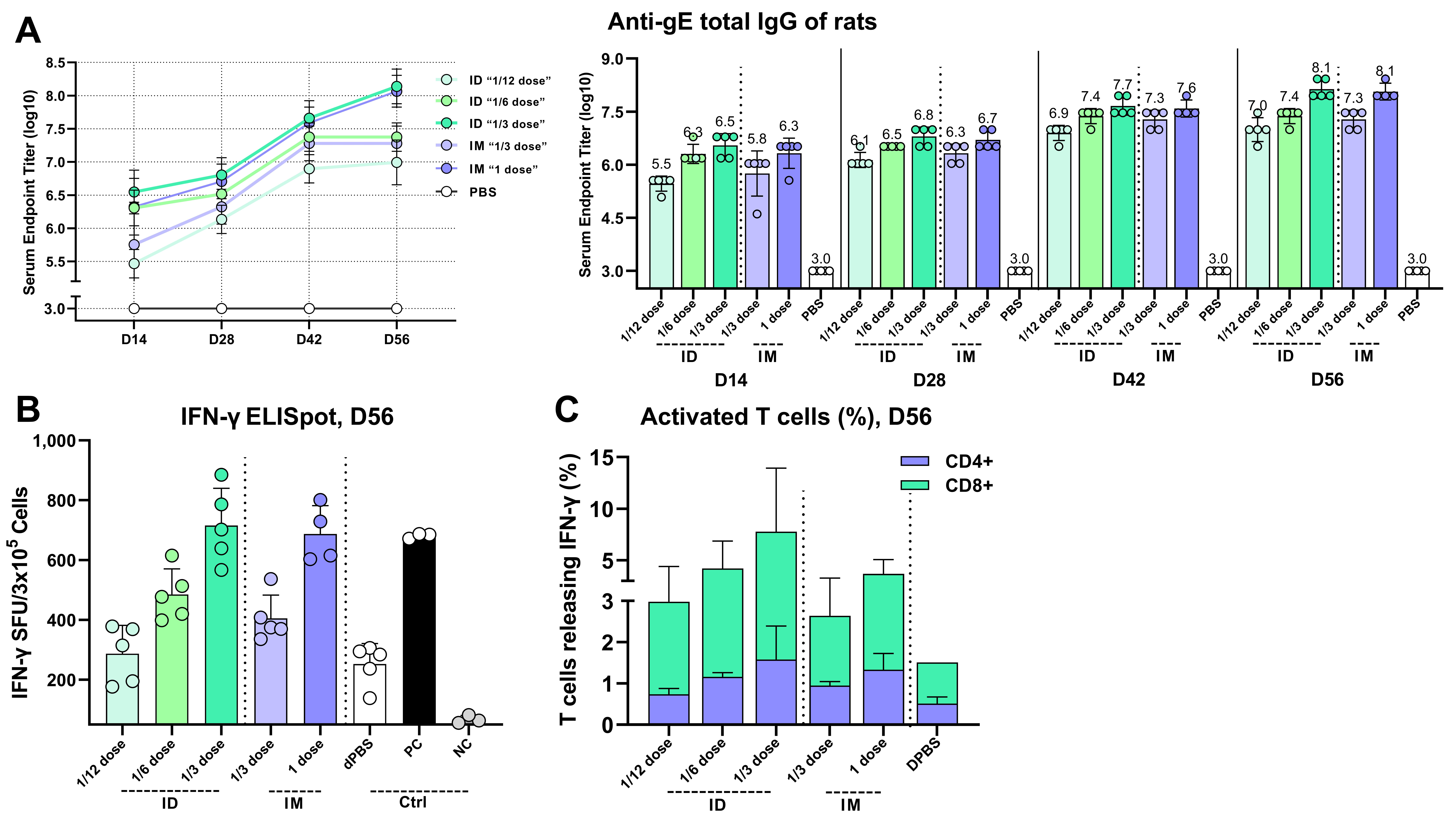
3.10. Intradermal Administration of CVG206 mRNA Vaccine Induced Comparable Robust Immune Responses at Much Lower Doses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, R.W.; Bouhassira, D.; Kassianos, G.; Leplège, A.; Schmader, K.E.; Weinke, T. The Impact of Herpes Zoster and Post-Herpetic Neuralgia on Quality-of-Life. BMC Med 2010, 8, 37. [Google Scholar] [CrossRef]
- Sen, N.; Arvin, A.M. Dissecting the Molecular Mechanisms of the Tropism of VaricellaZoster Virus for Human T Cells. J. Virol. 2016, 90, 3284–3287. [Google Scholar] [CrossRef] [PubMed]
- Zerboni, L.; Sen, N.; Oliver, S.L.; Arvin, A.M. Molecular Mechanisms of Varicella Zoster Virus Pathogenesis. Nat. Rev. Microbiol. 2014, 12, 197–210. [Google Scholar] [CrossRef]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.E.; Oxman, M.N.; et al. Varicella Zoster Virus Infection. Nat. Rev. Dis. Primers 2015, 1, 15016. [Google Scholar] [CrossRef]
- van Oorschot, D.; Vroling, H.; Bunge, E.; Diaz-Decaro, J.; Curran, D.; Yawn, B. A Systematic Literature Review of Herpes Zoster Incidence Worldwide. Hum. Vaccines Immunother. 2021, 17, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Arai, H.; Chen, L.-Y.; Chou, M.-Y.; Djauzi, S.; Dong, B.; Kojima, T.; Kwon, K.T.; Leong, H.N.; Leung, E.M.F.; et al. Looking Back to Move Forward: A Twenty-Year Audit of Herpes Zoster in Asia-Pacific. BMC Infect. Dis. 2017, 17, 213. [Google Scholar] [CrossRef]
- Yang, F.; Yu, S.; Fan, B.; Liu, Y.; Chen, Y.X.; Kudel, I.; Concialdi, K.; DiBonaventura, M.; Hopps, M.; Hlavacek, P.; et al. The Epidemiology of Herpes Zoster and Postherpetic Neuralgia in China: Results from a Cross-Sectional Study. Pain. Ther. 2019, 8, 249–259. [Google Scholar] [CrossRef]
- Patil, A.; Goldust, M.; Wollina, U. Herpes Zoster: A Review of Clinical Manifestations and Management. Viruses 2022, 14, 192. [Google Scholar] [CrossRef]
- van der Beek, M.; Vermont, C.; Bredius, R.; Marijt, E.; van der blij-de Brower, C.; Kroes, A.; Claas, E.; Vossen, A. Persistence and Antiviral Resistance of Varicella Zoster Virus in Hematological Patients. Clin. Infect. Dis. 2012, 56, 335–343. [Google Scholar] [CrossRef]
- Jeon, Y.H. Herpes Zoster and Postherpetic Neuralgia: Practical Consideration for Prevention and Treatment. Korean J. Pain. 2015, 28, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Asada, H. Recent Topics in the Management of Herpes Zoster. J. Dermatol. 2023, 50, 305–310. [Google Scholar] [CrossRef]
- Shingrix FDA Approval History. Available online: https://www.drugs.com/history/shingrix.html (accessed on 5 December 2024).
- Shah, R.A.; Limmer, A.L.; Nwannunu, C.E.; Patel, R.R.; Mui, U.N.; Tyring, S.K. Shingrix for Herpes Zoster: A Review. Ski. Ther. Lett. 2019, 24, 5–7. [Google Scholar]
- Guzmán, L.; Villalón, K.; Marchant, M.J.; Tarnok, M.E.; Cárdenas, P.; Aquea, G.; Acevedo, W.; Padilla, L.; Bernal, G.; Molinari, A.; et al. In Vitro Evaluation and Molecular Docking of QS-21 and Quillaic Acid from Quillaja Saponaria Molina as Gastric Cancer Agents. Sci. Rep. 2020, 10, 10534. [Google Scholar] [CrossRef] [PubMed]
- Dendouga, N.; Fochesato, M.; Lockman, L.; Mossman, S.; Giannini, S.L. Cell-Mediated Immune Responses to a Varicella-Zoster Virus Glycoprotein E Vaccine Using Both a TLR Agonist and QS21 in Mice. Vaccine 2012, 30, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Bartlett, J.; Fireman, B.; Marks, M.A.; Hansen, J.; Lewis, E.; Aukes, L.; Saddier, P. Effectiveness of the Live Zoster Vaccine during the 10 Years Following Vaccination: Real World Cohort Study Using Electronic Health Records. BMJ 2023, 383, e076321. [Google Scholar] [CrossRef]
- What Everyone Should Know About Zostavax. Available online: https://archive.cdc.gov/www_cdc_gov/vaccines/vpd/shingles/public/zostavax/index.html (accessed on 6 December 2024).
- Willis, E.D.; Woodward, M.; Brown, E.; Popmihajlov, Z.; Saddier, P.; Annunziato, P.W.; Halsey, N.A.; Gershon, A.A. Herpes Zoster Vaccine Live: A 10 Year Review of Post-Marketing Safety Experience. Vaccine 2017, 35, 7231–7239. [Google Scholar] [CrossRef]
- Lal, H.; Cunningham, A.L.; Godeaux, O.; Chlibek, R.; Diez-Domingo, J.; Hwang, S.-J.; Levin, M.J.; McElhaney, J.E.; Poder, A.; Puig-Barberà, J.; et al. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N. Engl. J. Med. 2015, 372, 2087–2096. [Google Scholar] [CrossRef]
- Wenbin Tuo, D.Z. QS-21: A Potent Vaccine Adjuvant. Nat. Prod. Chem. Res. 2015, 3, e113. [Google Scholar] [CrossRef]
- Monslow, M.A.; Elbashir, S.; Sullivan, N.L.; Thiriot, D.S.; Ahl, P.; Smith, J.; Miller, E.; Cook, J.; Cosmi, S.; Thoryk, E.; et al. Immunogenicity Generated by mRNA Vaccine Encoding VZV gE Antigen Is Comparable to Adjuvanted Subunit Vaccine and Better than Live Attenuated Vaccine in Nonhuman Primates. Vaccine 2020, 38, 5793–5802. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Kramps, T.; Elbers, K. RNA Vaccines: Methods and Protocols; Humana Press: New York, NY, USA, 2017; ISBN 978-1-4939-6479-6. [Google Scholar]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A Comprehensive Review of SARS-CoV-2 Vaccines: Pfizer, Moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar] [CrossRef]
- Hogan, M.J.; Pardi, N. mRNA Vaccines in the COVID-19 Pandemic and Beyond. Annu. Rev. Med. 2022, 73, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, Y.; Chong, K.; Cui, M.; Cao, Z.; Tang, C.; Tian, Z.; Hu, Y.; Zhao, Y.; Jiang, S. Recent Advances in Lipid Nanoparticles and Their Safety Concerns for mRNA Delivery. Vaccines 2024, 12, 1148. [Google Scholar] [CrossRef]
- Kozma, G.T.; Mészáros, T.; Berényi, P.; Facskó, R.; Patkó, Z.; Oláh, C.Z.; Nagy, A.; Fülöp, T.G.; Glatter, K.A.; Radovits, T.; et al. Role of Anti-Polyethylene Glycol (PEG) Antibodies in the Allergic Reactions to PEG-Containing Covid-19 Vaccines: Evidence for Immunogenicity of PEG. Vaccine 2023, 41, 4561–4570. [Google Scholar] [CrossRef]
- Pfizer and BioNTech Initiate Phase 1/2 Study of First mRNA-Based Shingles Vaccine Program. Available online: https://www.pfizer.com/news/announcements/pfizer-and-biontech-initiate-phase-12-study-first-mrna-based-shingles-vaccine (accessed on 8 January 2025).
- Moderna Advances Multiple Vaccine Programs to Late-Stage Clinical Trials. Available online: https://news.modernatx.com/news/news-details/2024/Moderna-Advances-Multiple-Vaccine-Programs-to-Late-Stage-Clinical-Trials/default.aspx (accessed on 8 January 2025).
- Bhattacharya, A.; Jan, L.; Burlak, O.; Li, J.; Upadhyay, G.; Williams, K.; Dong, J.; Rohrer, H.; Pynn, M.; Simon, A.; et al. Potent and Long-Lasting Humoral and Cellular Immunity against Varicella Zoster Virus Induced by mRNA-LNP Vaccine. npj Vaccines 2024, 9, 72. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, T.; Zhao, W.; Shao, A.; Zhao, H.; Ma, W.; Gong, Y.; Zeng, X.; Weng, C.; Bu, L.; et al. Herpes Zoster mRNA Vaccine Induces Superior Vaccine Immunity over Licensed Vaccine in Mice and Rhesus Macaques. Emerg. Microbes Infect. 2024, 13, 2309985. [Google Scholar] [CrossRef]
- Innorna Receives IND Clearance for IN001 Herpes Zoster mRNA Vaccine in China. Available online: https://www.innorna.com/news/316.html (accessed on 21 January 2025).
- Welsh, M.D.; Harper, D.R.; Garcia-Valcarcel, M.; Fowler, W.J.; Aitken, C.; Jeffries, D.J.; Layton, G.T. Ability of Yeast Ty-VLPs (Virus-like Particles) Containing Varicella-Zoster Virus (VZV)gE and Assembly Protein Fragments to Induce in Vitro Proliferation of Human Lymphocytes from VZV Immune Patients. J. Med. Virol. 1999, 59, 78–83. [Google Scholar] [CrossRef]
- Roos-Mattjus, P.; Sistonen, L. The Ubiquitin-proteasome Pathway. Ann. Med. 2004, 36, 285–295. [Google Scholar] [CrossRef]
- Zhang, M.; Obata, C.; Hisaeda, H.; Ishii, K.; Murata, S.; Chiba, T.; Tanaka, K.; Li, Y.; Furue, M.; Chou, B.; et al. A Novel DNA Vaccine Based on Ubiquitin–Proteasome Pathway Targeting ‘Self’-Antigens Expressed in Melanoma/Melanocyte. Gene Ther. 2005, 12, 1049–1057. [Google Scholar] [CrossRef]
- Rinaldi, M.; Fioretti, D.; Lurescia, S. (Eds.) DNA Vaccines: Methods and Protocols, 3rd ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; Volume 1143, ISBN 978-1-4939-0409-9. [Google Scholar]
- Hu, Y.-L.; Zhang, L.-Q.; Liu, X.-Q.; Ye, W.; Zhao, Y.-X.; Zhang, L.; Qiang, Z.-X.; Zhang, L.-X.; Lei, Y.-F.; Jiang, D.-B.; et al. Construction and Evaluation of DNA Vaccine Encoding Crimean Congo Hemorrhagic Fever Virus Nucleocapsid Protein, Glycoprotein N-Terminal and C-Terminal Fused with LAMP1. Front. Cell. Infect. Microbiol. 2023, 13, 1121163. [Google Scholar] [CrossRef] [PubMed]
- Kinchington, P.R.; Fite, K.; Turse, S.E. Nuclear Accumulation of IE62, the Varicella-Zoster Virus (VZV) Major Transcriptional Regulatory Protein, Is Inhibited by Phosphorylation Mediated by the VZV Open Reading Frame 66 Protein Kinase. J. Virol. 2000, 74, 2265–2277. [Google Scholar] [CrossRef] [PubMed]
- Fan, L. Codon Optimization. Available online: https://patents.google.com/patent/WO2020024917A1/en (accessed on 8 January 2025).
- Gaskill, B.N.; Karas, A.Z.; Garner, J.P.; Pritchett-Corning, K.R. Nest Building as an Indicator of Health and Welfare in Laboratory Mice. JoVE 2013, 82, 51012. [Google Scholar] [CrossRef]
- Brewer, J.M.; Tetley, L.; Richmond, J.; Liew, F.Y.; Alexander, J. Lipid Vesicle Size Determines the Th1 or Th2 Response to Entrapped Antigen. J. Immunol. 1998, 161, 4000–4007. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Bang, Y.-J.; Kwon, S.P.; Kwak, W.; Park, S.-I.; Roh, G.; Bae, S.-H.; Kim, J.-Y.; Kwak, H.W.; Kim, Y.; et al. Analyzing Immune Responses to Varied mRNA and Protein Vaccine Sequences. npj Vaccines 2023, 8, 84. [Google Scholar] [CrossRef]
- Yang, K.; Whalen, B.J.; Tirabassi, R.S.; Selin, L.K.; Levchenko, T.S.; Torchilin, V.P.; Kislauskis, E.H.; Guberski, D.L. A DNA Vaccine Prime Followed by a Liposome-Encapsulated Protein Boost Confers Enhanced Mucosal Immune Responses and Protection. J. Immunol. 2008, 180, 6159–6167. [Google Scholar] [CrossRef]
- Dorninger, F.; Zeitler, G.; Berger, J. Nestlet Shredding and Nest Building Tests to Assess Features of Psychiatric Disorders in Mice. BIO-PROTOCOL 2020, 10, e3863. [Google Scholar]
- Nussbaum, A.K.; Dick, T.P.; Keilholz, W.; Schirle, M.; Stevanović, S.; Dietz, K.; Heinemeyer, W.; Groll, M.; Wolf, D.H.; Huber, R.; et al. Cleavage Motifs of the Yeast 20S Proteasome β Subunits Deduced from Digests of Enolase 1. Proc. Natl. Acad. Sci. USA 1998, 95, 12504–12509. [Google Scholar] [CrossRef]
- Fishbain, S.; Prakash, S.; Herrig, A.; Elsasser, S.; Matouschek, A. Rad23 Escapes Degradation Because It Lacks a Proteasome Initiation Region. Nat. Commun. 2011, 2, 192. [Google Scholar] [CrossRef]
- Yu, H.; Kago, G.; Yellman, C.M.; Matouschek, A. Ubiquitin-like Domains Can Target to the Proteasome but Proteolysis Requires a Disordered Region. EMBO J. 2016, 35, 1522–1536. [Google Scholar] [CrossRef]
- Yu, H.; Matouschek, A. Recognition of Client Proteins by the Proteasome. Annu. Rev. Biophys. 2017, 46, 149–173. [Google Scholar] [CrossRef]
- Çetin, G.; Klafack, S.; Studencka-Turski, M.; Krüger, E.; Ebstein, F. The Ubiquitin–Proteasome System in Immune Cells. Biomolecules 2021, 11, 60. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Orsi, A.; Ansaldi, F.; Gasparini, R.; Icardi, G. Fluzone® Intra-Dermal (Intanza®/Istivac® Intra-Dermal): An Updated Overview. Hum. Vaccines Immunother. 2016, 12, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Doebel, T.; Voisin, B.; Nagao, K. Langerhans Cells—The Macrophage in Dendritic Cell Clothing. Trends Immunol. 2017, 38, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Kenney, R.T.; Villar, C.P. Dose Sparing with Intradermal Injection of Influenza Vaccine. N. Engl. J. Med. 2004, 351, 2295–2301. [Google Scholar]
- Prins, M.L.M.; Roozen, G.V.T.; Pothast, C.R.; Huisman, W.; Van Binnendijk, R.; Den Hartog, G.; Kuiper, V.P.; Prins, C.; Janse, J.J.; Lamers, O.A.C.; et al. Immunogenicity and Reactogenicity of Intradermal mRNA-1273 SARS-CoV-2 Vaccination: A Non-Inferiority, Randomized-Controlled Trial. npj Vaccines 2024, 9, 1. [Google Scholar] [CrossRef]
- Hickling, J.; Jones, K.; Friede, M.; Zehrung, D.; Chen, D.; Kristensen, D. Intradermal Delivery of Vaccines: Potential Benefits and Current Challenges. Bull. World Health Organ. 2011, 89, 221–226. [Google Scholar] [CrossRef]
- VASILAKOS, J.P. Addressing the Challenges of Vaccines and Intradermal Delivery. Available online: https://www.kindevadd.com/wp-content/uploads/2020/08/Addressing-the-Challenges-of-Vaccines-Intradermal-Delivery.pdf (accessed on 8 January 2025).
- Al Jarad, N.; Empey, D.W.; Duckworth, G. Administration of the BCG Vaccination Using the Multipuncture Method in Schoolchildren: A Comparison with the Intradermal Method. Thorax 1999, 54, 762–764. [Google Scholar] [CrossRef]
- Laurent, P.E.; Bonnet, S.; Alchas, P.; Regolini, P.; Mikszta, J.A.; Pettis, R.; Harvey, N.G. Evaluation of the Clinical Performance of a New Intradermal Vaccine Administration Technique and Associated Delivery System. Vaccine 2007, 25, 8833–8842. [Google Scholar] [CrossRef]
- Beaujean, M.; Uijen, R.F.; Langereis, J.D.; Boccara, D.; Dam, D.; Soria, A.; Veldhuis, G.; Adam, L.; Bonduelle, O.; Van Der Wel, N.N.; et al. The Immunological Effects of Intradermal Particle-Based Vaccine Delivery Using a Novel Microinjection Needle Studied in a Human Skin Explant Model. Vaccine 2023, 41, 2270–2279. [Google Scholar] [CrossRef]
- Moderna Receives, U.S. FDA Approval for RSV Vaccine mRESVIAR. Available online: https://news.modernatx.com/news/news-details/2024/Moderna-Receives-U.S.-FDA-Approval-for-RSV-Vaccine-mRESVIAR/default.aspx (accessed on 10 January 2025).
| Sample | Encapsulation Efficiency | Integrality | Particle Size (nm) | Zeta Potential | PDI (≤0.2) | dsRNA |
|---|---|---|---|---|---|---|
| CVG199 LNP | 95 | 90.1 | 92.3 | −2.4 | 0.17 | ≤0.40% |
| CVG200 LNP | 96 | 91.5 | 90.8 | −3.4 | 0.18 | ≤0.40% |
| CVG201 LNP | 95 | 91.8 | 95.8 | −2.8 | 0.20 | ≤0.40% |
| CVG202 LNP | 94 | 91.8 | 95.3 | −1.5 | 0.20 | ≤0.40% |
| CVG203 LNP | 91 | 94.2 | 111.0 | −6.4 | 0.25 | ≤0.40% |
| CVG204 LNP | 93 | 91.1 | 97.9 | −3.2 | 0.18 | ≤0.40% |
| CVG205 LNP | 88 | 78.8 | 110.7 | −3.6 | 0.22 | ≤0.40% |
| CVG206 LNP | 98 | 90.6 | 91.9 | −2.5 | 0.10 | ≤0.40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Zhou, W.; Liu, F.; Li, W.; Xu, Y.; Liang, Z.; Cao, M.; Hou, L.; Liu, P.; Wu, F.; et al. An mRNA Vaccine for Herpes Zoster and Its Efficacy Evaluation in Naïve/Primed Murine Models. Vaccines 2025, 13, 327. https://doi.org/10.3390/vaccines13030327
Jiang L, Zhou W, Liu F, Li W, Xu Y, Liang Z, Cao M, Hou L, Liu P, Wu F, et al. An mRNA Vaccine for Herpes Zoster and Its Efficacy Evaluation in Naïve/Primed Murine Models. Vaccines. 2025; 13(3):327. https://doi.org/10.3390/vaccines13030327
Chicago/Turabian StyleJiang, Linglei, Wenshuo Zhou, Fei Liu, Wenhui Li, Yan Xu, Zhenwei Liang, Man Cao, Li Hou, Pengxuan Liu, Feifei Wu, and et al. 2025. "An mRNA Vaccine for Herpes Zoster and Its Efficacy Evaluation in Naïve/Primed Murine Models" Vaccines 13, no. 3: 327. https://doi.org/10.3390/vaccines13030327
APA StyleJiang, L., Zhou, W., Liu, F., Li, W., Xu, Y., Liang, Z., Cao, M., Hou, L., Liu, P., Wu, F., Shen, A., Zhang, Z., Zhang, X., Zhao, H., Pan, X., Wu, T., Jia, W., & Zhang, Y. (2025). An mRNA Vaccine for Herpes Zoster and Its Efficacy Evaluation in Naïve/Primed Murine Models. Vaccines, 13(3), 327. https://doi.org/10.3390/vaccines13030327






