Mind the Age Gap: Expanding the Age Window for mRNA Vaccine Testing in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. LNP Preparation
2.3. LNP Characterization
2.4. In Vivo Expression and Antibody Responses
2.5. Statistical Analysis
3. Results
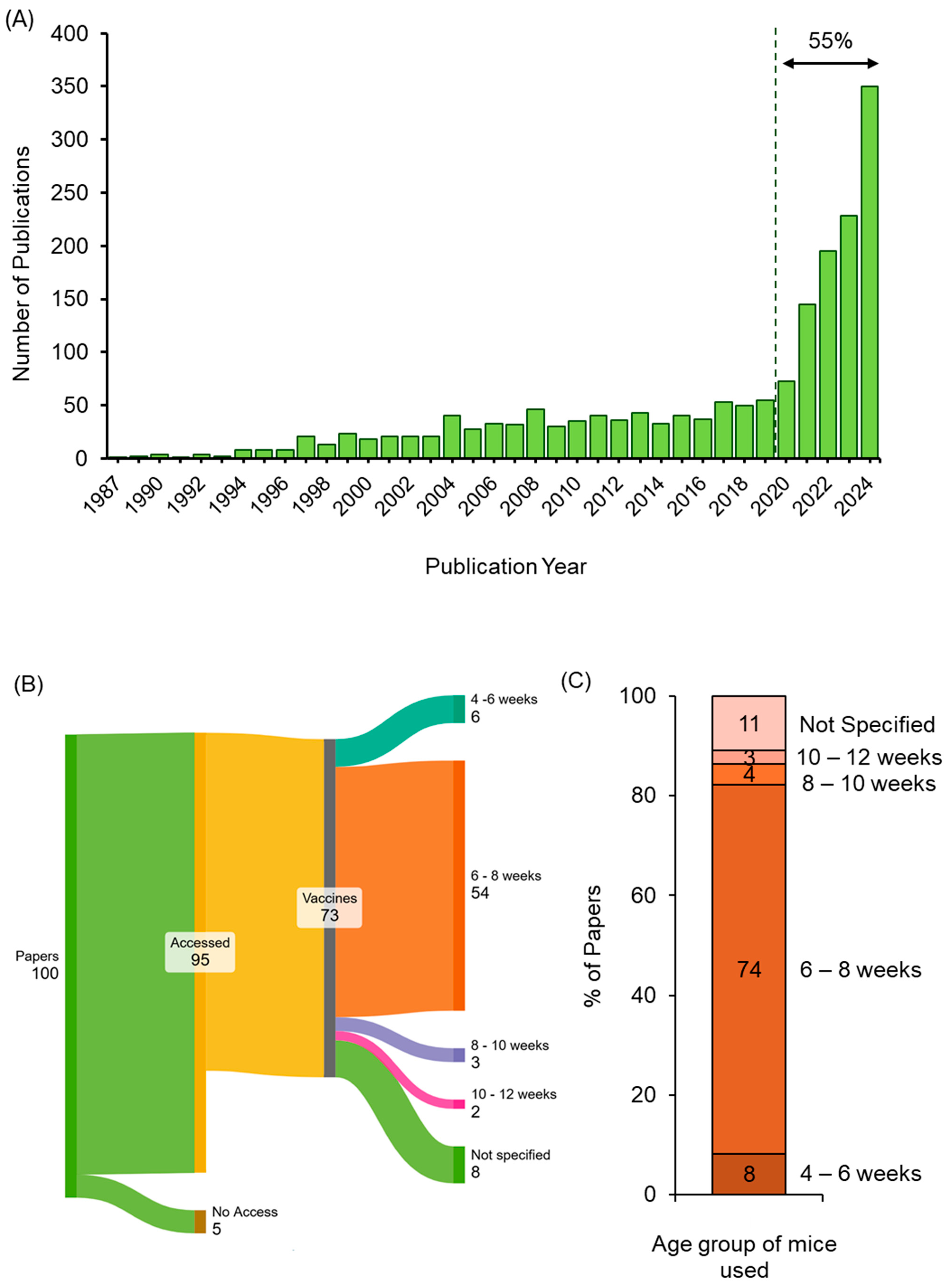
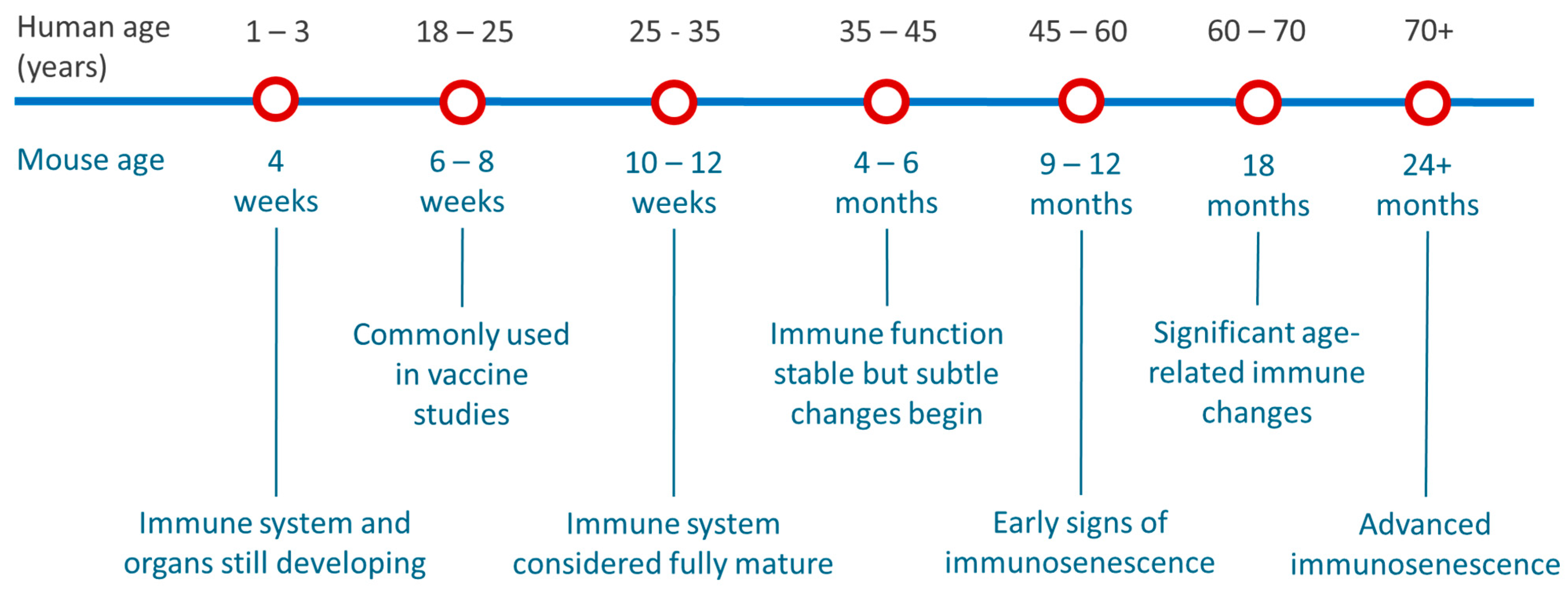
3.1. Assessing Age Selection Used in Published Pre-Clinical Mouse mRNA Vaccine Studies
3.2. LNP Formulation and Physicochemical Characterization
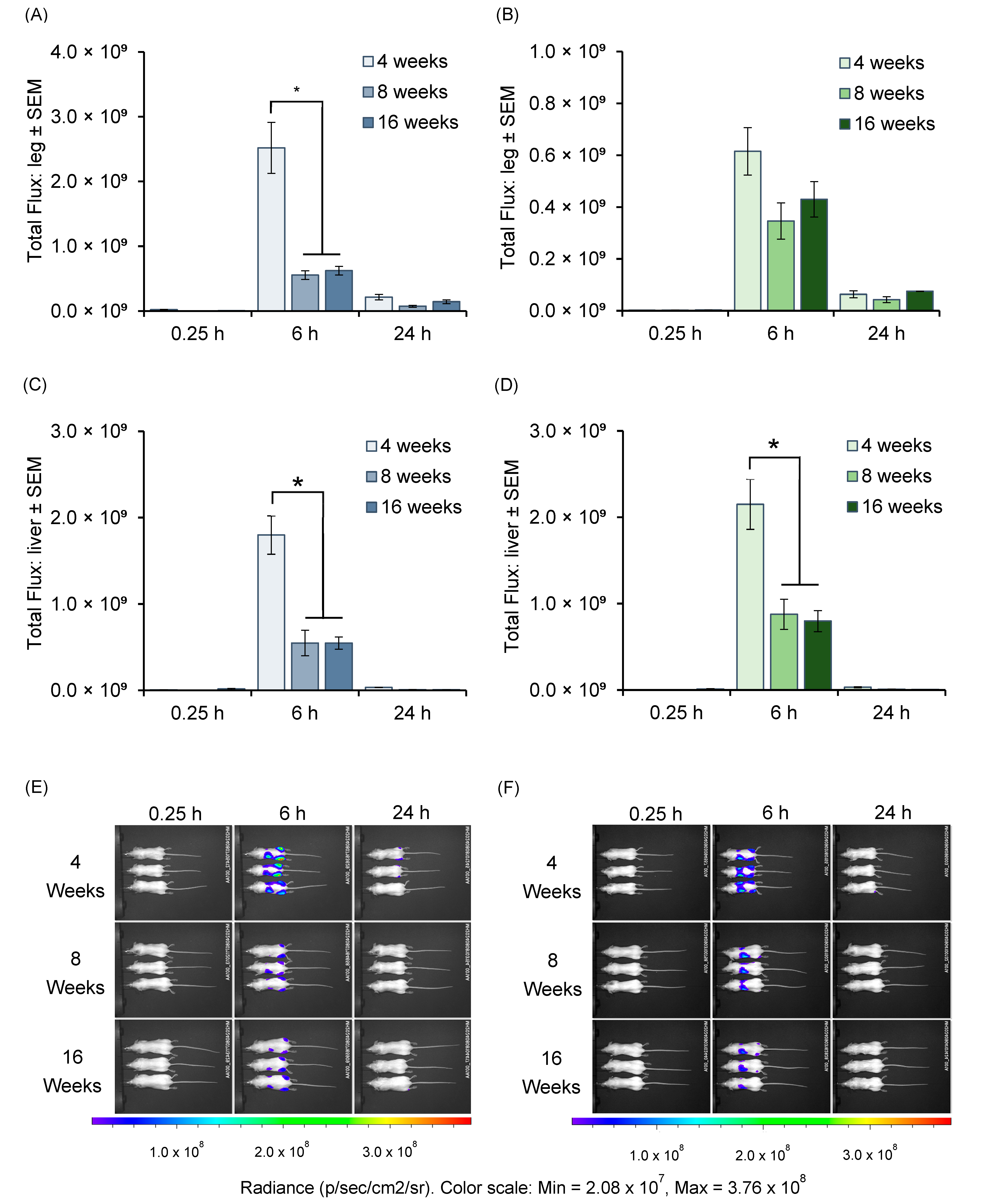
3.3. In Vivo mRNA Expression Across Age Groups
3.4. Antibody Responses in Different Age Groups of Mice Immunized with mRNA-LNPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiros, T.G.; Levast, B.; Auray, G.; Strom, S.; van Kessel, J.; Gerdts, V. The Importance of Animal Models in the Development of Vaccines. In Innovation in Vaccinology; Baschieri, S., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 251–264. [Google Scholar]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [PubMed]
- Zhang, L.; More, K.R.; Ojha, A.; Jackson, C.B.; Quinlan, B.D.; Li, H.; He, W.; Farzan, M.; Pardi, N.; Choe, H. Effect of mRNA-LNP components of two globally-marketed COVID-19 vaccines on efficacy and stability. npj Vaccines 2023, 8, 156. [Google Scholar] [CrossRef]
- Guimaraes, P.P.; Zhang, R.; Spektor, R.; Tan, M.; Chung, A.; Billingsley, M.M.; El-Mayta, R.; Riley, R.S.; Wang, L.; Wilson, J.M. Ionizable lipid nanoparticles encapsulating barcoded mRNA for accelerated in vivo delivery screening. J. Control. Release 2019, 316, 404–417. [Google Scholar]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther.-Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Zadory, M.; Lopez, E.; Babity, S.; Gravel, S.-P.; Brambilla, D. Current knowledge on the tissue distribution of mRNA nanocarriers for therapeutic protein expression. Biomater. Sci. 2022, 10, 6077–6115. [Google Scholar] [CrossRef]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef] [PubMed]
- McMillan, C.; Druschitz, A.; Rumbelow, S.; Borah, A.; Binici, B.; Rattray, Z.; Perrie, Y. Tailoring lipid nanoparticle dimensions through manufacturing processes. RSC Pharm. 2024, 1, 841–853. [Google Scholar] [CrossRef]
- Hussain, M.; Binici, B.; O’Connor, L.; Perrie, Y. Production of mRNA lipid nanoparticles using advanced crossflow micromixing. J. Pharm. Pharmacol. 2024, 76, 1572–1583. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Sellers, R.S.; Clifford, C.B.; Treuting, P.M.; Brayton, C. Immunological Variation Between Inbred Laboratory Mouse Strains:Points to Consider in Phenotyping Genetically Immunomodified Mice. Vet. Pathol. 2012, 49, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Binici, B.; Rattray, Z.; Schroeder, A.; Perrie, Y. The Role of Biological Sex in Pre-Clinical (Mouse) mRNA Vaccine Studies. Vaccines 2024, 12, 282. [Google Scholar] [CrossRef]
- Hu, J.G.; Yokoyama, T.; Kitagawa, T. Studies on the optimal immunization schedule of experimental animals. IV. The optimal age and sex of mice, and the influence of booster injections. Chem. Pharm. Bull. 1990, 38, 448–451. [Google Scholar] [CrossRef]
- Carter, K.C.; Ata, D.T.; Aruleba, R.T.; Hurdayal, R. Preclinical Testing of Vaccine Candidates in Animal Models. In System Vaccinology; Prajapati, V., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 257–280. [Google Scholar]
- Mähler Convenor, M.; Berard, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K.; Raspa, M. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab. Anim. 2014, 48, 178–192. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Yan, F.; Gao, Y.; Yang, S.; Xia, X. COVID-19 animal models and vaccines: Current landscape and future prospects. Vaccines 2021, 9, 1082. [Google Scholar] [CrossRef] [PubMed]
- Karekar, N.; Cahn, A.R.; Morla-Folch, J.; Saffon, A.; Ward, R.W.; Ananthanarayanan, A.; Teunissen, A.J.; Bhardwaj, N.; Vabret, N. Protocol for the development of mRNA lipid nanoparticle vaccines and analysis of immunization efficiency in mice. STAR Protoc. 2024, 5, 103087. [Google Scholar] [CrossRef]
- de Frel, D.L.; Atsma, D.E.; Pijl, H.; Seidell, J.C.; Leenen, P.J.M.; Dik, W.A.; van Rossum, E.F.C. The Impact of Obesity and Lifestyle on the Immune System and Susceptibility to Infections Such as COVID-19. Front. Nutr. 2020, 7, 597600. [Google Scholar] [CrossRef]
- Painter, S.D.; Ovsyannikova, I.G.; Poland, G.A. The weight of obesity on the human immune response to vaccination. Vaccine 2015, 33, 4422–4429. [Google Scholar] [CrossRef]
- Park, H.L.; Shim, S.H.; Lee, E.Y.; Cho, W.; Park, S.; Jeon, H.J.; Ahn, S.Y.; Kim, H.; Nam, J.H. Obesity-induced chronic inflammation is associated with the reduced efficacy of influenza vaccine. Hum. Vaccin. Immunother. 2014, 10, 1181–1186. [Google Scholar] [CrossRef]
- Piening, A.; Ebert, E.; Gottlieb, C.; Khojandi, N.; Kuehm, L.M.; Hoft, S.G.; Pyles, K.D.; McCommis, K.S.; DiPaolo, R.J.; Ferris, S.T.; et al. Obesity-related T cell dysfunction impairs immunosurveillance and increases cancer risk. Nat. Commun. 2024, 15, 2835. [Google Scholar] [CrossRef] [PubMed]
- Meade, C.J.; Sheena, J. Immunity in genetically obese rodents. In Animal Models of Obesity; Festing, M.F.W., Ed.; Palgrave Macmillan UK: London, UK, 1979; pp. 205–220. [Google Scholar] [CrossRef]
- Hagan, C. When are mice considered old. Jackson Lab. 2017, 649. Available online: https://www.jax.org/news-and-insights/jax-blog/2017/November/when-are-mice-considered-old (accessed on 21 March 2025).
- Blake, J.A.; Baldarelli, R.; Kadin, J.A.; Richardson, J.E.; Smith, C.L.; Bult, C.J. Mouse Genome Database (MGD): Knowledgebase for mouse–human comparative biology. Nucleic Acids Res. 2021, 49, D981–D987. [Google Scholar] [PubMed]
- Ghia, P.; ten Boekel, E.; Rolink, A.G.; Melchers, F. B-cell development: A comparison between mouse and man. Immunol. Today 1998, 19, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Holladay, S.D.; Smialowicz, R.J. Development of the murine and human immune system: Differential effects of immunotoxicants depend on time of exposure. Environ. Health Perspect. 2000, 108 (Suppl. S3), 463–473. [Google Scholar] [CrossRef]
- Landreth, K.S. Critical windows in development of the rodent immune system. Hum. Exp. Toxicol. 2002, 21, 493–498. [Google Scholar] [CrossRef]
- Aaberge, I.; North, R.; Groeng, E.C.; Løvik, M. Antibody response to pneumococcal polysaccharide vaccine in young, adult and old mice. Scand. J. Immunol. 1993, 38, 17–30. [Google Scholar]
- García, M.; Misplon, J.A.; Price, G.E.; Lo, C.-Y.; Epstein, S.L. Age dependence of immunity induced by a candidate universal influenza vaccine in mice. PLoS ONE 2016, 11, e0153195. [Google Scholar]
- Allen, J.C.; Toapanta, F.R.; Baliban, S.M.; Sztein, M.B.; Tennant, S.M. Reduced immunogenicity of a live Salmonella enterica serovar Typhimurium vaccine in aged mice. Front. Immunol. 2023, 14, 1190339. [Google Scholar]
- Castro, F.; Leal, B.; Denny, A.; Bahar, R.; Lampkin, S.; Reddick, R.; Lu, S.; Gravekamp, C. Vaccination with Mage-b DNA induces CD8 T-cell responses at young but not old age in mice with metastatic breast cancer. Br. J. Cancer 2009, 101, 1329–1337. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Montecino-Rodriguez, E.; Berent-Maoz, B.; Dorshkind, K. Causes, consequences, and reversal of immune system aging. J. Clin. Investig. 2013, 123, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Brook, B.; Fatou, B.; Checkervarty, A.K.; Barman, S.; Sweitzer, C.; Bosco, A.-N.; Sherman, A.C.; Baden, L.R.; Morrocchi, E.; Sanchez-Schmitz, G. The mRNA vaccine BNT162b2 demonstrates impaired TH1 immunogenicity in human elders in vitro and aged mice in vivo. Res. Sq. 2022, rs.3.rs-2395118. [Google Scholar] [CrossRef]

| Lipid | SM102 LNPs | ALC-0315 LNPs | |
|---|---|---|---|
| DSPC | 10% | 9.4% | |
| Cholesterol | 38.5% | 42.7% | |
| Ionizable lipid | SM102 | 50% | |
| ALC-0315 | 46.3% | ||
| PEG lipid | DMG-PEG | 1.5% | |
| ALC-0159 | 1.6% | ||
| EZ Cap™ Firefly Luciferase mRNA (5-moUTP) | ||
|---|---|---|
| LNP Physico-Chemical Attributes | SM102 LNPs | ALC-0315 LNPs |
| z-average diameter | 69 ± 1 | 89 ± 4 |
| PDI | 0.05 ± 0.01 | 0.02 ± 0.01 |
| Zeta Potential (mV) | −4 ± 3 | −4 ± 1 |
| mRNA Encapsulation (%) | 93 ± 1 | 92 ± 3 |
| mRNA Recovery (%) | 90 ± 8 | 92 ± 12 |
| Ovalbumin-Encoding mRNA Modified with 5-Methoxyuridine (5moU) | ||
|---|---|---|
| LNP Physicochemical Attributes | SM102 | ALC-0315 |
| z-average diameter | 84 ± 11 | 87 ± 8 |
| PDI | 0.04 ± 0.02 | 0.06 ± 0.05 |
| Zeta Potential (mV) | −2 ± 2 | −1 ± 1 |
| mRNA Encapsulation (%) | 92 ± 1 | 95 ± 1 |
| mRNA Recovery (%) | 91 ± 8 | 97 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.; Ferguson-Ugorenko, A.; Macfarlane, R.; Orr, N.; Clarke, S.; Wilkinson, M.J.A.; Horan, L.; Perrie, Y. Mind the Age Gap: Expanding the Age Window for mRNA Vaccine Testing in Mice. Vaccines 2025, 13, 370. https://doi.org/10.3390/vaccines13040370
Hussain M, Ferguson-Ugorenko A, Macfarlane R, Orr N, Clarke S, Wilkinson MJA, Horan L, Perrie Y. Mind the Age Gap: Expanding the Age Window for mRNA Vaccine Testing in Mice. Vaccines. 2025; 13(4):370. https://doi.org/10.3390/vaccines13040370
Chicago/Turabian StyleHussain, Muattaz, Agata Ferguson-Ugorenko, Rebecca Macfarlane, Natalie Orr, Samuel Clarke, Michael J. A. Wilkinson, Linda Horan, and Yvonne Perrie. 2025. "Mind the Age Gap: Expanding the Age Window for mRNA Vaccine Testing in Mice" Vaccines 13, no. 4: 370. https://doi.org/10.3390/vaccines13040370
APA StyleHussain, M., Ferguson-Ugorenko, A., Macfarlane, R., Orr, N., Clarke, S., Wilkinson, M. J. A., Horan, L., & Perrie, Y. (2025). Mind the Age Gap: Expanding the Age Window for mRNA Vaccine Testing in Mice. Vaccines, 13(4), 370. https://doi.org/10.3390/vaccines13040370







