From Antibodies to Immunity: Assessing Correlates of Flavivirus Protection and Cross-Reactivity
Abstract
1. Introduction
2. Methods
3. Current Understanding of Correlates of Protection Against Flaviviruses
3.1. Dengue Virus (DENV)
3.2. Yellow Fever Virus (YFV)
3.3. Japanese Encephalitis Virus (JEV)
3.4. West Nile Virus (WNV)
3.5. Zika Virus (ZIKV)
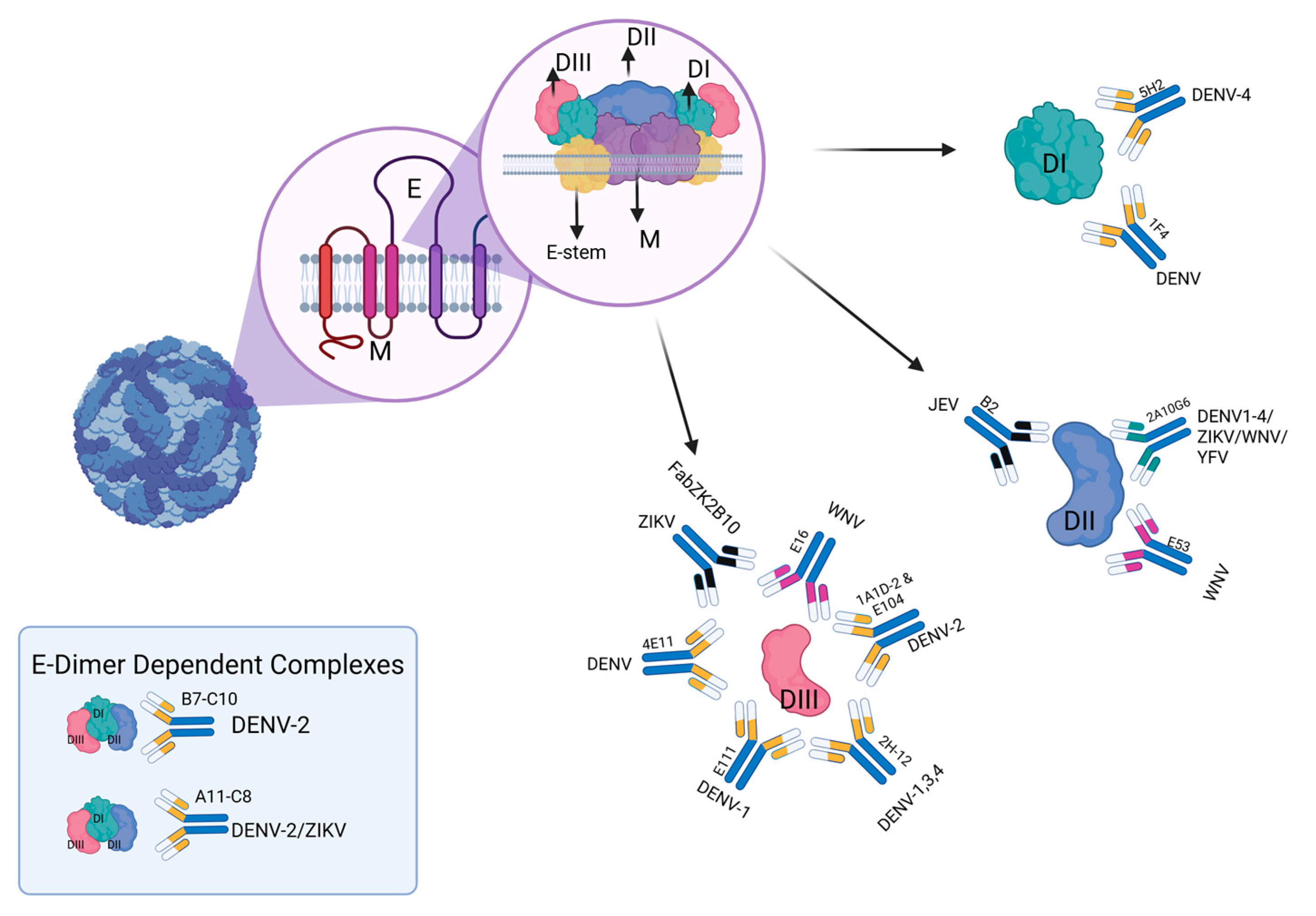
4. Cross-Reactive Immunity Between Flaviviruses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, J.E.; Dolin, R.; Blaser, M.J. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 9th ed.; Elsevier: Philadelphia, PA, USA, 2020. [Google Scholar]
- Heinz, F.X.; Stiasny, K. Flaviviruses and flavivirus vaccines. Vaccine 2012, 30, 4301–4306. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A. Chapter 1—Flaviviruses and where the Zika virus fits in: An overview. In Zika Virus Biology, Transmission, and Pathology; Martin, C.R., Martin, C.J.H., Preedy, V.R., Rajendram, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–18. [Google Scholar]
- Simon, L.V.; Hashmi, M.F.; Torp, K.D. Yellow Fever. In StatPearls; Treasure Island (FL) ineligible companies. Disclosure: Muhammad Hashmi declares no relevant financial relationships with ineligible companies. Disclosure: Klaus Torp declares no relevant financial relationships with ineligible companies; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Schaefer, T.J.; Panda, P.K.; Wolford, R.W. Dengue Fever. In StatPearls; Treasure Island (FL) ineligible companies. Disclosure: Prasan Panda declares no relevant financial relationships with ineligible companies. Disclosure: Robert Wolford declares no relevant financial relationships with ineligible companies; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Simon, L.V.; Sandhu, D.S.; Goyal, A.; Kruse, B. Japanese Encephalitis. In StatPearls; Treasure Island (FL) ineligible companies. Disclosure: Divyajot Sandhu declares no relevant financial relationships with ineligible companies. Disclosure: Amandeep Goyal declares no relevant financial relationships with ineligible companies. Disclosure: Brian Kruse declares no relevant financial relationships with ineligible companies; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Clark, M.B.; Schaefer, T.J. West Nile Virus. In StatPearls; Treasure Island (FL) ineligible companies. Disclosure: Timothy Schaefer declares no relevant financial relationships with ineligible companies; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Wolford, R.W.; Schaefer, T.J. Zika Virus. In StatPearls; Treasure Island (FL) ineligible companies. Disclosure: Timothy Schaefer declares no relevant financial relationships with ineligible companies; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- World Health Organization. Yellow Fever; World Health Organization: Geneva, Switzerland, 2023.
- Zhao, R.; Wang, M.; Cao, J.; Shen, J.; Zhou, X.; Wang, D.; Cao, J. Flavivirus: From Structure to Therapeutics Development. Life 2021, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Becnel, J.J.; Valles, S.M. Chapter 5—RNA Viruses Infecting Pest Insects. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Perera, R.; Kuhn, R.J. Structural proteomics of dengue virus. Curr. Opin. Microbiol. 2008, 11, 369–377. [Google Scholar] [CrossRef]
- van Leur, S.W.; Heunis, T.; Munnur, D.; Sanyal, S. Pathogenesis and virulence of flavivirus infections. Virulence 2021, 12, 2814–2838. [Google Scholar] [CrossRef] [PubMed]
- Kok, B.H.; Lim, H.T.; Lim, C.P.; Lai, N.S.; Leow, C.Y.; Leow, C.H. Dengue virus infection—A review of pathogenesis, vaccines, diagnosis and therapy. Virus Res. 2023, 324, 199018. [Google Scholar] [CrossRef]
- Goncalvez, A.P.; Chien, C.H.; Tubthong, K.; Gorshkova, I.; Roll, C.; Donau, O.; Schuck, P.; Yoksan, S.; Wang, S.D.; Purcell, R.H.; et al. Humanized monoclonal antibodies derived from chimpanzee Fabs protect against Japanese encephalitis virus in vitro and in vivo. J. Virol. 2008, 82, 7009–7021. [Google Scholar] [CrossRef]
- Dai, L.; Song, J.; Lu, X.; Deng, Y.Q.; Musyoki, A.M.; Cheng, H.; Zhang, Y.; Yuan, Y.; Song, H.; Haywood, J.; et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe 2016, 19, 696–704. [Google Scholar] [CrossRef]
- Cherrier, M.V.; Kaufmann, B.; Nybakken, G.E.; Lok, S.M.; Warren, J.T.; Chen, B.R.; Nelson, C.A.; Kostyuchenko, V.A.; Holdaway, H.A.; Chipman, P.R.; et al. Structural basis for the preferential recognition of immature flaviviruses by a fusion-loop antibody. Embo J. 2009, 28, 3269–3276. [Google Scholar] [CrossRef]
- Cockburn, J.J.; Navarro Sanchez, M.E.; Goncalvez, A.P.; Zaitseva, E.; Stura, E.A.; Kikuti, C.M.; Duquerroy, S.; Dussart, P.; Chernomordik, L.V.; Lai, C.J.; et al. Structural insights into the neutralization mechanism of a higher primate antibody against dengue virus. Embo J. 2012, 31, 767–779. [Google Scholar] [CrossRef]
- Fibriansah, G.; Tan, J.L.; Smith, S.A.; de Alwis, A.R.; Ng, T.S.; Kostyuchenko, V.A.; Ibarra, K.D.; Wang, J.; Harris, E.; de Silva, A.; et al. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO Mol. Med. 2014, 6, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sheng, J.; Austin, S.K.; Hoornweg, T.E.; Smit, J.M.; Kuhn, R.J.; Diamond, M.S.; Rossmann, M.G. Structure of acidic pH dengue virus showing the fusogenic glycoprotein trimers. J. Virol. 2015, 89, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, R.; Wang, L.; Ben, H.; Yu, L.; Gao, F.; Shi, X.; Yin, C.; Zhang, F.; Xiang, Y.; et al. Structural Basis for Neutralization and Protection by a Zika Virus-Specific Human Antibody. Cell Rep. 2019, 26, 3360–3368.e5. [Google Scholar] [CrossRef]
- Kaufmann, B.; Nybakken, G.E.; Chipman, P.R.; Zhang, W.; Diamond, M.S.; Fremont, D.H.; Kuhn, R.J.; Rossmann, M.G. West Nile virus in complex with the Fab fragment of a neutralizing monoclonal antibody. Proc. Natl. Acad. Sci. USA 2006, 103, 12400–12404. [Google Scholar] [CrossRef]
- Lok, S.-M.; Kostyuchenko, V.; Nybakken, G.E.; Holdaway, H.A.; Battisti, A.J.; Sukupolvi-Petty, S.; Sedlak, D.; Fremont, D.H.; Chipman, P.R.; Roehrig, J.T.; et al. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat. Struct. Mol. Biol. 2008, 15, 312–317. [Google Scholar] [CrossRef]
- Midgley, C.M.; Flanagan, A.; Tran, H.B.; Dejnirattisai, W.; Chawansuntati, K.; Jumnainsong, A.; Wongwiwat, W.; Duangchinda, T.; Mongkolsapaya, J.; Grimes, J.M.; et al. Structural analysis of a dengue cross-reactive antibody complexed with envelope domain III reveals the molecular basis of cross-reactivity. J. Immunol. 2012, 188, 4971–4979. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.K.; Dowd, K.A.; Shrestha, B.; Nelson, C.A.; Edeling, M.A.; Johnson, S.; Pierson, T.C.; Diamond, M.S.; Fremont, D.H. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog. 2012, 8, e1002930. [Google Scholar] [CrossRef]
- Cockburn, J.J.; Navarro Sanchez, M.E.; Fretes, N.; Urvoas, A.; Staropoli, I.; Kikuti, C.M.; Coffey, L.L.; Arenzana Seisdedos, F.; Bedouelle, H.; Rey, F.A. Mechanism of dengue virus broad cross-neutralization by a monoclonal antibody. Structure 2012, 20, 303–314. [Google Scholar] [CrossRef]
- Rouvinski, A.; Guardado-Calvo, P.; Barba-Spaeth, G.; Duquerroy, S.; Vaney, M.C.; Kikuti, C.M.; Navarro Sanchez, M.E.; Dejnirattisai, W.; Wongwiwat, W.; Haouz, A.; et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature 2015, 520, 109–113. [Google Scholar] [CrossRef]
- Barba-Spaeth, G.; Dejnirattisai, W.; Rouvinski, A.; Vaney, M.-C.; Medits, I.; Sharma, A.; Simon-Lorière, E.; Sakuntabhai, A.; Cao-Lormeau, V.-M.; Haouz, A.; et al. Structural basis of potent Zika–dengue virus antibody cross-neutralization. Nature 2016, 536, 48–53. [Google Scholar] [CrossRef]
- Kaufmann, B.; Chipman, P.R.; Holdaway, H.A.; Johnson, S.; Fremont, D.H.; Kuhn, R.J.; Diamond, M.S.; Rossmann, M.G. Capturing a flavivirus pre-fusion intermediate. PLoS Pathog. 2009, 5, e1000672. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.A. Recent updates on correlates of vaccine-induced protection. Front. Immunol. 2022, 13, 1081107. [Google Scholar] [CrossRef]
- Haks, M.C.; Bottazzi, B.; Cecchinato, V.; De Gregorio, C.; Del Giudice, G.; Kaufmann, S.H.E.; Lanzavecchia, A.; Lewis, D.J.M.; Maertzdorf, J.; Mantovani, A.; et al. Molecular Signatures of Immunity and Immunogenicity in Infection and Vaccination. Front. Immunol. 2017, 8, 1563. [Google Scholar] [CrossRef] [PubMed]
- Vashishtha, V.M.; Kumar, P. The durability of vaccine-induced protection: An overview. Expert Rev. Vaccines 2024, 23, 389–408. [Google Scholar] [CrossRef]
- Dutta, S.K.; Langenburg, T. A Perspective on Current Flavivirus Vaccine Development: A Brief Review. Viruses 2023, 15, 860. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yamanaka, A.; Konishi, E. A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine 2014, 32, 1326–1337. [Google Scholar] [CrossRef]
- Anantharaj, A.; Agrawal, T.; Shashi, P.K.; Tripathi, A.; Kumar, P.; Khan, I.; Pareek, M.; Singh, B.; Pattabiraman, C.; Kumar, S.; et al. Neutralizing antibodies from prior exposure to dengue virus negatively correlate with viremia on re-infection. Commun. Med. 2023, 3, 148. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; Adams, L.E.; Durbin, A.P.; Muñoz-Jordán, J.L.; Poehling, K.A.; Sánchez-González, L.M.; Volkman, H.R.; Paz-Bailey, G. Dengue: A Growing Problem with New Interventions. Pediatrics 2022, 149, e2021055522. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, E.D.; Martínez-Barnetche, J.; Juárez-Palma, L.; Alvarado-Delgado, A.; González-Bonilla, C.R.; Rodríguez, M.H. Genetic diversity and spatiotemporal dynamics of DENV-1 and DENV-2 infections during the 2012–2013 outbreak in Mexico. Virology 2022, 573, 141–150. [Google Scholar] [CrossRef]
- Inizan, C.; Minier, M.; Prot, M.; O’Connor, O.; Forfait, C.; Laumond, S.; Marois, I.; Biron, A.; Gourinat, A.C.; Goujart, M.A.; et al. Viral evolution sustains a dengue outbreak of enhanced severity. Emerg. Microbes Infect. 2021, 10, 536–544. [Google Scholar] [CrossRef]
- Bos, S.; Graber, A.L.; Cardona-Ospina, J.A.; Duarte, E.M.; Zambrana, J.V.; Ruíz Salinas, J.A.; Mercado-Hernandez, R.; Singh, T.; Katzelnick, L.C.; de Silva, A.; et al. Protection against symptomatic dengue infection by neutralizing antibodies varies by infection history and infecting serotype. Nat. Commun. 2024, 15, 382. [Google Scholar] [CrossRef]
- Halstead, S.B.; Rojanasuphot, S.; Sangkawibha, N. Original antigenic sin in dengue. Am. J. Trop. Med. Hyg. 1983, 32, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Montoya, M.; Gresh, L.; Balmaseda, A.; Harris, E. Neutralizing antibody titers against dengue virus correlate with protection from symptomatic infection in a longitudinal cohort. Proc. Natl. Acad. Sci. USA 2016, 113, 728–733. [Google Scholar] [CrossRef]
- Salje, H.; Cummings, D.A.T.; Rodriguez-Barraquer, I.; Katzelnick, L.C.; Lessler, J.; Klungthong, C.; Thaisomboonsuk, B.; Nisalak, A.; Weg, A.; Ellison, D.; et al. Reconstruction of antibody dynamics and infection histories to evaluate dengue risk. Nature 2018, 557, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue Antibody-Dependent Enhancement: Knowns and Unknowns. Microbiol. Spectr 2014, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Sabeena, S.P.; Varma, M.; Arunkumar, G. Current Understanding of the Pathogenesis of Dengue Virus Infection. Curr. Microbiol. 2021, 78, 17–32. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 2003, 100, 6986–6991. [Google Scholar] [CrossRef]
- Crill, W.D.; Roehrig, J.T. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 2001, 75, 7769–7773. [Google Scholar] [CrossRef]
- Sarker, A.; Dhama, N.; Gupta, R.D. Dengue virus neutralizing antibody: A review of targets, cross-reactivity, and antibody-dependent enhancement. Front. Immunol. 2023, 14, 1200195. [Google Scholar] [CrossRef]
- Beltramello, M.; Williams, K.L.; Simmons, C.P.; Macagno, A.; Simonelli, L.; Quyen, N.T.; Sukupolvi-Petty, S.; Navarro-Sanchez, E.; Young, P.R.; de Silva, A.M.; et al. The human immune response to Dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 2010, 8, 271–283. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Jumnainsong, A.; Onsirisakul, N.; Fitton, P.; Vasanawathana, S.; Limpitikul, W.; Puttikhunt, C.; Edwards, C.; Duangchinda, T.; Supasa, S.; et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science 2010, 328, 745–748. [Google Scholar] [CrossRef]
- Hadinegoro, S.R.; Arredondo-García, J.L.; Capeding, M.R.; Deseda, C.; Chotpitayasunondh, T.; Dietze, R.; Muhammad Ismail, H.I.; Reynales, H.; Limkittikul, K.; Rivera-Medina, D.M.; et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015, 373, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.; Dayan, G.H.; Arredondo-García, J.L.; Rivera, D.M.; Cunha, R.; Deseda, C.; Reynales, H.; Costa, M.S.; Morales-Ramírez, J.O.; Carrasquilla, G.; et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N. Engl. J. Med. 2015, 372, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Capeding, M.R.; Tran, N.H.; Hadinegoro, S.R.; Ismail, H.I.; Chotpitayasunondh, T.; Chua, M.N.; Luong, C.Q.; Rusmil, K.; Wirawan, D.N.; Nallusamy, R.; et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 2014, 384, 1358–1365. [Google Scholar] [CrossRef]
- Rosa, B.R.; Cunha, A.; Medronho, R.A. Efficacy, immunogenicity and safety of a recombinant tetravalent dengue vaccine (CYD-TDV) in children aged 2-17 years: Systematic review and meta-analysis. BMJ Open 2019, 9, e019368. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Ye, W.; Chen, J. Current Development and Challenges of Tetravalent Live-Attenuated Dengue Vaccines. Front. Immunol. 2022, 13, 840104. [Google Scholar] [CrossRef]
- Wilder-Smith, A. Dengue vaccine development: Challenges and prospects. Curr. Opin. Infect. Dis. 2022, 35, 390–396. [Google Scholar] [CrossRef]
- Mandaric, S.; Friberg, H.; Saez-Llorens, X.; Borja-Tabora, C.; Biswal, S.; Escudero, I.; Faccin, A.; Gottardo, R.; Brose, M.; Roubinis, N.; et al. Long term T cell response and safety of a tetravalent dengue vaccine in healthy children. NPJ Vaccines 2024, 9, 192. [Google Scholar] [CrossRef]
- Tricou, V.; Yu, D.; Reynales, H.; Biswal, S.; Saez-Llorens, X.; Sirivichayakul, C.; Lopez, P.; Borja-Tabora, C.; Bravo, L.; Kosalaraksa, P.; et al. Long-term efficacy and safety of a tetravalent dengue vaccine (TAK-003): 4·5-year results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Glob. Health 2024, 12, e257–e270. [Google Scholar] [CrossRef]
- Biswal, S.; Reynales, H.; Saez-Llorens, X.; Lopez, P.; Borja-Tabora, C.; Kosalaraksa, P.; Sirivichayakul, C.; Watanaveeradej, V.; Rivera, L.; Espinoza, F.; et al. Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents. N. Engl. J. Med. 2019, 381, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.F.; Long, C.M.; Poh, C.L. Current status of the development of dengue vaccines. Vaccine X 2025, 22, 100604. [Google Scholar] [CrossRef]
- Borja-Tabora, C.; Fernando, L.; Lopez Medina, E.; Reynales, H.; Rivera, L.; Saez-Llorens, X.; Sirivichayakul, C.; Yu, D.; Folschweiller, N.; Moss, K.J.; et al. Immunogenicity, Safety, and Efficacy of a Tetravalent Dengue Vaccine in Children and Adolescents: An Analysis by Age Group. Clin. Infect. Dis. 2024, 80, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kallas, E.G.; Precioso, A.R.; Palacios, R.; Thomé, B.; Braga, P.E.; Vanni, T.; Campos, L.M.A.; Ferrari, L.; Mondini, G.; da Graça Salomão, M.; et al. Safety and immunogenicity of the tetravalent, live-attenuated dengue vaccine Butantan-DV in adults in Brazil: A two-step, double-blind, randomised placebo-controlled phase 2 trial. Lancet Infect. Dis. 2020, 20, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.L.; Cintra, M.A.T.; Moreira, J.A.; Patiño, E.G.; Braga, P.E.; Tenório, J.C.V.; de Oliveira Alves, L.B.; Infante, V.; Silveira, D.H.R.; de Lacerda, M.V.G.; et al. Efficacy and safety of Butantan-DV in participants aged 2-59 years through an extended follow-up: Results from a double-blind, randomised, placebo-controlled, phase 3, multicentre trial in Brazil. Lancet Infect. Dis. 2024, 24, 1234–1244. [Google Scholar] [CrossRef]
- Hills, S.L.; Walter, E.B.; Atmar, R.L.; Fischer, M. Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunization Practices. Morb. Mortal. Wkly. Rep. 2019, 68, 1–33. [Google Scholar] [CrossRef]
- Firbas, C.; Jilma, B. Product review on the JE vaccine IXIARO. Hum. Vaccin Immunother. 2015, 11, 411–420. [Google Scholar] [CrossRef]
- Diamond, M.S.; Sitati, E.M.; Friend, L.D.; Higgs, S.; Shrestha, B.; Engle, M. A critical role for induced IgM in the protection against West Nile virus infection. J. Exp. Med. 2003, 198, 1853–1862. [Google Scholar] [CrossRef]
- Ben-Nathan, D.; Lustig, S.; Tam, G.; Robinzon, S.; Segal, S.; Rager-Zisman, B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J. Infect. Dis. 2003, 188, 5–12. [Google Scholar] [CrossRef]
- Theiler, M.; Smith, H.H. The use of yellow fever virus modified by in vitro cultivation for human immunization. J. Exp. Med. 1937, 65, 787–800. [Google Scholar] [CrossRef]
- Poland, J.D.; Calisher, C.H.; Monath, T.P.; Downs, W.G.; Murphy, K. Persistence of neutralizing antibody 30-35 years after immunization with 17D yellow fever vaccine. Bull. World Health Organ. 1981, 59, 895–900. [Google Scholar]
- de Melo, A.B.; da Silva Mda, P.; Magalhães, M.C.; Gonzales Gil, L.H.; Freese de Carvalho, E.M.; Braga-Neto, U.M.; Bertani, G.R.; Marques, E.T., Jr.; Cordeiro, M.T. Description of a prospective 17DD yellow fever vaccine cohort in Recife, Brazil. Am. J. Trop. Med. Hyg. 2011, 85, 739–747. [Google Scholar] [CrossRef]
- Amanna, I.J.; Slifka, M.K. Questions regarding the safety and duration of immunity following live yellow fever vaccination. Expert Rev. Vaccines 2016, 15, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
- Modjarrad, K.; Lin, L.; George, S.L.; Stephenson, K.E.; Eckels, K.H.; De La Barrera, R.A.; Jarman, R.G.; Sondergaard, E.; Tennant, J.; Ansel, J.L.; et al. Preliminary aggregate safety and immunogenicity results from three trials of a purified inactivated Zika virus vaccine candidate: Phase 1, randomised, double-blind, placebo-controlled clinical trials. Lancet 2018, 391, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Koren, M.A.; Lin, L.; Eckels, K.H.; De La Barrera, R.; Dussupt, V.; Donofrio, G.; Sondergaard, E.L.; Mills, K.T.; Robb, M.L.; Lee, C.; et al. Safety and immunogenicity of a purified inactivated Zika virus vaccine candidate in adults primed with a Japanese encephalitis virus or yellow fever virus vaccine in the USA: A phase 1, randomised, double-blind, placebo-controlled clinical trial. Lancet Infect. Dis. 2023, 23, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef]
- Kirkpatrick, B.D.; Durbin, A.P.; Pierce, K.K.; Carmolli, M.P.; Tibery, C.M.; Grier, P.L.; Hynes, N.; Diehl, S.A.; Elwood, D.; Jarvis, A.P.; et al. Robust and Balanced Immune Responses to All 4 Dengue Virus Serotypes Following Administration of a Single Dose of a Live Attenuated Tetravalent Dengue Vaccine to Healthy, Flavivirus-Naive Adults. J. Infect. Dis. 2015, 212, 702–710. [Google Scholar] [CrossRef]
- Thomas, S.J.; Nisalak, A.; Anderson, K.B.; Libraty, D.H.; Kalayanarooj, S.; Vaughn, D.W.; Putnak, R.; Gibbons, R.V.; Jarman, R.; Endy, T.P. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: How alterations in assay conditions impact performance. Am. J. Trop. Med. Hyg. 2009, 81, 825–833. [Google Scholar] [CrossRef]
- Erickson, A.K.; Pfeiffer, J.K. Spectrum of disease outcomes in mice infected with YFV-17D. J. Gen. Virol. 2015, 96 Pt 6, 1328–1339. [Google Scholar] [CrossRef]
- World Health, O. Vaccines and vaccination against yellow fever: WHO position paper—June 2013. Wkly. Epidemiol. Rec. 2013, 88, 269–283. [Google Scholar]
- Gotuzzo, E.; Yactayo, S.; Córdova, E. Efficacy and duration of immunity after yellow fever vaccination: Systematic review on the need for a booster every 10 years. Am. J. Trop. Med. Hyg. 2013, 89, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.H.; McGowan, E.; Jadi, R.; Young, E.; Lopez, C.A.; Baric, R.S.; Lazear, H.M.; de Silva, A.M. Lack of Durable Cross-Neutralizing Antibodies Against Zika Virus from Dengue Virus Infection. Emerg. Infect. Dis. 2017, 23, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Felipe, L.; Alpizar, Y.A.; Ma, J.; Coelmont, L.; Dallmeier, K. YF17D-based vaccines—Standing on the shoulders of a giant. Eur J. Immunol. 2024, 54, e2250133. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, S.; Valdés, O.; Pupo, M.; Delgado, I.; Alvarez, M.; Pelegrino, J.L.; Guzmán, M.G. MAC-ELISA and ELISA inhibition methods for detection of antibodies after yellow fever vaccination. J. Virol. Methods 2003, 110, 179–184. [Google Scholar] [CrossRef]
- Mason, R.A.; Tauraso, N.M.; Spertzel, R.O.; Ginn, R.K. Yellow fever vaccine: Direct challenge of monkeys given graded doses of 17D vaccine. Appl. Microbiol. 1973, 25, 539–544. [Google Scholar] [CrossRef]
- Jean, K.; Donnelly, C.A.; Ferguson, N.M.; Garske, T. A Meta-Analysis of Serological Response Associated with Yellow Fever Vaccination. Am. J. Trop. Med. Hyg. 2016, 95, 1435–1439. [Google Scholar] [CrossRef]
- Colin de Verdiere, N.; Durier, C.; Samri, A.; Meiffredy, V.; Launay, O.; Matheron, S.; Mercier-Delarue, S.; Even, S.; Aboulker, J.P.; Molina, J.M.; et al. Immunogenicity and safety of yellow fever vaccine in HIV-1-infected patients. Aids 2018, 32, 2291–2299. [Google Scholar] [CrossRef]
- Medina-Magües, L.G.; Mühe, J.; Jasny, E.; Medina-Magües, E.S.; Roth, N.; Lopera-Madrid, J.; Salas-Quinchucua, C.; Knuese, C.; Petsch, B.; Osorio, J.E. Immunogenicity and protective activity of mRNA vaccine candidates against yellow fever virus in animal models. NPJ Vaccines 2023, 8, 31. [Google Scholar] [CrossRef]
- Garg, H.; Mehmetoglu-Gurbuz, T.; Joshi, A. Virus Like Particles (VLP) as multivalent vaccine candidate against Chikungunya, Japanese Encephalitis, Yellow Fever and Zika Virus. Sci. Rep. 2020, 10, 4017. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, A.; Yadav, P.; Dubey, S.K.; Azhar, E.I.; Maitra, S.S.; Dwivedi, V.D. Molecular pathogenesis of Japanese encephalitis and possible therapeutic strategies. Arch. Virol. 2022, 167, 1739–1762. [Google Scholar] [CrossRef]
- Vannice, K.S.; Hills, S.L.; Schwartz, L.M.; Barrett, A.D.; Heffelfinger, J.; Hombach, J.; Letson, G.W.; Solomon, T.; Marfin, A.A.; Anderson, K.; et al. The future of Japanese encephalitis vaccination: Expert recommendations for achieving and maintaining optimal JE control. npj Vaccines 2021, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Japanese Encephalitis; World Health Organization: Geneva, Switzerland, 2024.
- Kim, J.-D.; Lee, A.-R.; Moon, D.-H.; Chung, Y.-U.; Hong, S.-Y.; Cho, H.J.; Kang, T.H.; Jang, Y.H.; Sohn, M.H.; Seong, B.-L.; et al. Efficacy of genotype-matched vaccine against re-emerging genotype V Japanese encephalitis virus. Emerg. Microbes Infect. 2024, 13, 2343910. [Google Scholar] [CrossRef]
- Letson, G.W.; Marfin, A.A.; Mooney, J.; Minh, H.V.; Hills, S.L. Impact of vaccination against Japanese encephalitis in endemic countries. PLoS Negl. Trop. Dis. 2024, 18, e0012390. [Google Scholar] [CrossRef] [PubMed]
- Kalimuddin, S.; Chan, Y.F.Z.; Sessions, O.M.; Chan, K.R.; Ong, E.Z.; Low, J.G.; Bertoletti, A.; Ooi, E.E. An experimental medicine decipher of a minimum correlate of cellular immunity: Study protocol for a double-blind randomized controlled trial. Front. Immunol. 2023, 14, 1135979. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.L.; Zou, W.W.; Chen, D.; Zhu, J.Y.; Wen, J.S. Japanese encephalitis virus E protein domain III immunization mediates cross-protection against Zika virus in mice via antibodies and CD8+ T cells. Virus Res. 2024, 345, 199376. [Google Scholar] [CrossRef]
- Mason, P.W.; Pincus, S.; Fournier, M.J.; Mason, T.L.; Shope, R.E.; Paoletti, E. Japanese encephalitis virus-vaccinia recombinants produce particulate forms of the structural membrane proteins and induce high levels of protection against lethal JEV infection. Virology 1991, 180, 294–305. [Google Scholar] [CrossRef]
- Dia, M.; Bob, N.S.; Talla, C.; Dupressoir, A.; Escadafal, C.; Thiam, M.S.; Diallo, A.; Ndiaye, O.; Heraud, J.M.; Faye, O.; et al. Performance assessment and validation of a plaque reduction neutralization test (PRNT) in support to yellow fever diagnostic and vaccine clinical trials. J. Med. Virol. 2023, 95, e28700. [Google Scholar] [CrossRef]
- Van Gessel, Y.; Klade, C.S.; Putnak, R.; Formica, A.; Krasaesub, S.; Spruth, M.; Cena, B.; Tungtaeng, A.; Gettayacamin, M.; Dewasthaly, S. Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO®) induced neutralizing antibody titers. Vaccine 2011, 29, 5925–5931. [Google Scholar] [CrossRef]
- Li, X.; Ma, S.J.; Liu, X.; Jiang, L.N.; Zhou, J.H.; Xiong, Y.Q.; Ding, H.; Chen, Q. Immunogenicity and safety of currently available Japanese encephalitis vaccines: A systematic review. Hum. Vaccin Immunother. 2014, 10, 3579–3593. [Google Scholar] [CrossRef]
- Markoff, L. Points to consider in the development of a surrogate for efficacy of novel Japanese encephalitis virus vaccines. Vaccine 2000, 18, 26–32. [Google Scholar] [CrossRef]
- Zhou, D.; Pei, C.; Liu, Z.; Yang, K.; Li, Q.; Chen, H.; Cao, S.; Song, Y. Identification of a protective epitope in Japanese encephalitis virus NS1 protein. Antivir. Res. 2020, 182, 104930. [Google Scholar] [CrossRef] [PubMed]
- Suthar, M.S.; Diamond, M.S.; Gale, M., Jr. West Nile virus infection and immunity. Nat. Rev. Microbiol. 2013, 11, 115–128. [Google Scholar] [CrossRef]
- Errett John, S.; Suthar Mehul, S.; McMillan, A.; Diamond Michael, S.; Gale, M. The Essential, Nonredundant Roles of RIG-I and MDA5 in Detecting and Controlling West Nile Virus Infection. J. Virol. 2013, 87, 11416–11425. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Johnson, J.R.; Truong, B.; Kim, G.; Weinbren, N.; Dittmar, M.; Shah, P.S.; Von Dollen, J.; Newton, B.W.; Jang, G.M.; et al. Identification of antiviral roles for the exon–junction complex and nonsense-mediated decay in flaviviral infection. Nat. Microbiol. 2019, 4, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Venter, M.; Myers, T.G.; Wilson, M.A.; Kindt, T.J.; Paweska, J.T.; Burt, F.J.; Leman, P.A.; Swanepoel, R. Gene expression in mice infected with West Nile virus strains of different neurovirulence. Virology 2005, 342, 119–140. [Google Scholar] [CrossRef]
- El Garch, H.; Minke, J.M.; Rehder, J.; Richard, S.; Edlund Toulemonde, C.; Dinic, S.; Andreoni, C.; Audonnet, J.C.; Nordgren, R.; Juillard, V. A West Nile virus (WNV) recombinant canarypox virus vaccine elicits WNV-specific neutralizing antibodies and cell-mediated immune responses in the horse. Vet. Immunol. Immunopathol. 2008, 123, 230–239. [Google Scholar] [CrossRef]
- Ng, T.; Hathaway, D.; Jennings, N.; Champ, D.; Chiang, Y.W.; Chu, H.J. Equine vaccine for West Nile virus. Dev. Biol. 2003, 114, 221–227. [Google Scholar]
- Naveed, A.; Eertink, L.G.; Wang, D.; Li, F. Lessons Learned from West Nile Virus Infection:Vaccinations in Equines and Their Implications for One Health Approaches. Viruses 2024, 16, 781. [Google Scholar] [CrossRef]
- A Study to Evaluate the Safety and Efficacy of a West Nile Virus Vaccine in Healthy Adults. Available online: https://clinicaltrials.gov/ct2/show/NCT00746798 (accessed on 22 April 2025).
- Gould, C.V.; Staples, J.E.; Huang, C.Y.; Brault, A.C.; Nett, R.J. Combating West Nile Virus Disease—Time to Revisit Vaccination. N. Engl. J. Med. 2023, 388, 1633–1636. [Google Scholar] [CrossRef]
- Barrett, P.N.; Terpening, S.J.; Snow, D.; Cobb, R.R.; Kistner, O. Vero cell technology for rapid development of inactivated whole virus vaccines for emerging viral diseases. Expert Rev. Vaccines 2017, 16, 883–894. [Google Scholar] [CrossRef]
- Infectious Diseases Clinical Research Consortium (IDCRC). IDCRC Launches Clinical Trial to Study the Safety and Immunogenicity of an Inactivated West Nile Virus vaccine. Infectious Diseases Clinical Research Consortium. Accessed 22 April 2025.
- Porterfield, J.S. A Simple Plaque Inhibition Test for Antiviral Agents: Application to Assay of Interferon. Lancet 1959, 274, 326–327. [Google Scholar] [CrossRef]
- Kalil, A.C.; Devetten, M.P.; Singh, S.; Lesiak, B.; Poage, D.P.; Bargenquast, K.; Fayad, P.; Freifeld, A.G. Use of Interferon-α in Patients with West Nile Encephalitis: Report of 2 Cases. Clin. Infect. Dis. 2005, 40, 764–766. [Google Scholar] [CrossRef]
- Khakoo, S.; Glue, P.; Grellier, L.; Wells, B.; Bell, A.; Dash, C.; Murray-Lyon, I.; Lypnyj, D.; Flannery, B.; Walters, K.; et al. Ribavirin and interferon alfa-2b in chronic hepatitis C: Assessment of possible pharmacokinetic and pharmacodynamic interactions. Br. J. Clin. Pharmacol. 1998, 46, 563–570. [Google Scholar] [CrossRef]
- Anderson, J.F.; Rahal, J.J. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg. Infect. Dis. 2002, 8, 107–108. [Google Scholar] [CrossRef]
- Oliphant, T.; Engle, M.; Nybakken, G.E.; Doane, C.; Johnson, S.; Huang, L.; Gorlatov, S.; Mehlhop, E.; Marri, A.; Chung, K.M.; et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 2005, 11, 522–530. [Google Scholar] [CrossRef]
- Diamond, M.S.; Shrestha, B.; Marri, A.; Mahan, D.; Engle, M. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J. Virol. 2003, 77, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lobigs, M.; Lee, E.; Müllbacher, A. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. J. Virol. 2003, 77, 13323–13334. [Google Scholar] [CrossRef]
- Lanteri, M.C.; O’Brien, K.M.; Purtha, W.E.; Cameron, M.J.; Lund, J.M.; Owen, R.E.; Heitman, J.W.; Custer, B.; Hirschkorn, D.F.; Tobler, L.H.; et al. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J. Clin. Investig. 2009, 119, 3266–3277. [Google Scholar] [CrossRef]
- Koblischke, M.; Spitzer, F.S.; Florian, D.M.; Aberle, S.W.; Malafa, S.; Fae, I.; Cassaniti, I.; Jungbauer, C.; Knapp, B.; Laferl, H.; et al. CD4 T Cell Determinants in West Nile Virus Disease and Asymptomatic Infection. Front. Immunol. 2020, 11, 16. [Google Scholar] [CrossRef]
- Brien, J.D.; Uhrlaub, J.L.; Nikolich-Zugich, J. West Nile virus-specific CD4 T cells exhibit direct antiviral cytokine secretion and cytotoxicity and are sufficient for antiviral protection. J. Immunol. 2008, 181, 8568–8575. [Google Scholar] [CrossRef]
- Koblischke, M.; Stiasny, K.; Aberle, S.W.; Malafa, S.; Tsouchnikas, G.; Schwaiger, J.; Kundi, M.; Heinz, F.X.; Aberle, J.H. Structural Influence on the Dominance of Virus-Specific CD4 T Cell Epitopes in Zika Virus Infection. Front. Immunol. 2018, 9, 1196. [Google Scholar]
- Simmons, C.P.; Dong, T.; Chau, N.V.; Dung, N.T.; Chau, T.N.; Thao, L.T.T.; Dung, N.T.; Hien, T.T.; Rowland-Jones, S.; Farrar, J. Early T-cell responses to dengue virus epitopes in Vietnamese adults with secondary dengue virus infections. J. Virol. 2005, 79, 5665–5675. [Google Scholar] [CrossRef]
- Purtha, W.E.; Tedder, T.F.; Johnson, S.; Bhattacharya, D.; Diamond, M.S. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. J. Exp. Med. 2011, 208, 2599–2606. [Google Scholar] [CrossRef] [PubMed]
- Allman, W.R.; Liu, L.; Coleman, A.S.; Akkoyunlu, M. MRL Strains Have a BAFFR Mutation without Functional Consequence. PLoS ONE 2016, 11, e0154518. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Draves, K.E.; Young, L.B.; Roe, K.; Bryan, M.A.; Dresch, C.; Richner, J.M.; Diamond, M.S.; Gale, M., Jr.; Clark, E.A. Protection of mice deficient in mature B cells from West Nile virus infection by passive and active immunization. PLoS Pathog. 2017, 13, e1006743. [Google Scholar] [CrossRef] [PubMed]
- Zika: Symptoms, Prevention and Treatments; Pan American Health Organization: Washington, DC, USA, 2015.
- Clinical Signs and Symptoms of Zika Virus Disease; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2025.
- Pattnaik, A.; Sahoo, B.R.; Pattnaik, A.K. Current Status of Zika Virus Vaccines: Successes and Challenges. Vaccines 2020, 8, 266. [Google Scholar] [CrossRef]
- Hamel, R.; Dejarnac, O.; Wichit, S.; Ekchariyawat, P.; Neyret, A.; Luplertlop, N.; Perera-Lecoin, M.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; et al. Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89, 8880–8896. [Google Scholar] [CrossRef]
- Kumar, A.; Hou, S.; Airo, A.M.; Limonta, D.; Mancinelli, V.; Branton, W.; Power, C.; Hobman, T.C. Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep. 2016, 17, 1766–1775. [Google Scholar] [CrossRef]
- Lazear, H.M.; Govero, J.; Smith, A.M.; Platt, D.J.; Fernandez, E.; Miner, J.J.; Diamond, M.S. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 2016, 19, 720–730. [Google Scholar] [CrossRef]
- Sapparapu, G.; Fernandez, E.; Kose, N.; Bin, C.; Fox, J.M.; Bombardi, R.G.; Zhao, H.; Nelson, C.A.; Bryan, A.L.; Barnes, T.; et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 2016, 540, 443–447. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Liu, X.; Dai, L.; Ma, T.; Qi, J.; Wong, G.; Peng, R.; Liu, S.; Li, J.; et al. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci. Transl. Med. 2016, 8, 369ra179. [Google Scholar] [CrossRef]
- Pardy, R.D.; Rajah, M.M.; Condotta, S.A.; Taylor, N.G.; Sagan, S.M.; Richer, M.J. Analysis of the T Cell Response to Zika Virus and Identification of a Novel CD8+ T Cell Epitope in Immunocompetent Mice. PLoS Pathog. 2017, 13, e1006184. [Google Scholar] [CrossRef] [PubMed]
- Elong Ngono, A.; Vizcarra, E.A.; Tang, W.W.; Sheets, N.; Joo, Y.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Mapping and Role of the CD8+ T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe 2017, 21, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.G.O.; Kitoko, J.Z.; Ferreira, F.M.; Suzart, V.G.; Papa, M.P.; Coelho, S.V.A.; Cavazzoni, C.B.; Paula-Neto, H.A.; Olsen, P.C.; Iwasaki, A.; et al. Critical role of CD4+ T cells and IFNγ signaling in antibody-mediated resistance to Zika virus infection. Nat. Commun. 2018, 9, 3136. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.A.; Ko, S.Y.; Morabito, K.M.; Yang, E.S.; Pelc, R.S.; DeMaso, C.R.; Castilho, L.R.; Abbink, P.; Boyd, M.; Nityanandam, R.; et al. Rapid development of a DNA vaccine for Zika virus. Science 2016, 354, 237–240. [Google Scholar] [CrossRef]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.L.; Kudchodkar, S.B.; Zaidi, F.I.; White, S.; Khan, A.S.; Racine, T.; Choi, H.; et al. Safety and Immunogenicity of an Anti-Zika Virus DNA Vaccine. N. Engl. J. Med. 2021, 385, e35. [Google Scholar] [CrossRef]
- Gaudinski, M.R.; Houser, K.V.; Morabito, K.M.; Hu, Z.; Yamshchikov, G.; Rothwell, R.S.; Berkowitz, N.; Mendoza, F.; Saunders, J.G.; Novik, L.; et al. Safety, tolerability, and immunogenicity of two Zika virus DNA vaccine candidates in healthy adults: Randomised, open-label, phase 1 clinical trials. Lancet 2018, 391, 552–562. [Google Scholar] [CrossRef]
- Essink, B.; Chu, L.; Seger, W.; Barranco, E.; Le Cam, N.; Bennett, H.; Faughnan, V.; Pajon, R.; Paila, Y.D.; Bollman, B.; et al. The safety and immunogenicity of two Zika virus mRNA vaccine candidates in healthy flavivirus baseline seropositive and seronegative adults: The results of two randomised, placebo-controlled, dose-ranging, phase 1 clinical trials. Lancet Infect. Dis. 2023, 23, 621–633. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. Zika Vaccine Development: Current Status. Mayo Clin. Proc. 2019, 94, 2572–2586. [Google Scholar] [CrossRef]
- Sirohi, D.; Chen, Z.; Sun, L.; Klose, T.; Pierson, T.C.; Rossmann, M.G.; Kuhn, R.J. The 3.8 Å resolution cryo-EM structure of Zika virus. Science 2016, 352, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.Q.; Dai, J.X.; Ji, G.H.; Jiang, T.; Wang, H.J.; Yang, H.O.; Tan, W.L.; Liu, R.; Yu, M.; Ge, B.X.; et al. A broadly flavivirus cross-neutralizing monoclonal antibody that recognizes a novel epitope within the fusion loop of E protein. PLoS ONE 2011, 6, e16059. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Tsai, W.Y.; Lin, S.R.; Kao, C.L.; Hu, H.P.; King, C.C.; Wu, H.C.; Chang, G.J.; Wang, W.K. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J. Virol. 2008, 82, 6631–6643. [Google Scholar] [CrossRef]
- Throsby, M.; Geuijen, C.; Goudsmit, J.; Bakker, A.Q.; Korimbocus, J.; Kramer, R.A.; Clijsters-van der Horst, M.; de Jong, M.; Jongeneelen, M.; Thijsse, S.; et al. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. J. Virol. 2006, 80, 6982–6992. [Google Scholar] [CrossRef]
- Oliphant, T.; Nybakken, G.E.; Engle, M.; Xu, Q.; Nelson, C.A.; Sukupolvi-Petty, S.; Marri, A.; Lachmi, B.E.; Olshevsky, U.; Fremont, D.H.; et al. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J. Virol. 2006, 80, 12149–12159. [Google Scholar] [CrossRef]
- Grifoni, A.; Pham, J.; Sidney, J.; O’Rourke, P.H.; Paul, S.; Peters, B.; Martini, S.R.; de Silva, A.D.; Ricciardi, M.J.; Magnani, D.M.; et al. Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Priyamvada, L.; Quicke, K.M.; Hudson, W.H.; Onlamoon, N.; Sewatanon, J.; Edupuganti, S.; Pattanapanyasat, K.; Chokephaibulkit, K.; Mulligan, M.J.; Wilson, P.C.; et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc. Natl. Acad. Sci. USA 2016, 113, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Young, G.; Zahralban-Steele, M.; Dean, H.J. Impact of prior flavivirus vaccination on immunogenicity and efficacy of an inactivated Zika vaccine in Indian rhesus macaques. Vaccine 2023, 41, 3024–3027. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Smatti, M.K.; Ouhtit, A.; Cyprian, F.S.; Almaslamani, M.A.; Thani, A.A.; Yassine, H.M. Antibody-Dependent Enhancement (ADE) and the role of complement system in disease pathogenesis. Mol. Immunol. 2022, 152, 172–182. [Google Scholar] [CrossRef]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef]
- Lobigs, M.; Pavy, M.; Hall, R. Cross-protective and infection-enhancing immunity in mice vaccinated against flaviviruses belonging to the Japanese encephalitis virus serocomplex. Vaccine 2003, 21, 1572–1579. [Google Scholar] [CrossRef]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.R.; Wang, X.; Saron, W.A.A.; Gan, E.S.; Tan, H.C.; Mok, D.Z.L.; Zhang, S.L.; Lee, Y.H.; Liang, C.; Wijaya, L.; et al. Cross-reactive antibodies enhance live attenuated virus infection for increased immunogenicity. Nat. Microbiol. 2016, 1, 16164. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Horton, D.L.; Johnson, N.; Li, L.; Barrett, A.D.T.; Smith, D.J.; Galbraith, S.E.; Solomon, T.; Fooks, A.R. Flavivirus-induced antibody cross-reactivity. J. Gen. Virol. 2011, 92 Pt 12, 2821–2829. [Google Scholar] [CrossRef]
- He, J.; Ding, X.; Zhao, J.; Zeng, J.; Zhou, Y.; Xiao, W.; Hua, D.; Liu, M.; Guo, H.; Zhang, Y.; et al. A novel pan-epitope based nanovaccine self-assembled with CpG enhances immune responses against flavivirus. J. Nanobiotechnol. 2024, 22, 738. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Bozzacco, L.; Keeffe, J.R.; Khouri, R.; Olsen, P.C.; Gazumyan, A.; Schaefer-Babajew, D.; Avila-Rios, S.; Nogueira, L.; Patel, R.; et al. Recurrent Potent Human Neutralizing Antibodies to Zika Virus in Brazil and Mexico. Cell 2017, 169, 597–609.e11. [Google Scholar] [CrossRef]
- Thomas, A.; Thiono, D.J.; Kudlacek, S.T.; Forsberg, J.; Premkumar, L.; Tian, S.; Kuhlman, B.; de Silva, A.M.; Metz, S.W. Dimerization of Dengue Virus E Subunits Impacts Antibody Function and Domain Focus. J. Virol. 2020, 94, e00745-20. [Google Scholar] [CrossRef]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Wongwiwat, W.; Supasa, S.; Zhang, X.; Dai, X.; Rouvinski, A.; Jumnainsong, A.; Edwards, C.; Quyen, N.T.H.; Duangchinda, T.; et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat. Immunol. 2015, 16, 170–177. [Google Scholar] [CrossRef] [PubMed]
| Disease Caused by Flaviviruses | Potential Correlate of Protection | Evidence | Reference |
|---|---|---|---|
| Dengue | Higher levels of nAb titer Anti-DENV (1:260) reduce by 10% the risk of symptomatic DENV infection in pediatric cohort (2–14 y). (Technique: PRNT 50 *) |
| [42] |
| CYD-TDV/Dengvaxia: Pediatric seropositive patients (2–16 y): GMT were DENV1 1:274, DENV2 1:445, DENV3 1:384, and DENV4 1:196.7, with an efficacy of 83.7%. (Technique: PRNT 50) |
| [52,61] | |
| TAK-003/Qdenga Pediatric seronegative patients (4–16 y): GMT were DENV1 1:87.8, DENV2 1:929.4, DENV3 1:71.7, and DENV4 1:64 with an efficacy of 66.2%. (Technique: MN 50 **) |
| [59,60,62] | |
| TV-003/TV-005/Butantan-DV In the seronegative population (2–59 y), the GMTs were DENV1 1:280.7, DENV2 1:167-5, DENV3 1:114-6, and DENV4 1:151-1, and the seropositive population was higher (DENV1 1:841.7, DENV2 1:732.3, DENV3 1:299.8, and DENV4 1:238.0), yielding an efficacy of 79.6%. (Technique: PRNT 50) |
| [63,64] | |
| Japanese B encephalitis | There is no efficacy data for JE-VC. However, the titer of ≥10 is an established immunologic correlate of protection. (Technique: PRNT 50) |
| [65,66] |
| West Nile viral encephalitis | Anti-WNV IgM titers of ≥1:45 (Technique: PRNT 50) |
| [67] |
| Anti-WNV IgG titers of 1:1600 to 1:3200 (Technique: ELISA) |
| [68] | |
| Yellow fever | Patients’ previous exposure to YFV; 4 weeks post-vaccination titers ranged from 1:8 to 1:125 (Technique: PRNT 50) | YFV-17D, 1937 | [69] |
| Subjects without exposure; 4 weeks post-vaccination titers were 1:10 (Technique: PRNT 50) | |||
| NT50 titers of >1:64 (37.9%) >1:8 in 68% and 16.4% were negative (<1:2) (Technique: PRNT 50) | Persistence of YFV-17D vaccine at 30–35 years | [70] | |
| Years post-vaccination; 1 year GMT 1046 (PRNT50 titer 1:160–2560), 1.5 GMT 452 (PRNT titer 1:160–640) at 5 and 10 years the PRNT titers < 100 (Technique: PRNT 50) | Persistence of YFV-17D vaccine | [71,72] | |
| Zika viral disease | Anti-ZIKV neutralizing antibody titers of >1:10 (Technique: MN 50) Anti-ZIKV neutralizing antibodies titers between >1:10 and >1:100 was evaluated (Technique: PRNT 50) |
| [73,74] |
| Anti-ZIKV neutralizing antibodies titers of ≥1300 (in BALB/C mice) and ≥400 (in Rhesus macaques) (Technique: PRNT 50) |
| [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, H.E.; Pinzon Burgos, E.F.; Camacho Ortega, S.; Heredia, A.; Chua, J.V. From Antibodies to Immunity: Assessing Correlates of Flavivirus Protection and Cross-Reactivity. Vaccines 2025, 13, 449. https://doi.org/10.3390/vaccines13050449
Flores HE, Pinzon Burgos EF, Camacho Ortega S, Heredia A, Chua JV. From Antibodies to Immunity: Assessing Correlates of Flavivirus Protection and Cross-Reactivity. Vaccines. 2025; 13(5):449. https://doi.org/10.3390/vaccines13050449
Chicago/Turabian StyleFlores, Hannah E., Eduar Fernando Pinzon Burgos, Sigrid Camacho Ortega, Alonso Heredia, and Joel V. Chua. 2025. "From Antibodies to Immunity: Assessing Correlates of Flavivirus Protection and Cross-Reactivity" Vaccines 13, no. 5: 449. https://doi.org/10.3390/vaccines13050449
APA StyleFlores, H. E., Pinzon Burgos, E. F., Camacho Ortega, S., Heredia, A., & Chua, J. V. (2025). From Antibodies to Immunity: Assessing Correlates of Flavivirus Protection and Cross-Reactivity. Vaccines, 13(5), 449. https://doi.org/10.3390/vaccines13050449





