Pertussis Vaccination Failure in the New Zealand Pediatric Population: Study Protocol
Abstract
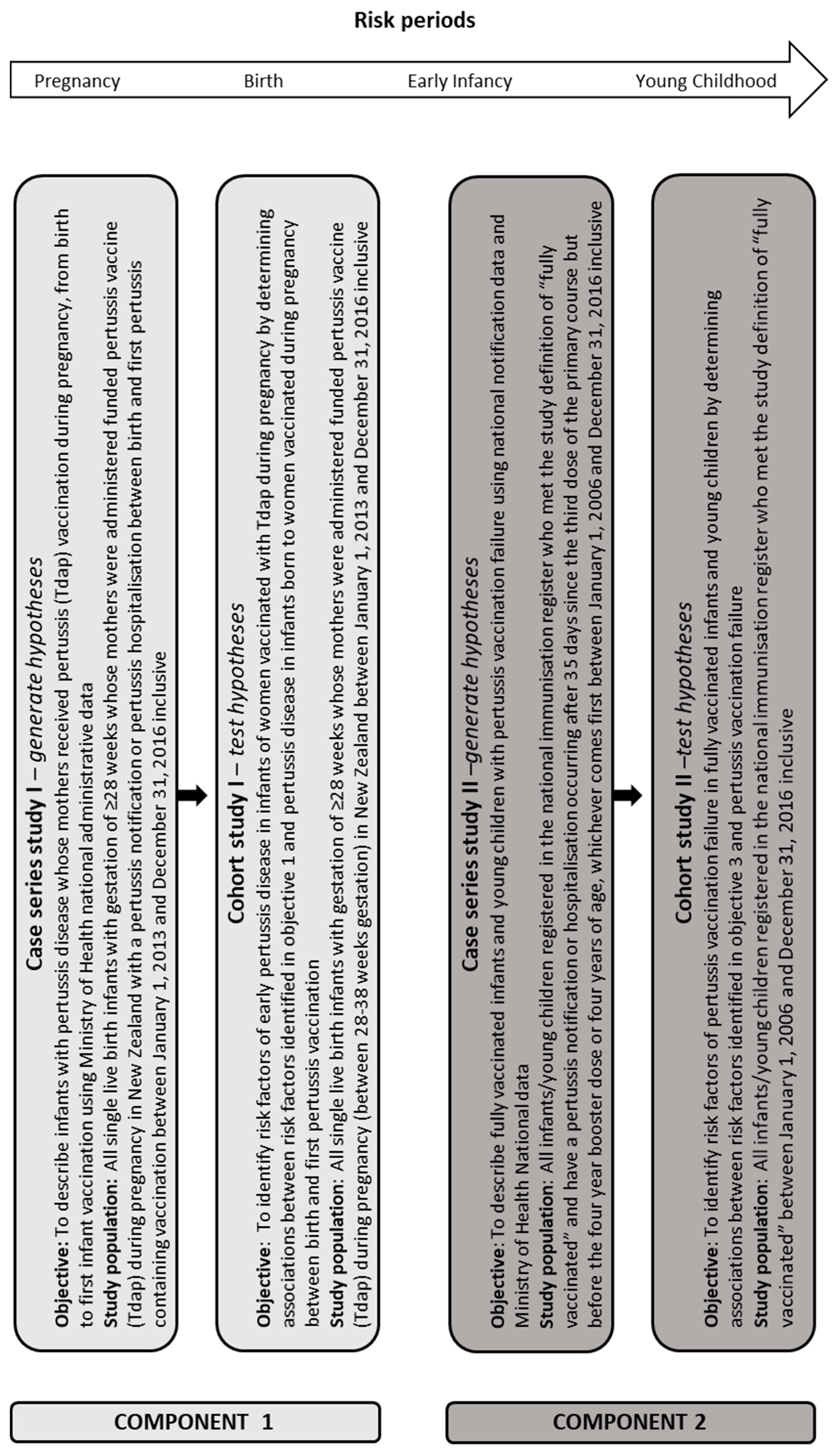
:1. Introduction
2. Materials and Methods
- (a)
- Between birth and first pertussis immunisation in infants born to mothers who received maternal pertussis vaccination during their pregnancy;
- (b)
- in fully vaccinated infants and young children before their four-year pertussis booster;
- (1)
- Describe infants born to mothers Tdap vaccinated during pregnancy with pertussis disease before their first pertussis vaccination, from birth to first pertussis vaccination using Ministry of Health national administrative data;
- (2)
- Identify risk factors for pertussis disease in infants of women Tdap vaccinated during pregnancy by determining associations between risk factors identified in Objective 1 and pertussis disease in infants born to women Tdap vaccinated during pregnancy between birth and first pertussis vaccination;
- (3)
- Describe fully vaccinated infants and young children with pertussis vaccination failure from birth to four year booster vaccination or four years old using Ministry of Health national administrative data and national notification data;
- (4)
- Identify risk factors of pertussis vaccination failure in fully vaccinated infants and young children by determining associations between risk factors identified in Objective 3 and pertussis vaccination failure.
2.1. Study Design
2.1.1. Objective One Inclusion Criteria
2.1.2. Objective Two Inclusion Criteria
2.1.3. Objective Three Inclusion Criteria
2.1.4. Objective Four Inclusion Criteria
2.1.5. Study Definitions
- (a)
- First pertussis-containing vaccination administered no earlier than 4 days (inclusive) before 6 weeks of age, and no later than 10 weeks of age;
- (b)
- Second pertussis-containing vaccination no later than six weeks after the scheduled age of 3 months;
- (c)
- Third pertussis-containing vaccination no later than six weeks after the scheduled age of 6 months;
- (d)
- Each pertussis-containing vaccination administered at least 3 weeks apart.
- (a)
- Clinically confirmed maternal pertussis vaccination failure in the infant:The occurrence of pertussis hospitalisation (ICD-AM 10 code A37.0 only) and or notification (“confirmed” status only) in the infant between birth and first pertussis vaccination.
- (b)
- Clinically suspected maternal vaccination pertussis vaccination failure in the infant:The occurrence of pertussis hospitalisation (ICD-AM 10 code A37.9 only) and or notification (“probable” and “suspected” status only) in the infant between birth and first pertussis vaccination.
- (a)
- Clinically confirmed primary series pertussis vaccination failure:The occurrence of pertussis hospitalisation (ICD-AM 10 code A37.0 only) and or notification (“confirmed” status only) more than 35 days after the last vaccination in the primary series but before four years of age or the receipt of the four-year pertussis booster vaccination. This timeframe aimed to capture only the individuals considered fully vaccinated at the time of exposure to B. pertussis who subsequently developed disease. Thirty-five days allowed 14 days for immune response to the pertussis vaccine and 21 days for the incubation period of pertussis.
- (b)
- Clinically suspected primary series pertussis vaccination failure:The occurrence of pertussis hospitalisation (ICD-AM 10 codes A37.8 and A37.9 only) and or notification (“probable” and “suspected” status only) more than 35 days after the last vaccination in the primary series but before four years of age or the receipt of the four-year pertussis booster vaccination.
2.2. Multiple Notification and Hospitalisation Events
2.3. Statistical Analysis Plan
Power Statement for Objective 4
3. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ministry of Health. Immunisation Handbook 2017; Ministry of Health: Wellington, New Zealand, 2017.
- World Health Organization. WHO-Recommended Surveillance Standard of Pertussis. 2017. Available online: http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/pertussis_standards/en/ (accessed on 8 July 2017).
- World Health Organization. Pertussis Vaccines: WHO Position Paper, August 2015—Recommendations. Vaccine 2016, 34, 1423–1425.
- World Health Organization. Pertussis. 2017. Available online: http://www.who.int/immunization/topics/pertussis/en/ (accessed on 1 July 2017).
- Sheridan, S.L.; Frith, K.; Snelling, T.L.; Grimwood, K.; McIntyre, P.B.; Lambert, S.B. Waning vaccine immunity in teenagers primed with whole cell and acellular pertussis vaccine: Recent epidemiology. Expert Rev. Vaccines 2014, 13, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Simondon, F.; Preziosi, M.; Yam, A.; Kane, C.; Chabirand, L.; Iteman, I.; Sanden, G.; Mboup, S.; Hoffenbach, A.; Knudsen, K.; et al. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 1997, 15, 1606–1612. [Google Scholar] [PubMed]
- Gustafsson, L.; Hessel, L.; Storsaeter, J.; Olin, P. Long-term Follow-up of Swedish Children Vaccinated with Acellular Pertussis Vaccines at 3, 5, and 12 Months of Age Indicates the Need for a Booster Dose at 5 to 7 Years of Age. Pediatrics 2006, 118, 978–984. [Google Scholar] [PubMed]
- Cherry, J.D. The History of Pertussis (Whooping Cough); 1906–2015: Facts, Myths, and Misconceptions. Curr. Epidemiol. Rep. 2015, 2, 120–130. [Google Scholar]
- Radke, S.; Petousis-Harris, H.; Watson, D.; Gentles, D.; Turner, N. Age-specific effectiveness following each dose of acellular pertussis vaccine among infants and young children. Vaccine 2017, 35, 177–183. [Google Scholar] [PubMed]
- Quinn, H.E.; Snelling, T.L.; Macartney, K.K.; McIntyre, P.B. Duration of protection after first dose of acellular pertussis vaccine in infants. Pediatrics 2014, 133, e513–e519. [Google Scholar] [CrossRef]
- Cherry, J.D.; Tan, T.; Wirsing von Konig, C.H.; Forsyth, K.D.; Thisyakorn, U.; Greenber, D.; Johnson, D.; Marchant, C.; Plotkin, S. Clinical definitions of pertussis: Summary of a global pertussis initiative roundtable meeting, February 2011. Clin. Infect. Dis. 2012, 54, 1756–1764. [Google Scholar] [CrossRef]
- McNamara, L.A.; Skoff, T.; Faulkner, A.; Miller, L.; Kudish, K.; Kenyon, C.; Bargsten, M.; Zansky, S.; Sullivan, A.D.; Martin, S.; et al. Reduced Severity of Pertussis in Persons with Age-Appropriate Pertussis Vaccination—United States, 2010–2012. Clin. Infect. Dis. 2017, 65, 811–818. [Google Scholar]
- Tozzi, A.E.; Pastore Celentano, L.; Ciofi degli Atti, M.L.; Salmaso, S. Diagnosis and management of pertussis. Can. Med. Assoc. J. 2005, 172, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Crowcroft, N.S.; Klein, N.P. A framework for research on vaccine effectiveness. Vaccine 2018, 36, 7286–7293. [Google Scholar] [CrossRef] [PubMed]
- Heininger, U.; Bachtiar, N.S.; Bahri, P.; Dana, A.; Dodoo, A.; Gidudu, J.; Matos dos Santos, E. The concept of vaccine failure. Vaccine 2011, 30, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, Y.; Liu, F.; Luo, Y.; Zhang, J.; Peng, X.; Zhang, Y.; Han, S.; Zhao, J.; He, Y. The epidemiology and risk factors for breakthrough varicella in Beijing Fengtai district. Vaccine 2012, 30, 6186–6189. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hsieh, Y.-C.; Liu, C.-C.; Huang, Y.-C.; Chang, K.-Y.; Chi, H.; Chang, L.-Y.; Huang, Y.-C.; Huang, L.-M. Invasive pneumococcal pneumonia caused by 13-valent pneumococcal conjugate vaccine types in children with different schedules. J. Microbiol. Immunol. Infect. 2017, 51, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Riise, Ø.R.; Laake, I.; Vestrheim, D.; Flem, E.; Moster, D.; Riise Bergsaker, M.A.; Storsæter, J. Risk of pertissis in relation to degree of prematurity in children less than 2 years old. Pediatr. Infect. Dis. J. 2017, 36, e151–e156. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics. Pertussis (Whooping Cough). In Red Book: 2012 Report of the Committee on Infectious Diseases; Pickering, L.K., Baker, Kimberlin, D.W., Long, S.S., Eds.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2012; pp. 553–566. [Google Scholar]
- Chan, K.N.; Elliman, A.; Bryan, E.; Silverman, M. Respiratory symptoms in children of low birth weight. Arch. Dis. Child. 1989, 64, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Haberling, D.L.; Holman, R.C.; Paddock, C.D.; Murphy, T.V. Infant and Maternal Risk Factors for Pertussis-Related Infant Mortality in the United States, 1999 to 2004. Pediatr. Infect. Dis. J. 2009, 28, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Langkamp, D.L.; Davis, J.P. Increased risk of reported pertussis and hospitalization associated with pertussis in low birth weight children. J. Pediatr. 1996, 128, 654–659. [Google Scholar] [CrossRef]
- Saari, T.N. Immunization of preterm and low birth weight infants. Pedatrics 2003, 112, 193–198. [Google Scholar] [CrossRef]
- Vitek, C.R.; Pascual, F.B.; Baughman, A.L.; Murphy, T.V. Increase in deaths from pertussis among young infants in the United States in the 1990s. Pediatr. Infect. Dis. J. 2003, 22, 628–634. [Google Scholar] [CrossRef]
- Zamir, C.S.; Dahan, D.B.; Shoob, H. Pertussis in infants under one year old: Risk markers and vaccination status—A case-control study. Vaccine 2015, 33, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.; Davidson, A.; Kafadar, K.; Norris, J.M.; Todd, J.; Steiner, J. Geographic Analysis of Pertussis Infection in an Urban Area: A Tool for Health Services Planning. Am. J. Public Health 1997, 87, 2022–2026. [Google Scholar] [CrossRef] [PubMed]
- Broutin, H.; Simondon, F.; Rohani, P.; Guégan, J.-F.; Grenfell, B.T. Loss of immunity to pertussis in a rural community in Senegal. Vaccine 2004, 22, 594–596. [Google Scholar] [CrossRef] [PubMed]
- Marie-Pierre, P.; Halloran, M.E. Effects of Pertussis Vaccination on Disease: Vaccine Efficacy in Reducing Clinical Severity. Clin. Infect. Dis. 2003, 37, 772–779. [Google Scholar] [Green Version]
- Gaayeb, L.; Pinçon, C.; Cames, C.; Sarr, J.-B.; Seck, M.; Schacht, A.-M.; Remoué, F.; Hermann, E.; Riveau, G. Immune response to Bordetella pertussis is associated with season and undernutrition in Senegalese children. Vaccine 2014, 32, 3431–3437. [Google Scholar] [CrossRef]
- Lacombe, K.; Yam, A.; Simondon, K.; Pinichiant, S.; Simondon, F. Risk factors for acellular and whole-cell pertussis vaccine failure in Senegalese children. Vaccine 2004, 23, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, N.; Debrie, A.; Raze, D.; Bertout, J.; Rouanet, C.; Younes, A.; Creusy, C.; Engle, J.; Goldman, W.; Locht, C. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2006, 2, e65. [Google Scholar] [CrossRef]
- Thorstensson, R.; Trollfors, B.; Al-Tawil, N.; Jahnmatz, M.; Bergstrom, J.; Ljungman, M.; Torner, A.; Wehlin, L.; Van Broekhoven, A.; Bosman, F.; et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine--BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS ONE 2014, 9, e83449. [Google Scholar] [CrossRef]
- Diavatopoulos, D.A.; Mills, K.H.G.; Kester, K.E.; Kampmann, B.; Silerova, M.; Heininger, U.; van Dongen, J.J.M.; van der Most, R.G.; Huijnen, M.A.; Siena, E.; et al. PERISCOPE: road towards effective control of pertussis. Lancet Infect. Dis. 2019, 19, e179–e186. [Google Scholar] [CrossRef]
- Roberts, R.; Moreno, G.; Bottero, D.; Gaillard, M.E.; Fingermann, M.; Graieb, A.; Rumbo, M.; Hozbor, D. Outer membrane vesicles as acellular vaccine against pertussis. Vaccine 2008, 26, 4639–4646. [Google Scholar] [CrossRef]
- Rumbo, M.; Hozbor, D. Development of improved pertussis vaccine. Hum. Vaccin. Immunother. 2014, 10, 2450–2453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health. Immunisation Handbook 2006; Ministry of Health: Wellington, New Zealand, 2006.
- Ministry of Health. National Health Index 2017. Available online: http://www.health.govt.nz/our-work/health-identity/national-health-index (accessed on 21 April 2017).
- Ministry of Health. National Immunisation Register. 2015. Available online: http://www.health.govt.nz/our-work/preventative-health-wellness/immunisation/national-immunisation-register (accessed on 23 April 2017).
- Ministry of Health. National Minimum Dataset (Hospital Events). 2014. Available online: http://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/national-minimum-dataset-hospital-events (accessed on 22 April 2017).
- Ministry of Health. National Maternity Collection. 2011. Available online: http://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/national-maternity-collection (accessed on 21 April 2017).
- Ministry of Health. Pharmaceutical Collection. 2017. Available online: http://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/pharmaceutical-collection (accessed on 22 September 2017).
- Institute of Environmental Sciences and Research Limited & Ministry of Health. Episurv. Available online: https://surv.esr.cri.nz/episurv/index.php (accessed on 1 June 2017).
- Plotkin, S.A.; Orenstein, W.A.; Offit, P.A.; Edwards, K.M. Plotkin’s Vaccines, 7th ed.; Elsevier: Philadelphia, PA, USA, 2018. [Google Scholar]
- Atkinson, W.L.; Pickering, L.K.; Schwartz, B.; Weniger, B.G.; Iskander, J.K.; Watson, J.C.; Centers for Disease Control and Prevention. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). Morb. Mortal. Rep. 2002, 51, 1–35. [Google Scholar]
- Grant, C.C.; Turner, N.M.; York, D.G.; Goodyear-Smith, F.; Petousis-Harris, H.A. Factors associated with immunisation coverage and timeliness in New Zealand. Br. J. Gen. Pract. 2010, 60, e113–e120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Institute of Environmental Sciences and Research Limited and Ministry of Health. Manual for Public Health Surveillance in New Zealand; Institute of Environmental Sciences and Research Ltd.: Porirua, New Zealand, 2017. [Google Scholar]
- Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases, 13th ed.; Public Health Foundation: Washington, DC, USA, 2015. [Google Scholar]
- Soe, M.M.; Sullivan, K.M. Open Source Epidemiologic Statistics for Public Health. 2005. Available online: http://www.openepi.com/Power/PowerCohort.htm (accessed on 22 February 2018).
- Baker, M.G.; Barnard, L.T.; Kvalsvig, A.; Verrall, A.; Zhang, J.; Keall, M.; Wilson, N.; Wall, T.; Howden-Chapman, P. Increasing incidence of serious infectious diseases and inequalities in New Zealand: A national epidemiological study. Lancet 2012, 379, 1112–1119. [Google Scholar] [CrossRef]
- Baker, M.G.; McDonald, A.; Zhang, J.; Howden-Chapman, P. Infectious Diseases Attributable to Crowding in New Zealand: A Systematic Review and Burden of Disease Estimate; He Kainga Oranga/Housing and Health Research Programme: Wellington, New Zealand, 2013. [Google Scholar]

| Data Source | Information | Relevant Fields |
|---|---|---|
| National Health Index database | The national health index (NHI) database holds static demographic information such as the NHI number, sex, date of birth, socioeconomic deprivation and ethnicity [37]. Some demographic information such as address, level of socioeconomic deprivation and ethnicity are changeable and therefore amenable to regular updates in real-time (at every presentation to health care services). The NHI number is a unique randomly assigned alphanumeric identifier that enables accurate identification of individuals for medical care and administrative records in New Zealand. The NHI number links all patient information from national and regional health services [37]. | Encrypted NHI number; Date of birth; Sex; New Zealand Deprivation Index 2013 (NZDep13) decile; Ethnicity |
| National Immunisation Register | An electronic collection of all registered immunisation enrolments and events of children in New Zealand and from 2005, these are updated weekly [38]. This collection does not necessarily contain all infants and young children who were vaccinated, parents or legal guardians can withdraw their child′s information from the national immunisation register collection. | Encrypted NHI number; Vaccine type; Antigen type; Vaccination date; Vaccination status; |
| National Minimum Dataset | A health statistic collection dataset containing clinical and other information about public and private hospital discharges for inpatients and day patients [39]. | Encrypted NHI; Admission and discharge date; Length of stay; ICD-10 diagnosis code(s) |
| Maternity collections | A collection of the demographic and clinical features of women in New Zealand using publicly funded maternity/new-born services from 9 months before birth to 3 months after [40]. | Encrypted NHI; Birth weight; Gestational age; Other inpatient and day-patient event data across pregnancy, birth and postnatal periods pertaining to both fetus/infant and mother |
| Pharmaceutical collection | A system used for management of subsidised pharmaceuticals in New Zealand [41]. | Encrypted NHI; Anatomical Class; Chemical name; Duration of supplied pharmaceuticals |
| National Notifiable Diseases Database (EpiSurv) | EpiSurv is a web assessable application operated by the Institute of Environmental Science and Research built using the Surveillance information New Zealand framework [42]. EpiSurv holds the national notifiable disease database and allows the real time recording of notifiable disease cases across New Zealand from public health services in New Zealand. EpiSurv is under contract from the Ministry of Health to collect demographic, clinical and risk factor information of reported cases. | Encrypted NHI; Disease; Notification status; Risk factors for disease; Report date; Onset date; |
| Classification | Definition |
|---|---|
| Suspect | Idiopathic presentation of any paroxysmal cough with whoop, vomit or apnoea |
| Probable | Presentation clinically compatible with pertussis with a high B. pertussis IgA test or a significant (fourfold increase in titres) in antibody levels between paired sera at the same laboratory |
| Confirmed | Clinically compatible presentation with either laboratory confirmed pertussis infection (B. pertussis only) or epidemiologically linked to a confirmed case |
| ICD10-AM Code | Description |
|---|---|
| A37.0 | Whooping cough due to B. pertussis |
| A37.8 | Whooping cough due to Bordetella species |
| A37.9 | Whooping cough, unspecified |
| Exposed (Deprived, NZDep13 Deciles 7–10) | Not-Exposed (Not Deprived, NZDep13 Deciles 1–6) | |
|---|---|---|
| Sample size | 219,814 | 285,800 |
| Risk of disease | 53 cases ÷ 219,814 × 100 = 0.02% | 32 cases ÷ 285,800 × 100 = 0.01% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisholm, H.; Howe, A.; Best, E.; Petousis-Harris, H. Pertussis Vaccination Failure in the New Zealand Pediatric Population: Study Protocol. Vaccines 2019, 7, 65. https://doi.org/10.3390/vaccines7030065
Chisholm H, Howe A, Best E, Petousis-Harris H. Pertussis Vaccination Failure in the New Zealand Pediatric Population: Study Protocol. Vaccines. 2019; 7(3):65. https://doi.org/10.3390/vaccines7030065
Chicago/Turabian StyleChisholm, Hannah, Anna Howe, Emma Best, and Helen Petousis-Harris. 2019. "Pertussis Vaccination Failure in the New Zealand Pediatric Population: Study Protocol" Vaccines 7, no. 3: 65. https://doi.org/10.3390/vaccines7030065
APA StyleChisholm, H., Howe, A., Best, E., & Petousis-Harris, H. (2019). Pertussis Vaccination Failure in the New Zealand Pediatric Population: Study Protocol. Vaccines, 7(3), 65. https://doi.org/10.3390/vaccines7030065





