Evaluation of the Potency of the First Commercial Vaccine for Clostridioides difficile Infection in Piglets and Comparison with the Humoral Response in Rabbits
Abstract
1. Introduction
2. Materials and Methods
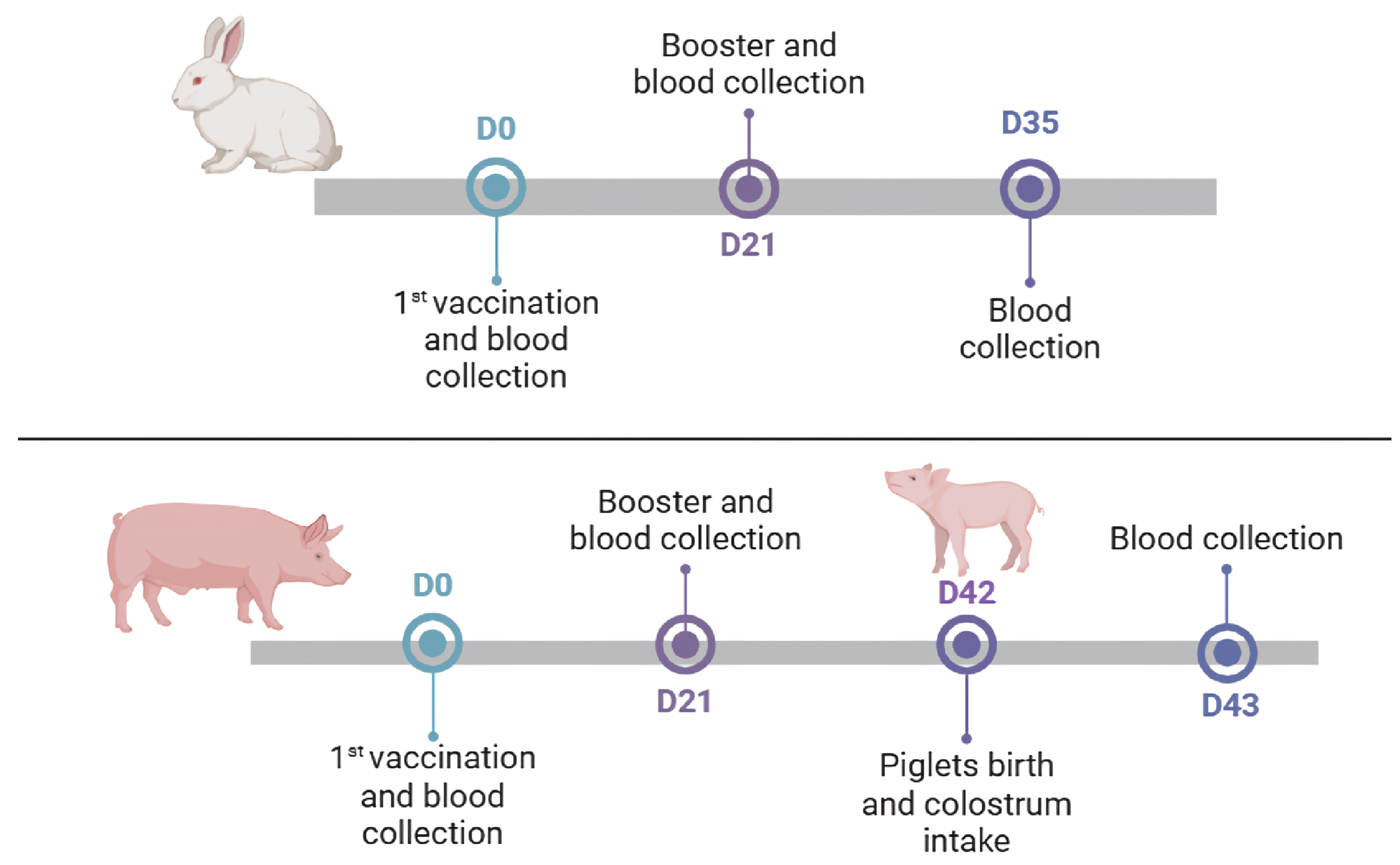
2.1. Experimental Design
2.2. Vaccination of Rabbits
2.3. Vaccination of Sows
2.4. Passive Immunity Assessment
2.5. Sera Titration
2.6. Statistical Analysis
3. Results
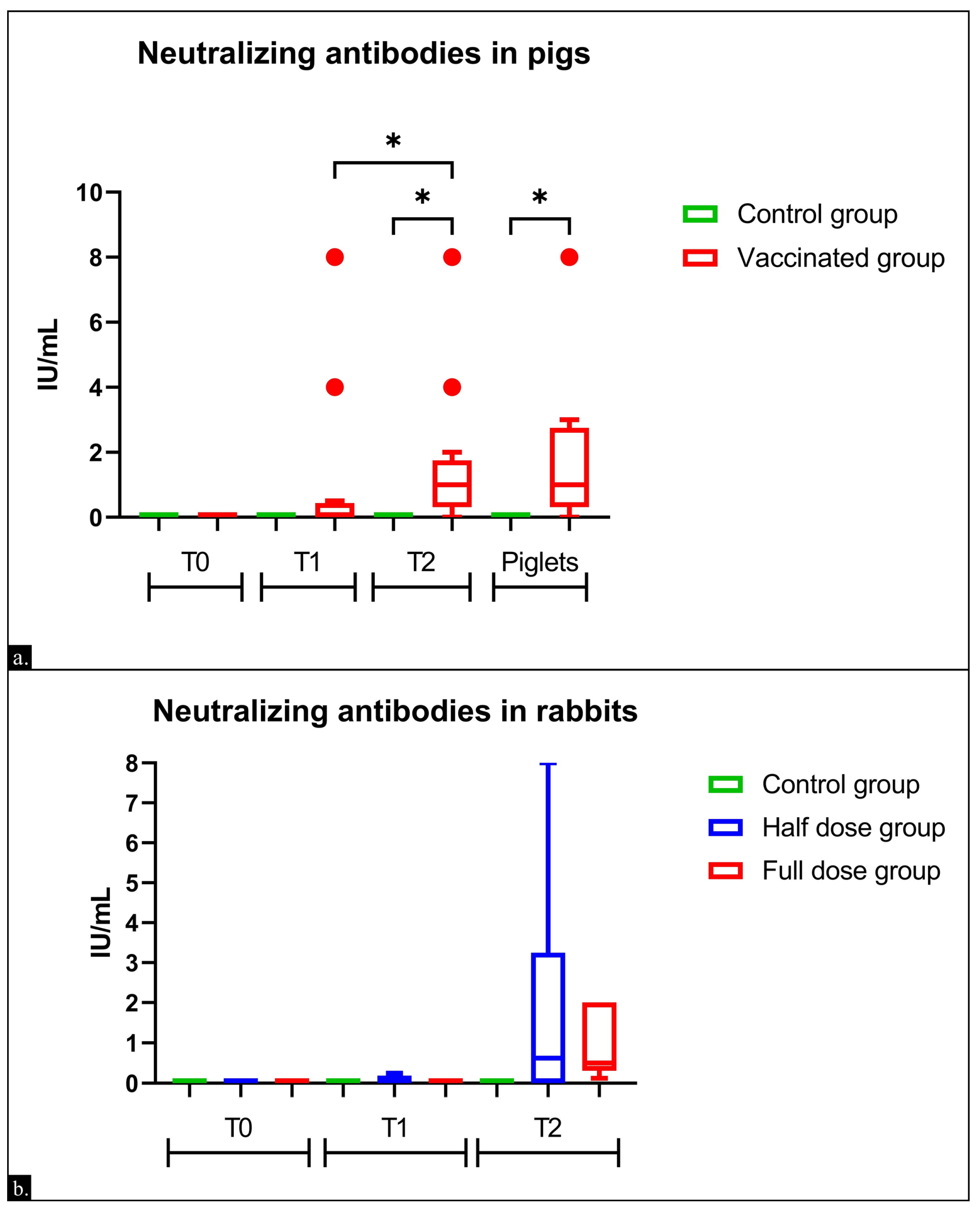
3.1. Immunogenicity in Sows
3.2. Immunogenicity in Rabbits
3.3. Comparison of Humoral Responses in Rabbits, Sows, and Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruxelle, J.-F.; Péchiné, S.; Collignon, A. Immunization Strategies Against Clostridium Difficile. In Updates on Clostridium difficile in Europe; Mastrantonio, P., Rupnik, M., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; Volume 1050, pp. 197–225. ISBN 978-3-319-72798-1. [Google Scholar]
- Diab, S.S.; Uzal, F.A.; Songer, J.G. Diseases Produced by Clostridium Difficile. In Clostridial Diseases of Animals; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Lawson, P.A.; Citron, D.M.; Tyrrell, K.L.; Finegold, S.M. Reclassification of Clostridium difficile as Clostridioides Difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe 2016, 40, 95–99. [Google Scholar] [CrossRef]
- Licciardi, C.; Primavilla, S.; Roila, R.; Lupattelli, A.; Farneti, S.; Blasi, G.; Petruzzelli, A.; Drigo, I.; Di Raimo Marrocchi, E. Prevalence, Molecular Characterization and Antimicrobial Susceptibility of Clostridioides Difficile Isolated from Pig Carcasses and Pork Products in Central Italy. Int. J. Environ. Res. Public Health 2021, 18, 11368. [Google Scholar] [CrossRef]
- Hall, I.C.; O’Toole, E. Intestinal Flora in New-Born Infants: With a Description of a New Pathogenic Anaerobe, Bacillus Difficilis. Am. J. Dis. Child. 1935, 49, 390. [Google Scholar] [CrossRef]
- Freeman, J.; Bauer, M.P.; Baines, S.D.; Corver, J.; Fawley, W.N.; Goorhuis, B.; Kuijper, E.J.; Wilcox, M.H. The Changing Epidemiology of Clostridium difficile Infections. Clin. Microbiol. Rev. 2010, 23, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.S.H.; Monaghan, T.M.; Wilcox, M.H. Clostridium Difficile Infection: Epidemiology, Diagnosis and Understanding Transmission. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Trindade, C.N.R.; Domingues, R.M.C.P.; Ferreira, E.O. The Epidemiology of Clostridioides Difficile Infection in Brazil: A Systematic Review Covering Thirty Years. Anaerobe 2019, 58, 13–21. [Google Scholar] [CrossRef]
- Proctor, A.; Cornick, N.A.; Wang, C.; Mooyottu, S.; Arruda, P.A.; Kobs, K.; Phillips, G.J. Neonatal Piglets Are Protected from Clostridioides Difficile Infection by Age-Dependent Increase in Intestinal Microbial Diversity. Microbiol. Spectr. 2021, 9, e0124321. [Google Scholar] [CrossRef]
- Diniz, A.N.; Moura, L.N.F.; Cruz, D.S.G.; Oliveira Junior, C.A.; Figueiredo, H.C.P.; Cunha, J.L.R.; Vilela, E.G.; Kuijper, E.J.; Wilcox, M.H.; Lobato, F.C.F.; et al. Characterization of the Virulence of Three Novel Clade 2 Clostridioides (Clostridium) Difficile Strains and a Two-Year Screening in Animals and Humans in Brazil. PLoS ONE 2022, 17, e0273013. [Google Scholar] [CrossRef]
- Buddle, J.E.; Fagan, R.P. Pathogenicity and Virulence of Clostridioides difficile. Virulence 2023, 14, 2150452. [Google Scholar] [CrossRef]
- Simor, A.E. Diagnosis, Management, and Prevention of Clostridium difficile Infection in Long-Term Care Facilities: A Review: Clostridium difficile Infection. J. Am. Geriatr. Soc. 2010, 58, 1556–1564. [Google Scholar] [CrossRef]
- Goorhuis, A.; Bakker, D.; Corver, J.; Debast, S.B.; Harmanus, C.; Notermans, D.W.; Bergwerff, A.A.; Dekker, F.W.; Kuijper, E.J. Emergence of Clostridium difficile Infection Due to a New Hypervirulent Strain, Polymerase Chain Reaction Ribotype 078. Clin. Infect. Dis. 2008, 47, 1162–1170. [Google Scholar] [CrossRef]
- Keel, K.; Brazier, J.S.; Post, K.W.; Weese, S.; Songer, J.G. Prevalence of PCR Ribotypes among Clostridium difficile Isolates from Pigs, Calves, and Other Species. J. Clin. Microbiol. 2007, 45, 1963–1964. [Google Scholar] [CrossRef]
- Koene, M.G.J.; Mevius, D.; Wagenaar, J.A.; Harmanus, C.; Hensgens, M.P.M.; Meetsma, A.M.; Putirulan, F.F.; Van Bergen, M.A.P.; Kuijper, E.J. Clostridium Difficile in Dutch Animals: Their Presence, Characteristics and Similarities with Human Isolates. Clin. Microbiol. Infect. 2012, 18, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Taminiau, B.; Van Broeck, J.; Avesani, V.; Delmée, M.; Daube, G. Clostridium Difficile in Young Farm Animals and Slaughter Animals in Belgium. Anaerobe 2012, 18, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Wakeford, T.; Reid-Smith, R.; Rousseau, J.; Friendship, R. Longitudinal Investigation of Clostridium difficile Shedding in Piglets. Anaerobe 2010, 16, 501–504. [Google Scholar] [CrossRef]
- Bauer, M.P.; Notermans, D.W.; Van Benthem, B.H.; Brazier, J.S.; Wilcox, M.H.; Rupnik, M.; Monnet, D.L.; Van Dissel, J.T.; Kuijper, E.J. Clostridium Difficile Infection in Europe: A Hospital-Based Survey. Lancet 2011, 377, 63–73. [Google Scholar] [CrossRef]
- Lim, S.C.; Knight, D.R.; Riley, T.V. Clostridium Difficile and One Health. Clin. Microbiol. Infect. 2020, 26, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Patterson, L.; Wilcox, M.H.; Fawley, W.N.; Verlander, N.Q.; Geoghegan, L.; Patel, B.C.; Wyatt, T.; Smyth, B. Morbidity and Mortality Associated with Clostridium difficile Ribotype 078: A Case–Case Study. J. Hosp. Infect. 2012, 82, 125–128. [Google Scholar] [CrossRef]
- O’Shaughnessy, R.A.; Habing, G.G.; Gebreyes, W.A.; Bowman, A.S.; Weese, J.S.; Rousseau, J.; Stull, J.W. Clostridioides Difficile on Ohio Swine Farms (2015): A Comparison of Swine and Human Environments and Assessment of On-farm Risk Factors. Zoonoses Public Health 2019, 66, 861–870. [Google Scholar] [CrossRef]
- Putsathit, P.; Neela, V.K.; Joseph, N.M.S.; Ooi, P.T.; Ngamwongsatit, B.; Knight, D.R.; Riley, T.V. Molecular Epidemiology of Clostridium difficile Isolated from Piglets. Vet. Microbiol. 2019, 237, 108408. [Google Scholar] [CrossRef]
- Schneeberg, A.; Neubauer, H.; Schmoock, G.; Baier, S.; Harlizius, J.; Nienhoff, H.; Brase, K.; Zimmermann, S.; Seyboldt, C. Clostridium Difficile Genotypes in Piglet Populations in Germany. J. Clin. Microbiol. 2013, 51, 3796–3803. [Google Scholar] [CrossRef] [PubMed]
- Yaeger, M.J.; Kinyon, J.M.; Songer, J.G. A Prospective, Case Control Study Evaluating the Association between Clostridium difficile Toxins in the Colon of Neonatal Swine and Gross and Microscopic Lesions. J. Vet. Diagn. Investig. 2007, 19, 52–59. [Google Scholar] [CrossRef]
- Songer, J.G.; Anderson, M.A. Clostridium Difficile: An Important Pathogen of Food Animals. Anaerobe 2006, 12, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.L.L.; Albano, M.O.; Martins, C.D.S.; Warren, C.A.; Brito, G.A.D.C. Role of Probiotics in Preventing Clostridioides Difficile Infection in Older Adults: An Integrative Review. Front. Med. 2023, 10, 1219225. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Lamont, J.T. Clostridium difficile Infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef]
- Vernaya, M.; McAdam, J.; Hampton, M.D. Effectiveness of Probiotics in Reducing the Incidence of Clostridium Difficile-Associated Diarrhea in Elderly Patients: A Systematic Review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 140–164. [Google Scholar] [CrossRef]
- Merrigan, M.M.; Sambol, S.P.; Johnson, S.; Gerding, D.N. Prevention of Fatal Clostridium difficile—Associated Disease during Continuous Administration of Clindamycin in Hamsters. J. Infect. Dis. 2003, 188, 1922–1927. [Google Scholar] [CrossRef]
- Nagaro, K.J.; Phillips, S.T.; Cheknis, A.K.; Sambol, S.P.; Zukowski, W.E.; Johnson, S.; Gerding, D.N. Nontoxigenic Clostridium difficile Protects Hamsters against Challenge with Historic and Epidemic Strains of Toxigenic BI/NAP1/027 C. Difficile. Antimicrob. Agents Chemother. 2013, 57, 5266–5270. [Google Scholar] [CrossRef]
- Oliveira Júnior, C.A.; Silva, R.O.S.; Lage, A.P.; Coura, F.M.; Ramos, C.P.; Alfieri, A.A.; Guedes, R.M.C.; Lobato, F.C.F. Non-Toxigenic Strain of Clostridioides Difficile Z31 Reduces the Occurrence of C. Difficile Infection (CDI) in One-Day-Old Piglets on a Commercial Pig Farm. Vet. Microbiol. 2019, 231, 1–6. [Google Scholar] [CrossRef]
- Bézay, N.; Ayad, A.; Dubischar, K.; Firbas, C.; Hochreiter, R.; Kiermayr, S.; Kiss, I.; Pinl, F.; Jilma, B.; Westritschnig, K. Safety, Immunogenicity and Dose Response of VLA84, a New Vaccine Candidate against Clostridium Difficile, in Healthy Volunteers. Vaccine 2016, 34, 2585–2592. [Google Scholar] [CrossRef]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium Difficile Infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Leav, B.A.; Blair, B.; Leney, M.; Knauber, M.; Reilly, C.; Lowy, I.; Gerding, D.N.; Kelly, C.P.; Katchar, K.; Baxter, R.; et al. Serum Anti-Toxin B Antibody Correlates with Protection from Recurrent Clostridium difficile Infection (CDI). Vaccine 2010, 28, 965–969. [Google Scholar] [CrossRef]
- Lobato, F.C.F. Clostridioses Dos Animais de Produção. Vet. Zootec. 2013, 20, 29–48. [Google Scholar]
- Chai, J.; Lee, C.H. Management of Primary and Recurrent Clostridium difficile Infection: An Update. Antibiotics 2018, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, G.; Gordon, D.L.; Steiner, T.; Tambyah, P.; Cosgrove, C.; Martens, M.; Bassily, E.; Chan, E.-S.; Patel, D.; Chen, J.; et al. Safety, Immunogenicity, and Efficacy of a Clostridioides Difficile Toxoid Vaccine Candidate: A Phase 3 Multicentre, Observer-Blind, Randomised, Controlled Trial. Lancet Infect. Dis. 2021, 21, 252–262. [Google Scholar] [CrossRef]
- Gibert, X.; Puig, A.; Sabaté, D.; Vidal-Mas, J.; March, R. Effects of a New Vaccine against Clostridioides Difficile and Clostridium Perfringens Type A. In Proceedings of the 12 European Symposium of Porcine Health Management (ESPHM), Bern, Switzerland, 14–16 April 2021. [Google Scholar]
- Silva, R.O.S.; Duarte, M.C.; Oliveira Junior, C.A.; De Assis, R.A.; Lana, A.M.Q.; Lobato, F.C.F. Comparison of Humoral Neutralizing Antibody Response in Rabbits, Guinea Pigs, and Cattle Vaccinated with Epsilon and Beta Toxoids from Clostridium Perfringens and C. Botulinum Types C and D Toxoids. Anaerobe 2018, 54, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Federal Government of the United States. USA Code of Federal Regulations; Federal Government of the United States: Washington, DC, USA, 2005. [Google Scholar]
- Van Soest, B.; Weber Nielsen, M.; Moeser, A.J.; Abuelo, A.; VandeHaar, M.J. Transition Milk Stimulates Intestinal Development of Neonatal Holstein Calves. J. Dairy. Sci. 2022, 105, 7011–7022. [Google Scholar] [CrossRef]
- Giannasca, P.J.; Zhang, Z.; Lei, W.; Boden, J.A.; Giel, M.A.; Monath, T.P.; Thomas, W.D. Serum Antitoxin Antibodies Mediate Systemic and Mucosal Protection from Clostridium difficile Disease in Hamsters. Infect. Immun. 1999, 67, 527–538. [Google Scholar] [CrossRef]
- Best, E.L.; Freeman, J.; Wilcox, M.H. Models for the Study of Clostridium difficile Infection. Gut Microbes 2012, 3, 145–167. [Google Scholar] [CrossRef]
- Siddiqui, F.; O’Connor, J.R.; Nagaro, K.; Cheknis, A.; Sambol, S.P.; Vedantam, G.; Gerding, D.N.; Johnson, S. Vaccination With Parenteral Toxoid B Protects Hamsters Against Lethal Challenge With Toxin A–Negative, Toxin B–Positive Clostridium difficile but Does Not Prevent Colonization. J. Infect. Dis. 2012, 205, 128–133. [Google Scholar] [CrossRef]
- Donald, R.G.K.; Flint, M.; Kalyan, N.; Johnson, E.; Witko, S.E.; Kotash, C.; Zhao, P.; Megati, S.; Yurgelonis, I.; Lee, P.K.; et al. A Novel Approach to Generate a Recombinant Toxoid Vaccine against Clostridium Difficile. Microbiology 2013, 159, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.S.; Salvarani, F.M.; Cruz Júnior, E.C.D.C.; Pires, P.S.; Santos, R.L.R.; Assis, R.A.D.; Guedes, R.M.D.C.; Lobato, F.C.F. Detection of Enterotoxin A and Cytotoxin B, and Isolation of Clostridium difficile in Piglets in Minas Gerais, Brazil. Cienc. Rural. 2011, 41, 1430–1435. [Google Scholar] [CrossRef]
- Salvarani, F.M.; Conceição, F.R.; Cunha, C.E.P.; Moreira, G.M.S.G.; Pires, P.S.; Silva, R.O.S.; Alves, G.G.; Lobato, F.C.F. Vaccination with Recombinant Clostridium Perfringens Toxoids α and β Promotes Elevated Antepartum and Passive Humoral Immunity in Swine. Vaccine 2013, 31, 4152–4155. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum Intake: Influence on Piglet Performance and Factors of Variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- King’ori, A.M. The Pre-Weaning Piglet: Colostrum and Milk Intake: A Review. J. Anim. Prod. Adv. 2012, 2, 277–283. [Google Scholar]
- Oliveira Júnior, C.A.D.; Duarte, M.C.; Antunes De Assis, R.; Alves, G.G.; Silva, R.O.S.; Faria Lobato, F.C. Humoral Responses in Cattle to Commercial Vaccines Containing Clostridium Perfringens Epsilon Toxoid and C. Botulinum Types C and D Toxoids Last Less than a-Year. Anaerobe 2019, 59, 72–75. [Google Scholar] [CrossRef]
- Fu, S.-W. Simplified Purification Method for Clostridium difficile Toxin A. World J. Gastroenterol. 2004, 10, 2756. [Google Scholar] [CrossRef]
- Popoff, M.R.; Rubin, E.J.; Gill, D.M.; Boquet, P. Actin-Specific ADP-Ribosyltransferase Produced by a Clostridium difficile Strain. Infect. Immun. 1988, 56, 2299–2306. [Google Scholar] [CrossRef]
- De Oliveira, C.A.; De Paula Gabardo, M.; Guedes, R.M.C.; Poncet, F.; Blanc, D.S.; Lobato, F.C.F.; Silva, R.O.S. Rodents Are Carriers of Clostridioides Difficile Strains Similar to Those Isolated from Piglets. Anaerobe 2018, 51, 61–63. [Google Scholar] [CrossRef]
- Delmée, M. Laboratory Diagnosis of Clostridium difficile Disease. Clin. Microbiol. Infect. 2001, 7, 411–416. [Google Scholar] [CrossRef]
- Payne, A.M.; Zorman, J.; Horton, M.; Dubey, S.; Ter Meulen, J.; Vora, K.A. Caspase Activation as a Versatile Assay Platform for Detection of Cytotoxic Bacterial Toxins. J. Clin. Microbiol. 2013, 51, 2970–2976. [Google Scholar] [CrossRef]
- Souza Júnior, M.F.; Lobato, Z.I.P.; Pires, P.S.; Silva, R.O.S.; Salvarani, F.M.; Assis, R.A.D.; Lobato, F.C.F. Padronização Da Titulação Da Toxina Épsilon de Clostridium Perfringens Tipo D Em Linhagem Contínua de Células Como Alternativa Ao Bioensaio Animal. Cienc. Rural. 2010, 40, 600–603. [Google Scholar] [CrossRef]
- Silva, R.O.S.; Guedes, R.M.D.C.; Lobato, F.C.F. Clostridium Difficile Infection: Main Features and Occurrence in Domestic Species in Brazil. Cienc. Rural. 2012, 43, 73–80. [Google Scholar] [CrossRef]
- Cruz Junior, E.C.; Salvarani, F.M.; Silva, R.O.S.; Silva, M.X.; Lobato, F.C.F.; Guedes, R.M.C. A Surveillance of Enteropathogens in Piglets from Birth to Seven Days of Age in Brazil. Pesq. Vet. Bras. 2013, 33, 963–969. [Google Scholar] [CrossRef]
- Lippke, R.T.; Borowski, S.M.; Marques, S.M.T.; Paesi, S.O.; Almeida, L.L.; Moreno, A.M.; Corbellini, L.G.; Barcellos, D.E.S.N.D. Matched Case-Control Study Evaluating the Frequency of the Main Agents Associated with Neonatal Diarrhea in Piglets. Pesq. Vet. Bras. 2011, 31, 505–510. [Google Scholar] [CrossRef]
- Farzan, A.; Kircanski, J.; DeLay, J.; Soltes, G.; Songer, J.G.; Friendship, R.; Prescott, J.F. An Investigation into the Association between Cpb2-Encoding Clostridium Perfringens Type A and Diarrhea in Neonatal Piglets. Can. J. Vet. Res. 2013, 77, 45–53. [Google Scholar] [PubMed]
- Keessen, E.C.; Gaastra, W.; Lipman, L.J.A. Clostridium Difficile Infection in Humans and Animals, Differences and Similarities. Vet. Microbiol. 2011, 153, 205–217. [Google Scholar] [CrossRef]
- Knetsch, C.W.; Connor, T.R.; Mutreja, A.; Van Dorp, S.M.; Sanders, I.M.; Browne, H.P.; Harris, D.; Lipman, L.; Keessen, E.C.; Corver, J.; et al. Whole Genome Sequencing Reveals Potential Spread of Clostridium difficile between Humans and Farm Animals in the Netherlands, 2002 to 2011. Eurosurveillance 2014, 19, 20954. [Google Scholar] [CrossRef]
- Redding, L.; Huang, E.; Ryave, J.; Webb, T.; Barnhart, D.; Baker, L.; Bender, J.; Kristula, M.; Kelly, D. Clostridioides Difficile on Dairy Farms and Potential Risk to Dairy Farm Workers. Anaerobe 2021, 69, 102353. [Google Scholar] [CrossRef]
- Debast, S.B.; Van Leengoed, L.A.M.G.; Goorhuis, A.; Harmanus, C.; Kuijper, E.J.; Bergwerff, A.A. Clostridium difficile PCR Ribotype 078 Toxinotype V Found in Diarrhoeal Pigs Identical to Isolates from Affected Humans. Environ. Microbiol. 2009, 11, 505–511. [Google Scholar] [CrossRef]
- Garza, M.A.; Thomas, B.; Saleh, A.; Nabbout, L.; Quigley, E.M.M.; Mathur, N. Look What the Cat Dragged in! Recurrent Clostridioides Difficile from a Household Cat. Am. J. Case Rep. 2023, 24, e940923-1–e940923-4. [Google Scholar] [CrossRef]
- Alexiou, S.; Diakou, A.; Kachrimanidou, M. The Role of Clostridioides Difficile Within the One Health Framework: A Review. Microorganisms 2025, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Quemeneur, L.; Petiot, N.; Arnaud-Barbe, N.; Hessler, C.; Pietrobon, P.J.; Londoño-Hayes, P. Clostridium Difficile Toxoid Vaccine Candidate Confers Broad Protection against a Range of Prevalent Circulating Strains in a Nonclinical Setting. Infect. Immun. 2018, 86, e00742-17. [Google Scholar] [CrossRef]
- De Arriba, M.L.; Carvajal, A.; Pozo, J.; Rubio, P. Mucosal and Systemic Isotype-Specific Antibody Responses and Protection in Conventional Pigs Exposed to Virulent or Attenuated Porcine Epidemic Diarrhoea Virus. Vet. Immunol. Immunopathol. 2002, 85, 85–97. [Google Scholar] [CrossRef]
- Park, J.-E.; Kang, K.-J.; Ryu, J.-H.; Park, J.-Y.; Jang, H.; Sung, D.-J.; Kang, J.-G.; Shin, H.-J. Porcine Epidemic Diarrhea Vaccine Evaluation Using a Newly Isolated Strain from Korea. Vet. Microbiol. 2018, 221, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Songer, J.G. Clostridia as Agents of Zoonotic Disease. Vet. Microbiol. 2010, 140, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Moxley, R.A.; Olson, L.R. Lesions of Transmissible Gastroenteritis Virus Infection in Experimentally Inoculated Pigs Suckling Immunized Sows. Am. J. Vet. Res. 1989, 50, 708–716. [Google Scholar] [CrossRef]
- Odendaal, M.W.; Visser, J.J.; Botha, W.J.; Prinsloo, H. The Passive Protection of Lambs against Clostridium Perfringens Type D with Semi-Purified Hyperimmune Serum. Onderstepoort J. Vet. Res. 1988, 55, 47–50. [Google Scholar]
- Yang, F.; Zhu, Z.; Liu, H.; Cao, W.; Zhang, W.; Wei, T.; Zheng, M.; Zhang, K.; Tian, H.; Zeng, Q.; et al. Evaluation of Antibody Response in Sows after Vaccination with Senecavirus A Vaccine and the Effect of Maternal Antibody Transfer on Antibody Dynamics in Offspring. Vaccines 2021, 9, 1066. [Google Scholar] [CrossRef]
- Hipra Suiseng® Diff/A. Available online: https://www.hipra.com/pt/suiseng-diffa (accessed on 27 May 2024).
- Cohen, O.R.; Steele, J.A.; Zhang, Q.; Schmidt, D.J.; Wang, Y.; Hamel, P.E.S.; Beamer, G.; Xu, B.; Tzipori, S. Systemically Administered IgG Anti-Toxin Antibodies Protect the Colonic Mucosa during Infection with Clostridium difficile in the Piglet Model. PLoS ONE 2014, 9, e111075. [Google Scholar] [CrossRef]
- Steele, J.; Mukherjee, J.; Parry, N.; Tzipori, S. Antibody Against TcdB, but Not TcdA, Prevents Development of Gastrointestinal and Systemic Clostridium difficile Disease. J. Infect. Dis. 2013, 207, 323–330. [Google Scholar] [CrossRef]
- Inoue, R.; Tsukahara, T. Composition and Physiological Functions of the Porcine Colostrum. Anim. Sci. J. 2021, 92, e13618. [Google Scholar] [CrossRef]
- Cabrera, R.A.; Lin, X.; Campbell, J.M.; Moeser, A.J.; Odle, J. Influence of Birth Order, Birth Weight, Colostrum and Serum Immunoglobulin G on Neonatal Piglet Survival. J. Anim. Sci. Biotechnol. 2012, 3, 42. [Google Scholar] [CrossRef] [PubMed]
- Dellagostin, D.; Klein, R.L.; Giacobbo, I.; Guizzo, J.A.; Dazzi, C.C.; Prigol, S.R.; Martín, C.B.G.; Kreutz, L.C.; Schryvers, A.B.; Frandoloso, R. TbpBY167A-Based Vaccine Is Safe in Pregnant Sows and Induces High Titers of Maternal Derived Antibodies That Reduce Glaesserella Parasuis Colonization in Piglets. Vet. Microbiol. 2023, 276, 109630. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, Y.; Wang, C.; Zhao, Y.; Yang, S.; Guo, R.; Hu, M.; Sun, M.; Zhang, G.; Li, Y.; et al. Efficacy Evaluation of a Bivalent Subunit Vaccine against Epidemic PEDV Heterologous Strains with Low Cross-Protection. J. Virol. 2024, 98, e01309–e01324. [Google Scholar] [CrossRef]
- Agenbag, B.; Swinbourne, A.M.; Petrovski, K.; Van Wettere, W.H.E.J. Validation of a Handheld Refractometer to Assess Merino Ewe Colostrum and Transition Milk Quality. J. Dairy Sci. 2023, 106, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, G.; Saleh, J.; Workman, D.; Pollak, R.; Elinoff, V.; Fraser, N.J.; Lefebvre, G.; Martens, M.; Mills, R.E.; Nathan, R.; et al. Defining the Optimal Formulation and Schedule of a Candidate Toxoid Vaccine against Clostridium difficile Infection: A Randomized Phase 2 Clinical Trial. Vaccine 2016, 34, 2170–2178. [Google Scholar] [CrossRef]
- Danz, H.R.; Lee, S.; Chapman-Bonofiglio, S.P.; Ginese, M.; Beamer, G.; Girouard, D.J.; Tzipori, S. The Impact of Actotoxumab Treatment of Gnotobiotic Piglets Infected With Different Clostridium difficile Isogenic Mutants. J. Infect. Dis. 2020, 221, 276–284. [Google Scholar] [CrossRef]
- Kelly, C.R.; Fischer, M.; Allegretti, J.R.; LaPlante, K.; Stewart, D.B.; Limketkai, B.N.; Stollman, N.H. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides Difficile Infections. Am. J. Gastroenterol. 2021, 116, 1124–1147. [Google Scholar] [CrossRef]
- Riley, T.V.; Lyras, D.; Douce, G.R. Status of Vaccine Research and Development for Clostridium Difficile. Vaccine 2019, 37, 7300–7306. [Google Scholar] [CrossRef]
- Alameh, M.-G.; Semon, A.; Bayard, N.U.; Pan, Y.-G.; Dwivedi, G.; Knox, J.; Glover, R.C.; Rangel, P.C.; Tanes, C.; Bittinger, K.; et al. A Multivalent mRNA-LNP Vaccine Protects against Clostridioides Difficile Infection. Science 2024, 386, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, A.; Tiwari, M.K.; Mamah Mustapha, S.S.; Navarrete, S.J.; Henriksen, A.B.; Møller, I.M.; Krogfelt, K.A.; Bjerrum, M.J.; Jørgensen, R. Detoxification of Toxin A and Toxin B by Copper Ion-Catalyzed Oxidation in Production of a Toxoid-Based Vaccine against Clostridioides Difficile. Free Radic. Biol. Med. 2020, 160, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Anosova, N.G.; Brown, A.M.; Li, L.; Liu, N.; Cole, L.E.; Zhang, J.; Mehta, H.; Kleanthous, H. Systemic Antibody Responses Induced by a Two-Component Clostridium difficile Toxoid Vaccine Protect against C. Difficile-Associated Disease in Hamsters. J. Med. Microbiol. 2013, 62, 1394–1404. [Google Scholar] [CrossRef]
- Lima, C.; Lobato, Z.; Pires, P.; Silva, R.; Salvarani, F.; Assis, R.; Lobato, F. Padronização de Teste de Potência in Vitro Para Vacinas Que Contenham Toxoide Alfa de Clostridium Novyi Tipo B. Arq. Inst. Biol. 2011, 78, 507–512. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Yan, Y.-X.; Kim, H.B.; Ju, X.; Zhao, S.; Zhang, K.; Tzipori, S.; Sun, X. A Chimeric Protein Comprising the Glucosyltransferase and Cysteine Proteinase Domains of Toxin B and the Receptor Binding Domain of Toxin A Induces Protective Immunity against Clostridium difficile Infection in Mice and Hamsters. Hum. Vaccines Immunother. 2015, 11, 2215–2222. [Google Scholar] [CrossRef]
- Lobato, F.C.F.; Moro, E.; Umehara, O.; Assis, R.A.; Martins, N.E.; Gonçalves, L.C.B. Avaliação Da Resposta de Antitoxinas Beta e Épsilon de Clostridium Perfringens Induzidas Em Bovinos e Coelhos Por Seis Vacinas Comerciais No Brasil. Arq. Bras. Med. Vet. Zootec. 2000, 52, 313–318. [Google Scholar] [CrossRef]
- Nascimento, R.A.P.; Lobato, F.C.F.; Abreu, V.L.V.; Martins, N.E.; Assis, R.A.; Carvalho Filho, M.B. Avaliação de Vacinas Contra Clostridium Novyi Tipo B. Arq. Bras. Med. Vet. Zootec. 2004, 56, 1–6. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, H.; Yang, Q.; Zhang, H.; Fan, G. Establishment of a Method for Evaluation of the Efficacy of a Classical Swine Fever Virus Subunit Vaccine in Rabbits. Am. J. Vet. Res. 2020, 81, 521–526. [Google Scholar] [CrossRef]
- Saadh, M.J.; Lafi, F.F.; Dahadha, A.A.; Albannan, M.S. Immunogenicity of a Newly Developed Vaccine against Clostridium Perfringens Alpha-Toxin in Rabbits and Cattle. Vet. World 2022, 15, 1617–1623. [Google Scholar] [CrossRef]
- Department of Health and Social Security. British Pharmacopeia Veterinary Antisera and Veterinary Vaccines; Department of Health and Social Security: London, UK; Medicines Commission: London, UK, 1998. [Google Scholar]
- Kiros, T.G.; Levast, B.; Auray, G.; Strom, S.; Van Kessel, J.; Gerdts, V. The Importance of Animal Models in the Development of Vaccines. In Innovation in Vaccinology; Baschieri, S., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 251–264. ISBN 978-94-007-4542-1. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amarante, V.S.d.; Campos, J.V.F.; Souza, T.G.V.d.; de Castro, Y.G.; Godoy, K.M.G.; Silva, R.O.S. Evaluation of the Potency of the First Commercial Vaccine for Clostridioides difficile Infection in Piglets and Comparison with the Humoral Response in Rabbits. Vaccines 2025, 13, 438. https://doi.org/10.3390/vaccines13050438
Amarante VSd, Campos JVF, Souza TGVd, de Castro YG, Godoy KMG, Silva ROS. Evaluation of the Potency of the First Commercial Vaccine for Clostridioides difficile Infection in Piglets and Comparison with the Humoral Response in Rabbits. Vaccines. 2025; 13(5):438. https://doi.org/10.3390/vaccines13050438
Chicago/Turabian StyleAmarante, Victor Santos do, João Victor Ferreira Campos, Thayanne Gabryelle Viana de Souza, Yasmin Gonçalves de Castro, Kelly Mara Gomes Godoy, and Rodrigo Otávio Silveira Silva. 2025. "Evaluation of the Potency of the First Commercial Vaccine for Clostridioides difficile Infection in Piglets and Comparison with the Humoral Response in Rabbits" Vaccines 13, no. 5: 438. https://doi.org/10.3390/vaccines13050438
APA StyleAmarante, V. S. d., Campos, J. V. F., Souza, T. G. V. d., de Castro, Y. G., Godoy, K. M. G., & Silva, R. O. S. (2025). Evaluation of the Potency of the First Commercial Vaccine for Clostridioides difficile Infection in Piglets and Comparison with the Humoral Response in Rabbits. Vaccines, 13(5), 438. https://doi.org/10.3390/vaccines13050438






