A Phase 1/2 Randomized Study to Evaluate the Safety, Tolerability, and Immunogenicity of Nucleoside-Modified Messenger RNA Influenza Vaccines in Healthy Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Intervention
2.3. Objectives and Endpoints
2.3.1. Safety
2.3.2. Immunogenicity
2.4. Statistical Analyses
3. Results
3.1. Phase 1 Substudy A
3.1.1. Participants
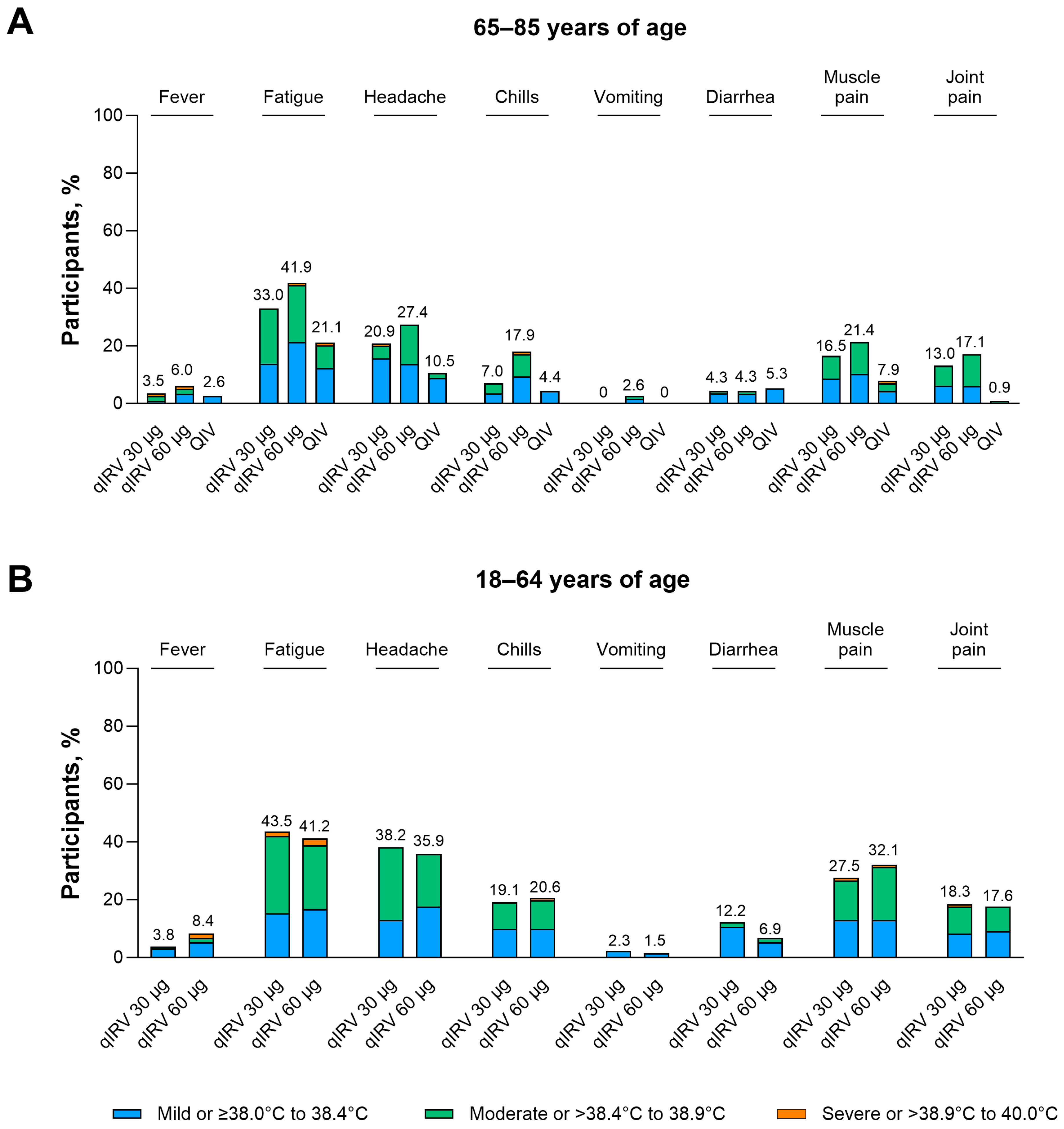
3.1.2. Safety
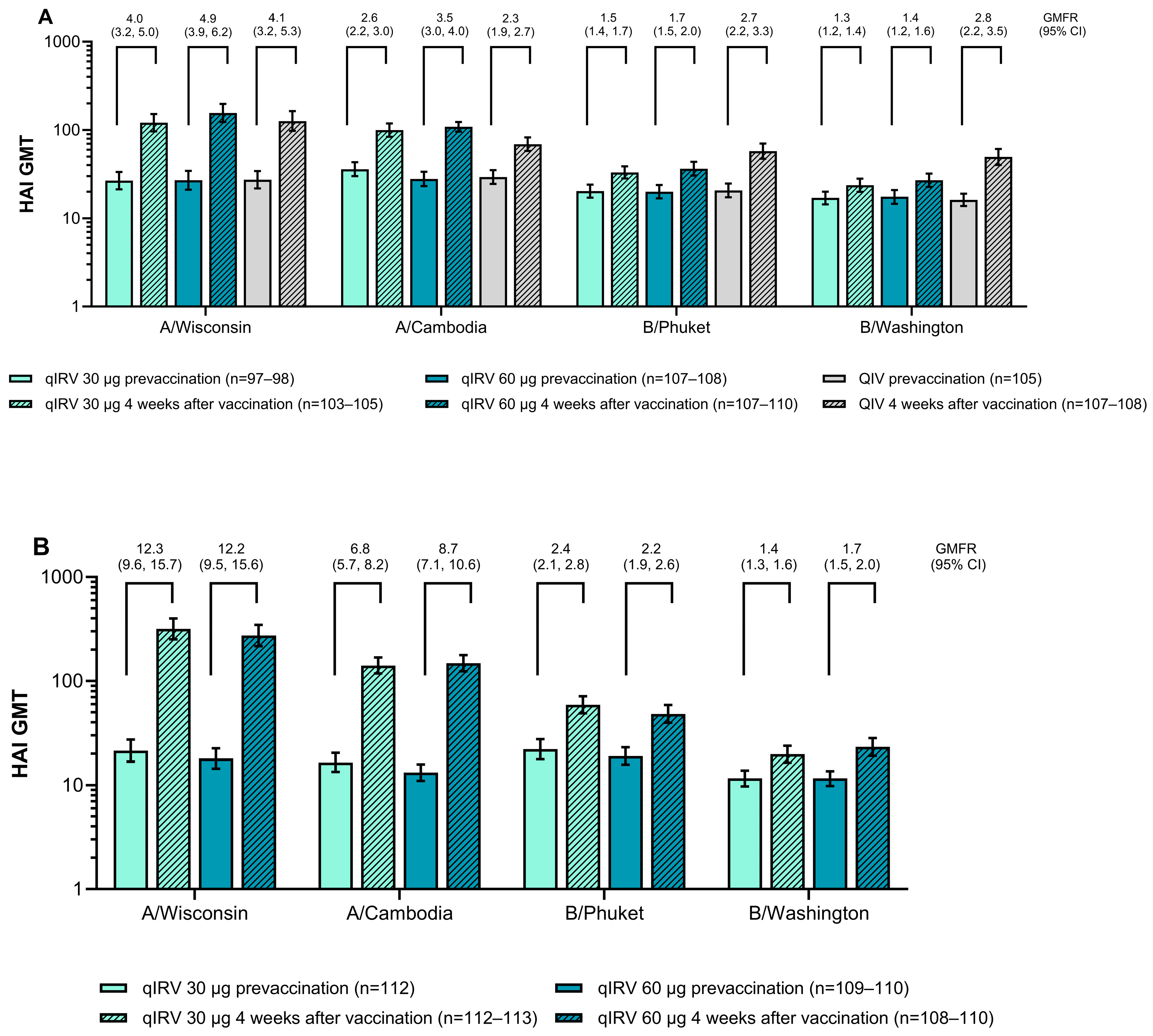
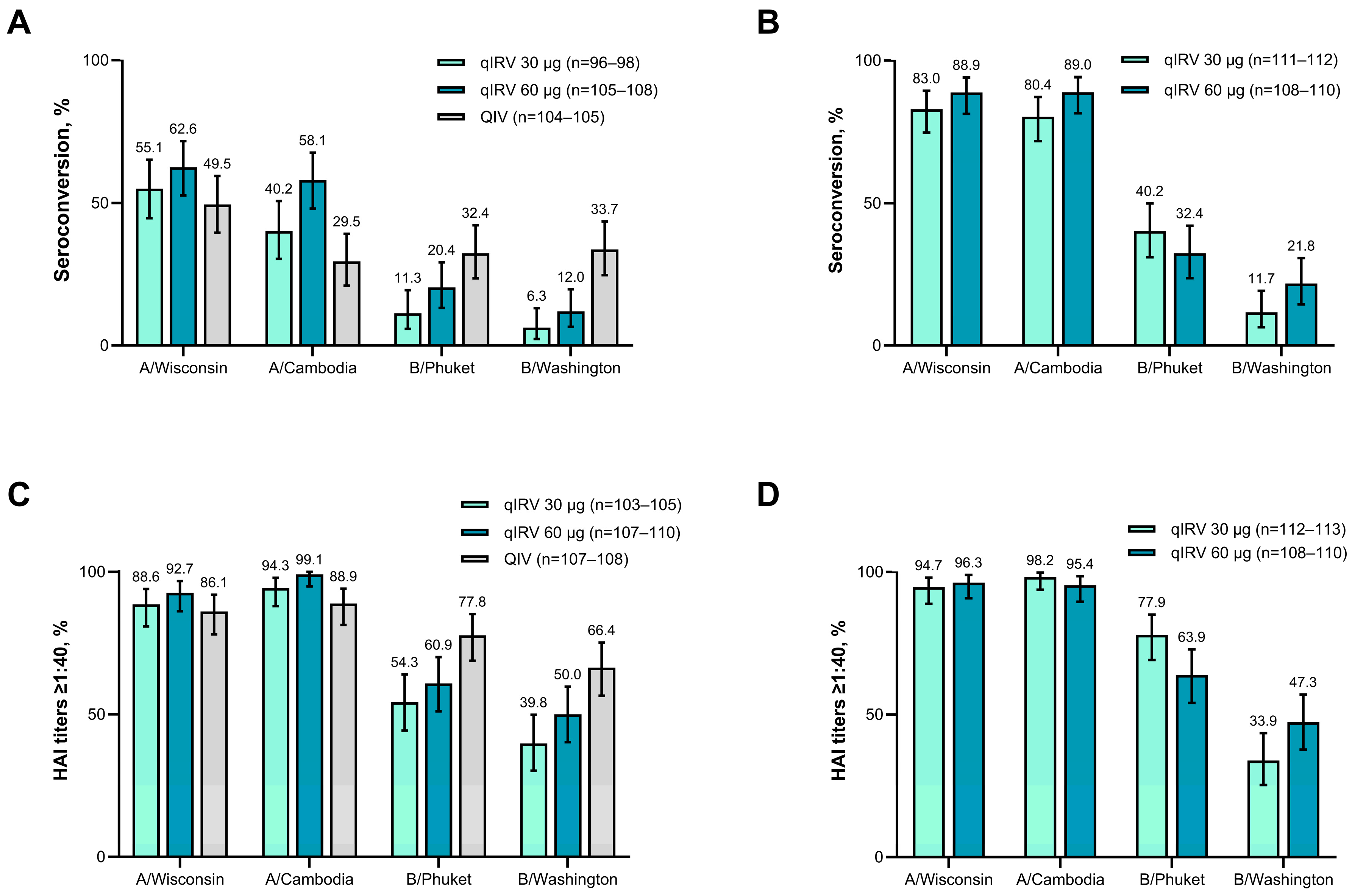
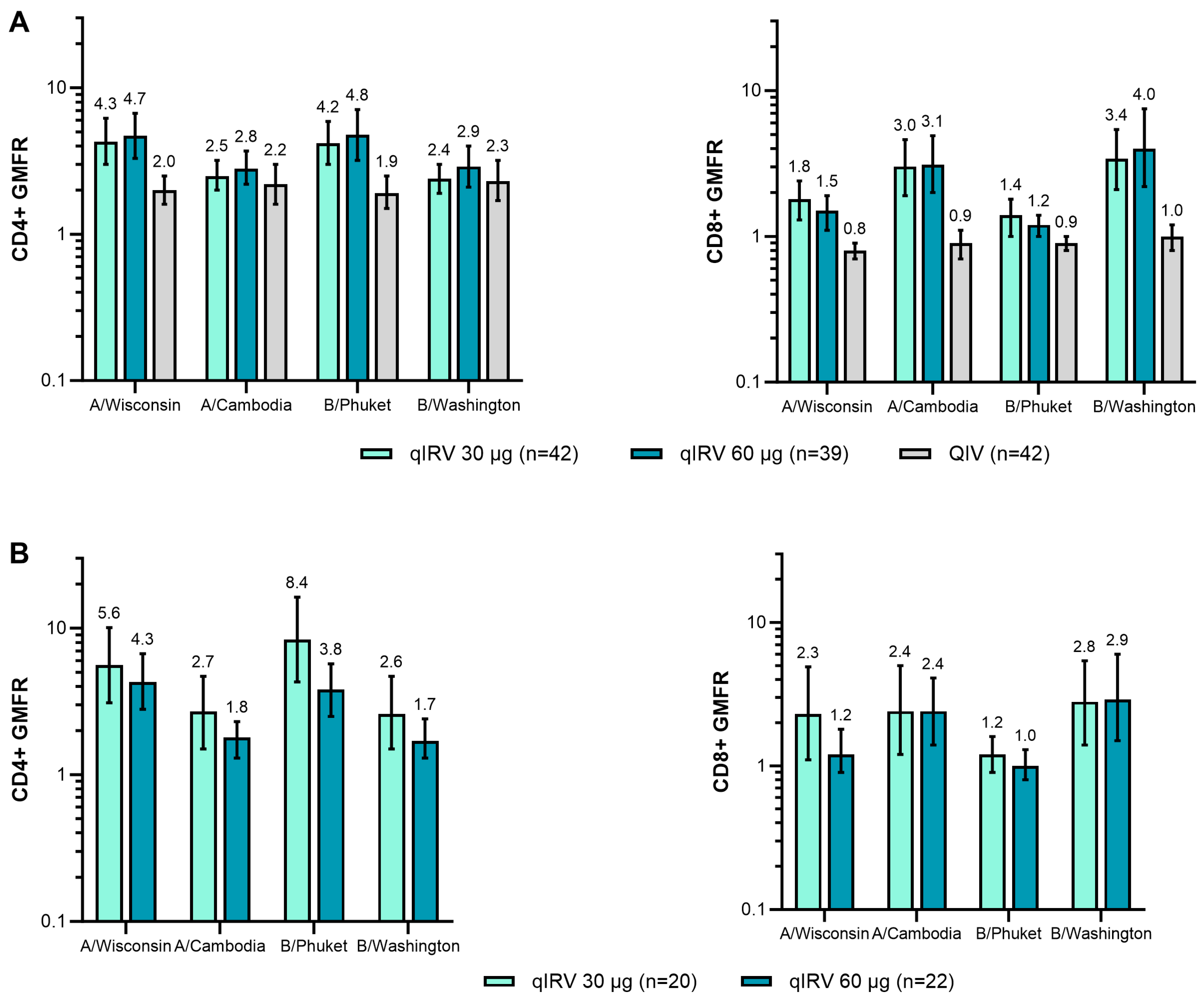
3.1.3. Immunogenicity
3.2. Phase 2 Substudy B
3.2.1. Participants
3.2.2. Safety
3.2.3. Immunogenicity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers. 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Eccles, R. Understanding the symptoms of the common cold and influenza. Lancet Infect. Dis. 2005, 5, 718–725. [Google Scholar] [CrossRef]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention. Preliminary Estimated Flu Disease Burden 2023–2024 Flu Season. Available online: https://www.cdc.gov/flu-burden/php/data-vis/2023-2024.html (accessed on 25 September 2024).
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Walter, E.B.; Bresee, J.S.; Fry, A.M.; Jernigan, D.B. Prevention and control of seasonal influenza with vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2017–18 Influenza Season. MMWR Recomm. Rep. 2017, 66, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.; Vesikari, T.; Szymczakiewicz-Multanowska, A.; Lattanzi, M.; Izu, A.; Groth, N.; Holmes, S. Clinical efficacy of cell culture–derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin. Infect. Dis. 2010, 51, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Tosh, P.K.; Jacobson, R.M.; Poland, G.A. Influenza vaccines: From surveillance through production to protection. Mayo Clin. Proc. 2010, 85, 257–273. [Google Scholar] [CrossRef]
- Agor, J.K.; Özaltın, O.Y. Models for predicting the evolution of influenza to inform vaccine strain selection. Hum. Vaccines Immunother. 2018, 14, 678–683. [Google Scholar] [CrossRef]
- Liang, W.; Tan, T.J.C.; Wang, Y.; Lv, H.; Sun, Y.; Bruzzone, R.; Mok, C.K.P.; Wu, N.C. Egg-adaptive mutations of human influenza H3N2 virus are contingent on natural evolution. PLoS Pathog. 2022, 18, e1010875. [Google Scholar] [CrossRef]
- Hall, E. Chapter 12: Influenza. In Epidemiology and Prevention of Vaccine-Preventable Diseases, 14th ed.; Hall, E., Wodi, A.P., Hamborsky, J., Morelli, V., Schillie, S., Eds.; Public Health Foundation: Washington, DC, USA, 2021. [Google Scholar]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Kackos, C.M.; DeBeauchamp, J.; Davitt, C.J.H.; Lonzaric, J.; Sealy, R.E.; Hurwitz, J.L.; Samsa, M.M.; Webby, R.J. Seasonal quadrivalent mRNA vaccine prevents and mitigates influenza infection. npj Vaccines 2023, 8, 157. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Hauguel, T.; Sharma, A.; Mastrocola, E.; Lowry, S.; Maddur, M.S.; Hu, C.H.; Rajput, S.; Vitsky, A.; Choudhary, S.; Manickam, B.; et al. Preclinical immunogenicity and safety of hemagglutinin-encoding modRNA influenza vaccines. npj Vaccines 2024, 9, 183. [Google Scholar] [CrossRef]
- Hsu, D.; Jayaraman, A.; Pucci, A.; Joshi, R.; Mancini, K.; Chen, H.L.; Koslovsky, K.; Mao, X.; Choi, A.; Henry, C.; et al. Safety and immunogenicity of mRNA-based seasonal influenza vaccines formulated to include multiple A/H3N2 strains with or without the B/Yamagata strain in US adults aged 50–75 years: A phase 1/2, open-label, randomised trial. Lancet Infect. Dis. 2025, 25, 25–35. [Google Scholar] [CrossRef]
- Lee, I.T.; Nachbagauer, R.; Ensz, D.; Schwartz, H.; Carmona, L.; Schaefers, K.; Avanesov, A.; Stadlbauer, D.; Henry, C.; Chen, R.; et al. Safety and immunogenicity of a phase 1/2 randomized clinical trial of a quadrivalent, mRNA-based seasonal influenza vaccine (mRNA-1010) in healthy adults: Interim analysis. Nat. Commun. 2023, 14, 3631. [Google Scholar] [CrossRef]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Herve, C.; Laupeze, B.; Del Giudice, G.; Didierlaurent, A.M.; Tavares Da Silva, F. The how’s and what’s of vaccine reactogenicity. npj Vaccines 2019, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Co, M.D.; Orphin, L.; Cruz, J.; Pazoles, P.; Green, K.M.; Potts, J.; Leporati, A.M.; Babon, J.A.; Evans, J.E.; Ennis, F.A.; et al. In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses. Vaccine 2009, 27, 319–327. [Google Scholar] [CrossRef]
- Jones, C.H.; Hauguel, T.; Beitelshees, M.; Davitt, M.; Welch, V.; Lindert, K.; Allen, P.; True, J.M.; Dolsten, M. Deciphering immune responses: A comparative analysis of influenza vaccination platforms. Drug Discov. Today 2024, 29, 104125. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Perini, D.; Mather, S.; Temperton, N.; Montomoli, E. Overview of serological techniques for influenza vaccine evaluation: Past, present and future. Vaccines 2014, 2, 707–734. [Google Scholar] [CrossRef] [PubMed]
- Monto, A.S.; Maassab, H.F. Ether treatment of type B influenza virus antigen for the hemagglutination inhibition test. J. Clin. Microbiol. 1981, 13, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.C.; Perera, R.; Fang, V.J.; Luk, L.H.; Chu, D.K.W.; Wu, P.; Barr, I.G.; Peiris, J.S.M.; Cowling, B.J. Variation by lineage in serum antibody responses to influenza B virus infections. PLoS ONE 2020, 15, e0241693. [Google Scholar] [CrossRef]
- Carlock, M.A.; Ingram, J.G.; Clutter, E.F.; Cecil, N.C.; Ramgopal, M.; Zimmerman, R.K.; Warren, W.; Kleanthous, H.; Ross, T.M. Impact of age and pre-existing immunity on the induction of human antibody responses against influenza B viruses. Hum. Vaccines Immunother. 2019, 15, 2030–2043. [Google Scholar] [CrossRef]
- Cowling, B.J.; Lim, W.W.; Perera, R.; Fang, V.J.; Leung, G.M.; Peiris, J.S.M.; Tchetgen Tchetgen, E.J. Influenza hemagglutination-inhibition antibody titer as a mediator of vaccine-induced protection for influenza B. Clin. Infect. Dis. 2019, 68, 1713–1717. [Google Scholar] [CrossRef]


| 65–85 Years of Age | 18–64 Years of Age | ||||
|---|---|---|---|---|---|
| qIRV 30 μg N = 116 | qIRV 60 μg N = 117 | Licensed QIV N = 115 | qIRV 30 μg N = 131 | qIRV 60 μg N = 131 | |
| Sex, n (%) | |||||
| Male | 55 (47.4) | 57 (48.7) | 48 (41.7) | 54 (41.2) | 57 (43.5) |
| Female | 61 (52.6) | 60 (51.3) | 67 (58.3) | 77 (58.8) | 74 (56.5) |
| Age at vaccination, mean (SD), years | 71.4 (5.03) | 71.9 (5.05) | 71.6 (4.59) | 42.8 (13.52) | 44.7 (12.91) |
| Race, n (%) | |||||
| White | 92 (79.3) | 101 (86.3) | 96 (83.5) | 98 (74.8) | 107 (81.7) |
| Black | 21 (18.1) | 12 (10.3) | 14 (12.2) | 20 (15.3) | 20 (15.3) |
| Asian | 1 (0.9) | 2 (1.7) | 1 (0.9) | 6 (4.6) | 1 (0.8) |
| Native Hawaiian, other Pacific Islander, American Indian, or Alaska native | 2 (1.7) | 0 | 1 (0.9) | 1 (0.8) | 1 (0.8) |
| Multiracial | 0 | 1 (0.9) | 3 (2.6) | 4 (3.1) | 0 |
| Not reported | 0 | 1 (0.9) | 0 | 2 (1.5) | 2 (1.5) |
| Ethnicity, n (%) | |||||
| Hispanic/Latino | 50 (43.1) | 60 (51.3) | 55 (47.8) | 74 (56.5) | 77 (58.8) |
| Non-Hispanic/non-Latino | 65 (56.0) | 56 (47.9) | 60 (52.2) | 57 (43.5) | 54 (41.2) |
| Not reported | 1 (0.9) | 1 (0.9) | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branche, A.; Mulligan, M.J.; Maniar, A.; Puente, O.; Oladipupo, I.; Crowther, G.; Zareba, A.M.; Yi, Z.; Scully, I.; Gomme, E.; et al. A Phase 1/2 Randomized Study to Evaluate the Safety, Tolerability, and Immunogenicity of Nucleoside-Modified Messenger RNA Influenza Vaccines in Healthy Adults. Vaccines 2025, 13, 383. https://doi.org/10.3390/vaccines13040383
Branche A, Mulligan MJ, Maniar A, Puente O, Oladipupo I, Crowther G, Zareba AM, Yi Z, Scully I, Gomme E, et al. A Phase 1/2 Randomized Study to Evaluate the Safety, Tolerability, and Immunogenicity of Nucleoside-Modified Messenger RNA Influenza Vaccines in Healthy Adults. Vaccines. 2025; 13(4):383. https://doi.org/10.3390/vaccines13040383
Chicago/Turabian StyleBranche, Angela, Mark J. Mulligan, Alok Maniar, Orlando Puente, Islamiat Oladipupo, Graham Crowther, Agnieszka M. Zareba, Zhuobiao Yi, Ingrid Scully, Emily Gomme, and et al. 2025. "A Phase 1/2 Randomized Study to Evaluate the Safety, Tolerability, and Immunogenicity of Nucleoside-Modified Messenger RNA Influenza Vaccines in Healthy Adults" Vaccines 13, no. 4: 383. https://doi.org/10.3390/vaccines13040383
APA StyleBranche, A., Mulligan, M. J., Maniar, A., Puente, O., Oladipupo, I., Crowther, G., Zareba, A. M., Yi, Z., Scully, I., Gomme, E., Koury, K., Kitchin, N., Allen, P. S., Anderson, A. S., Gurtman, A., & Lindert, K. (2025). A Phase 1/2 Randomized Study to Evaluate the Safety, Tolerability, and Immunogenicity of Nucleoside-Modified Messenger RNA Influenza Vaccines in Healthy Adults. Vaccines, 13(4), 383. https://doi.org/10.3390/vaccines13040383







