Identification of Potential Amblyomma americanum Antigens After Vaccination with Tick Extracellular Vesicles in White-Tailed Deer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Label-Free Quantitative Proteomic Analysis
2.1.1. Sample Preparation
2.1.2. Mass Spectrometry
2.1.3. Data Analysis
2.2. Extracellular Vesicle Cargo Comparison and Pathway Enrichment Analysis
2.3. Western Blot Analysis
2.4. Immunoprecipitation and Label-Free Quantification of Antigenic Proteins
2.4.1. Immunoprecipitation
2.4.2. Mass Spectrometry
2.4.3. Data Analysis
2.5. Data Availability
2.6. Antigens Classification
3. Results
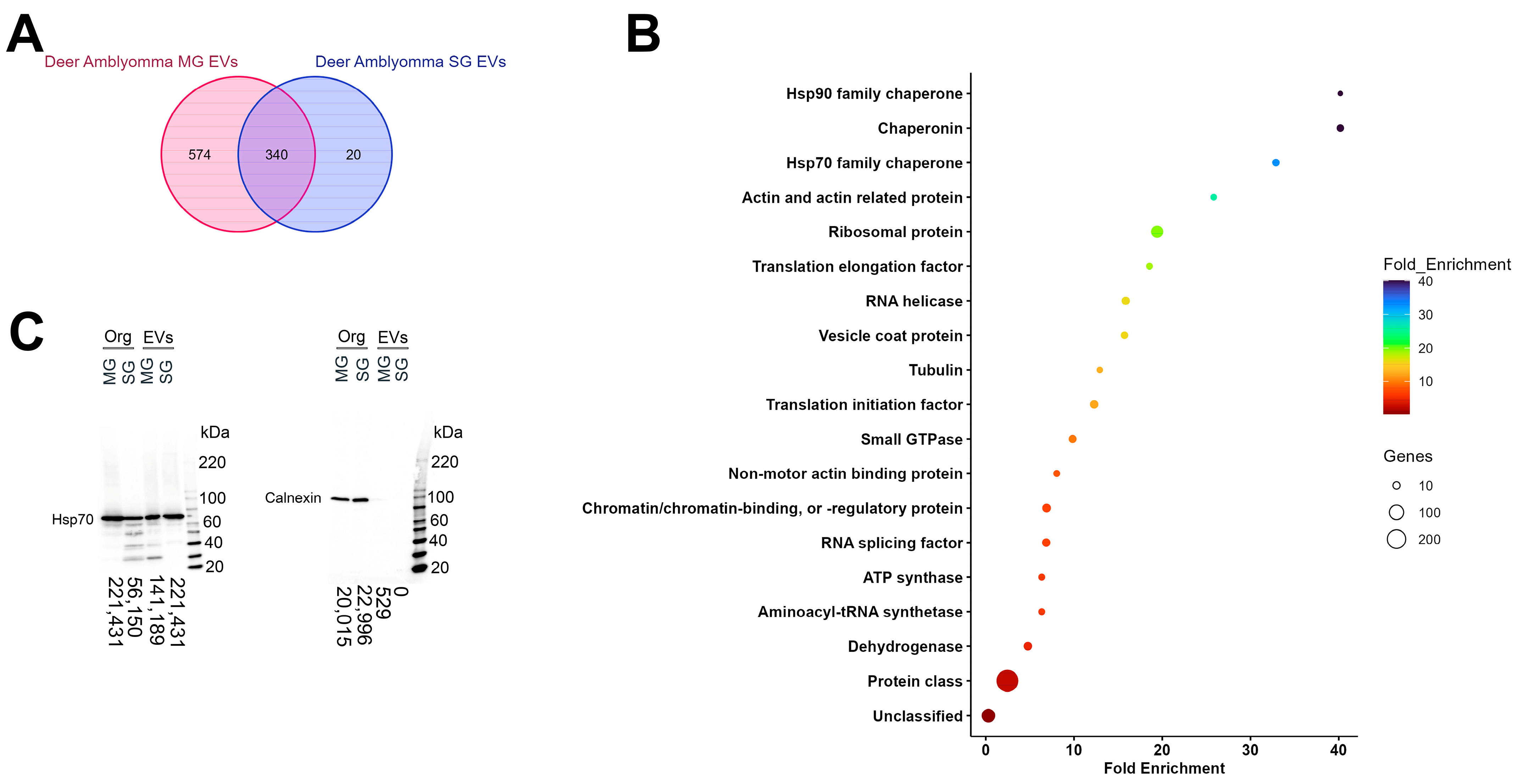
3.1. Extracellular Vesicles Secreted by Different Organs Share a Core Set of Proteins
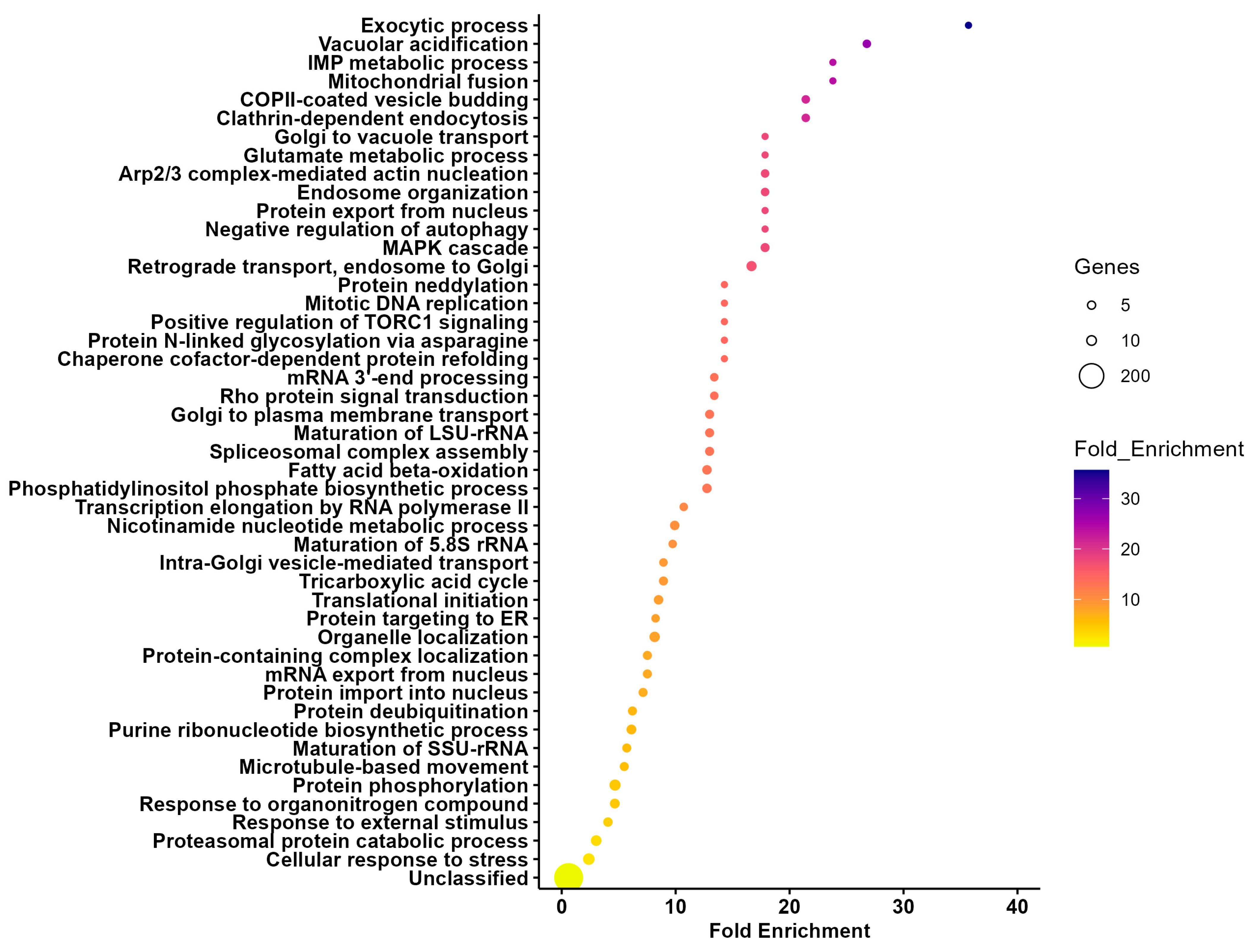
3.2. Unique and Differentially Expressed Proteins Within Midgut Extracellular Vesicles Mirror Organ Functions
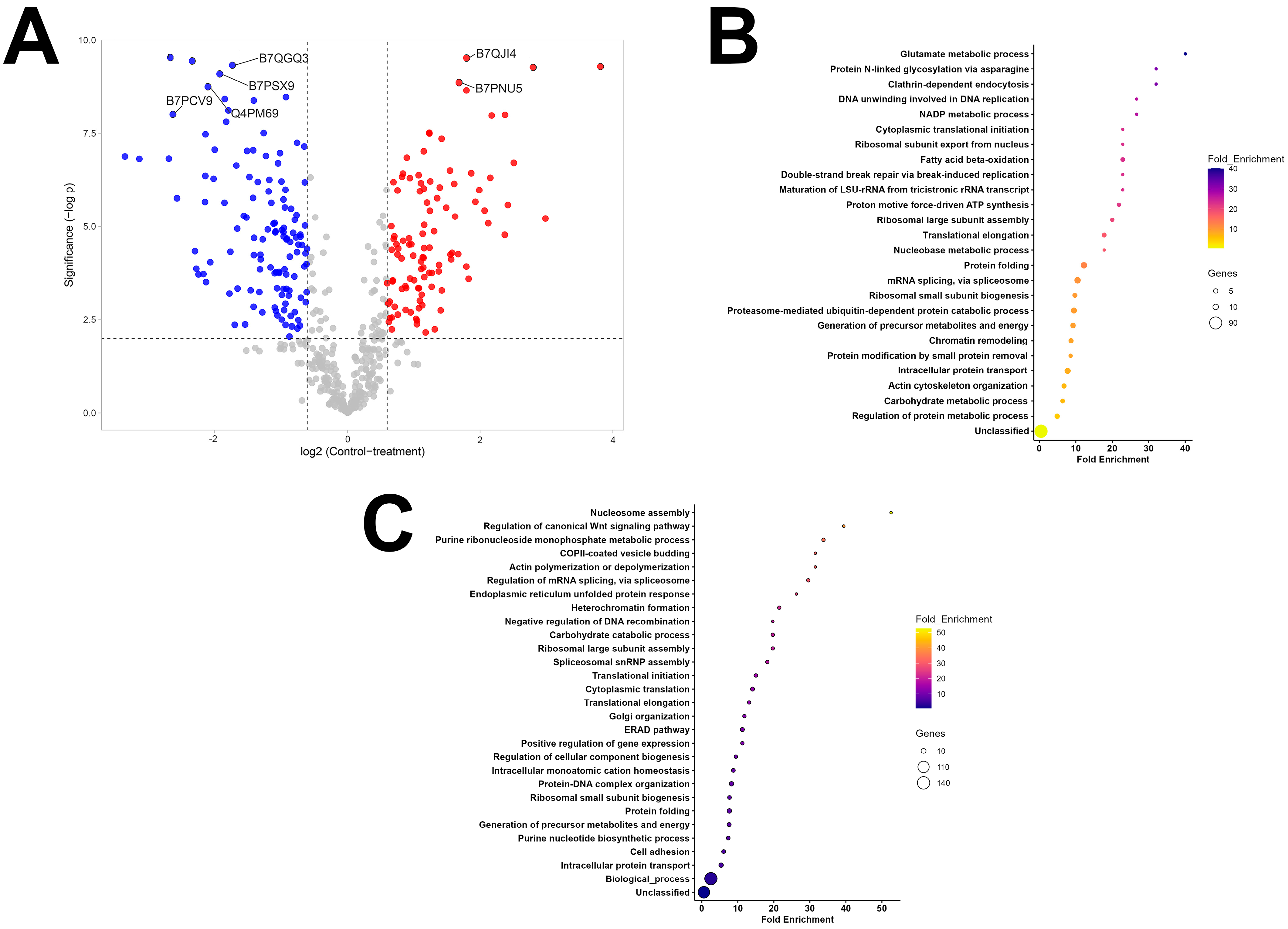
3.3. Salivary Gland-Derived Extracellular Vesicles Contain More Antigenic Proteins than Midgut Vesicles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| US | United States |
| WTD | White-tailed deer |
| SG | Salivary |
| MG | Midgut |
| EVs | Extracellular vesicles |
| PBS | Phosphate-buffered saline |
| LC | Liquid chromatography |
| DDA | Data-dependent acquisition |
| PASEF | Parallel accumulation–serial fragmentation |
| MS | Mass spectrometry |
| TIMS | Trapped ion mobility spectrometry |
| LFQ | Label-free quantification |
| MBR | Match between runs |
| NCBI | National Center for Biotechnology Information |
| PSM | Peptide–spectrum match |
| Da | Daltons |
| PVDF | Polyvinylidene fluoride |
| SDS | Sodium dodecyl sulfate |
| PAGE | Polyacrylamide gel electrophoresis |
| HRP | Horse radish peroxidase |
| DIA | Data-independent acquisition |
| CID | Collision-induced dissociation |
| FDR | False discovery rate |
| PSI | Position specific iterated |
| BLAST | Basic Local Alignment Search Tool |
| ATP | Adenosine triphosphate |
References
- CDC. Tickborne Disease Surveillance Data Summary. Available online: https://www.cdc.gov/ticks/data-research/facts-stats/tickborne-disease-surveillance-data-summary.html (accessed on 22 November 2024).
- Deshpande, G.; Beetch, J.E.; Heller, J.G.; Naqvi, O.H.; Kuhn, K.G. Assessing the Influence of Climate Change and Environmental Factors on the Top Tick-Borne Diseases in the United States: A Systematic Review. Microorganisms 2023, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L. Tick species infesting humans in the United States. Ticks Tick-Borne Dis. 2022, 13, 102025. [Google Scholar] [CrossRef] [PubMed]
- Teel, P.D.; Fuchs, T.W.; Huston, J.E.; Longnecker, M.T.; Pickel, S.L. Effects of sequential infestations of Dermacentor albipictus and Amblyomma americanum (Acari: Ixodidae) on overwintering beef cows in west-central Texas. J. Med. Entomol. 1990, 27, 632–641. [Google Scholar] [CrossRef]
- Higuita, N.I.A.; Franco-Paredes, C.; Henao-Martínez, A.F. The expanding spectrum of disease caused by the Lone Star Tick, Amblyomma americanum. Infez. Med. 2021, 29, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Sagurova, I.; Ludwig, A.; Ogden, N.H.; Pelcat, Y.; Dueymes, G.; Gachon, P. Predicted Northward Expansion of the Geographic Range of the Tick Vector Amblyomma americanum in North America under Future Climate Conditions. Environ. Health Perspect. 2019, 127, 107014. [Google Scholar] [CrossRef]
- Tardy, O.; Acheson, E.S.; Bouchard, C.; Chamberland, É.; Fortin, A.; Ogden, N.H.; Leighton, P.A. Mechanistic movement models to predict geographic range expansions of ticks and tick-borne pathogens: Case studies with Ixodes scapularis and Amblyomma americanum in eastern North America. Ticks Tick-Borne Dis. 2023, 14, 102161. [Google Scholar] [CrossRef]
- Koch, H.G. Suitability of White-Tailed Deer, Cattle, and Goats as Hosts for the Lone Star Tick, Amblyomma americanum (Acari: Ixodidae). J. Kans. Entomol. Soc. 1988, 61, 251–257. [Google Scholar]
- Hofmeester, T.R.; Sprong, H.; Jansen, P.A.; Prins, H.H.T.; van Wieren, S.E. Deer presence rather than abundance determines the population density of the sheep tick, Ixodes ricinus, in Dutch forests. Parasites Vectors 2017, 10, 433. [Google Scholar] [CrossRef]
- Larson, S.R.; Sabo, A.E.; Kruger, E.; Jones, P.; Paskewitz, S.M. Ixodes scapularis density in US temperate forests shaped by deer, earthworms, and disparate factors at two scales. Ecosphere 2022, 13, e3932. [Google Scholar] [CrossRef]
- Bloemer, S.R.; Snoddy, E.L.; Cooney, J.C.; Fairbanks, K. Influence of Deer Exclusion on Populations of Lone Star Ticks and American Dog Ticks (Acari: Ixodidae). J. Econ. Entomol. 1986, 79, 679–683. [Google Scholar] [CrossRef]
- Paddock, C.D.; Yabsley, M.J. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr. Top. Microbiol. Immunol. 2007, 315, 289–324. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.D.; Cheng, C.; Jaworski, D.C.; Willard, L.H.; Sanderson, M.W.; Ganta, R.R. Ehrlichia chaffeensis infection in the reservoir host (white-tailed deer) and in an incidental host (dog) is impacted by its prior growth in macrophage and tick cell environments. PLoS ONE 2014, 9, e109056. [Google Scholar] [CrossRef]
- Yabsley, M.J.; Varela, A.S.; Tate, C.M.; Dugan, V.G.; Stallknecht, D.E.; Little, S.E.; Davidson, W.R. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus). Emerg. Infect. Dis. 2002, 8, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Buller, R.S.; Arens, M.; Hmiel, S.P.; Paddock, C.D.; Sumner, J.W.; Rikhisa, Y.; Unver, A.; Gaudreault-Keener, M.; Manian, F.A.; Liddell, A.M.; et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 1999, 341, 148–155. [Google Scholar] [CrossRef]
- Tsao, J.I.; Hamer, S.A.; Han, S.; Sidge, J.L.; Hickling, G.J. The Contribution of Wildlife Hosts to the Rise of Ticks and Tick-Borne Diseases in North America. J. Med. Entomol. 2021, 58, 1565–1587. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.C.; Stafford, K.C.; Linske, M.A.; Brackney, D.E.; LaBonte, A.M.; Stuber, H.R.; Cozens, D.W. Effective control of the motile stages of Amblyomma americanum and reduced Ehrlichia spp. prevalence in adults via permethrin treatment of white-tailed deer in coastal Connecticut, USA. Ticks Tick-Borne Dis. 2021, 12, 101675. [Google Scholar] [CrossRef]
- Wong, T.J.; Schramm, P.J.; Foster, E.; Hahn, M.B.; Schafrick, N.H.; Conlon, K.C.; Cameron, L. The Effectiveness and Implementation of 4-Poster Deer Self-Treatment Devices for Tick-Borne Disease Prevention; CDC: Atlanta, GA, USA, 2017; p. 32. [Google Scholar]
- Nawrocki, C.C.; Piedmonte, N.; Niesobecki, S.A.; Rowe, A.; Hansen, A.P.; Kaufman, A.; Foster, E.; Meek, J.I.; Niccolai, L.; White, J.; et al. Acceptability of 4-poster deer treatment devices for community-wide tick control among residents of high Lyme disease incidence counties in Connecticut and New York, USA. Ticks Tick-Borne Dis. 2023, 14, 102231. [Google Scholar] [CrossRef]
- Kaplan, Z.D.; Richardson, E.A.; Taylor, C.E.; Kaufman, P.E.; Weeks, E.N.I. Determination of the Discriminating Concentration Towards Permethrin for Surveying Resistance in Amblyomma americanum. J. Med. Entomol. 2022, 59, 922–929. [Google Scholar] [CrossRef]
- Rosario-Cruz, R.; Domínguez-García, D.I.; Almazán, C. Inclusion of Anti-Tick Vaccines into an Integrated Tick Management Program in Mexico: A Public Policy Challenge. Vaccines 2024, 12, 403. [Google Scholar] [CrossRef]
- Gonzalez, J.; Harvey, C.; Ribeiro-Silva, C.d.S.; Leal-Galvan, B.; Persinger, K.A.; Olafson, P.U.; Johnson, T.L.; Oliva Chavez, A. Evaluation of tick salivary and midgut extracellular vesicles as anti-tick vaccines in White-tailed deer (Odocoileus virginianus). Ticks Tick-Borne Dis. 2025, 16, 102420. [Google Scholar] [CrossRef]
- Nawaz, M.; Malik, M.I.; Zhang, H.; Hassan, I.A.; Cao, J.; Zhou, Y.; Hameed, M.; Hussain Kuthu, Z.; Zhou, J. Proteomic Analysis of Exosome-Like Vesicles Isolated from Saliva of the Tick Haemaphysalis longicornis. Front. Cell. Infect. Microbiol. 2020, 10, 542319. [Google Scholar] [CrossRef]
- Oliva Chávez, A.S.; Wang, X.; Marnin, L.; Archer, N.K.; Hammond, H.L.; Carroll, E.E.M.; Shaw, D.K.; Tully, B.G.; Buskirk, A.D.; Ford, S.L.; et al. Tick extracellular vesicles enable arthropod feeding and promote distinct outcomes of bacterial infection. Nat. Commun. 2021, 12, 3696. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Y.; Sun, M.; Zhou, Y.; Cao, J.; Zhang, H.; Xuan, X.; Zhou, J. Proteomic analysis of extracellular vesicles from tick hemolymph and uptake of extracellular vesicles by salivary glands and ovary cells. Parasit Vectors 2023, 16, 125. [Google Scholar] [CrossRef]
- Butler, L.R.; Singh, N.; Marnin, L.; Valencia, L.M.; O’Neal, A.J.; Paz, F.E.C.; Shaw, D.K.; Chavez, A.S.O.; Pedra, J.H.F. The role of Rab27 in tick extracellular vesicle biogenesis and pathogen infection. Parasit Vectors 2024, 17, 57. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Li, J.; Smith, L.S.; Zhu, H.J. Data-independent acquisition (DIA): An emerging proteomics technology for analysis of drug-metabolizing enzymes and transporters. Drug Discov. Today Technol. 2021, 39, 49–56. [Google Scholar] [CrossRef]
- Domingues, L.N.; Bendele, K.G.; Bodine, D.M.; Halos, L.; Cutolo, A.A.; Liebstein, M.; Widener, J.; Figueiredo, M.; Moreno, Y.; Epe, C.; et al. A reverse vaccinology approach identified novel recombinant tick proteins with protective efficacy against Rhipicephalus microplus infestation. Ticks Tick-Borne Dis. 2024, 15, 102403. [Google Scholar] [CrossRef]
- Rodríguez-Mallon, A. The Bm86 Discovery: A Revolution in the Development of Anti-Tick Vaccines. Pathogens 2023, 12, 231. [Google Scholar] [CrossRef] [PubMed]
- Ndekezi, C.; Nkamwesiga, J.; Ochwo, S.; Kimuda, M.P.; Mwiine, F.N.; Tweyongyere, R.; Amanyire, W.; Muhanguzi, D. Identification of Ixodid Tick-Specific Aquaporin-1 Potential Anti-tick Vaccine Epitopes: An in-silico Analysis. Front. Bioeng. Biotechnol. 2019, 7, 236. [Google Scholar] [CrossRef]
- Costa, G.C.A.; Ribeiro, I.C.T.; Melo-Junior, O.; Gontijo, N.F.; Sant’Anna, M.R.V.; Pereira, M.H.; Pessoa, G.C.D.; Koerich, L.B.; Oliveira, F.; Valenzuela, J.G.; et al. Amblyomma sculptum Salivary Protease Inhibitors as Potential Anti-Tick Vaccines. Front. Immunol. 2020, 11, 611104. [Google Scholar] [CrossRef]
- Palmer, M.V.; Whipple, D.L.; Olsen, S.C.; Jacobson, R.H. Cell mediated and humoral immune responses of white-tailed deer experimentally infected with Mycobacterium bovis. Res. Vet. Sci. 2000, 68, 95–98. [Google Scholar] [CrossRef]
- Waters, W.R.; Palmer, M.V.; Pesch, B.A.; Olsen, S.C.; Wannemuehler, M.J.; Whipple, D.L. MHC class II-restricted, CD4(+) T-cell proliferative responses of peripheral blood mononuclear cells from Mycobacterium bovis-infected white-tailed deer. Vet. Immunol. Immunopathol. 2000, 76, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Ditchkoff, S.S.; Lochmiller, R.L.; Masters, R.E.; Hoofer, S.R.; Van Den Bussche, R.A. Major-histocompatibility-complex-associated variation in secondary sexual traits of white-tailed deer (Odocoileus virginianus): Evidence for good-genes advertisement. Evolution 2001, 55, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Ivy-Israel, N.M.D.; Moore, C.E.; Schwartz, T.S.; Ditchkoff, S.S. Characterization of two MHC II genes (DOB, DRB) in white-tailed deer (Odocoileus virginianus). BMC Genet. 2020, 21, 83. [Google Scholar] [CrossRef]
- Parvizpour, S.; Pourseif, M.M.; Razmara, J.; Rafi, M.A.; Omidi, Y. Epitope-based vaccine design: A comprehensive overview of bioinformatics approaches. Drug Discov. Today 2020, 25, 1034–1042. [Google Scholar] [CrossRef]
- Paul, S.; Sidney, J.; Sette, A.; Peters, B. TepiTool: A Pipeline for Computational Prediction of T Cell Epitope Candidates. Curr. Protoc. Immunol. 2016, 114, 18.19.11–18.19.24. [Google Scholar] [CrossRef]
- Kar, P.P.; Araveti, P.B.; Kuriakose, A.; Srivastava, A. Design of a multi-epitope protein as a subunit vaccine against lumpy skin disease using an immunoinformatics approach. Sci. Rep. 2022, 12, 19411. [Google Scholar] [CrossRef]
- Knorr, S.; Reissert-Oppermann, S.; Tomás-Cortázar, J.; Barriales, D.; Azkargorta, M.; Iloro, I.; Elortza, F.; Pinecki-Socias, S.; Anguita, J.; Hovius, J.W.; et al. Identification and Characterization of Immunodominant Proteins from Tick Tissue Extracts Inducing a Protective Immune Response against Ixodes ricinus in Cattle. Vaccines 2021, 9, 636. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.R.; Crispell, G.; Mohamed, A.; Cox, C.; Lange, J.; Choudhary, S.; Commins, S.P.; Karim, S. Alpha-Gal Syndrome: Involvement of Amblyomma americanum α-D-Galactosidase and β-1,4 Galactosyltransferase Enzymes in α-Gal Metabolism. Front Cell. Infect. Microbiol. 2021, 11, 775371. [Google Scholar] [CrossRef]
- Vechtova, P.; Sterbova, J.; Sterba, J.; Vancova, M.; Rego, R.O.M.; Selinger, M.; Strnad, M.; Golovchenko, M.; Rudenko, N.; Grubhoffer, L. A bite so sweet: The glycobiology interface of tick-host-pathogen interactions. Parasit Vectors 2018, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Leyva-Castillo, J.M.; Narasimhan, S. Tick salivary glycans—A sugar-coated tick bite. Trends Parasitol. 2023, 39, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Miyake, K.; Kamiya, A.; Karasuyama, H. The role of basophils in acquired protective immunity to tick infestation. Parasite Immunol. 2021, 43, e12804. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S. Insect chitinase and chitinase-like proteins. Cell. Mol. Life Sci. 2010, 67, 201–216. [Google Scholar] [CrossRef]
- You, M.; Fujisaki, K. Vaccination effects of recombinant chitinase protein from the hard tick Haemaphysalis longicornis (Acari: Ixodidae). J. Vet. Med. Sci. 2009, 71, 709–712. [Google Scholar] [CrossRef]
- Franta, Z.; Frantová, H.; Konvičková, J.; Horn, M.; Sojka, D.; Mareš, M.; Kopáček, P. Dynamics of digestive proteolytic system during blood feeding of the hard tick Ixodes ricinus. Parasit Vectors 2010, 3, 119. [Google Scholar] [CrossRef]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar] [CrossRef]
- Ndawula, C., Jr.; Tabor, A.E. Cocktail Anti-Tick Vaccines: The Unforeseen Constraints and Approaches toward Enhanced Efficacies. Vaccines 2020, 8, 457. [Google Scholar] [CrossRef]
- Luo, X.; McAndrews, K.M.; Arian, K.A.; Morse, S.J.; Boeker, V.; Kumbhar, S.V.; Hu, Y.; Mahadevan, K.K.; Church, K.A.; Chitta, S.; et al. Development of an engineered extracellular vesicles-based vaccine platform for combined delivery of mRNA and protein to induce functional immunity. J. Control. Release 2024, 374, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Douanne, N.; Wu, T.; Kaur, I.; Tsering, T.; Erzingatzian, A.; Nadeau, A.; Juncker, D.; Nerguizian, V.; Burnier, J.V. Leveraging nature’s nanocarriers: Translating insights from extracellular vesicles to biomimetic synthetic vesicles for biomedical applications. Sci. Adv. 2025, 11, eads5249. [Google Scholar] [CrossRef] [PubMed]
- Gai, C.; Pomatto, M.A.C.; Deregibus, M.C.; Dieci, M.; Piga, A.; Camussi, G. Edible Plant-Derived Extracellular Vesicles for Oral mRNA Vaccine Delivery. Vaccines 2024, 12, 200. [Google Scholar] [CrossRef]
- Voordouw, M.J.; Tupper, H.; Önder, Ö.; Devevey, G.; Graves, C.J.; Kemps, B.D.; Brisson, D. Reductions in human Lyme disease risk due to the effects of oral vaccination on tick-to-mouse and mouse-to-tick transmission. Vector-Borne Zoonotic Dis. 2013, 13, 203–214. [Google Scholar] [CrossRef] [PubMed]
- USDA-APHIS. Oral Rabies Vaccination. Available online: https://www.aphis.usda.gov/national-wildlife-programs/rabies/vaccine (accessed on 24 March 2025).
| Protein Accession | Protein Name (Tick Species) | Vesicle Type |
|---|---|---|
| JAT97670.1 | Putative alpha-macroglobulin, partial (Amblyomma aureolatum) | Salivary |
| JAT93800.1 | Putative alpha-macroglobulin, partial (Amblyomma aureolatum) | Salivary |
| AEO35522.1 | Hypothetical protein (Amblyomma maculatum) | Salivary |
| JAT93667.1 | Putative conserved secreted protein precursor (Amblyomma aureolatum) | Salivary |
| AXP34687.1 | Vitellogenin-1 (Haemaphysalis flava) | Salivary |
| XP_029834972.1 | Lysosomal alpha-glucosidase-like, partial (Ixodes scapularis) | Salivary |
| JAU01362.1 | Putative vitellogenin-2, partial (Amblyomma sculptum) | Midgut |
| JAT99430.1 | Putative lysosomal acid phosphatase, partial (Amblyomma sculptum) | Midgut |
| JAU00577.1 | Putative conserved secreted protein precursor, partial (Amblyomma sculptum) | Midgut |
| JAP80714.1 | Hypothetical protein (Rhipicephalus appendiculatus) | Midgut |
| Protein Accession # | Protein Name (Tick Species) | Vesicle Type |
|---|---|---|
| JAT95206.1 | Hypothetical protein, partial (Amblyomma aureolatum) | Midgut |
| JAG92630.1 | Putative peptidase family m2 angiotensin converting enzyme, partial (Amblyomma aureolatum) | Salivary |
| JAT95172.1 | Putative catalytically inactive chitinase-like lectin, partial (Amblyomma aureolatum) | Salivary |
| JAT95331.1 | Hypothetical protein, partial (Amblyomma aureolatum) | Salivary |
| JAU00577.1 | Putative conserved secreted protein precursor, partial (Amblyomma sculptum) | Salivary |
| JAU00888.1 | Putative alpha-d-galactosidase melibiase, partial (Amblyomma sculptum) | Salivary |
| JAU00890.1 | Protein in meprin, partial (Amblyomma sculptum) | Salivary |
| JAP80714.1 | Hypothetical protein (Rhipicephalus appendiculatus) | Salivary |
| Protein Accession # | Protein Name (Tick Species) | Vesicle Type |
|---|---|---|
| JAT94005.1 | Putative secreted mucin muc17, partial (Amblyomma aureolatum) | Midgut |
| JAU02543.1 | Hypothetical protein, partial (Amblyomma sculptum) | Midgut |
| JAT97490.1 | N-acylsphingosine amidohydrolase acid ceramidase, partial (Amblyomma aureolatum) | Salivary |
| XP_029824064.1 | Hemicentin-2 putative, partial (Ixodes scapularis) | Salivary |
| JAP66750.1 | Metalloprotease atp-dependent zinc metalloprotease yme1 protein isoform x1, partial (Hyalomma excavatum) | Salivary |
| JAP71591.1 | LOW glucosidase ii catalytic alpha subunit, partial (Ixodes ricinus) | Salivary |
| JAU01951.1 | Putative conserved plasma membrane protein, partial (Amblyomma sculptum) | Salivary |
| Protein Accession # | Protein Name (Tick Species) | Vesicle Type |
|---|---|---|
| JAT93800.1 | Putative alpha-macroglobulin, partial (Amblyomma aureolatum) | Salivary |
| JAP82974.1 | Uncharacterized alpha-mannosidase (Rhipicephalus appendiculatus) | Salivary |
| JAT95210.1 | Putative glucosidase ii catalytic alpha subunit, partial (Amblyomma aureolatum) | Salivary |
| JAU00361.1 | Hypothetical protein, partial (Amblyomma sculptum) | Salivary |
| AEO36445.1 | Endochitinase-like protein (Amblyomma maculatum) | Salivary |
| JAP83809.1 | Hypothetical protein (Rhipicephalus appendiculatus) | Salivary |
| JAT95556.1 | Putative aminopeptidase (Amblyomma aureolatum) | Salivary |
| JAU00184.1 | Putative catalytic domain of lysosomal alpha-mannosidase, partial (Amblyomma sculptum) | Salivary |
| JAP80906.1 | Lysosomal acid phosphatase (Rhipicephalus appendiculatus) | Salivary |
| JAG92577.1 | Putative tetraspanin (Amblyomma americanum) | Salivary |
| JAU01916.1 | Putative vitellogenin-b (Amblyomma sculptum) | Salivary |
| JAT97770.1 | Putative leucine aminopeptidase, partial (Amblyomma aureolatum) | Salivary |
| JAT97963.1 | Putative alpha-amylase, partial (Amblyomma aureolatum) | Salivary |
| AJR36491.1 | Vitellogenin-6 CP3 (Ixodes ricinus) | Salivary |
| JAT92299.1 | Putative secreted protein, partial (Amblyomma aureolatum) | Salivary |
| AEO35082.1 | Putative protein (Amblyomma maculatum) | Salivary |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva Chávez, A.; Gonzalez, J.; Harvey, C.; Ribeiro-Silva, C.d.S.; Leal-Galvan, B.; Persinger, K.A.; Durski, S.; Olafson, P.U.; Johnson, T.L. Identification of Potential Amblyomma americanum Antigens After Vaccination with Tick Extracellular Vesicles in White-Tailed Deer. Vaccines 2025, 13, 355. https://doi.org/10.3390/vaccines13040355
Oliva Chávez A, Gonzalez J, Harvey C, Ribeiro-Silva CdS, Leal-Galvan B, Persinger KA, Durski S, Olafson PU, Johnson TL. Identification of Potential Amblyomma americanum Antigens After Vaccination with Tick Extracellular Vesicles in White-Tailed Deer. Vaccines. 2025; 13(4):355. https://doi.org/10.3390/vaccines13040355
Chicago/Turabian StyleOliva Chávez, Adela, Julia Gonzalez, Cristina Harvey, Cárita de Souza Ribeiro-Silva, Brenda Leal-Galvan, Kelly A. Persinger, Sarah Durski, Pia U. Olafson, and Tammi L. Johnson. 2025. "Identification of Potential Amblyomma americanum Antigens After Vaccination with Tick Extracellular Vesicles in White-Tailed Deer" Vaccines 13, no. 4: 355. https://doi.org/10.3390/vaccines13040355
APA StyleOliva Chávez, A., Gonzalez, J., Harvey, C., Ribeiro-Silva, C. d. S., Leal-Galvan, B., Persinger, K. A., Durski, S., Olafson, P. U., & Johnson, T. L. (2025). Identification of Potential Amblyomma americanum Antigens After Vaccination with Tick Extracellular Vesicles in White-Tailed Deer. Vaccines, 13(4), 355. https://doi.org/10.3390/vaccines13040355











