Developing a Vaccine Against Human Cytomegalovirus: Identifying and Targeting HCMV’s Immunological Achilles’ Heel
Abstract
1. Clinical Burden of HCMV and the Need for Vaccine Development
2. HCMV Virion Presents Many Possible Vaccine Targets
3. The Journey and Challenges of HCMV Vaccine Development
4. Glycoprotein B as an Immune Target
5. Successes and Lessons of gB/MF59
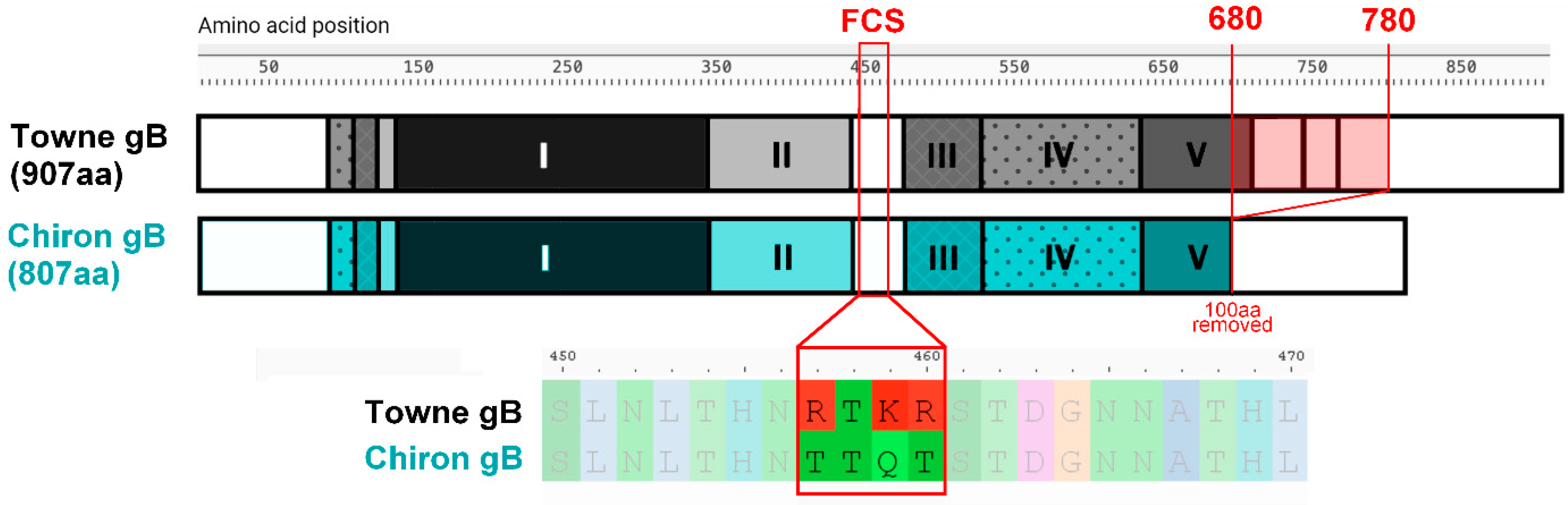
6. Recent Advances in gB/MF59 Analyses
6.1. Neutralising vs. Non-Neutralising Antibodies
6.2. Does the Virus Hide Its Important Epitopes?
6.3. Are Neutralising Antibodies Against gB Not Important?
7. Other Major Trials
8. Discussion and Future Directions
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Antigenic Domain |
| EBV | Epstein–Barr Virus |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HCMV | Human Cytomegalovirus |
| HIV | Human Immunodeficiency Virus |
| HSV-1 | Herpes Simplex Virus |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| FL | Fusion Loop |
| FCS | Furin Cleavage Site |
| PC | Pentameric Complex |
| UK | United Kingdom |
| US | United States |
| VSV | Vesicular Stomatitis Virus |
References
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A systematic literature review of the global seroprevalence of cytomegalovirus: Possible implications for treatment, screening, and vaccine development. BMC Public Health 2022, 22, 1659. [Google Scholar] [CrossRef]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Jackson, S.E.; Mason, G.M.; Wills, M.R. Human cytomegalovirus immunity and immune evasion. Virus Res. 2011, 157, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.D. Burden of disease associated with human cytomegalovirus and prospects for elimination by universal immunisation. Lancet Infect. Dis. 2012, 12, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Shepp, D.H.; Moses, J.E.; Kaplan, M.H. Seroepidemiology of Cytomegalovirus in Patients with Advanced HIV Disease: Influence on Disease Expression and Survival. JAIDS J. Acquir. Immune Defic. Syndr. 1996, 11, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.B.; Rowlands, D.T.; Rifkind, D. Infectious Pulmonary Disease in Patients Receiving Immunosuppressive Therapy for Organ Transplantation. N. Engl. J. Med. 1964, 271, 1021–1027. [Google Scholar] [CrossRef]
- Ljungman, P.; Hakki, M.; Boeckh, M. Cytomegalovirus in Hematopoietic Stem Cell Transplant Recipients. Hematol./Oncol. Clin. N. Am. 2011, 25, 151. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pierrotti, L.C.; Abdala, E.; Costa, S.F.; Strabelli, T.M.; Campos, S.V.; Ramos, J.F.; Latif, A.Z.; Litvinov, N.; Maluf, N.Z.; et al. Cytomegalovirus infection in transplant recipients. Clinics 2015, 70, 515–523. [Google Scholar] [CrossRef]
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512. [Google Scholar] [CrossRef]
- Ishikawa, S.; Tasaki, M.; Saito, K.; Nakagawa, Y.; Ikeda, M.; Takahashi, K.; Tomita, Y. Long-term CMV monitoring and chronic rejection in renal transplant recipients. Front. Cell. Infect. Microbiol. 2023, 13, 1190794. [Google Scholar] [CrossRef]
- Griffiths, P.; Reeves, M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat. Rev. Microbiol. 2021, 19, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.A.; Chang, S.-H.; Lentine, K.L.; Schnitzler, M.A.; Norman, D.; Olyaei, A.; Malinoski, D.; Dharnidharka, V.; Segev, D.; Istre, G.R.; et al. The Clinical and Economic Benefit of CMV Matching in Kidney Transplant: A Decision Analysis. Transplantation 2022, 106, 1227–1232. [Google Scholar] [CrossRef]
- Korndewal, M.J.; Oudesluys-Murphy, A.M.; Kroes, A.C.M.; van der Sande, M.A.B.; de Melker, H.E.; Vossen, A.C.T.M. Long-term impairment attributable to congenital cytomegalovirus infection: A retrospective cohort study. Dev. Med. Child Neurol. 2017, 59, 1261–1268. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Bialek, S.; Cannon, M.J. Attribution of Congenital Cytomegalovirus Infection to Primary Versus Non-Primary Maternal Infection. Clin. Infect. Dis. 2010, 52, e11–e13. [Google Scholar] [CrossRef]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Marsico, C.; Kimberlin, D.W. Congenital Cytomegalovirus infection: Advances and challenges in diagnosis, prevention and treatment. Ital. J. Pediatr. 2017, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Zelini, P.; d’Angelo, P.; De Cicco, M.; Achille, C.; Sarasini, A.; Fiorina, L.; Cirasola, D.; Marazzi, V.; Piccini, S.; Furione, M.; et al. Human cytomegalovirus non-primary infection during pregnancy: Antibody response, risk factors and newborn outcome. Clin. Microbiol. Infect. 2022, 28, 1375–1381. [Google Scholar] [CrossRef]
- Coppola, T.; Mangold, J.F.; Cantrell, S.; Permar, S.R. Impact of Maternal Immunity on Congenital Cytomegalovirus Birth Prevalence and Infant Outcomes: A Systematic Review. Vaccines 2019, 7, 129. [Google Scholar] [CrossRef]
- Noyola, D.E.; Demmler, G.J.; Williamson, D.W.; Griesser, C.; Sellers, S.; Llorente, A.; Littman, T.; Williams, S.; Jarrett, L.; Yow, M.D. Cytomegalovirus urinary excretion and long term outcome in children with congenital cytomegalovirus infection. Pediatr. Infect. Dis. J. 2000, 19, 505–510. [Google Scholar] [CrossRef]
- Williams, E.J.; Gray, J.; Luck, S.; Atkinson, C.; Embleton, N.D.; Kadambari, S.; Davis, A.; Griffiths, P.; Sharland, M.; Berrington, J.E.; et al. First estimates of the potential cost and cost saving of protecting childhood hearing from damage caused by congenital CMV infection. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F501–F506. [Google Scholar] [CrossRef]
- Retzler, J.; Hex, N.; Bartlett, C.; Webb, A.; Wood, S.; Star, C.; Griffiths, P.; Jones, C.E. Economic cost of congenital CMV in the UK. Arch. Dis. Child. 2018, 104, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Dollard, S.C.; Ortega-Sanchez, I.R. Economic assessments of the burden of congenital cytomegalovirus infection and the cost-effectiveness of prevention strategies. Semin. Perinatol. 2021, 45, 151393. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.; Sinha, A.; Samant, S.; Candrilli, S. The Economic Burden of Congenital Cytomegalovirus Disease in the First Year of Life: A Retrospective Analysis of Health Insurance Claims Data in the United States. Clin. Ther. 2019, 41, 1040–1056.e3. [Google Scholar] [CrossRef]
- Inagaki, K.; Blackshear, C.; Palmer, A.; Hobbs, C.V. Risk Factors, Geographic Distribution, and Healthcare Burden of Symptomatic Congenital Cytomegalovirus Infection in the United States: Analysis of a Nationally Representative Database, 2000–2012. J. Pediatr. 2018, 199, 118–123.e1. [Google Scholar] [CrossRef] [PubMed]
- Wilski, N.A.; Snyder, C.M. From Vaccine Vector to Oncomodulation: Understanding the Complex Interplay between CMV and Cancer. Vaccines 2019, 7, 62. [Google Scholar] [CrossRef]
- Wang, H.; Peng, G.; Bai, J.; He, B.; Huang, K.; Hu, X.; Liu, D. Cytomegalovirus Infection and Relative Risk of Cardiovascular Disease (Ischemic Heart Disease, Stroke, and Cardiovascular Death): A Meta-Analysis of Prospective Studies Up to 2016. J. Am. Heart Assoc. 2017, 6, e005025. [Google Scholar] [CrossRef]
- Permar, S.R.; Schleiss, M.R.; Plotkin, S.A. A vaccine against cytomegalovirus: How close are we? J. Clin. Investig. 2025, 135, e182317. [Google Scholar] [CrossRef] [PubMed]
- Nowlin, D.M.; Cooper, N.R.; Compton, T. Expression of a human cytomegalovirus receptor correlates with infectibility of cells. J. Virol. 1991, 65, 3114–3121. [Google Scholar] [CrossRef]
- Sinzger, C.; Grefte, A.; Plachter, B.; Gouw, A.S.H.; The, T.H.; Jahn, G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 1995, 76, 741–750. [Google Scholar] [CrossRef]
- Dupont, L.; Reeves, M.B. Cytomegalovirus latency and reactivation: Recent insights into an age old problem. Rev. Med. Virol. 2015, 26, 75–89. [Google Scholar] [CrossRef]
- Gerna, G.; Kabanova, A.; Lilleri, D. Human Cytomegalovirus Cell Tropism and Host Cell Receptors. Vaccines 2019, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Wille, P.T.; Wisner, T.W.; Ryckman, B.; Johnson, D.C. Human Cytomegalovirus (HCMV) Glycoprotein gB Promotes Virus Entry In Trans Acting as the Viral Fusion Protein Rather than as a Receptor-Binding Protein. mBio 2013, 4, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Wisner, T.W.; Johnson, D.C.; Heldwein, E.E. HCMV gB shares structural and functional properties with gB proteins from other herpesviruses. Virology 2013, 435, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, M.K.; Compton, T. Human Cytomegalovirus Glycoprotein B Is Required for Virus Entry and Cell-to-Cell Spread but Not for Virion Attachment, Assembly, or Egress. J. Virol. 2009, 83, 3891–3903. [Google Scholar] [CrossRef]
- Varnum, S.M.; Streblow, D.N.; Monroe, M.E.; Smith, P.; Auberry, K.J.; Pasa-Tolic, L.; Wang, D.; Camp, D.G.; Rodland, K.; Wiley, S.; et al. Identification of Proteins in Human Cytomegalovirus (HCMV) Particles: The HCMV Proteome. J. Virol. 2004, 78, 10960–10966. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Kamil, J.P. Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism. Viruses 2018, 10, 704. [Google Scholar] [CrossRef]
- Shimamura, M.; Mach, M.; Britt, W.J. Human Cytomegalovirus Infection Elicits a Glycoprotein M (gM)/gN-Specific Virus-Neutralizing Antibody Response. J. Virol. 2006, 80, 4591–4600. [Google Scholar] [CrossRef]
- Krzyzaniak, M.; Mach, M.; Britt, W.J. The Cytoplasmic Tail of Glycoprotein M (gpUL100) Expresses Trafficking Signals Required for Human Cytomegalovirus Assembly and Replication. J. Virol. 2007, 81, 10316–10328. [Google Scholar] [CrossRef]
- Mach, M.; Osinski, K.; Kropff, B.; Schloetzer-Schrehardt, U.; Krzyzaniak, M.; Britt, W. The Carboxy-Terminal Domain of Glycoprotein N of Human Cytomegalovirus Is Required for Virion Morphogenesis. J. Virol. 2007, 81, 5212–5224. [Google Scholar] [CrossRef] [PubMed]
- Kabanova, A.; Marcandalli, J.; Zhou, T.; Bianchi, S.; Baxa, U.; Tsybovsky, Y.; Lilleri, D.; Silacci-Fregni, C.; Foglierini, M.; Fernandez-Rodriguez, B.M.; et al. Platelet-derived growth factor-alpha receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat. Microbiol. 2016, 1, 1–8. [Google Scholar]
- Vanarsdall, A.L.; Johnson, D.C. Human cytomegalovirus entry into cells. Curr. Opin. Virol. 2012, 2, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.; Chou, C.; Li, H.; Hai, R.; Patterson, D.; Stolc, V.; Zhu, H.; Liu, F. Functional profiling of a human cytomegalovirus genome. Proc. Natl. Acad. Sci. USA 2003, 100, 14223–14228. [Google Scholar] [CrossRef]
- Martinez-Martin, N.; Marcandalli, J.; Huang, C.S.; Arthur, C.P.; Perotti, M.; Foglierini, M.; Ho, H.; Dosey, A.M.; Shriver, S.; Payandeh, J.; et al. An Unbiased Screen for Human Cytomegalovirus Identifies Neuropilin-2 as a Central Viral Receptor. Cell 2018, 174, 1158–1171.e19. [Google Scholar] [CrossRef]
- Stanton, R.J.; Baluchova, K.; Dargan, D.J.; Cunningham, C.; Sheehy, O.; Seirafian, S.; McSharry, B.P.; Neale, M.L.; Davies, J.A.; Tomasec, P.; et al. Reconstruction of the complete human cytomegalovirus genome in a BAC reveals RL13 to be a potent inhibitor of replication. J. Clin. Investig. 2010, 120, 3191–3208. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Ye, X.; Ku, Z.; Su, H.; Jones, H.G.; Wang, N.; Mishra, A.K.; Freed, D.C.; Li, F.; Tang, A.; et al. Structural basis for HCMV Pentamer recognition by neuropilin 2 and neutralizing antibodies. Sci. Adv. 2022, 8, eabm2546. [Google Scholar] [CrossRef]
- Vanarsdall, A.L.; Chin, A.L.; Liu, J.; Jardetzky, T.S.; Mudd, J.O.; Orloff, S.L.; Streblow, D.; Mussi-Pinhata, M.M.; Yamamoto, A.Y.; Duarte, G.; et al. HCMV trimer- and pentamer-specific antibodies synergize for virus neutralization but do not correlate with congenital transmission. Proc. Natl. Acad. Sci. USA 2019, 116, 3728–3733. [Google Scholar] [CrossRef] [PubMed]
- Biggs, A.T.; Littlejohn, L.F. Vaccination and natural immunity: Advantages and risks as a matter of public health policy. Lancet Reg. Health–Am. 2022, 8, 100242. [Google Scholar] [CrossRef]
- Plotkin, S.A.; Furukawa, T.; Zygraich, N.; Huygelen, C. Candidate cytomegalovirus strain for human vaccination. Infect. Immun. 1975, 12, 521–527. [Google Scholar] [CrossRef]
- Plotkin, S.; Friedman, H.; Fleisher, G.; Dafoe, D.; Grossman, R.; Lynn Smiley, M.; Starr, S.; Wlodaver, C.; Friedman, A.; Barker, C. Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet 1984, 323, 528–530. [Google Scholar] [CrossRef]
- Adler, S.P.; Starr, S.E.; Plotkin, S.A.; Hempfling, S.H.; Buis, J.; Manning, M.L.; Best, A.M. Immunity Induced By Primary Human Cytomegalovirus Infection Protects Against Secondary Infection Among Women of Childbearing Age. J. Infect. Dis. 1995, 171, 26–32. [Google Scholar] [CrossRef]
- Adler, S.P.; Manganello, A.-M.; Lee, R.; McVoy, M.A.; Nixon, D.E.; Plotkin, S.; Mocarski, E.; Cox, J.H.; Fast, P.E.; Nesterenko, P.A.; et al. A Phase 1 Study of 4 Live, Recombinant Human Cytomegalovirus Towne/Toledo Chimera Vaccines in Cytomegalovirus–Seronegative Men. J. Infect. Dis. 2016, 214, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Dargan, D.J.; Douglas, E.; Cunningham, C.; Jamieson, F.; Stanton, R.J.; Baluchova, K.; McSharry, B.P.; Tomasec, P.; Emery, V.C.; Percivalle, E.; et al. Sequential mutations associated with adaptation of human cytomegalovirus to growth in cell culture. J. Gen. Virol. 2010, 91, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.S.; Baraniak, I.; Lilleri, D.; Reeves, M.B.; Griffiths, P.D.; Permar, S.R. Immune Correlates of Protection Against Human Cytomegalovirus Acquisition, Replication, and Disease. J. Infect. Dis. 2020, 221 (Suppl. S1), S45–S59. [Google Scholar] [CrossRef] [PubMed]
- Anderholm, K.M.; Bierle, C.J.; Schleiss, M.R. Cytomegalovirus Vaccines: Current Status and Future Prospects. Drugs 2016, 76, 1625–1645. [Google Scholar] [CrossRef]
- Bernstein, D.I.; Munoz, F.M.; Callahan, S.T.; Rupp, R.; Wootton, S.H.; Edwards, K.M.; Turley, C.B.; Stanberry, L.R.; Patel, S.M.; Mcneal, M.M.; et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine 2016, 34, 313–319. [Google Scholar] [CrossRef]
- Griffiths, P.D.; Stanton, A.; McCarrell, E.; Smith, C.; Osman, M.; Harber, M.; Davenport, A.; Jones, G.; Wheeler, D.C.; O’Beirne, J.; et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: A phase 2 randomised placebo-controlled trial. Lancet 2011, 377, 1256–1263. [Google Scholar] [CrossRef]
- Pass, R.F.; Zhang, C.; Evans, A.; Simpson, T.; Andrews, W.; Huang, M.-L.; Corey, L.; Hill, J.; Davis, E.; Flanigan, C.; et al. Vaccine Prevention of Maternal Cytomegalovirus Infection. N. Engl. J. Med. 2009, 360, 1191–1199. [Google Scholar] [CrossRef]
- Rieder, F.; Steininger, C. Cytomegalovirus vaccine: Phase II clinical trial results. Clin. Microbiol. Infect. 2014, 20, 95–102. [Google Scholar] [CrossRef]
- Schleiss, M.R.; Permar, S.R.; Plotkin, S.A. Progress toward Development of a Vaccine against Congenital Cytomegalovirus Infection. Clin. Vaccine Immunol. 2017, 24, e00268-17. [Google Scholar] [CrossRef]
- Ljungman, P.; Bermudez, A.; Logan, A.C.; Kharfan-Dabaja, M.A.; Chevallier, P.; Martino, R.; Wulf, G.; Selleslag, D.; Kakihana, K.; Langston, A.; et al. A randomised, placebo-controlled phase 3 study to evaluate the efficacy and safety of ASP0113, a DNA-based CMV vaccine, in seropositive allogeneic haematopoietic cell transplant recipients. EClinicalMedicine 2021, 33, 100787. [Google Scholar] [CrossRef]
- Langley, J.M.; Gantt, S.; Halperin, S.A.; Ward, B.; McNeil, S.; Ye, L.; Cai, Y.; Smith, B.; Anderson, D.E.; Mitoma, F.D. An enveloped virus-like particle alum-adjuvanted cytomegalovirus vaccine is safe and immunogenic: A first-in-humans Canadian Immunization Research Network (CIRN) study. Vaccine 2024, 42, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Berencsi, K.; Gyulai, Z.; Gonczol, E.; Pincus, S.; Cox, W.I.; Michelson, S.; Kari, L.; Meric, C.; Cadoz, M.; Zahradnik, J.; et al. A Canarypox Vector–Expressing Cytomegalovirus (CMV) Phosphoprotein 65 Induces Long-Lasting Cytotoxic T Cell Responses in Human CMV-Seronegative Subjects. J. Infect. Dis. 2001, 183, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.I.; Reap, E.A.; Katen, K.; Watson, A.; Smith, K.; Norberg, P.; Olmsted, R.A.; Hoeper, A.; Morris, J.; Negri, S.; et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine 2009, 28, 484–493. [Google Scholar] [CrossRef]
- Aldoss, I.; La Rosa, C.; Baden, L.R.; Longmate, J.; Ariza-Heredia, E.J.; Rida, W.N.; Lingaraju, C.R.; Zhou, Q.; Martinez, J.; Kaltcheva, T.; et al. Poxvirus Vectored Cytomegalovirus Vaccine to Prevent Cytomegalovirus Viremia in Transplant Recipients: A Phase 2, Randomized Clinical Trial. Ann. Intern. Med. 2020, 172, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Schwendinger, M.; Thiry, G.; De Vos, B.; Leroux-Roels, G.; Bruhwyler, J.; Huygens, A.; Ganeff, C.; Buchinger, H.; Orlinger, K.K.; Pinschewer, D.D.; et al. A Randomized Dose-Escalating Phase I Trial of a Replication-Deficient Lymphocytic Choriomeningitis Virus Vector-Based Vaccine Against Human Cytomegalovirus. J. Infect. Dis. 2020, 225, 1399–1410. [Google Scholar] [CrossRef]
- Bunse, L.; Sommerer, C.; Tan, C.L.; Korell, F.; Schmitt, A.; Huckelhoven-Krauss, A.; Neuber, B.; Mertens, T.; Platten, M.; Green, E.W.; et al. Common T-Cell-Receptor Motifs and Features in Patients with Cytomegalovirus (CMV)-Seronegative End-Stage Renal Disease Receiving a Peptide Vaccination against CMV. Int. J. Mol. Sci. 2022, 23, 1029. [Google Scholar] [CrossRef]
- Nakamura, R.; Rosa, C.L.; Longmate, J.; Drake, J.; Slape, C.; Zhou, Q.; Lampa, M.G.; O’Donnell, M.; Cai, J.-L.; Farol, L.; et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: Randomised phase 1b trial. Lancet Haematol. 2016, 3, e87–e98. [Google Scholar] [CrossRef]
- Fierro, C.; Brune, D.; Shaw, M.; Schwartz, H.; Knightly, C.; Lin, J.; Carfi, A.; Natenshon, A.; Kalidindi, S.; Reuter, C.; et al. Safety and Immunogenicity of a Messenger RNA–Based Cytomegalovirus Vaccine in Healthy Adults: Results From a Phase 1 Randomized Clinical Trial. J. Infect. Dis. 2024, 230, e668–e678. [Google Scholar] [CrossRef]
- Brito, L.A.; Chan, M.; Shaw, C.A.; Hekele, A.; Carsillo, T.; Schaefer, M.; Archer, J.; Seubert, A.; Otten, G.R.; Beard, C.W.; et al. A Cationic Nanoemulsion for the Delivery of Next-generation RNA Vaccines. Mol. Ther. 2014, 22, 2118–2129. [Google Scholar] [CrossRef]
- Foglierini, M.; Marcandalli, J.; Perez, L. HCMV Envelope Glycoprotein Diversity Demystified. Front. Microbiol. 2019, 10, 1005. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, W.; Krummenacher, C.; Chen, Y.; Zheng, Q.; Zhao, Q.; Zeng, M.-S.; Xia, N.; Zeng, Y.-X.; Xu, M.; et al. Targeting herpesvirus entry complex and fusogen glycoproteins with prophylactic and therapeutic agents. Trends Microbiol. 2023, 31, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.; Kropff, B.; Kalbacher, H.; Britt, W.; Sundqvist, V.A.; Ostberg, L.; Mach, M. A continuous sequence of more than 70 amino acids is essential for antibody binding to the dominant antigenic site of glycoprotein gp58 of human cytomegalovirus. J. Virol. 1992, 66, 5290–5297. [Google Scholar] [CrossRef] [PubMed]
- Speckner, A.; Glykofrydes, D.; Ohlin, M.; Mach, M. Antigenic domain 1 of human cytomegalovirus glycoprotein B induces a multitude of different antibodies which, when combined, results in incomplete virus neutralization. J. Gen. Virol. 1999, 80, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Ohlin, M.; Sundqvist, V.A.; Mach, M.; Wahren, B.; Borrebaeck, C.A. Fine specificity of the human immune response to the major neutralization epitopes expressed on cytomegalovirus gp58/116 (gB), as determined with human monoclonal antibodies. J. Virol. 1993, 67, 703–710. [Google Scholar] [CrossRef]
- McVoy, M.M.; Tenorio, E.; Kauvar, L.M. A Native Human Monoclonal Antibody Targeting HCMV gB (AD-2 Site I). Int. J. Mol. Sci. 2018, 19, 3982. [Google Scholar] [CrossRef]
- McLean, G.R.; Olsen, O.A.; Watt, I.N.; Rathanaswami, P.; Leslie, K.B.; Babcook, J.S.; Schrader, J.W. Recognition of Human Cytomegalovirus by Human Primary Immunoglobulins Identifies an Innate Foundation to an Adaptive Immune Response. J. Immunol. 2005, 174, 4768–4778. [Google Scholar] [CrossRef]
- Harnois, M.J.; Dennis, M.; Stohr, D.; Valencia, S.M.; Rodgers, N.; Semmes, E.C.; Webster, H.S.; Jenks, J.A.; Barfield, R.; Pollara, J.; et al. Characterization of Plasma Immunoglobulin G Responses in Elite Neutralizers of Human Cytomegalovirus. J. Infect. Dis. 2022, 226, 1667–1677. [Google Scholar] [CrossRef]
- Meyer, H.; Sundqvist, V.-A.; Pereira, L.; Mach, M. Glycoprotein gp116 of human cytomegalovirus contains epitopes for strain-common and strain-specific antibodies. J. Gen. Virol. 1992, 73, 2375–2383. [Google Scholar] [CrossRef]
- Kniess, N.; Mach, M.; Fay, J.; Britt, W.J. Distribution of linear antigenic sites on glycoprotein gp55 of human cytomegalovirus. J. Virol. 1991, 65, 138–146. [Google Scholar] [CrossRef]
- Potzsch, S.; Spindler, N.; Wiegers, A.-K.; Fisch, T.; Rucker, P.; Sticht, H.; Grieb, N.; Baroti, T.; Weisel, F.; Stamminger, T.; et al. B Cell Repertoire Analysis Identifies New Antigenic Domains on Glycoprotein B of Human Cytomegalovirus which Are Target of Neutralizing Antibodies. PLoS Pathog. 2011, 7, e1002172. [Google Scholar] [CrossRef]
- Spindler, N.; Rucker, P.; Potzsch, S.; Diestel, U.; Sticht, H.; Martin-Parras, L.; Winkler, T.H.; Mach, M. Characterization of a Discontinuous Neutralizing Epitope on Glycoprotein B of Human Cytomegalovirus. J. Virol. 2013, 87, 8927–8939. [Google Scholar] [CrossRef] [PubMed]
- Chandramouli, S.; Ciferri, C.; Nikitin, P.A.; Caló, S.; Gerrein, R.; Balabanis, K.; Monroe, J.; Hebner, C.; Lilja, A.E.; Settembre, E.C.; et al. Structure of HCMV glycoprotein B in the postfusion conformation bound to a neutralizing human antibody. Nat. Commun. 2015, 6, 8176. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Baraniak, I.A.; Lankina, A.; Moulder, Z.; Holenya, P.; Atkinson, C.; Tang, G.; Mahungu, T.; Kern, F.; Griffiths, P.D.; et al. The cytomegalovirus gB/MF59 vaccine candidate induces antibodies against an antigenic domain controlling cell-to-cell spread. Nat. Commun. 2023, 14, 1041. [Google Scholar] [CrossRef]
- Spaete, R.R.; Saxena, A.; Scott, P.I.; Song, G.J.; Probert, W.S.; Britt, W.J.; Gibson, W.; Rasmussen, L.; Pachl, C. Sequence requirements for proteolytic processing of glycoprotein B of human cytomegalovirus strain Towne. J. Virol. 1990, 64, 2922–2931. [Google Scholar] [CrossRef] [PubMed]
- Frey, S.E.; Harrison, C.; Pass, R.F.; Yang, E.; Boken, D.; Sekulovich, R.E.; Percell, S.; Izu, A.E.; Hirabayashi, S.; Burke, R.L.; et al. Effects of Antigen Dose and Immunization Regimens on Antibody Responses to a Cytomegalovirus Glycoprotein B Subunit Vaccine. J. Infect. Dis. 1999, 180, 1700–1703. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; Nest, G.V.; Rappuoli, R.; Giudice, G.D. The history of MF59((R)) adjuvant: A phoenix that arose from the ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef]
- Drulak, M.W.; Malinoski, F.J.; Fuller, S.A.; Stewart, S.S.; Hoskin, S.; Duliege, A.-M.; Sekulovich, R.; Burke, R.; Winston, S. Vaccination of Seropositive Subjects with CHIRON CMV gB Subunit Vaccine Combined with MF59 Adjuvant for Production of CMV Immune Globulin. Viral Immunol. 2000, 13, 49–56. [Google Scholar] [CrossRef]
- Schleiss, M.R.; Bourne, N.; Bernstein, D.I. Preconception Vaccination with a Glycoprotein B (gB) DNA Vaccine Protects against Cytomegalovirus (CMV) Transmission in the Guinea Pig Model of Congenital CMV Infection. J. Infect. Dis. 2003, 188, 1868–1874. [Google Scholar] [CrossRef]
- Fowler, K.B.; Stagno, S.; Pass, R.F. Maternal Immunity and Prevention of Congenital Cytomegalovirus Infection. JAMA 2003, 289, 1008–1011. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Gastanaduy, P.A.; Gambhir, M.; Plotkin, S.A. Review of Mathematical Models of Vaccination for Preventing Congenital Cytomegalovirus Infection. J. Infect. Dis. 2020, 221 (Suppl. S1), S86–S93. [Google Scholar] [CrossRef]
- Shi, S.; Zhu, H.; Xia, X.; Liang, Z.; Ma, X.; Sun, B. Vaccine adjuvants: Understanding the structure and mechanism of adjuvanticity. Vaccine 2019, 37, 3167–3178. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Cao, Z.; Wang, S.; Lee, R.B.; Wang, X.; Murata, H.; Adler, S.P.; McVoy, M.A.; Snapper, C.M. Novel trimeric human cytomegalovirus glycoprotein B elicits a high-titer neutralizing antibody response. Vaccine 2018, 36, 5580–5590. [Google Scholar] [CrossRef]
- Baraniak, I.; Kropff, B.; McLean, G.R.; Pichon, S.; Piras-Douce, F.; Milne, R.S.B.; Smith, C.; Mach, M.; Griffiths, P.D.; Reeves, M.B. Epitope-Specific Humoral Responses to Human Cytomegalovirus Glycoprotein-B Vaccine With MF59: Anti-AD2 Levels Correlate With Protection From Viremia. J. Infect. Dis. 2018, 217, 1907–1917. [Google Scholar] [CrossRef]
- Bialas, K.M.; Westreich, D.; Cisneros de la Rosa, E.; Nelson, C.S.; Kauvar, L.M.; Fu, T.-M.; Permar, S.R. Maternal Antibody Responses and Nonprimary Congenital Cytomegalovirus Infection of HIV-1–Exposed Infants. J. Infect. Dis. 2016, 214, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Baraniak, I.; Kropff, B.; Ambrose, L.; McIntosh, M.; McLean, G.R.; Pichon, S.; Atkinson, C.; Milne, R.S.B.; Mach, M.; Griffiths, P.D.; et al. Protection from cytomegalovirus viremia following glycoprotein B vaccination is not dependent on neutralizing antibodies. Proc. Natl. Acad. Sci. USA 2018, 115, 6273–6278. [Google Scholar] [CrossRef]
- Bootz, A.; Karbach, A.; Spindler, J.; Kropff, B.; Reuter, N.; Sticht, H.; Winkler, T.H.; Britt, W.J.; Mach, M. Protective capacity of neutralizing and non-neutralizing antibodies against glycoprotein B of cytomegalovirus. PLoS Pathog. 2017, 13, e1006601. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, M.L.; Webster, H.S.; Wang, H.-Y.; Jenks, J.A.; Nelson, C.S.; Tu, J.J.; Mangold, J.F.; Valencia, S.; Pollara, J.; Edwards, W.; et al. Specificity and effector functions of non-neutralizing gB-specific monoclonal antibodies isolated from healthy individuals with human cytomegalovirus infection. Virology 2020, 548, 182–191. [Google Scholar] [CrossRef]
- Nelson, C.S.; Huffman, T.; Jenks, J.A.; Cisneros de la Rosa, E.; Xie, G.; Vandergrift, N.; Pass, R.F.; Pollara, J.; Permar, S.R. HCMV glycoprotein B subunit vaccine efficacy mediated by nonneutralizing antibody effector functions. Proc. Natl. Acad. Sci. USA 2018, 115, 6267–6272. [Google Scholar] [CrossRef]
- Semmes, E.C.; Miller, I.G.; Wimberly, C.E.; Phan, C.T.; Jenks, J.A.; Harnois, M.J.; Berendam, S.J.; Webster, H.; Hurst, J.H.; Kurtzberg, J.; et al. Maternal Fc-mediated non-neutralizing antibody responses correlate with protection against congenital human cytomegalovirus infection. J. Clin. Investig. 2022, 132, e156827. [Google Scholar] [CrossRef]
- Chandler, T.L.; Yang, A.; Otero, C.E.; Permar, S.R.; Caddy, S.L. Protective mechanisms of nonneutralizing antiviral antibodies. PLoS Pathog. 2023, 19, e1011670. [Google Scholar] [CrossRef]
- Mader, K.; Dustin, L.B. Beyond bNAbs: Uses, Risks, and Opportunities for Therapeutic Application of Non-Neutralising Antibodies in Viral Infection. Antibodies 2024, 13, 28. [Google Scholar] [CrossRef]
- Reinig, S.; Shih, S.R. Non-neutralizing functions in anti-SARS-CoV-2 IgG antibodies. Biomed. J. 2024, 47, 100666. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.P.; Lewis, N.; Conlon, A.; Christiansen, M.P.; Al-Ibrahim, M.; Rupp, R.; Fu, T.-M.; Bautista, O.; Tang, H.; Wang, D.; et al. Phase 1 Clinical Trial of a Conditionally Replication-Defective Human Cytomegalovirus (CMV) Vaccine in CMV-Seronegative Subjects. J. Infect. Dis. 2019, 220, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Jenks, J.A.; Nelson, C.S.; Roark, H.K.; Goodwin, M.L.; Pass, R.F.; Bernstein, D.I.; Walter, E.B.; Edwards, K.M.; Wang, D.; Fu, T.-M.; et al. Antibody binding to native cytomegalovirus glycoprotein B predicts efficacy of the gB/MF59 vaccine in humans. Sci. Transl. Med. 2020, 12, eabb3611. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R. Recombinant cytomegalovirus glycoprotein B vaccine: Rethinking the immunological basis of protection. Proc. Natl. Acad. Sci. USA 2018, 115, 6110–6112. [Google Scholar] [CrossRef]
- Perotti, M.; Marcandalli, J.; Demurtas, D.; Sallusto, F.; Perez, L. Rationally designed Human Cytomegalovirus gB nanoparticle vaccine with improved immunogenicity. PLoS Pathog. 2020, 16, e1009169. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Freed, D.C.; Tang, A.; Rustandi, R.R.; Troutman, M.C.; Espeseth, A.S.; Zhang, N.; An, Z.; McVoy, M.; Zhu, H.; et al. Complement enhances in vitro neutralizing potency of antibodies to human cytomegalovirus glycoprotein B (gB) and immune sera induced by gB/MF59 vaccination. npj Vaccines 2017, 2, 36. [Google Scholar] [CrossRef]
- Baraniak, I.; Gomes, A.C.; Sodi, I.; Langstone, T.; Rothwell, E.; Atkinson, C.; Pichon, S.; Piras-Douce, F.; Griffiths, P.D.; Reeves, M.B. Seronegative patients vaccinated with cytomegalovirus gB-MF59 vaccine have evidence of neutralising antibody responses against gB early post-transplantation. EBioMedicine 2019, 50, 45–54. [Google Scholar] [CrossRef]
- Ishibashi, K.; Tokumoto, T.; Shirakawa, H.; Hashimoto, K.; Ikuta, K.; Kushida, N.; Yanagida, T.; Shishido, K.; Aikawa, K.; Toma, H.; et al. Lack of antibodies against the antigen domain 2 epitope of cytomegalovirus (CMV) glycoprotein B is associated with CMV disease after renal transplantation in recipients having the same glycoprotein H serotypes as their donors. Transpl. Infect. Dis. 2010, 13, 318–323. [Google Scholar] [CrossRef]
- Finnefrock, A.C.; Freed, D.C.; Tang, A.; Li, F.; He, X.; Wu, C.; Nahas, D.; Wang, D.; Fu, T.-M. Preclinical evaluations of peptide-conjugate vaccines targeting the antigenic domain-2 of glycoprotein B of human cytomegalovirus. Hum. Vaccines Immunother. 2016, 12, 2106–2112. [Google Scholar] [CrossRef]
- Sponholtz, M.R.; Byrne, P.O.; Lee, A.G.; Ramamohan, A.R.; Goldsmith, J.A.; McCool, R.S.; Zhou, L.; Johnson, N.V.; Hsieh, C.-L.; Connors, M.; et al. Structure-based design of a soluble human cytomegalovirus glycoprotein B antigen stabilized in a prefusion-like conformation. Proc. Natl. Acad. Sci. USA 2024, 121, e2404250121. [Google Scholar] [CrossRef] [PubMed]
- Karthigeyan, K.P.; Connors, M.; Binuya, C.R.; Gross, M.; Fuller, A.S.; Crooks, C.M.; Wang, H.-Y.; Sponholtz, M.R.; Byrne, P.O.; Herbek, S.; et al. A Human Cytomegalovirus Prefusion-like Glycoprotein B Subunit Vaccine Elicits Similar Humoral Immunity to That of Postfusion gB in Mice; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2024. [Google Scholar]
- Hu, X.; Karthigeyan, K.P.; Herbek, S.; Valencia, S.M.; Jenks, J.A.; Webster, H.; Miller, I.G.; Connors, M.; Pollara, J.; Andy, C.; et al. Human Cytomegalovirus mRNA-1647 Vaccine Candidate Elicits Potent and Broad Neutralization and Higher Antibody-Dependent Cellular Cytotoxicity Responses Than the gB/MF59 Vaccine. J. Infect. Dis. 2024, 230, 455–466. [Google Scholar] [CrossRef]
- Vlahava, V.M.; Murrell, I.; Zhuang, L.; Aicheler, R.J.; Lim, E.; Miners, K.L.; Ladell, K.; Suarez, N.M.; Price, D.A.; Davison, A.J.; et al. Monoclonal antibodies targeting nonstructural viral antigens can activate ADCC against human cytomegalovirus. J. Clin. Investig. 2021, 131, e139296. [Google Scholar] [CrossRef] [PubMed]
- Panther, L.; Basnet, S.; Fierro, C.; Brune, D.; Leggett, R.; Peterson, J.; Pickrell, P.; Lin, J.; Wu, K.; Lee, H.; et al. 2892. Safety and Immunogenicity of mRNA-1647, an mRNA-Based Cytomegalovirus Vaccine in Healthy Adults: Results of a Phase 2, Randomized, Observer-Blind, Placebo-Controlled, Dose-Finding Trial. Open Forum Infect. Dis. 2023, 10 (Suppl. S2), ofad500-2475. [Google Scholar]
- Wang, D.; Freed, D.C.; He, X.; Li, F.; Tang, A.; Cox, K.S.; Dubey, S.A.; Cole, S.; Medi, M.B.; Liu, Y.; et al. A replication-defective human cytomegalovirus vaccine for prevention of congenital infection. Sci. Transl. Med. 2016, 8, 362ra145. [Google Scholar] [CrossRef]
- Ye, X.; Shih, D.J.H.; Ku, Z.; Hong, J.; Barrett, D.F.; Rupp, R.E.; Zhang, N.; Fu, T.-M.; Zheng, W.J.; An, Z. Transcriptional signature of durable effector T cells elicited by a replication defective HCMV vaccine. npj Vaccines 2024, 9, 70. [Google Scholar] [CrossRef]
- Das, R.; Blázquez-Gamero, D.; Bernstein, D.I.; Gantt, S.; Bautista, O.; Beck, K.; Conlon, A.; Rosenbloom, D.I.S.; Wang, D.; Ritter, M.; et al. Safety, efficacy, and immunogenicity of a replication-defective human cytomegalovirus vaccine, V160, in cytomegalovirus-seronegative women: A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Infect. Dis. 2023, 23, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Taher, H.; Kreklywich, C.N.; Schmidt, K.A.; Scheef, E.A.; Barfield, R.; Otero, C.E.; Valencia, S.M.; Crooks, C.M.; Mirza, A.; et al. The pentameric complex is not required for vertical transmission of cytomegalovirus in seronegative pregnant rhesus macaques. bioRxiv 2023. [Google Scholar] [CrossRef]
- Pereira, L.; Petitt, M.; Tabata, T. Cytomegalovirus Infection and Antibody Protection of the Developing Placenta. Clin. Infect. Dis. 2013, 57 (Suppl. S4), S174–S177. [Google Scholar] [CrossRef][Green Version]
- Martins, J.P.; Andoniou, C.E.; Fleming, P.; Kuns, R.D.; Schuster, I.S.; Voigt, V.; Daly, S.; Varelias, A.; Tey, S.-K.; Degli-Esposti, M.A.; et al. Strain-specific antibody therapy prevents cytomegalovirus reactivation after transplantation. Science 2019, 363, 288–293. [Google Scholar] [CrossRef]
- Slezak, S.L.; Bettinotti, M.; Selleri, S.; Adams, S.; Marincola, F.M.; Stroncek, D.F. CMV pp65 and IE-1 T cell epitopes recognized by healthy subjects. J. Transl. Med. 2007, 5, 1–16. [Google Scholar] [CrossRef]
- Lilleri, D.; Fornara, C.; Revello, M.G.; Gerna, G. Human cytomegalovirus-specific memory CD8+ and CD4+ T cell differentiation after primary infection. J. Infect. Dis. 2008, 198, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Gomes, A.; Cubells, A.; Pallares, C.; Corpas-Burgos, F.; Berenguer, M.; Aguilera, V.; Lopez-Labrador, F.X. Cytomegalovirus specific polyfunctional T-cell responses expressing CD107a predict control of CMV infection after liver transplantation. Cell Immunol. 2022, 371, 104455. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Sedikides, G.X.; Mason, G.M.; Okecha, G.; Wills, M.R. Human Cytomegalovirus (HCMV)-Specific CD4+ T Cells Are Polyfunctional and Can Respond to HCMV-Infected Dendritic Cells In Vitro. J. Virol. 2017, 91, e02128-16. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Singh, M.P. Immuno-informatics approach to design a multi-epitope vaccine to combat cytomegalovirus infection. Eur. J. Pharm. Sci. 2020, 147, 105279. [Google Scholar] [CrossRef]
- Schleiss, M.R.; Bourne, N.; Stroup, G.; Bravo, F.J.; Jensen, N.J.; Bernstein, D.I. Protection against Congenital Cytomegalovirus Infection and Disease in Guinea Pigs, Conferred by a Purified Recombinant Glycoprotein B Vaccine. J. Infect. Dis. 2004, 189, 1374–1381. [Google Scholar] [CrossRef]
- Sabbaj, S.; Pass, R.F.; Pichon, S.; Goepfert, P.A. Glycoprotein B Vaccine Is Capable of Boosting Both Antibody and CD4 T-Cell Responses to Cytomegalovirus in Chronically Infected Women. J. Infect. Dis. 2011, 203, 1534–1541. [Google Scholar] [CrossRef]

| Vaccine Name (Company/Developer) | Antigen(s) | Antigen Presentation/Delivery | Most Advanced Clinical Trial Stage | Reported Efficacy for Protection(for Phase II and Up) | Reference(s) |
|---|---|---|---|---|---|
| gB/MF59 (Sanofi Pasteur and Chiron, now Novartis—adjuvant) | gB | Modified (Chiron) gB—mutated furin cleavage site and removed transmembrane domain. Adjuvanted with MF59 | Phase II | 43–50% | [55,56,57] |
| GSK1492903A (GSK) | gB | Chimeric HCMV gB fused with HSV-1 gD | Phase I | NA | [58,59] |
| ASP0113 (VCL-CB01) (Astellas Pharma and Vical) | gB, pp65 | Two plasmids that encode HCMV pp65 and gB adjuvanted with poloxamer CRL1005 and benzalkonium chloride | Phase III | Exact number not reported. Not better than placebo | [60] |
| VBI-1501A (VBI Vaccines) | gB | Enveloped virus-like particle expressing HCMV gB modified to have VSV G transmembrane domain | Phase I | NA | [61] |
| ALVAC-pp65 (Sanofi Pasteur) | pp65 | Replication-deficient canarypox vector | Phase II | Number not reported | [62] |
| ALVAC-gB (Sanofi Pasteur) | gB | Replication-deficient canarypox vector | Phase I | NA | [62] |
| AVX601 (AlphaVax) | gB, pp65, IE1 | Aphavirus replicon particle expressing gB and a pp65-IE1 fusion protein | Phase I | NA | [63] |
| Triplex (Helocyte) | pp65, IE1, IE2 | Poxvirus vector (MVA) expressing pp65, IE1-exon4, and IE2-exon5 | Phase II (ongoing) | To be reported | [64] |
| HB-101 (Hoopkia) | gB, pp65 | Two replication-deficient choriomeningitis viruses expressing gB and pp65 | Phase II (ongoing) | To be reported | [65] |
| CMVPepVac (Renal Center Heidelberg) | pp65 | HCMV pp65 peptide with Aldara adjuvant | Phase I | NA | [66] |
| CMVPepVax (City of Hope and The National Cancer Institute) | pp65 | Chimeric peptide consisting of CD8+ T-cell epitope of pp65 and a tetanus T-helper epitope | Phase Ib | NA | [67] |
| mRNA-1647 (Moderna) | gB and PC | mRNAs encoding for gB and five proteins comprising PC within lipid nanoparticles | Phases II and III (both ongoing) | To be reported | [68] |
| HCMV SAM (Novartis Vaccines and Diagnostics) | gB, pp65, IE1 | Self-amplifying RNA encoding for gB and pp65-IE1 fusion protein in a cationic nanoemulsion | Phase I | NA | [69] |
| Trials | Target Population | Age | Vaccination Schedule | Time of Follow-Up After First Vaccination | Measured Objective(s) + Method | gB/MF59 Efficacy |
|---|---|---|---|---|---|---|
| [57] | Seronegative postpartum women | 14–40 | Vaccine or placebo at 0, 1, and 6 months | 42 months | Rate of seroconversion: testing for IgG against non-gB HCMV proteins, further confirmed with q-RT PCR. | 50% |
| [55] | Seronegative adolescent girls | 12–17 | Vaccine or placebo at 0, 1, and 6 months | 34 months | Rate of seroconversion: gB-adsorption assay followed by sera analysis Viral shedding: q-RT PCR on various body fluid samples gB IgG levels: ELISA | 43–45% |
| [56] | Seronegative and seropositive solid-organ transplant recipients | 50.5 (mean age) | Vaccine or placebo at 0, 1, and 6 months | Up to 13 months | Presence of HCMV DNA in blood: q-RT PCR gB IgG levels: ELISA Neutralising antibody levels: neutralisation assay using human fibroblasts | 50% |
| References | Study Analysed | Neutralisation Measured How? | Other Antiviral Activity Measured? | Conclusions |
|---|---|---|---|---|
| [98] | Phase I trial in healthy adults | Direct neutralisation against autologous (Towne) and heterologous (AD169, TB40e) strains | Neutralising ability with the addition of complement. Later studies also explored ADCP, ADCC, and NK cell degranulation. | Superior neutralisation ability (with and without the complement) when compared to the sera from phase II trial on postpartum women, possibly due to physiological differences influenced by pregnancy and childbirth [104] |
| Phase II trial in postpartum women | Limited neutralising ability pre- and post-vaccination against heterologous strains, slight increase in neutralising ability against Towne. Possible ADCP and ADCC mechanism of protection. Total titre of gB-specific antibodies is the correlate of protection, despite limited neutralising titre. | |||
| [95] | Phase II trial in transplant patients | Direct neutralisation against clinical Merlin strain. | Neutralising ability with the addition of complement. Also explored NK cell degranulation. | Limited neutralising ability pre- and post-vaccination against Merlin with and without the addition of the complement. No effect of sera on NK cell degranulation. Limited influence of ADCC on vaccine-induced protection. Total titre of gB-specific antibodies is the correlate of protection, despite limited neutralising titre. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lankina, A.; Raposo, M.; Hargreaves, A.; Atkinson, C.; Griffiths, P.; Reeves, M.B. Developing a Vaccine Against Human Cytomegalovirus: Identifying and Targeting HCMV’s Immunological Achilles’ Heel. Vaccines 2025, 13, 435. https://doi.org/10.3390/vaccines13050435
Lankina A, Raposo M, Hargreaves A, Atkinson C, Griffiths P, Reeves MB. Developing a Vaccine Against Human Cytomegalovirus: Identifying and Targeting HCMV’s Immunological Achilles’ Heel. Vaccines. 2025; 13(5):435. https://doi.org/10.3390/vaccines13050435
Chicago/Turabian StyleLankina, Anastasia, Marta Raposo, Alexander Hargreaves, Claire Atkinson, Paul Griffiths, and Matthew B. Reeves. 2025. "Developing a Vaccine Against Human Cytomegalovirus: Identifying and Targeting HCMV’s Immunological Achilles’ Heel" Vaccines 13, no. 5: 435. https://doi.org/10.3390/vaccines13050435
APA StyleLankina, A., Raposo, M., Hargreaves, A., Atkinson, C., Griffiths, P., & Reeves, M. B. (2025). Developing a Vaccine Against Human Cytomegalovirus: Identifying and Targeting HCMV’s Immunological Achilles’ Heel. Vaccines, 13(5), 435. https://doi.org/10.3390/vaccines13050435






