Neutralizing Antibody Response to the AreXvy Respiratory Syncytial Virus Vaccine in Lung Transplant Recipients: Assessment Against Reference and Seasonal Strains
Abstract
:1. Introduction
2. Methods
2.1. Study Design, Population and Follow-Up Protocol
2.2. Clinical Care of Lung Transplant Recipients
2.2.1. Immunosuppression Protocol
2.2.2. Antimicrobial Prophylaxis
2.3. Microbiological Materials and Methods
2.3.1. Viral Isolation of RSV Variants
2.3.2. ELISA
2.3.3. RSV Micro-Neutralization Assay
2.3.4. Sample Preparation for Sequencing
2.3.5. Phylogenetic Analysis
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Lung Transplant Recipients
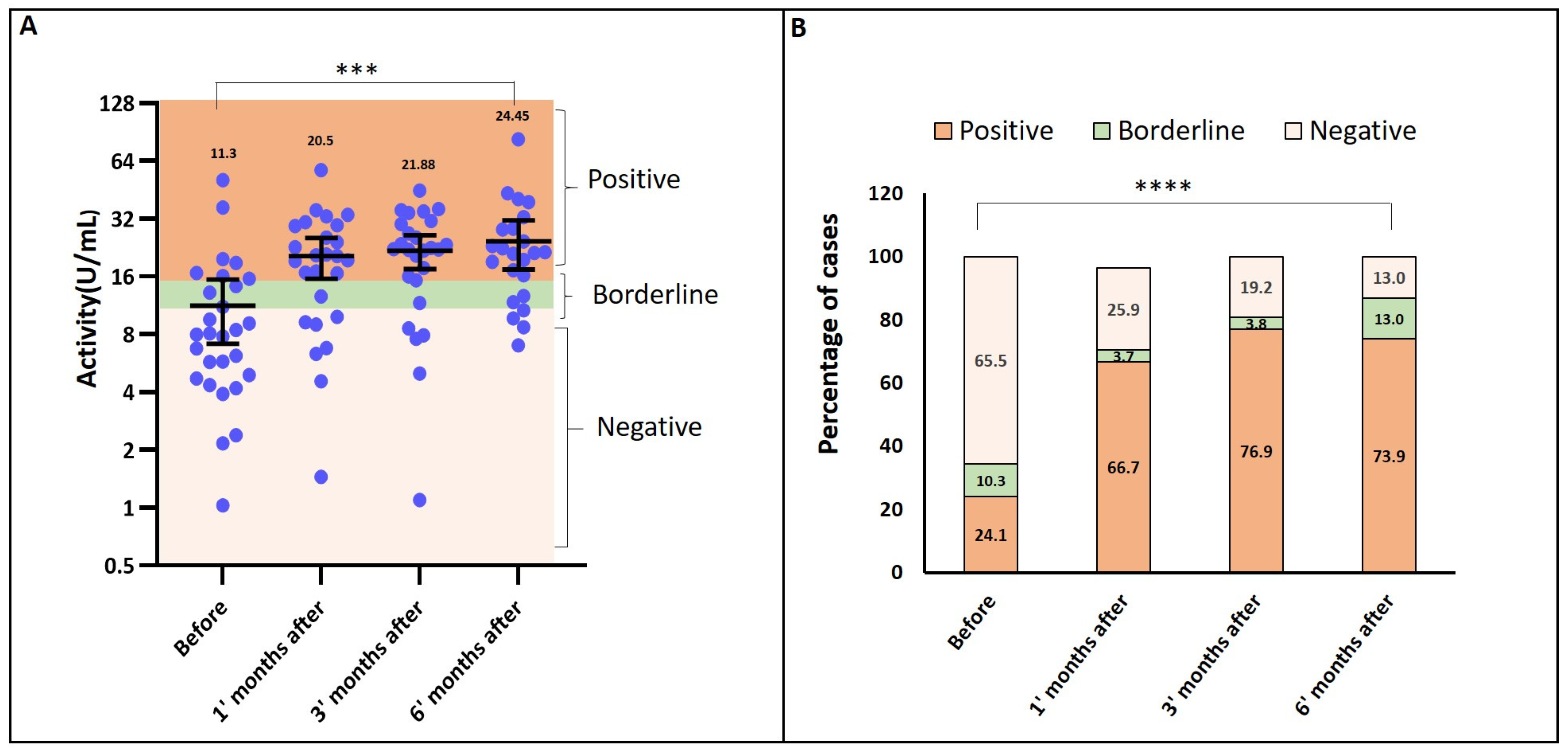
3.2. Seropositivity and Antibody Activity (ELISA Results)
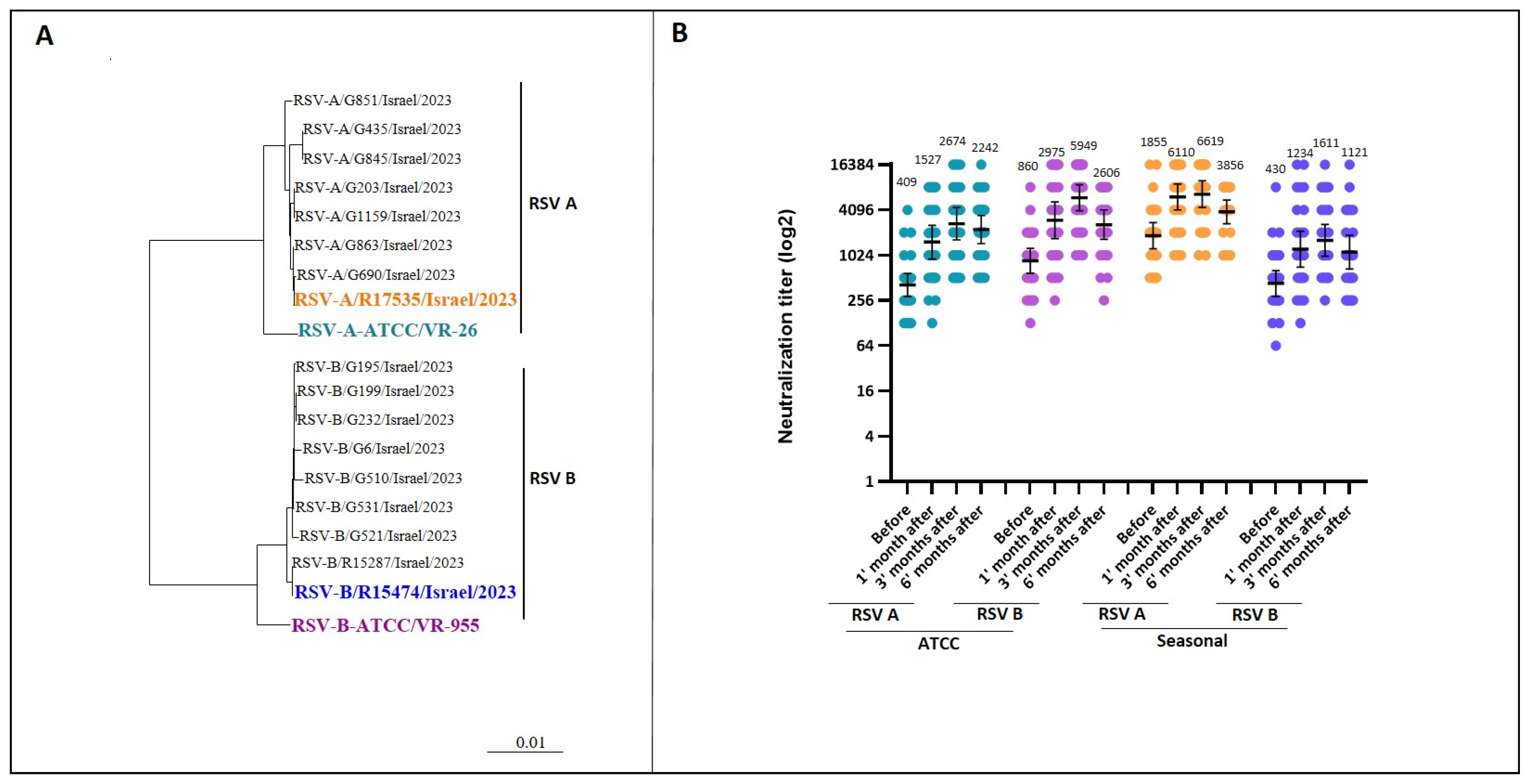
3.3. Neutralizing Antibody Response (Micro-Neutralization Assay)
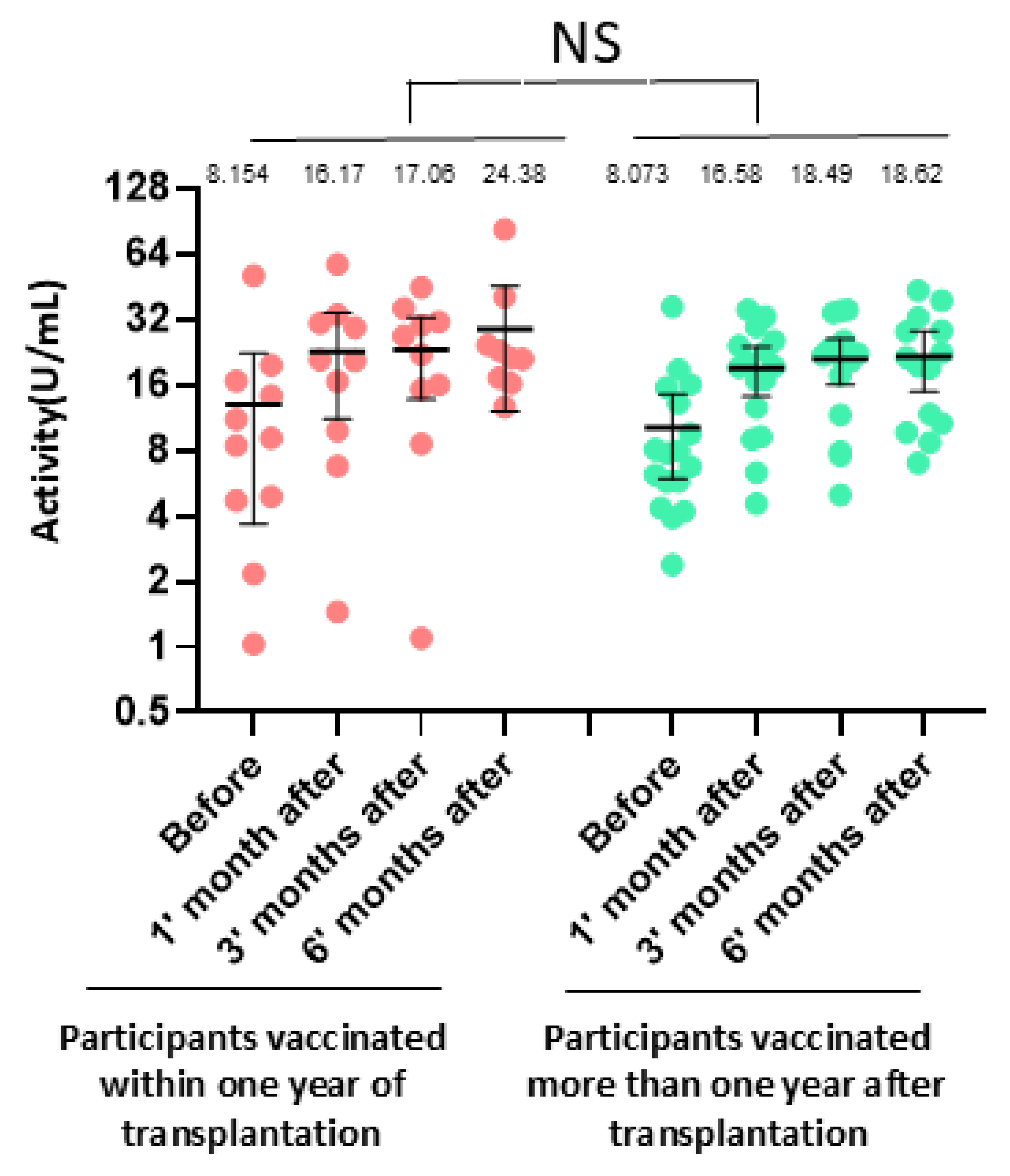
3.4. Impact of Clinical Factors on Antibody Response
3.5. Vaccine Tolerability and Reactogenicity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declaration of AI Usage
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Srikantiah, P.; Vora, P.; Klugman, K.P. Assessing the Full Burden of Respiratory Syncytial Virus in Young Infants in Low- and Middle-Income Countries: The Importance of Community Mortality Studies. Clin. Infect. Dis. 2021, 73, S177–S179. [Google Scholar] [CrossRef] [PubMed]
- Ackerson, B.; Tseng, H.F.; Sy, L.S.; Solano, Z.; Slezak, J.; Luo, Y.; Fischetti, C.A.; Shinde, V. Severe Morbidity and Mortality Associated With Respiratory Syncytial Virus Versus Influenza Infection in Hospitalized Older Adults. Clin. Infect. Dis. 2019, 69, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Korsten, K.; Adriaenssens, N.; Coenen, S.; Butler, C.; Ravanfar, B.; Rutter, H.; Allen, J.; Falsey, A.; Pircon, J.Y.; Gruselle, O.; et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): An international prospective cohort study. Eur. Respir. J. 2021, 57, 2002688. [Google Scholar] [CrossRef]
- Munting, A.; Manuel, O. Viral infections in lung transplantation. J. Thorac. Dis. 2021, 13, 6673–6694. [Google Scholar] [CrossRef]
- Trang, T.P.; Whalen, M.; Hilts-Horeczko, A.; Doernberg, S.B.; Liu, C. Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: A single-center retrospective cohort study and review of the literature. Transpl. Infect. Dis. 2018, 20, e12844. [Google Scholar] [CrossRef]
- Hopkins, P.; McNeil, K.; Kermeen, F.; Musk, M.; McQueen, E.; Mackay, I.; Sloots, T.; Nissen, M. Human metapneumovirus in lung transplant recipients and comparison to respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 2008, 178, 876–881. [Google Scholar] [CrossRef]
- Pelaez, A.; Lyon, G.M.; Force, S.D.; Ramirez, A.M.; Neujahr, D.C.; Foster, M.; Naik, P.M.; Gal, A.A.; Mitchell, P.O.; Lawrence, E.C. Efficacy of oral ribavirin in lung transplant patients with respiratory syncytial virus lower respiratory tract infection. J. Heart Lung Transplant. 2009, 28, 67–71. [Google Scholar] [CrossRef]
- Mombelli, M.; Lang, B.M.; Neofytos, D.; Aubert, J.D.; Benden, C.; Berger, C.; Boggian, K.; Egli, A.; Soccal, P.M.; Kaiser, L.; et al. Burden, epidemiology, and outcomes of microbiologically confirmed respiratory viral infections in solid organ transplant recipients: A nationwide, multi-season prospective cohort study. Am. J. Transplant. 2021, 21, 1789–1800. [Google Scholar] [CrossRef]
- Paulsen, G.C.; Danziger-Isakov, L. Respiratory Viral Infections in Solid Organ and Hematopoietic Stem Cell Transplantation. Clin. Chest Med. 2017, 38, 707–726. [Google Scholar] [PubMed]
- Li, L.; Avery, R.; Budev, M.; Mossad, S.; Danziger-Isakov, L. Oral versus inhaled ribavirin therapy for respiratory syncytial virus infection after lung transplantation. J. Heart Lung Transplant. 2012, 31, 839–844. [Google Scholar] [PubMed]
- Permpalung, N.; Thaniyavarn, T.; Saullo, J.L.; Arif, S.; Miller, R.A.; Reynolds, J.M.; Alexander, B.D. Oral and Inhaled Ribavirin Treatment for Respiratory Syncytial Virus Infection in Lung Transplant Recipients. Transplantation 2020, 104, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Burrows, F.S.; Carlos, L.M.; Benzimra, M.; Marriott, D.J.; Havryk, A.P.; Plit, M.L.; Malouf, M.A.; Glanville, A.R. Oral ribavirin for respiratory syncytial virus infection after lung transplantation: Efficacy and cost-efficiency. J. Heart Lung Transplant. 2015, 34, 958–962. [Google Scholar] [CrossRef]
- Manuel, O.; Estabrook, M.; American Society of Transplantation Infectious Diseases Community of Practice. RNA respiratory viral infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13511. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Approves First Respiratory Syncytial Virus (RSV) Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-respiratory-syncytial-virus-rsv-vaccine (accessed on 1 January 2024).
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef]
- Vigano, M.; Beretta, M.; Lepore, M.; Abete, R.; Benatti, S.V.; Grassini, M.V.; Camagni, S.; Chiodini, G.; Vargiu, S.; Vittori, C.; et al. Vaccination Recommendations in Solid Organ Transplant Adult Candidates and Recipients. Vaccines 2023, 11, 1611. [Google Scholar] [CrossRef]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007, 26, 1229–1242. [Google Scholar] [CrossRef]
- Tapia, L.I.; Shaw, C.A.; Aideyan, L.O.; Jewell, A.M.; Dawson, B.C.; Haq, T.R.; Piedra, P.A. Gene sequence variability of the three surface proteins of human respiratory syncytial virus (HRSV) in Texas. PLoS ONE 2014, 9, e90786. [Google Scholar] [CrossRef]
- Trubin, P.; Azar, M.M.; Kotton, C.N. The respiratory syncytial virus vaccines are here: Implications for solid organ transplantation. Am. J. Transplant. 2024, 24, 897–904. [Google Scholar]
- Olmsted, R.A.; Elango, N.; Prince, G.A.; Murphy, B.R.; Johnson, P.R.; Moss, B.; Chanock, R.M.; Collins, P.L. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccinia virus: Comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc. Natl. Acad. Sci. USA 1986, 83, 7462–7466. [Google Scholar] [CrossRef] [PubMed]
- Magro, M.; Mas, V.; Chappell, K.; Vazquez, M.; Cano, O.; Luque, D.; Terron, M.C.; Melero, J.A.; Palomo, C. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. Proc. Natl. Acad. Sci. USA 2012, 109, 3089–3094. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Raghunandan, R.; Wu, Y.; Liu, Y.; Massare, M.; Nathan, M.; Zhou, B.; Lu, H.; Boddapati, S.; Li, J.; et al. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS ONE 2012, 7, e50852. [Google Scholar] [CrossRef]
- McLellan, J.S.; Ray, W.C.; Peeples, M.E. Structure and function of respiratory syncytial virus surface glycoproteins. Curr. Top. Microbiol. Immunol. 2013, 372, 83–104. [Google Scholar] [CrossRef]
- Ngwuta, J.O.; Chen, M.; Modjarrad, K.; Joyce, M.G.; Kanekiyo, M.; Kumar, A.; Yassine, H.M.; Moin, S.M.; Killikelly, A.M.; Chuang, G.Y.; et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci. Transl. Med. 2015, 7, 309ra162. [Google Scholar] [CrossRef]
- Dagnew, A.F.; Ilhan, O.; Lee, W.S.; Woszczyk, D.; Kwak, J.Y.; Bowcock, S.; Sohn, S.K.; Rodriguez Macias, G.; Chiou, T.J.; Quiel, D.; et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: A phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect. Dis. 2019, 19, 988–1000. [Google Scholar] [CrossRef]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Ramet, M.; et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
| N = 28 | |
|---|---|
| Baseline clinical characteristics | |
| Recipient age at transplant, years, median (IQR) | 59 (50–65) |
| Recipient age at time of vaccination, years, median (IQR) | 62 (53–67) |
| Time from transplant to vaccination, days, median (IQR) | 486 (243–966) |
| Sex, male, n (%) | 21 (75) |
| Lung transplant type | |
| Bilateral lung transplant | 25 (89.3) |
| Single lung transplant | 3 (10.7) |
| Native lung disease, n (%) | |
| Pulmonary fibrosis | 19 (67.9) |
| Chronic obstructive pulmonary disease | 4 (14.3) |
| Cystic fibrosis | 2 (7.1) |
| Other | 3 (10.7) |
| CMV serology, n (%) | |
| D+R− | 2 (7.1) |
| D+R+ | 20 (71.4) |
| D−R+ | 6 (21.4) |
| D−R− | 0 |
| Injection-site reaction, n (%) | |
| Pain | 8 (28.6) |
| Swelling or erythema | 4 (14.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levy, L.; Yahav, D.; Benzimra, M.; Bezalel, Y.; Hoffman, T.; Shirin, N.; Sinai, T.; Jurkowicz, M.; Deri, O.; Matalon, N.; et al. Neutralizing Antibody Response to the AreXvy Respiratory Syncytial Virus Vaccine in Lung Transplant Recipients: Assessment Against Reference and Seasonal Strains. Vaccines 2025, 13, 398. https://doi.org/10.3390/vaccines13040398
Levy L, Yahav D, Benzimra M, Bezalel Y, Hoffman T, Shirin N, Sinai T, Jurkowicz M, Deri O, Matalon N, et al. Neutralizing Antibody Response to the AreXvy Respiratory Syncytial Virus Vaccine in Lung Transplant Recipients: Assessment Against Reference and Seasonal Strains. Vaccines. 2025; 13(4):398. https://doi.org/10.3390/vaccines13040398
Chicago/Turabian StyleLevy, Liran, Dafna Yahav, Mark Benzimra, Yael Bezalel, Tomer Hoffman, Neta Shirin, Tomer Sinai, Menucha Jurkowicz, Ofir Deri, Noa Matalon, and et al. 2025. "Neutralizing Antibody Response to the AreXvy Respiratory Syncytial Virus Vaccine in Lung Transplant Recipients: Assessment Against Reference and Seasonal Strains" Vaccines 13, no. 4: 398. https://doi.org/10.3390/vaccines13040398
APA StyleLevy, L., Yahav, D., Benzimra, M., Bezalel, Y., Hoffman, T., Shirin, N., Sinai, T., Jurkowicz, M., Deri, O., Matalon, N., Saute, M., Lustig, Y., Nachum, E., Peled, M., Nemet, I., & Mandelboim, M. (2025). Neutralizing Antibody Response to the AreXvy Respiratory Syncytial Virus Vaccine in Lung Transplant Recipients: Assessment Against Reference and Seasonal Strains. Vaccines, 13(4), 398. https://doi.org/10.3390/vaccines13040398





