Innovations, Challenges, and Future Prospects for Combination Vaccines Against Human Infections
Abstract
:1. Introduction
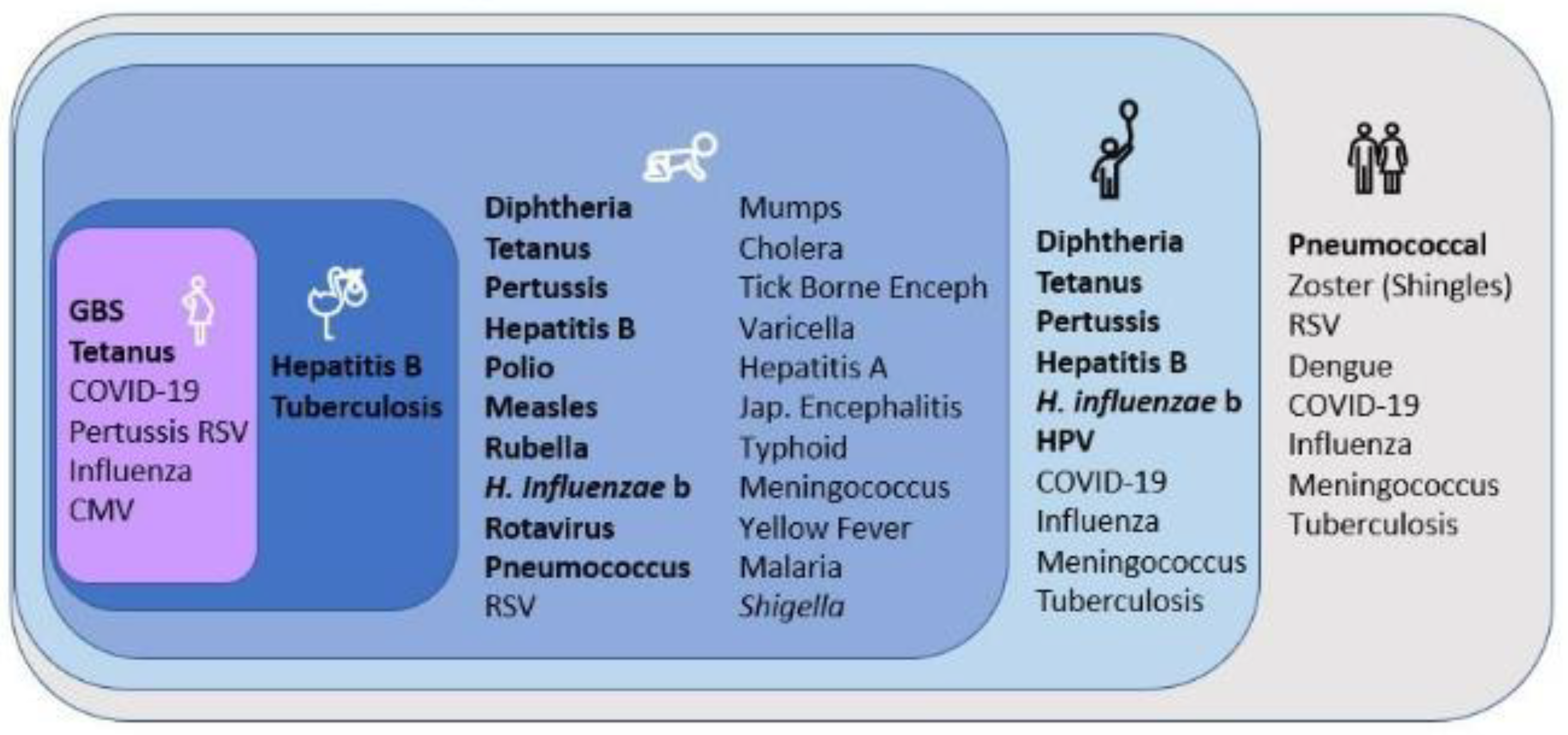
2. Currently Used Combination Vaccines
3. Upcoming Combination Vaccine Innovations
3.1. Combined mRNA Vaccines
3.2. Recombinant Combination Vaccines
3.3. Live Attenuated Combination Vaccines
3.4. Virus-Based Combination Vaccines
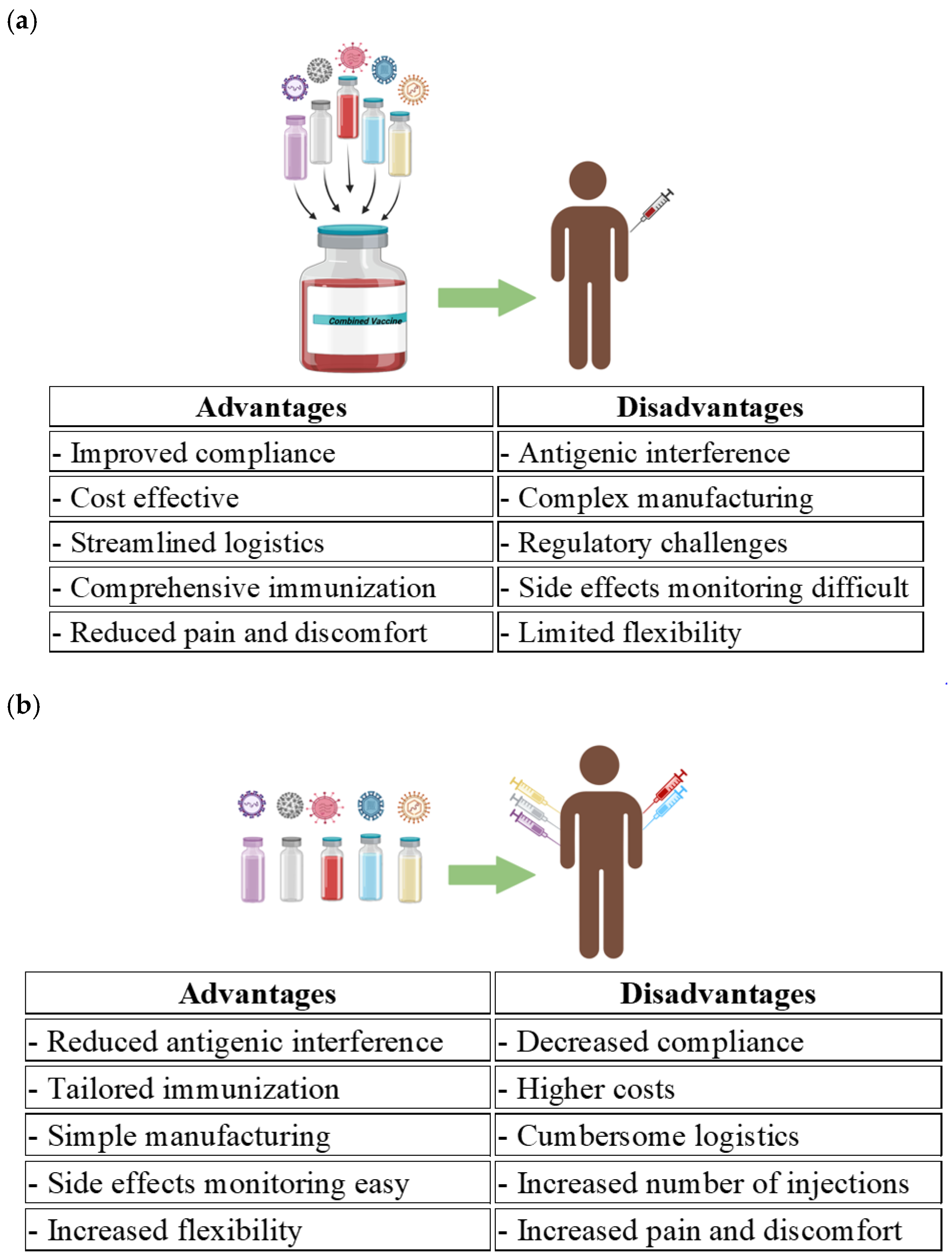
4. Advantages of Combination Vaccines
5. Challenges and Future Prospects
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shattock, A.J.; Johnson, H.C.; Sim, S.Y.; Carter, A.; Lambach, P.; Hutubessy, R.C.W.; Thompson, K.M.; Badizadegan, K.; Lambert, B.; Ferrari, M.J.; et al. Contribution of vaccination to improved survival and health: Modelling 50 years of the Expanded Programme on Immunization. Lancet 2024, 403, 2307–2316. [Google Scholar] [PubMed]
- WHO. Global Immunization Efforts Have Saved at Least 154 Million Lives over the Past 50 Years; WHO: Geneva, Switzerland, 2024; Available online: https://www.who.int/news/item/24-04-2024-global-immunization-efforts-have-saved-at-least-154-million-lives-over-the-past-50-years (accessed on 20 January 2025).
- Mullins, L.P.; Mason, E.; Winter, K.; Sadarangani, M. Vaccination is an integral strategy to combat antimicrobial resistance. PLoS Pathog. 2023, 19, e1011379. [Google Scholar]
- Humphries, S.; Humphries, S.; Bystrianyk, R. Dissolving Illusions: Disease, Vaccines, and the Forgotten History; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2013. [Google Scholar]
- Harrison, J.A. Wrong About Polio: A Review of Suzanne Humphries, MD and Roman Bystrianyk’s “Dissolving Illusions”; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2018; pp. 1–59. [Google Scholar]
- PGEI. Routine Vaccines, Extraordinary Impact. 2022. Available online: https://polioeradication.org/news/routine-vaccines-extraordinary-impact-polio/polio (accessed on 13 March 2025).
- Dattani, S.; Spooner, F.; Ochmann, S.; Roser, M. Polio. 2024. Available online: https://ourworldindata.org/polio (accessed on 13 March 2025).
- PGEI. History of Polio; PGEI: Geneva, Switzerland, 2025; Available online: https://polioeradication.org/about-polio/history-of-polio/ (accessed on 14 March 2025).
- Dall, C. Five Countries Report New Polio Cases; Center for Infectious Disease Research and Policy (CIDRAP): Minneapolis, MN, USA, 2025; Available online: https://www.cidrap.umn.edu/polio/five-countries-report-new-polio-cases (accessed on 14 March 2025).
- Kennedy, R.; Poland, G.A. Chapter 37—Smallpox. In Vaccines for Biodefense and Emerging and Neglected Diseases; Barrett, A.D.T., Stanberry, L.R., Eds.; Academic Press: London, UK, 2009; pp. 685–711. [Google Scholar]
- WHO. Smallpox; WHO: Geneva, Switzerland, 2025; Available online: https://www.who.int/health-topics/smallpox#tab=tab_1 (accessed on 16 March 2025).
- Liu, B.; Cao, B.; Wang, C.; Han, B.; Sun, T.; Miao, Y.; Lu, Q.; Cui, F. Immunogenicity and Safety of Childhood Combination Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 472. [Google Scholar] [CrossRef]
- Scorza, F.B.; Martin, L.B.; Podda, A.; Rappuoli, R. A strategic model for developing vaccines against neglected diseases: An example of industry collaboration for sustainable development. Hum. Vaccines Immunother. 2022, 18, 2136451. [Google Scholar]
- Aga, A.M.; Kelel, M.; Gemeda, M.T. Recent advances in mRNA vaccine development. J. Microbiol. Biotechnol. 2023, 8, 000275. [Google Scholar]
- PATH. Combination Vaccines Could be the Future of Immunization Programs; PATH: Seattle, WA, USA, 2024; Available online: https://www.path.org/our-impact/articles/combination-vaccines-could-be-the-future-of-immunization-programs/ (accessed on 20 January 2025).
- Ogden, S.A.; Ludlow, J.T.; Alsayouri, K. Diphtheria Tetanus Pertussis (DTaP) Vaccine. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Roush, S.W.; Murphy, T.V. Historical Comparisons of Morbidity and Mortality for Vaccine-Preventable Diseases in the United States. JAMA 2007, 298, 2155–2163. [Google Scholar] [PubMed]
- Lubanga, A.F.; Bwanali, A.N.; Kangoma, M.; Matola, Y.; Moyo, C.; Kaonga, B.; Ssebibubbu, S.; Makole, T.J.; Kambili, F.; Chumbi, G.D.; et al. Addressing the re-emergence and resurgence of vaccine-preventable diseases in Africa: A health equity perspective. Hum. Vaccines Immunother. 2024, 20, 2375081. [Google Scholar]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar]
- Samarasekera, U. Diphtheria outbreak in west Africa. Lancet Infect. Dis. 2024, 24, e87. [Google Scholar] [CrossRef]
- Diphtheria Outbreaks Reported in Multiple Countries. Available online: https://www.vax-before-travel.com/2023/09/22/diphtheria-outbreaks-reported-multiple-countries (accessed on 14 March 2023).
- Africa CDC. Diphtheria Outbreak in Africa: Strengthening Response Capacities; Africa CDC: Addis Ababa, Ethiopia, 2023; Available online: https://africacdc.org/news-item/diphtheria-outbreak-in-africa-strengthening-response-capacities/ (accessed on 14 March 2025).
- Préziosi, M.-P.; Yam, A.; Wassilak, S.G.F.; Chabirand, L.; Simaga, A.; Ndiaye, M.; Dia, M.; Dabis, F.; Simondon, F. Epidemiology of pertussis in a West African community before and after introduction of a widespread vaccination program. Am. J. Epidemiol. 2002, 155, 891–896. [Google Scholar] [CrossRef]
- Fanget, F. Pertussis: A Tale of Two Vaccines. 2020. Available online: https://www.nature.com/articles/d42859-020-00013-8 (accessed on 14 March 2025).
- Vashistha, V.M. Pertussis outbreaks in the developed world: Are acellular pertussis vaccines ineffective? Counterpoint. Indian Pediatr. 2013, 50, 1111–1112. [Google Scholar]
- Quinn, M.; Edmond, K.M.; Fawzi, W.W.; Hurt, L.; Kirkwood, B.R.; Masanja, H.; Muhihi, A.J.; Newton, S.; A Noor, R.; Williams, P.L.; et al. Non-specific effects of BCG and DTP vaccination on infant mortality: An analysis of birth cohorts in Ghana and Tanzania. Vaccine 2022, 40, 3737–3745. [Google Scholar] [CrossRef] [PubMed]
- Aaby, P.; Ravn, H.; Fisker, A.B.; Rodrigues, A.; Benn, C.S. Is diphtheria-tetanus-pertussis (DTP) associated with increased female mortality? A meta-analysis testing the hypotheses of sex-differential non-specific effects of DTP vaccine. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 570–581. [Google Scholar]
- WHO. Nonspecific Effects of Childhood Immunization; WHO: Geneva, Switzerland, 2025; Available online: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/diphtheria-tetanus-and-pertussis-vaccines#cms (accessed on 9 March 2025).
- Halsey, N.A. Combination vaccines: Defining and addressing current safety concerns. Clin. Infect. Dis. 2001, 33 (Suppl. S4), S312–S318. [Google Scholar] [CrossRef]
- Kostinov, M.P. Immunoprophylaxis of measles with the use of combined vaccines. Epidemiol. Vaccin. 2020, 19, 57–62. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Measles, Mumps, and Rubella (MMR) Vaccination: What Everyone Should Know; Centers for Disease Control and Prevention: Atlanta, Georgia, 2025. Available online: https://www.cdc.gov/vaccines/vpd/mmr/public/index.html (accessed on 9 March 2025).
- Shah, N.; Parikh, R.; Casabona, G.; Kolhapure, S. A new combined vaccine against measles, mumps, rubella and varicella in India. Indian Pediatr. 2017, 54, 1041–1046. [Google Scholar] [CrossRef]
- Marin, M.; Broder, K.R.; Temte, J.L.; E Snider, D.; Seward, J.F. Use of combination measles, mumps, rubella, and varicella vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2010, 59, 1–12. [Google Scholar] [PubMed]
- Ma, S.J.; Li, X.; Xiong, Y.Q.; Chen, Q. Combination Measles-Mumps-Rubella-Varicella Vaccine in Healthy Children: A Systematic Review and Meta-analysis of Immunogenicity and Safety. Medicine 2015, 94, e1721. [Google Scholar] [CrossRef]
- Bower, H. MMR vaccine policy is backed. BMJ 1998, 316, 955. [Google Scholar] [CrossRef]
- Yang, K.; Kim, H.; Ortiz, E.; Huoi, C.; Kang, J. Post-Marketing Safety Surveillance of a Childhood Pentavalent Diphtheria–Tetanus–Acellular Pertussis–Polio and Haemophilus influenzae Type B (DTaP-IPV//Hib) Vaccine in South Korea. Infect. Dis. Ther. 2023, 12, 499–511. [Google Scholar] [CrossRef]
- Esposito, S.; Tagliabue, C.; Bosis, S.; Ierardi, V.; Gambino, M.; Principi, N. Hexavalent vaccines for immunization in paediatric age. Clin. Microbiol. Infect. 2014, 20, 76–85. [Google Scholar] [PubMed]
- Zepp, F.; Schmitt, H.J.; Cleerbout, J.; Verstraeten, T.; Schuerman, L.; Jacquet, J.M. Review of 8 years of experience with Infanrix hexa™ (DTPa–HBV–IPV/Hib hexavalent vaccine). Expert Rev. Vaccines 2009, 8, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Lalwani, S.; Parekh, S.; Pujari, P.; Shewale, S.; Palkar, S.; Hanumante, N.; Gokhale, S.; Ks, J.; Kumar, R.; et al. A phase I, open label, clinical study to assess the safety and immunogenicity of indigenously developed liquid (DTwP-HepB-IPV-Hib) hexavalent combination vaccine in healthy toddlers aged 16–24 months. Hum. Vaccines Immunother. 2022, 18, 2146435. [Google Scholar] [CrossRef]
- Sharma, H.; Parekh, S.; Pujari, P.; Shewale, S.; Desai, S.; Kawade, A.; Lalwani, S.; Ravi, M.D.; Kamath, V.; Mahopatra, J.; et al. A phase III randomized-controlled study of safety and immunogenicity of DTwP-HepB-IPV-Hib vaccine (HEXASIIL®) in infants. NPJ Vaccines 2024, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Boisnard, F.; Manson, C.; Serradell, L.; Macina, D. DTaP-IPV-HB-Hib vaccine (Hexaxim): An update 10 years after first licensure. Expert Rev. Vaccines 2023, 22, 1196–1213. [Google Scholar]
- Cho, H.-K.; Park, S.E.; Kim, Y.-J.; Jo, D.S.; Kim, Y.-K.; Eun, B.-W.; Lee, T.-J.; Lee, J.; Lee, H.; Kim, K.H.; et al. Recommendation for use of diphtheria and tetanus toxoids and acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b conjugate, and hepatitis B vaccine in infants. Clin. Exp. Pediatr. 2021, 64, 602–607. [Google Scholar]
- Combination vaccines for childhood immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP). Am. Fam. Physician 1999, 59, 2565–2574. [Google Scholar]
- Skibinski, D.A.; Baudner, B.C.; Singh, M.; T O’Hagan, D. Combination vaccines. J. Glob. Infect. Dis. 2011, 3, 63–72. [Google Scholar]
- Moderna. Moderna Announces Positive Phase 3 Data for Combination Vaccine Against Influenza and COVID-19. 2024. Available online: https://investors.modernatx.com/news/news-details/2024/Moderna-Announces-Positive-Phase-3-Data-for-Combination-Vaccine-Against-Influenza-and-COVID-19-/default.aspx (accessed on 8 March 2025).
- Pfizer. Pfizer and BioNTech Provide Update on mRNA-Based Combination Vaccine Program Against Influenza and COVID-19 in Individuals 18–64 Years of Age. 2024. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-mrna-based-combination (accessed on 8 March 2025).
- Snape, M.D.; Ghamloush, S.S.; Chen, G.L.; Dhar, R.; Mithani, R.; Righi, V.; Morsy, L.; Kapoor, A.; Girard, B.; El Asmar, L.; et al. Phase 1 safety and immunogenicity results of two investigational mRNA vaccines, mRNA-1345, a respiratory syncytial virus vaccine, and mRNA-1653, a human metapneumovirus and parainfluenza virus type 3 combination vaccine in seropositive young children. OFID 2023, 10 (Suppl. S2), S1163. [Google Scholar]
- Wang, Y.; Ma, Q.; Li, M.; Mai, Q.; Ma, L.; Zhang, H.; Zhong, H.; Mai, K.; Cheng, N.; Feng, P.; et al. A decavalent composite mRNA vaccine against both influenza and COVID-19. bioRxiv 2024, 15, e0066824. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, M.; Zhou, C.; Lu, X.; Huang, B.; Zhang, N.; Zhao, H.; Chi, H.; Zhang, X.; Ling, D.; et al. Rational development of a combined mRNA vaccine against COVID-19 and influenza. NPJ Vaccines 2022, 7, 84. [Google Scholar] [PubMed]
- Mazunina, E.P.; Gushchin, V.A.; Bykonia, E.N.; Kleymenov, D.A.; Siniavin, A.E.; Kozlova, S.R.; Mukasheva, E.A.; Shidlovskaya, E.V.; Kuznetsova, N.A.; Usachev, E.V.; et al. Immunogenicity and Efficacy of Combined mRNA Vaccine Against Influenza and SARS-CoV-2 in Mice Animal Models. Vaccines 2024, 12, 1206. [Google Scholar] [CrossRef]
- Wolf, M.A.; O’hara, J.M.; Bitzer, G.J.; Narayanan, E.; Boehm, D.T.; Bevere, J.R.; DeJong, M.A.; Hall, J.M.; Wong, T.Y.; Falcone, S.; et al. Multivalent mRNA-DTP vaccines are immunogenic and provide protection from Bordetella pertussis challenge in mice. NPJ Vaccines 2024, 9, 103. [Google Scholar] [PubMed]
- Rybicki, E.P. First WHO/MPP mRNA Technology Transfer Programme Meeting. Lancet Microbe 2023, 4, e564–e566. [Google Scholar] [PubMed]
- Hulscher, N.; Bowden, M.; McCullough, P. Review: Calls for Market Removal of COVID-19 Vaccines Intensify as Risks Far Outweigh Theoretical Benefits. Sci. Public Health Policy Law 2025, 6, 1–17. [Google Scholar]
- Rubio-Casillas, A.; Cowley, D.; Raszek, M.; Uversky, V.N.; Redwan, E.M. Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer? Int. J. Biol. Macromol. 2024, 267, 131427. [Google Scholar]
- Mulroney, T.E.; Pöyry, T.; Yam-Puc, J.C.; Rust, M.; Harvey, R.F.; Kalmar, L.; Horner, E.; Booth, L.; Ferreira, A.P.; Stoneley, M.; et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 2024, 625, 189–194. [Google Scholar]
- Segalla, G. Apparent Cytotoxicity and Intrinsic Cytotoxicity of Lipid Nanomaterials Contained in a COVID-19 mRNA Vaccine. Int. J. Vaccine Theory Pract. Res. 2023, 3, 957–972. [Google Scholar]
- Shinde, V.; Woo, W.; Liu, S.; Cook, S.; Santiago, Z.; Neal, S.; Plested, J.S.; Vincent, T.; Zhu, M.; Cloney-Clark, S.; et al. 2142. Safety and immunogenicity of COVID Influenza Combination Vaccine. Open Forum Infect. Dis. 2022, 9 (Suppl. S2), ofac492.1762. [Google Scholar]
- Sanofi. Press Release: Two Combination Vaccine Candidates for Prevention of Influenza and COVID-19 Granted Fast Track Designation in the US. 2025. Available online: https://www.sanofi.com/en/media-room/press-releases/2024/2024-12-11-06-00-00-2995072 (accessed on 3 March 2025).
- Tzenios, N.; Tazanios, M.E.; Chahine, M. Combining Influenza and COVID-19 Booster Vaccination Strategy to Improve Vaccination Uptake Necessary for Managing the Health Pandemic: A Systematic Review and Meta-Analysis. Vaccines 2022, 11, 16. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, H.; Forgacs, D.; Ross, T.M. Flu-COVID combo recombinant protein vaccines elicited protective immune responses against both influenza and SARS-CoV-2 viruses infection. Vaccine 2024, 42, 1184–1192. [Google Scholar] [PubMed]
- Chai, P.; Pu, X.; Ge, J.; Ren, S.; Xia, X.; Luo, A.; Wang, S.; Wang, X.; Li, J. The recombinant protein combined vaccine based on the fragment C of tetanus toxin and the cross-reacting material 197. Appl. Microbiol. Biotechnol. 2021, 105, 1683–1692. [Google Scholar]
- Wüthrich, M.; Dobson, H.E.; Taira, C.L.; Okaa, U.J.; Dias, L.d.S.; Isidoro-Ayza, M.; Petrovsky, N.; Klein, B.S. Combination Adjuvants Enhance Recombinant Protein Vaccine Protection against Fungal Infection. mBio 2021, 12, e0201821. [Google Scholar]
- Lee, S.; Nguyen, M.T. Recent Advances of Vaccine Adjuvants for Infectious Diseases. Immune Netw. 2015, 15, 51–57. [Google Scholar]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [PubMed]
- Mehla, R.; Kokate, P.; Bhosale, S.R.; Vaidya, V.; Narayanan, S.; Shandil, R.K.; Singh, M.; Rudramurthy, G.R.; Naveenkumar, C.N.; Bharathkumar, K.; et al. A Live Attenuated COVID-19 Candidate Vaccine for Children: Protection against SARS-CoV-2 Challenge in Hamsters. Vaccines 2023, 11, 255. [Google Scholar] [CrossRef]
- Fidel, P.L.; Noverr, M.C. Could an unrelated live attenuated vaccine serve as a preventive measure to dampen septic inflammation associated with COVID-19 infection? mBio 2020, 11, e00907-20. [Google Scholar]
- MacDonald, S.E.; Tough, S.; Guo, X.; Kellner, J.D. Impact of combination MMRV vaccine on first-dose coverage for measles and varicella: A population-based study. J. Public Health 2020, 30, 1063–1068. [Google Scholar]
- Matushkina, A.; Isakova-Sivak, I.; Kiseleva, I.; Leontieva, G.; Suvorov, A.; Rudenko, L. Development of a Recombinant Live Attenuated Influenza Vaccine Virus Expressing Pneumococcal Surface Antigen A as a Strategy for Combined Protection Against Influenza and Bacterial Coinfection Caused by Streptococcus pneumoniae. Open Microbiol. J. 2024, 18, e18742858303845. [Google Scholar]
- Ogonczyk-Makowska, D.; Brun, P.; Vacher, C.; Chupin, C.; Droillard, C.; Carbonneau, J.; Laurent, E.; Dulière, V.; Traversier, A.; Terrier, O.; et al. Mucosal bivalent live attenuated vaccine protects against human metapneumovirus and respiratory syncytial virus in mice. NPJ Vaccines 2024, 9, 111. [Google Scholar]
- Flaxman, A.; Sebastian, S.; Appelberg, S.; Cha, K.M.; Ulaszewska, M.; Purushotham, J.; Gilbride, C.; Sharpe, H.; Spencer, A.J.; Bibi, S.; et al. Potent immunogenicity and protective efficacy of a multi-pathogen vaccination targeting Ebola, Sudan, Marburg and Lassa viruse. PLoS Pathog. 2024, 20, e1012262. [Google Scholar]
- Stepanova, E.; Isakova-Sivak, I.; Rudenko, L. Options for the development of a bivalent vaccine against SARS-CoV-2 and influenza. Expert Rev. Vaccines 2022, 21, 1533–1535. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Wang, X.; Peng, H.; Ding, L.; Wang, X.; Hu, Y.; Dong, L.; Yang, T.; Hong, X.; Xing, M.; et al. A Single Vaccine Protects against SARS-CoV-2 and Influenza Virus in Mice. J. Virol. 2022, 96, e0157821. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Ouyang, M.J.; Olukitibi, T.A.; Warner, B.; Vendramelli, R.; Truong, T.; Zhang, M.; Kung, S.; Fowke, K.R.; Kobasa, D.; et al. Development and Characterization of Recombinant Vesicular Stomatitis Virus (rVSV)-based Bivalent Vaccine Against COVID-19 Delta Variant and Influenza Virus. BioRxiv 2021. [Google Scholar] [CrossRef]
- Mendiretta, S.K.; Glueck, R.; Giannino, V.; Cantarella, G.; Scuderi, F.; Billeter, M.; Fazzio, A. Combined Measles-Human Papilloma Vaccine. U.S. Patent No. US 9,623,098 B2, 18 April 2017. [Google Scholar]
- McCann, N.; O’Connor, D.; Lambe, T.; Pollard, A.J. Viral vector vaccines. Curr. Opin. Immunol. 2022, 77, 102210. [Google Scholar] [CrossRef]
- Isakova-Sivak, I.; Stepanova, E.; Matyushenko, V.; Niskanen, S.; Mezhenskaya, D.; Bazhenova, E.; Krutikova, E.; Kotomina, T.; Prokopenko, P.; Neterebskii, B.; et al. Development of a T Cell-Based COVID-19 Vaccine Using a Live Attenuated Influenza Vaccine Viral Vector. Vaccines 2022, 10, 1142. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; He, J.; Chen, J.; Qi, R.; Yuan, L.; Shao, T.; Zhao, H.; Chen, C.; Chen, Y.; et al. Intranasal influenza-vectored COVID-19 vaccine restrains the SARS-CoV-2 inflammatory response in hamsters. Nat. Commun. 2023, 14, 4117. [Google Scholar]
- Stepanova, E.; Isakova-Sivak, I.; Matyushenko, V.; Mezhenskaya, D.; Kudryavtsev, I.; Kostromitina, A.; Chistiakova, A.; Rak, A.; Bazhenova, E.; Prokopenko, P.; et al. Safety and Immunogenicity Study of a Bivalent Vaccine for Combined Prophylaxis of COVID-19 and Influenza in Non-Human Primates. Vaccines 2024, 12, 1099. [Google Scholar] [CrossRef]
- Bommireddy, R.; Stone, S.; Bhatnagar, N.; Kumari, P.; E Munoz, L.; Oh, J.; Berry, J.L.; Jacobson, K.M.; Jafaar, L.; Naing, S.-H.; et al. Influenza virus-like particle-based hybrid vaccine containing RBD induces immunity against influenza and SARS-CoV-2 viruses. J. Immunol. 2022, 208 (Suppl. S1), 64.01. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, R.; Xu, L.; Li, Y.; Li, S.; Yu, H.; Li, S.; Zhu, H.; Cheng, T.; Xia, N. A novel combined vaccine based on monochimeric VLP co-displaying multiple conserved epitopes against enterovirus 71 and varicella-zoster virus. Vaccine 2017, 35, 2728–2735. [Google Scholar] [CrossRef]
- Rothen, D.A.; Dutta, S.K.; Krenger, P.S.; Pardini, A.; Vogt, A.-C.S.; Josi, R.; Lieknina, I.; Osterhaus, A.D.M.E.; Mohsen, M.O.; Vogel, M.; et al. Preclinical Development of a Novel Zika Virus-like Particle Vaccine in Combination with Tetravalent Dengue Virus-like Particle Vaccines. Vaccines 2024, 12, 1053. [Google Scholar] [CrossRef]
- Moderna. The Potential Impact of Combination Vaccines on Public Health. Available online: https://www.modernatx.com/media-center/all-media/blogs/impact-combination-vaccines?fromLocale=ko-KR (accessed on 15 January 2025).
- Centers for Disease Control and Prevention. Combination Vaccines; Centers for Disease Control and Prevention: Atlanta, Georgia, 2025. Available online: https://www.cdc.gov/vaccines-children/about/combination-vaccines.html (accessed on 9 March 2025).
- Elliman, D.; Bedford, H. Safety and efficacy of combination vaccines. BMJ 2003, 326, 995–996. [Google Scholar] [CrossRef] [PubMed]
- Offit, P.A.; Quarles, J.; Gerber, M.A.; Hackett, C.J.; Marcuse, E.K.; Kollman, T.R.; Gellin, B.G.; Landry, S. Addressing Parents’ Concerns: Do Multiple Vaccines Overwhelm or Weaken the Infant’s Immune System? Pediatrics 2002, 109, 124–129. [Google Scholar] [PubMed]
- Tamminen, K.; Lappalainen, S.; Huhti, L.; Vesikari, T.; Blazevic, V. Trivalent Combination Vaccine Induces Broad Heterologous Immune Responses to Norovirus and Rotavirus in Mice. PLoS ONE 2013, 8, e70409. [Google Scholar] [CrossRef] [PubMed]
- Bodas-Pinedo, A.; Lafuente, E.M.; Pelaez-Prestel, H.F.; Ras-Carmona, A.; Subiza, J.L.; Reche, P.A. Combining different bacteria in vaccine formulations enhances the chance for antiviral cross-reactive immunity: A detailed in silico analysis for influenza A virus. Front. Immunol. 2023, 14, 1235053. [Google Scholar] [CrossRef]
- Zhu, F.; Tan, C.; Li, C.; Ma, S.; Wen, H.; Yang, H.; Rao, M.; Zhang, P.; Peng, W.; Cui, Y.; et al. Design of a multi-epitope vaccine against six Nocardia species based on reverse vaccinology combined with immunoinformatics. Front. Immunol. 2023, 14, 1100188. [Google Scholar]
- Jiang, F.; Liu, Y.; Xue, Y.; Cheng, P.; Wang, J.; Lian, J.; Gong, W. Developing a multiepitope vaccine for the prevention of SARS-CoV-2 and monkeypox virus co-infection: A reverse vaccinology analysis. Int. Immunopharmacol. 2023, 115, 109728. [Google Scholar]
- Vidor, E.; Soubeyrand, B. Manufacturing DTaP-based combination vaccines: Industrial challenges around essential public health tools. Expert Rev. Vaccines 2016, 15, 1575–1582. [Google Scholar]
- Kana, B.D.; Arbuthnot, P.; Botwe, B.K.; E Choonara, Y.; Hassan, F.; Louzir, H.; Matsoso, P.; Moore, P.L.; Muhairwe, A.; Naidoo, K.; et al. Opportunities and challenges of leveraging COVID-19 vaccine innovation and technologies for developing sustainable vaccine manufacturing capabilities in Africa. Lancet Infect. Dis. 2023, 23, e288–e300. [Google Scholar]
- Akegbe, H.; Onyeaka, H.; Mazi, I.M.; Olowolafe, O.A.; Omotosho, A.D.; Oladunjoye, I.O.; Tajudeen, Y.A.; Ofeh, A.S. The need for Africa to develop capacity for vaccinology as a means of curbing antimicrobial resistance. Vaccine X 2023, 14, 100320. [Google Scholar]
- McCoy, J.R.; Mendoza, J.M.; Spik, K.W.; Badger, C.; Gomez, A.F.; Schmaljohn, C.S.; Sardesai, N.Y.; E Broderick, K. A multi-head intradermal electroporation device allows for tailored and increased dose DNA vaccine delivery to the skin. Hum. Vaccines Immunother. 2014, 10, 3039–3047. [Google Scholar]
- Walker, R.I.; Clifford, A. Recommendations regarding the development of combined enterotoxigenic Eschericha coli and Shigella vaccines for infants. Vaccine 2015, 33, 946–953. [Google Scholar] [PubMed]
- Healthcare, G.D. Novavax Employs AI-Based Dose Selection for Covid-Influenza Vaccine Trials. 2025. Available online: https://www.clinicaltrialsarena.com/analyst-comment/novavax-ai-covid-influenza-vaccine/ (accessed on 3 March 2025).
- Falk, L.A.; Arciniega, J.; McVittie, L. Manufacturing Issues with Combining Different Antigens: A Regulatory Perspective. Clin. Infect. Dis. 2001, 33 (Suppl. S4), S351–S355. [Google Scholar] [PubMed]
- Maman, K.; Zöllner, Y.; Greco, D.; Duru, G.; Sendyona, S.; Remy, V. The value of childhood combination vaccines: From beliefs to evidence. Hum. Vaccines Immunother. 2015, 11, 2132–2141. [Google Scholar]
| Combination | Brand Name | Vaccine | Coverage |
|---|---|---|---|
| Hexavalent | Infanrix hexa® | DTaP–HepB–Hib/IPV | Diphtheria, tetanus, acellular pertussis, poliovirus, Haemophilus influenzae Type b, and Hepatitis B |
| Vaxelis® | DTaP–IPV–Hib–HepB | ||
| HEXASIIL® | DTwP–HepB–IPV–Hib vaccine | Diphtheria, tetanus, whole-cell pertussis, Hepatitis B, poliovirus, Haemophilus Influenzae Type B | |
| Pentavalent | Pediarix™ | DTaP–HepB–IPV | Diphtheria, tetanus, acellular pertussis, Hepatitis B, poliovirus |
| Pentacel™ | DTaP–IPV–Hib | Diphtheria, tetanus, acellular pertussis, poliovirus, Haemophilus influenzae Type B | |
| Tetravalent | Kinrix™ | DTaP–IPV | Diphtheria, tetanus, acellular pertussis, poliovirus |
| TriHIBit | DTaP–Hib | Diphtheria, tetanus, acellular pertussis, Haemophilus influenzae Type B | |
| ProQuad® | MMRV | Measles, mumps, rubella, varicella | |
| Trivalent | Tripedia™ Daptacel™ Infanrix™ | DTaP | Diphtheria, tetanus, acellular pertussis |
| Adacel™ | DTaP (Adult) | ||
| Boostrix™ | |||
| M-M-R® II | MMR | Measles, mumps, rubella | |
| PRIORIX® | |||
| Bivalent | COMVAX® | Hib–Hep B | Haemophilus influenzae Type B, Hepatitis B |
| Twinrix® | HepA–HepB (Adult) | Hepatitis A, Hepatitis B |
| Platform | Target | Antigens | Trial Phase | CTN | Sponsor (Vaccine) |
|---|---|---|---|---|---|
| mRNA | Influenza and SARS-CoV-2 | HA of Influenza A (H1N1, H3N2), and Influenza B (Victoria and Yamagata lineage), RBD and NTD of spike protein of SARS-CoV-2 omicron BA.4/BA.5 subvariants | 1, 2, 3 | NCT05827926 NCT06097273 NCT06694389 NCT06508320 | Moderna (mRNA-1083) |
| mRNA | Influenza and SARS-CoV-2 | HA of Influenza A (H1N1, H3N2), and Influenza B (Victoria and Yamagata lineage), S-2P prefusion stabilized spike protein of SARS-CoV-2 original Wuhan-Hu-1 | 1, 2 | NCT05375838 | Moderna (mRNA-1073) |
| mRNA | Influenza and SARS-CoV-2 | HA of Influenza A (H1N1, H3N2), and Influenza B (Victoria and Yamagata lineage), spike protein of SARS-CoV-2 original Wuhan-Hu-1 and omicron BA.4/BA.5 subvariants | 1, 2 | NCT06696734 | BioNTech SE (qIRV(22/23)/ bivalentBNT162b2) |
| mRNA | Influenza and SARS-CoV-2 | HA of tIRV (H1N1, H3N2, Victoria lineage) or HA of qIRV (H1N1, H3N2, Victoria and Yamagata lineage), spike protein of SARS-CoV-2 original Wuhan-Hu-1 and Omicron BA.4/BA.5 subvariants | 3 | NCT06178991 | BioNTech SE (Combination A, Combination B) |
| mRNA | Influenza, RSV, and SARS-CoV-2 | mRNA-1045: HA of 4 Influenza A (H1N1, H3N2), and Influenza B (Victoria and Yamagata linages), prefusion fusion protein of RSV; mRNA-1230: an additional spike of SARS-CoV-2 | 1 | NCT05585632 | Moderna (mRNA-1045, mRNA-1230) |
| mRNA | hMPV and PIV3 | Fusion protein of hMPV, fusion protein of PIV3 | 1 | NCT04144348 NCT03392389 | Moderna (mRNA-1653) |
| Viral vector | Ebola and Marburg | Chimpanzee adenoviral vector containing GP of Sudan ebola, chimpanzee adenoviral vector containing Marburg Angola GP | 1 | NCT04723602 | Albert B. Sabin vaccine Institute (cAd3-EBO-S and cAd3 Marburg) |
| Nanoparticle vaccine | Influenza and SARS-CoV-2 | SARS-CoV-2 recombinant spike nanoparticle, quadrivalent HA nanoparticle influenza combination vaccine with Matrix-M adjuvant | 1, 2 | NCT04961541 NCT05519839 | Novavax (qNIV/CoV2373) |
| Recombinant | Influenza and SARS-CoV-2 | Recombinant influenza vaccine + adjuvanted recombinant COVID-19 vaccine | 1, 2 | NCT06695130 | RIV + rC19 |
| Inactivated/recombinant | Influenza and SARS-CoV-2 | Inactivated influenza vaccine + adjuvanted recombinant COVID-19 vaccine | 1, 2 | NCT06695117 | IIV-HD + rC19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, M.; Hong, K.-J. Innovations, Challenges, and Future Prospects for Combination Vaccines Against Human Infections. Vaccines 2025, 13, 335. https://doi.org/10.3390/vaccines13040335
Fatima M, Hong K-J. Innovations, Challenges, and Future Prospects for Combination Vaccines Against Human Infections. Vaccines. 2025; 13(4):335. https://doi.org/10.3390/vaccines13040335
Chicago/Turabian StyleFatima, Munazza, and Kee-Jong Hong. 2025. "Innovations, Challenges, and Future Prospects for Combination Vaccines Against Human Infections" Vaccines 13, no. 4: 335. https://doi.org/10.3390/vaccines13040335
APA StyleFatima, M., & Hong, K.-J. (2025). Innovations, Challenges, and Future Prospects for Combination Vaccines Against Human Infections. Vaccines, 13(4), 335. https://doi.org/10.3390/vaccines13040335





