Foot-and-Mouth Disease Virus: Immunobiology, Advances in Vaccines and Vaccination Strategies Addressing Vaccine Failures—An Indian Perspective
Abstract
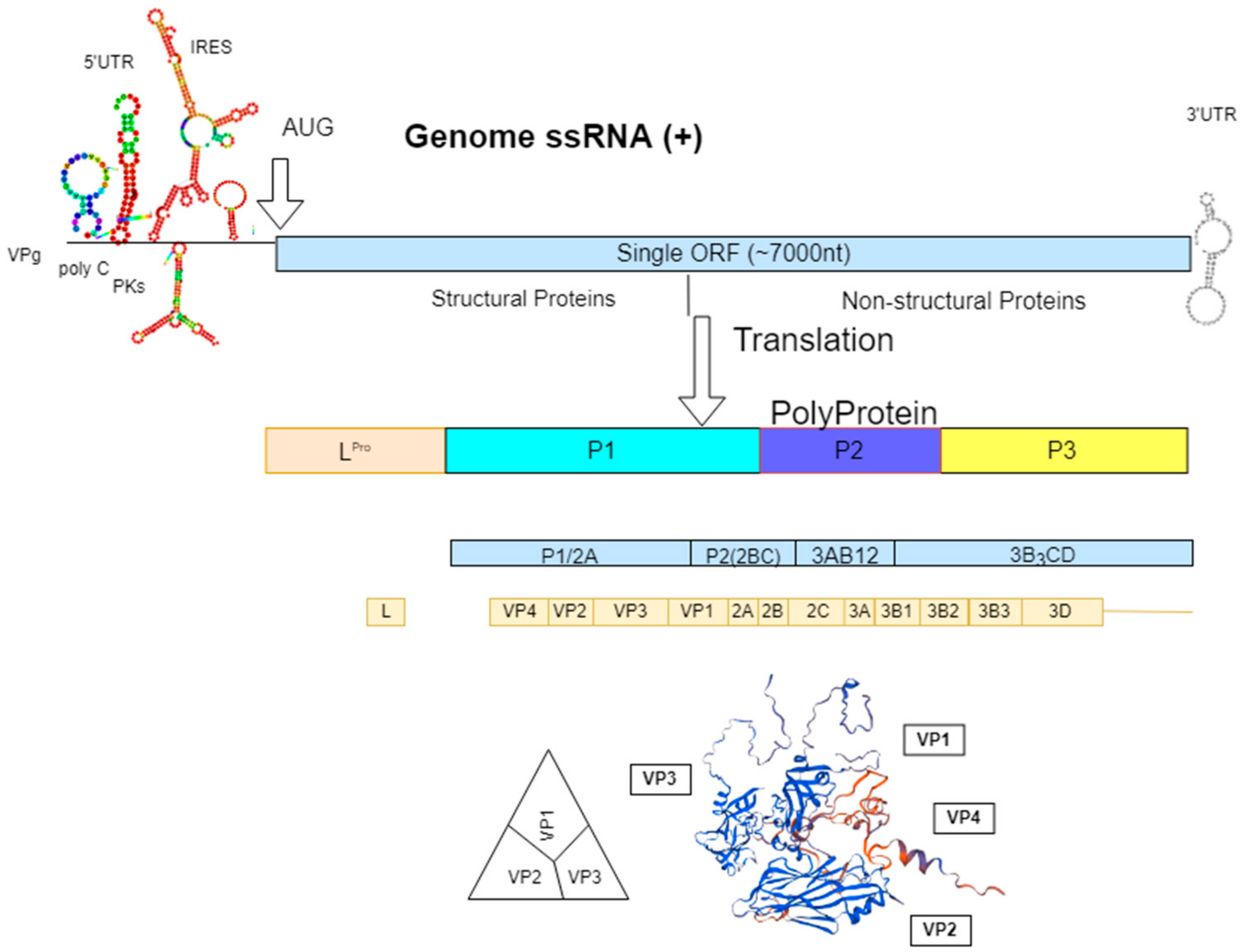
:1. Introduction
2. Trends and Advances in Vaccines against FMDV
2.1. Inactivated Whole Virus Vaccines
2.2. Modified Virus Inactivated Vaccines
2.3. Live Attenuated Vaccines
2.4. Viral Vector Vaccines
2.5. Virus-Like Particle Vaccines
2.6. DNA Vaccines
2.7. Peptide Vaccines
2.8. Plant Based Recombinant Vaccines
2.9. Potential Use of Immunomodulatory Molecules
3. Epidemiology of FMD in India
3.1. Circulating Virus Pool
3.2. Serotype O
3.3. Serotype A
3.4. Serotype Asia 1
4. FMD Monitoring in India
5. Immunobiology of FMDV and Vaccine Failures: Challenges and Solutions
5.1. Failure to Vaccinate
5.2. Vaccination Failure
5.2.1. Vaccine Strain Matching with Circulating Virus
5.2.2. Vaccine Quality
5.2.3. Impaired Immune Response to Vaccine
5.2.4. Break in the Herd Immunity
5.2.5. Duration of Protective Immunity
5.2.6. Maternally Derived Antibody Inhibition
5.2.7. FMDV Persistence in Recovered Animals
5.2.8. Other Factors
Virus Circulation in Other Ruminant Species
Animal Stressors
Amount of FMDV Exposure to Vaccinated Animals
6. Tackling the Challenges
7. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grubman, M.J.; Baxt, B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004, 17, 465–493. [Google Scholar] [CrossRef]
- Racaniello, R.R. Picornaviridae: The Viruses and Their Replication. In Fields Virology, 4th ed.; Fields, B.N., Knipe, D.M., Howleym, P.M., Eds.; Lippincott Williams and Wilkins, Lippincott-Raven Publishers: Philadelphia, PA, USA, 2001; pp. 685–722. [Google Scholar]
- Arzt, J.; Juleff, N.; Zhang, Z.; Rodriguez, L.L. The pathogenesis of foot-and-mouth disease I: Viral pathways in cattle. Transbound. Emerg. Dis. 2011, 58, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Arzt, J.; Baxt, B.; Grubman, M.J.; Jackson, T.; Juleff, N.; Rhyan, J.; Rieder, E.; Waters, R.; Rodriguez, L.L. The pathogenesis of foot-and-mouth disease II: Viral pathways in swine, small ruminants, and wildlife; myotropism, chronic syndromes, and molecular virus-host interactions. Transbound. Emerg. Dis. 2011, 58, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Mahy, B.W. Overview of Foot-and-Mouth Disease and Its Impact as Re-Emerging Viral Infection. In Foot and Mouth Disease Current Perspectives; Sobrino, F., Domingo, E., Eds.; Horizon Biosciences: Norfolk, UK, 2004; pp. 437–446. [Google Scholar]
- Arzt, J.; Belsham, G.J.; Lohse, L.; Bøtner, A.; Stenfeldt, C. Transmission of Foot-and-Mouth Disease from Persistently Infected Carrier Cattle to Naive Cattle via Transfer of Oropharyngeal Fluid. Msphere 2018, 3, e00365-18. [Google Scholar] [CrossRef] [Green Version]
- Maree, F.; de Klerk-Lorist, L.-M.; Gubbins, S.; Zhang, F.; Seago, J.; Pérez-Martín, E.; Reid, L.; Scott, K.; van Schalkwyk, L.; Bengis, R.; et al. Differential persistence of foot-and-mouth disease virus in African buffalo is related to virus virulence. J. Virol. 2016, 90, 5132–5140. [Google Scholar] [CrossRef]
- Stenfeldt, C.; Diaz-San Segundo, F.; de Los Santos, T.; Rodriguez, L.L.; Arzt, J. The Pathogenesis of Foot-and-Mouth Disease in Pigs. Front. Vet. Sci. 2016, 3, 41. [Google Scholar] [CrossRef]
- Mason, P.W.; Grubman, M.J.; Baxt, B. Molecular basis of pathogenesis of FMDV. Virus Res. 2003, 91, 9–32. [Google Scholar] [CrossRef]
- Carrillo, C.; Tulman, E.R.; Delhon, G.; Lu, Z.; Carreno, A.; Vagnozzi, A.; Kutish, G.F.; Rock, D.L. Comparative genomics of foot-and-mouth disease virus. J. Virol. 2005, 79, 6487–6504. [Google Scholar] [CrossRef] [PubMed]
- Serrano, P.; Pulido, M.R.; Sáiz, M.; Martínez-Salas, E. The 3′ end of the foot-and-mouth disease virus genome establishes two distinct long-range RNA-RNA interactions with the 5′ end region. J. Gen. Virol. 2006, 87, 3013–3022. [Google Scholar] [CrossRef]
- Sáiz, M.; Gómez, S.; Martínez-Salas, E.; Sobrino, F. Deletion or substitution of the aphthovirus 3′ NCR abrogates infectivity and virus replication. J. Gen. Virol. 2001, 82, 93–101. [Google Scholar] [CrossRef]
- Fry, E.E.; Lea, S.M.; Jackson, T.; Newman, J.W.; Ellard, F.M.; Blakemore, W.E.; Abu-Ghazaleh, R.; Samuel, A.; King, A.M.; Stuart, D.I. The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex. EMBO J. 1999, 18, 543–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrero, R.; Limardo, R.R.; Carrillo, E.; König, G.A.; Turjanski, A.G. A computational study of the interaction of the foot and mouth disease virus VP1 with monoclonal antibodies. J. Immunol. Methods 2015, 425, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sainz, I.; Gavitt, T.D.; Koster, M.; Ramirez-Medina, E.; Rodriguez, Y.Y.; Wu, P.; Silbart, L.K.; de Los Santos, T.; Szczepanek, S.M. The VP1 G-H loop hypervariable epitope contributes to protective immunity against Foot and Mouth Disease Virus in swine. Vaccine 2019, 37, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Burman, A.; Clark, S.; Abrescia, N.G.A.; Fry, E.E.; Stuart, D.I.; Jackson, T. Specificity of the VP1 GH loop of Foot-and-Mouth Disease virus for alphav integrins. J. Virol. 2006, 80, 9798–9810. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, E.; Ruiz-Jarabo, C.M.; Sevilla, N.; Andreu, D.; Beck, E.; Domingo, E. Cell recognition by foot-and-mouth disease virus that lacks the RGD integrinbinding motif: Flexibility in aphthovirus receptor usage. J. Virol. 2000, 74, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.J.; Sumption, K.J.; Charleston, B. Options for control of foot-and-mouth disease: Knowledge, capability and policy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2657–2667. [Google Scholar] [CrossRef] [PubMed]
- Kamel, M.; El-Sayed, A.; Castañeda Vazquez, H. Foot-and-mouth disease vaccines: Recent updates and future perspectives. Arch. Virol. 2019, 164, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Parida, S. Vaccination against foot-and-mouth disease virus: Strategies and effectiveness. Expert Rev. Vaccines. 2009, 8, 347–365. [Google Scholar] [CrossRef]
- Segundo, D.F.; Montiel, N.A.; Sturza, D.F.; Perez-Martin, E.; Hickman, D.; Ramirez-Medina, E.; Grubman, M.J.; de Los Santos, T. Combination of Adt-O1Manisa and Ad5-boIFNλ3 induces early protective immunity against foot-and-mouth disease in cattle. Virology 2016, 499, 340–349. [Google Scholar] [CrossRef]
- Biswal, J.K.; Sanyal, A.; Rodriguez, L.L.; Subramaniam, S.; Arzt, J.; Sharma, G.K.; Hammond, J.M.; Parida, S.; Mohapatra, J.K.; Mathapati, B.S.; et al. Foot-and-mouth disease: Global status and Indian perspective. Indian J. Anim. Sci. 2012, 82, 109–131. [Google Scholar]
- Prabhu, M.; Safuullah, A.M.; Selvam, S. Evaluation of Economic losses due to foot and mouth disease in Bovines of Salem district. Agric. Econ. Res. Rev. 2004, 17, 77–84. [Google Scholar]
- Venkataramanan, R.; Hemadri, D.; Bandyopadhyay, S.K.; Taneja, V.K. Foot-and mouth Disease in India: Present status. In Proceedings of the Workshop on Global Roadmap for Improving the Tools to Control Foot-and-Mouth Disease in Endemic Settings, Agra, India, 29 December–1 November 2006. [Google Scholar]
- Singh, B.; Prasad, S.; Sinha, D.K.; Verma, M.R. Estimation of economic losses due to foot and mouth disease in India. Indian J. Anim. Sci. 2013, 83, 964–970. [Google Scholar]
- Pattnaik, B.; Subramaniam, S.; Sanyal, A.; Mohapatra, J.K.; Dash, B.B.; Ranjan, R.; Rout, M. Foot-and-mouth Disease: Global Status and Future Road Map for Control and Prevention in India. Agric. Res. 2012, 1, 132–147. [Google Scholar] [CrossRef] [Green Version]
- Sumption, K.; Domenech, J.; Ferrari, G. Progressive control of FMD on a global scale. Vet. Rec. 2012, 170, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.K.; Mahajan, S.; Matura, R.; Biswal, J.K.; Ranjan, R.; Subramaniam, S.; Misri, J.; Bambal, R.G.; Pattnaik, B. Herd Immunity Against Foot-and-Mouth Disease Under Different Vaccination Practices in India. Transbound. Emerg. Dis. 2017, 64, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Annual Reports. Directorate on Foot and Mouth Disease, Mukteswar, 2003–2017. Available online: www.pdfmd.ernet.in (accessed on 2 December 2018).
- Sharma, G.K.; Mahajan, S.; Verma, B.; Matura, R.; Subramaniam, S.; Mohapatra, J.K.; Dash, B.B.; Pattnaik, B. Epidemiological investigation of Foot-and-Mouth Disease incidences in southern peninsular India during 2013. In Proceedings of the Open Session of the Standing Technical and Research Committees of the EuFMD, Cavtat, Croatia, 29–31 October 2014. [Google Scholar]
- Subramaniam, S.; Mohapatra, J.K.; Das, B.; Sanyal, A.; Pattnaik, B. Genetic and antigenic analysis of foot-and-mouth disease virus serotype O responsible for outbreaks in India during 2013. Infect. Genet. Evol. 2015, 30, 59–64. [Google Scholar] [CrossRef]
- Woolhouse, M.E.; Haydon, D.T.; Pearson, A.; Kitching, R.P. Failure of vaccination to prevent outbreaks of foot-and-mouth disease. Epidemiol. Infect. 1996, 116, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Sharma, G.K.; Mohapatra, J.K.; Mahajan, S.; Matura, R.; Subramaniam, S.; Pattnaik, B. Comparative evaluation of non-structural protein-antibody detecting ELISAs for foot-and-mouth disease sero-surveillance under intensive vaccination. J. Virol. Methods 2014, 207, 22–28. [Google Scholar] [CrossRef]
- Lyons, N.A.; Lyoo, Y.S.; King, D.P.; Paton, D.J. Challenges of Generating and Maintaining Protective Vaccine-Induced Immune Responses for Foot-and-Mouth Disease Virus in Pigs. Front. Vet. Sci. 2016, 30, 102. [Google Scholar] [CrossRef]
- Knight-Jones, T.J.; Rushton, J. The economic impacts of foot and mouth disease—What are they, how big are they and where do they occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef]
- International Office of Epizootics; Biological Standards Commission; International Office of Epizootics; International Committee. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: Mammals, Birds and Bees; Office International Des Epizooties: Paris, France, 2018. [Google Scholar]
- Stenfeldt, C.; Pacheco, J.; Smoliga, G.; Bishop, E.; Pauszek, S.; Hartwig, E.; Rodriguez, L.; Arzt, J. Detection of foot-and-mouth disease virus RNA and capsid protein in lymphoid tissues of convalescent pigs does not indicate existence of a carrier state. Transbound. Emerg Dis. 2016, 63, 152–164. [Google Scholar] [CrossRef]
- Lombard, M.; Fussel, A.E. Antigen and vaccine banks: Technical requirements and the role of the European antigen bank in emergency foot and mouth disease vaccination. Rev. Sci. Tech. 2007, 26, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Grubman, M.J.; Mason, P.W. Prospects, including time-frames, for improved foot and mouth disease vaccines. Rev. Sci. Tech. 2002, 21, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Chen, H.T.; Zhou, J.H.; Ma, L.N.; Ding, Y.Z.; Liu, Y.S. Research in advance for FMD novel vaccines. Virol. J. 2011, 8, 268. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.L.; Gay, C.G. Development of vaccines toward the global control and eradication of foot-and-mouth disease. Expert Rev. Vaccines 2011, 10, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Knight-Jones, T.J.; Charleston, B.; Rodriguez, L.L.; Gay, C.G.; Sumption, K.J.; Vosloo, W. Global Foot-and-Mouth Disease Research Update and Gap Analysis: 3-Vaccines. Transbound. Emerg. Dis. 2016, 63, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Mahendran, M.; Gupta, P.K.; Rai, A. DNA Vaccines and their applications in Veterinary Practice: Current Perspectives. Vet. Res. Commun. 2008, 32, 341–356. [Google Scholar] [CrossRef]
- Dhama, K.; Wani, M.Y.; Deb, R.; Karthik, K.; Tiwari, R.; Barathidasan, R.; Kumar, A.; Mahima; Verma, A.K.; Singh, S.D. Plant based oral vaccines for human and animal pathogens—A new era of prophylaxis: Current and future perspectives. J. Exp. Biol. Agric. Sci. 2013, 1, 1–12. [Google Scholar]
- Singh, R.K.; Badasara, S.K.; Dhama, K.; Malik, Y.P.S. Progress and Prospects in Vaccine Research. In National Workshop on “Current Trends and Future Research Challenges in Vaccines and Adjuvants”; Chapter, Organized at ICAR, Bareilly, Uttar Pradesh, India, 19–20 November 2015; Indian Veterinary Research Institute: Bareilly, Uttar Pradesh, India, 2015; pp. 1–19. [Google Scholar]
- Robinson, L.; Knight-Jones, T.J.; Charleston, B.; Rodriguez, L.L.; Gay, C.G.; Sumption, K.J.; Vosloo, W. Global Foot-and-Mouth Disease Research Update and Gap Analysis: 6-Immunology. Transbound. Emerg. Dis. 2016, 63, 56–62. [Google Scholar] [CrossRef]
- Shahriari, A.G.; Habibi-Pirkoohi, M. Developing Vaccines Against Foot-and-Mouth Disease: A Biotechnological Approach. Arch. Razi Inst. 2018, 73, 1–9. [Google Scholar]
- Sammin, D.J.; Paton, D.J.; Parida, S.; Ferris, N.P.; Hutchings, G.H.; Reid, S.M.; Shaw, A.E.; Holmes, C.; Gibson, D.; Corteyn, M.; et al. Evaluation of laboratory tests for SAT serotypes of foot-and-mouth disease virus with specimens collected from convalescent cattle in Zimbabwe. Vet. Rec. 2005, 160, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Brocchi, E.; Bergmann, I.E.; Dekker, A.; Paton, D.J.; Sammin, D.J.; Greiner, M.; Grazioli, S.; De Simone, F.; Yadin, H.; Haas, B.; et al. Comparative evaluation of six ELISAs for the detection of antibodies to the non-structural proteins of foot-and-mouth disease virus. Vaccine 2006, 24, 6966–6979. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.J.; de Clercq, K.; Greiner, M.; Dekker, A.; Brocchi, E.; Bergmann, I.; Sammin, S.; Gubbins, D.J.; Parida, S. Application of non-structural protein antibody tests in substantiating freedom from foot-and-mouth disease virus infection after emergency vaccination of cattle. Vaccine 2006, 24, 6503–6512. [Google Scholar] [CrossRef] [PubMed]
- Robiolo, B.; Seki, C.; Fondevilla, N.; Grigera, P.; Scodeller, E.; Periolo, O.; La Torre, J.; Mattion, N. Analysis of the immune response to FMDV structural and non-structural proteins in cattle in Argentina by the combined use of liquid phase and 3ABC-ELISA tests. Vaccine 2006, 24, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.; Mohapatra, J.K.; Pandey, L.K.; Mohanty, N.N.; Das, B.; Prusty, B.R.; Pattnaik, B. Mutational analysis of foot and mouth disease virus nonstructural polyprotein 3AB-coding region to design a negative marker virus. Virus Res. 2018, 243, 36–43. [Google Scholar] [CrossRef]
- Biswal, J.K.; Subramaniam, S.; Ranjan, R.; Sharma, G.K.; Misri, J.; Pattnaik, B. Marker vaccine potential of foot-and-mouth disease virus with large deletion in the non-structural proteins 3A and 3B. Biologicals 2015, 43, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Chinsangaram, J.; Beard, C.; Mason, P.W.; Zellner, M.K.; Ward, G.; Grubman, M.J. Antibody response in mice inoculated with DNA expressing foot-and-mouth disease virus capsid proteins. J. Virol. 1998, 72, 4454–4457. [Google Scholar]
- Mason, P.W.; Piccone, M.E.; McKenna, T.S.; Chinsangaram, J.; Grubman, M.J. Evaluation of a live-attenuated foot-and-mouth disease virus as a vaccine candidate. Virology 1997, 227, 96–102. [Google Scholar] [CrossRef]
- Uddowla, S.; Hollister, J.; Pacheco, J.M.; Rodriguez, L.L.; Rieder, E. A safe foot-and mouth disease vaccine platform with two negative markers for differentiating infected from vaccinated animals. J. Virol. 2012, 86, 11675–11685. [Google Scholar] [CrossRef]
- Li, P.; Lu, Z.; Bai, X.; Li, D.; Sun, P.; Bao, H.; Fu, Y.; Cao, Y.; Chen, Y.; Xie, B.; et al. Evaluation of a 3A-truncated foot-and-mouth disease virus in pigs for its potential as a marker vaccine. Vet. Res. 2014, 1, 45–51. [Google Scholar] [CrossRef]
- Biswal, J.K.; Bisht, P.; Subramaniam, S.; Ranjan, R.; Sharma, G.K.; Pattnaik, B. Engineering foot-and-mouth disease virus serotype O IND R2/1975 for one-step purification by immobilized metal affinity chromatography. Biologicals 2015, 43, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Bachrach, H.L. Foot-and-mouth disease. Annu. Rev. Microbiol. 1968, 22, 201–244. [Google Scholar] [CrossRef] [PubMed]
- Segundo, F.D.; Weiss, M.; Perez-Martin, E.; Dias, C.C.; Grubman, M.J.; Santos Tde, L. Inoculation of swine with foot-and-mouth disease SAP-mutant virus induces early protection against disease. J. Virol. 2012, 86, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- De los Santos, T.; Segundo, F.D.; Zhu, J.; Koster, M.; Dias, C.C.; Grubman, M.J. A conserved domain in the leader proteinase of foot-and-mouth disease virus is required for proper subcellular localization and function. J. Virol. 2009, 83, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Arzt, J.; Pacheco, J.M.; Smoliga, G.R.; Tucker, M.T.; Bishop, E.; Pauszek, S.J.; Hartwig, E.J.; de los Santos, T.; Rodriguez, L.L. Foot-and-mouth disease virus virulence in cattle is co-determined by viral replication dynamics and route of infection. Virology 2014, 452, 12–22. [Google Scholar] [CrossRef]
- Piccone, M.E.; Pacheco, J.M.; Pauszek, S.J.; Kramer, E.; Rieder, E.; Borca, M.V.; Rodriguez, L.L. The region between the two polyprotein initiation codons of foot-and-mouth disease virus is critical for virulence in cattle. Virology 2010, 396, 152–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Bai, X.; Li, P.; Zhang, M.; Bao, H.; Sun, P.; Lu, Z.; Cao, Y.; Chen, Y.; Li, D.; et al. Influence of Foot-and-Mouth Disease Virus O/CHN/Mya98/33-P Strain Leader Protein on Viral Replication and Host Innate Immunity. Viral Immunol. 2015, 28, 360–366. [Google Scholar] [CrossRef]
- Rodríguez Pulido, M.; Sobrino, F.; Borrego, B.; Sáiz, M. Attenuated foot-and-mouth disease virus RNA carrying a deletion in the 3′ noncoding region can elicit immunity in swine. J. Virol. 2009, 83, 3475–3485. [Google Scholar] [CrossRef]
- Burns, C.C.; Shaw, J.; Campagnoli, R.; Jorba, J.; Vincent, A.; Quay, J.; Kew, O. Modulation of poliovirus replicative fitness in HeLa cells by deoptimization of synonymous codon usage in the capsid region. J. Virol. 2006, 80, 3259–3272. [Google Scholar] [CrossRef]
- Mueller, S.; Papamichail, D.; Coleman, J.R.; Skiena, S.; Wimmer, E. Reduction of the rate of poliovirus protein synthesis through large-scale codon deoptimization causes attenuation of viral virulence by lowering specific infectivity. J. Virol. 2006, 80, 9687–9696. [Google Scholar] [CrossRef]
- Segundo, F.; Medina, G.N.; Ramirez-Medina, E.; Velazquez-Salinas, L.; Koster, M.; Grubman, M.J.; de los Santos, T. Synonymous Deoptimization of Foot-and-Mouth Disease Virus Causes Attenuation In Vivo while Inducing a Strong Neutralizing Antibody Response. J. Virol. 2015, 90, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Berinstein, A.; Tami, C.; Taboga, O.; Smitsaart, E.; Carrillo, E. Protective immunityagainst foot-and-mouth disease virus induced by a recombinant vaccinia virus. Vaccine 2000, 18, 2231–2238. [Google Scholar] [CrossRef]
- Zheng, M.; Jin, N.; Zhang, H.; Jin, M.; Lu, H.; Ma, M.; Li, C.; Yin, G.; Wang, R.; Liu, Q. Construction and immunogenicity of a recombinant fowlpox virus containing the capsid and 3C protease coding regions of foot-and-mouth disease virus. J. Virol. Methods 2006, 136, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, M.; Polacek, C.; Bøtner, A.; Belsham, G.J. Processing of the VP1/2A junction is not necessary for production of foot-and-mouth disease virus empty capsids and infectious viruses: Characterization of self-tagged particles. J. Virol. 2013, 87, 11591–11603. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, M.; Muszynski, B.; Organtini, L.J.; Ashley, R.E.; Hafenstein, S.L.; Belsham, G.J.; Polacek, C. Assembly and characterization of foot-and-mouth disease virus empty capsid particles expressed within mammalian cells. J. Gen. Virol. 2013, 94, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Qian, P.; Li, X.M.; Yu, X.L.; Chen, H.C. A recombinant pseudorabies virus co-expressing capsid proteins precursor P1-2A of FMDV and VP2 protein of porcine parvovirus: A trivalent vaccine candidate. Biotechnol. Lett. 2007, 29, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- De Avila Botton, S.; Brum, M.C.; Bautista, E.; Koster, M.; Weiblen, R.; Golde, W.T.; Grubman, M.J. Immunopotentiation of a foot-and-mouth disease virus subunit vaccine by interferon alpha. Vaccine 2006, 24, 3446–3456. [Google Scholar] [CrossRef] [PubMed]
- Mayr, G.A.; Chinsangaram, J.; Grubman, M.J. Development of replication-defective adenovirus serotype 5 containing the capsid and 3C protease coding regions of foot-and mouth disease virus as a vaccine candidate. Virology 1999, 263, 496–506. [Google Scholar] [CrossRef]
- Mayr, G.A.; O’Donnell, V.; Chinsangaram, J.; Mason, P.W.; Grubman, M.J. Immune responses and protection against foot-and-mouth disease virus (FMDV) challenge in swine vaccinated with adenovirus-FMDV constructs. Vaccine 2001, 19, 2152–2162. [Google Scholar] [CrossRef]
- Moraes, M.P.; Mayr, G.A.; Mason, P.W.; Grubman, M.J. Early protection against homologous challenge after a single dose of replication-defective human adenovirus type 5 expressing capsid proteins of foot-and-mouth disease virus (FMDV) strain A24. Vaccine 2002, 20, 1631–1639. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Brum, M.C.; Moraes, M.P.; Golde, W.T.; Grubman, M.J. Rapid protection of cattle from direct challenge with foot-and-mouth disease virus (FMDV) by a single inoculation with an adenovirus-vectored FMDV subunit vaccine. Virology 2005, 337, 205–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraes, M.P.; Segundo, F.D.; Dias, C.C.; Pena, L.; Grubman, M.J. Increased efficacy of an adenovirus-vectored foot-and-mouth disease capsid subunit vaccine expressing nonstructural protein 2B is associated with a specific T cell response. Vaccine 2011, 29, 9431–9440. [Google Scholar] [CrossRef] [PubMed]
- Pena, L.; Moraes, M.P.; Koster, M.; Burrage, T.; Pacheco, J.M.; Segundo, F.D.; Grubman, M.J. Delivery of a foot-and-mouth disease virus empty capsid subunit antigen with nonstructural protein 2B improves protection of swine. Vaccine 2008, 26, 5689–5699. [Google Scholar] [CrossRef] [PubMed]
- Medina, G.N.; Montiel, N.; Diaz-San Segundo, F.; Sturza, D.; Ramirez-Medina, E.; Grubman, M.J.; de Los Santos, T. Evaluation of a Fiber-Modified Adenovirus Vector Vaccine against Foot-and-Mouth Disease in Cattle. Clin. Vaccine Immunol. 2015, 23, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasa, B.P.; Mohapatra, J.K.; Pauszek, S.J.; Koster, M.; Dhanya, V.C.; Tamil Selvan, R.P.; Hosamani, M.; Saravanan, P.; Basagoudanavar, S.H.; de Los Santos, T.; et al. Recombinant human adenovirus-5 expressing capsid proteins of Indian vaccine strains of foot-and-mouth disease virus elicits effective antibody response in cattle. Vet. Microbiol. 2017, 203, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.A.; Saravanan, P.; Hosamani, M.; Basagoudanavar, S.H.; Sreenivasa, B.P.; Tamilselvan, R.P.; Venkataramanan, R. Novel immunogenic baculovirus expressed virus-like particles of foot-and-mouth disease (FMD) virus protect guinea pigs against challenge. Res. Vet. Sci. 2013, 95, 1217–1223. [Google Scholar] [CrossRef]
- Ruiz, V.; Mignaqui, A.C.; Nunez, M.C.; Reytor, E.; Escribano, J.M.; Wigdorovitz, A. Comparison of strategies for the production of FMDV empty capsids using the baculovirus vector system. Mol. Biotechnol. 2014, 56, 963–970. [Google Scholar] [CrossRef]
- Srinivas, M.V.; Basagoudanavar, S.H.; Tamil Selvan, R.P.; Hosamani, M. Modulating the level of expression of 3C protease in relation to capsid precursor (P1) of FMD virus enhances the yield of empty capsids. Int. J. Curr. Biotechnol. 2015, 3, 1–7. [Google Scholar]
- Ganji, V.K.; Biswal, J.K.; Lalzampuia, H.; Basagoudanavar, S.H.; Saravanan, P.; Tamil Selvan, R.P.; Umapathi, V.; Reddy, G.R.; Sanyal, A.; Dechamma, H.J. Mutation in the VP2 gene of P1-2A capsid protein increases the thermostability of virus-like particles of foot-and-mouth disease virus serotype O. Appl. Microbiol. Biotechnol. 2018, 102, 8883–8893. [Google Scholar] [CrossRef]
- Crisci, E.; Fraile, L.; Moreno, N.; Blanco, E.; Cabezón, R.; Costa, C.; Mussá, T.; Baratelli, M.; Martinez-Orellana, P.; Ganges, L.; et al. Chimeric calicivirus-like particles elicit specific immune responses in pigs. Vaccine 2012, 23, 2427–2439. [Google Scholar] [CrossRef]
- Li, Z.; Yi, Y.; Yin, X.; Zhang, Y.; Liu, M.; Liu, H.; Li, X.; Li, Y.; Zhang, Z.; Liu, J. Development of a foot-and-mouth disease virus serotype A empty capsid subunit vaccine using silkworm (Bombyx mori) pupae. PLoS ONE 2012, 7, e43849. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Saravanan, P.; Jalali, S.K. Expression and purification of virus like particles (VLPs) of foot-and-mouth disease virus in Eri silkworm (Samiacynthiaricini) larvae. Virus Dis. 2016, 27, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, H.Y.; Wang, Y.; Yin, B.; Lv, C.; Mo, X.; Yan, H.; Xuan, Y.; Huang, Y.; Pang, W.; et al. Large-scale production of foot-and-mouth disease virus (serotype Asia 1) VLP vaccine in Escherichia coli and protection potency evaluation in cattle. BMC Biotechnol. 2016, 16, 56. [Google Scholar] [CrossRef] [PubMed]
- Cedillo-Barron, L.; Foster-Cuevas, M.; Belsham, G.J.; Lefevre, F.; Parkhouse, R.M. Induction of a protective response in swine vaccinated with DNA encoding foot-and mouth disease virus empty capsid proteins and the 3D RNA polymerase. J. Gen. Virol. 2001, 82, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Borrego, B.; Argilaguet, J.M.; Pérez-Martín, E.; Dominguez, J.; Pérez-Filgueira, M.; Escribano, J.M.; Sobrino, F.; Rodriguez, F. A DNA vaccine encoding foot-and-mouth disease virus B and T-cell epitopes targeted to class II swine leukocyte antigens protects pigs against viral challenge. Antivir. Res. 2011, 92, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Ganges, L.; Borrego, B.; Fernández-Pacheco, P.; Revilla, C.; Fernández-Borges, N.; Domínguez, J.; Sobrino, F.; Rodriguez, F. DNA immunization of pigs with foot-and-mouth disease virus minigenes: From partial protection to disease exacerbation. Virus Res. 2011, 157, 121–125. [Google Scholar] [CrossRef] [PubMed]
- İz, S.G.; Döşkaya, M.; Borrego, B.; Rodriguez, F.; Gürüz, Y.; Gürhan, I.D. Co-expression of the Bcl-xL antiapoptotic protein enhances the induction of Th1-like immune responses in mice immunized with DNA vaccines encoding FMDV B and T cell epitopes. Vet. Res. Commun. 2013, 37, 187–196. [Google Scholar]
- Kotla, S.; Vishanath, B.S.; Dechamma, H.J.; Ganesh, K.; Suryanarayana, V.S.S.; Reddy, G.R. DNA vaccine (P1-2A-3C-pCDNA) co-administered with Bovine IL-18 gives protective immune response against Foot and Mouth Disease in cattle. Vet. Microbiol. 2016, 193, 106–115. [Google Scholar] [CrossRef]
- Dar, P.A.; Suryanaryana, V.S.; Nagarajan, G.; Reddy, G.R.; Dechamma, H.J.; Kondabattula, G. DNA prime-protein boost strategy with replicase-based DNA vaccine against foot-and-mouth disease in bovine calves. Vet. Microbiol. 2013, 163, 62–70. [Google Scholar] [CrossRef]
- Wong, H.T.; Cheng, S.C.; Chan, E.W.; Sheng, Z.T.; Yan, W.Y.; Zheng, Z.X.; Xie, Y. Plasmids encoding foot-and-mouth disease virus VP1 epitopes elicited immune responses in mice and swine and protected swine against viral infection. Virology 2000, 278, 27–35. [Google Scholar] [CrossRef]
- Shao, J.J.; Wang, J.F.; Chang, H.Y.; Liu, J.X. Immune potential of a novel multiple epitope vaccine to FMDV type Asia 1 in guinea pigs and sheep. Virol. Sin. 2011, 26, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.L.; Barrera, J.; Kramer, E.; Lubroth, J.; Brown, F.; Golde, W.T. A synthetic peptide containing the consensus sequence of the G-H loop region of foot-and-mouth disease virus type-O VP1 and a promiscuous T-helper epitope induces peptide-specific antibodies but fails to protect cattle against viral challenge. Vaccine 2003, 21, 3751–3756. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, L.; Ding, Y.; Zhou, P.; Lv, J.; Chen, H.; Fang, Y.; Liu, X.; Chang, H.; Zhang, J.; et al. Efficacy of synthetic peptide candidate vaccines against serotype-A foot-and-mouth disease virus in cattle. Appl. Microbiol. Biotechnol. 2015, 99, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Cubillos, C.; de la Torre, B.G.; Jakab, A.; Clementi, G.; Borrás, E.; Bárcena, J.; Andreu, D.; Sobrino, F.; Blanco, E. Enhanced mucosal immunoglobulin A response and solid protection against foot-and-mouth disease virus challenge induced by a novel dendrimeric peptide. J. Virol. 2008, 82, 7223–7230. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Guerra, B.; de la Torre, B.G.; Defaus, S.; Dekker, A.; Andreu, D.; Sobrino, F. Full protection of swine against foot-and-mouth disease by a bivalent B-cell epitope dendrimer peptide. Antivir. Res. 2016, 129, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, A.G.; Bagheri, A.; Bassami, M.R.; Malekzadeh-Shafaroudi, S.; Afsharifar, A.; Niazi, A. Expression of Hemagglutinin—Neuraminidase and fusion epitopes of Newcastle Disease Virus in transgenic tobacco. Electron. J. Biotechnol. 2016, 22, 38–43. [Google Scholar] [CrossRef]
- Wigdorovitz, A.; Carrillo, C.; Dus Santos, M.J.; Trono, K.; Peralta, A.; Gómez, M.C.; Ríos, R.D.; Franzone, P.M.; Sadir, A.M.; Escribano, J.M.; et al. Induction of a protective antibody response to foot and mouth disease virus in mice following oral or parenteral immunization with alfalfa transgenic plants expressing the viral structural protein VP1. Virology 1999, 255, 347–353. [Google Scholar] [CrossRef]
- Dus Santos, M.J.; Wigdorovitz, A.; Trono, K.; Rios, R.D.; Franzone, P.M.; Gil, F.; Moreno, J.; Carrillo, C.; Escribano, J.M.; Borca, M.V. A novel methodology to develop a foot and mouth disease virus (FMDV) peptide-based vaccine in transgenic plants. Vaccine 2002, 20, 1141–1147. [Google Scholar] [CrossRef]
- Li, Z.; Yi, Y.; Yin, X.; Zhang, Z.; Liu, J. Expression of Foot-and-Mouth Disease Virus Capsid Proteins in Silkworm-Baculovirus Expression System and Its Utilization as a Subunit Vaccine. PLoS ONE 2008, 3, e2273. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Zheng, Q.; Hou, L.; Du, L.; Zhang, Y.; Qiao, X.; Hou, J.; Huang, K. The immunopotentiator CVC1302 enhances immune efficacy and protective ability of foot-and-mouth disease virus vaccine in pigs. Vaccine 2018, 36, 7929–7935. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, Z.; Li, Y.; Sun, P.; Li, D.; Li, P.; Bai, X.; Fu, Y.; Bao, H.; Zhou, C.; et al. Poly(I:C) combined with multi-epitope protein vaccine completely protects against virulent foot-and-mouth disease virus challenge in pigs. Antivir. Res. 2013, 97, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Terhuja, M.; Saravanan, P.; Tamilselvan, R.P. Comparative efficacy of virus like particle (VLP) vaccine of foot-and-mouth-disease virus (FMDV) type O adjuvanted with poly I:C or CpG in guinea pigs. Biologicals 2015, 43, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Sreenivasa, B.P.; Selvan, R.P.; Basagoudanavar, S.H.; Hosamani, M.; Reddy, N.D.; Nathanielsz, J.; Derozier, C.; Venkataramanan, R. Protective immune response to liposome adjuvanted high potency foot-and-mouth disease vaccine in Indian cattle. Vaccine 2015, 33, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhao, X.; Yan, W.; Wang, W.; Zuo, X.; Huang, K.; Liu, Y.; Chen, J.; Wang, J.; Cong, W.; et al. Alpha interferon is a powerful adjuvant for a recombinant protein vaccine against foot-and-mouth disease virus in swine, and an effective stimulus of in vivo immune response. Vaccine 2007, 25, 5199–5208. [Google Scholar] [CrossRef] [PubMed]
- Dias, C.C.; Moraes, M.P.; Weiss, M.; Diaz-San Segundo, F.; Perez-Martin, E.; Salazar, A.M.; de los Santos, T.; Grubman, M.J. Novel antiviral therapeutics to control foot-and-mouth disease. J. Interferon Cytokine Res. 2012, 32, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Borrego, B.; Blanco, E.; Rodríguez Pulido, M.; Mateos, F.; Lorenzo, G.; Cardillo, S.; Smitsaart, E.; Sobrino, F.; Sáiz, M. Combined administration of synthetic RNA and a conventional vaccine improves immune responses and protection against foot-and-mouth disease virus in swine. Antivir. Res. 2017, 142, 30–36. [Google Scholar] [CrossRef]
- Borrego, B.; Rodríguez-Pulido, M.; Mateos, F.; de la Losa, N.; Sobrino, F.; Sáiz, M. Delivery of synthetic RNA can enhance the immunogenicity of vaccines against foot-and-mouth disease virus (FMDV) in mice. Vaccine 2013, 31, 4375–4381. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.M.; Park, J.H.; Lee, K.N.; Kim, S.K.; You, S.H.; Kim, T.; Tark, D.; Lee, H.S.; Seo, M.G.; Kim, B. Robust Protection against Highly Virulent Foot-and-Mouth Disease Virus in Swine by Combination Treatment with Recombinant Adenoviruses Expressing Porcine Alpha and Gamma Interferons and Multiple Small Interfering RNAs. J. Virol. 2015, 89, 8267–8279. [Google Scholar] [CrossRef] [Green Version]
- You, S.H.; Kim, T.; Choi, J.H.; Park, G.; Lee, K.N.; Kim, B.; Lee, M.H.; Kim, H.S.; Kim, S.M.; Park, J.H. Coinjection of a vaccine and anti-viral agents can provide fast-acting protection from foot-and-mouth disease. Antivir. Res. 2017, 143, 195–204. [Google Scholar] [CrossRef]
- Rodríguez Pulido, M.; Del Amo, L.; Sobrino, F.; Sáiz, M. Synthetic RNA derived from the foot-and-mouth disease virus genome elicits antiviral responses in bovine and porcine cells through IRF3 activation. Vet. Microbiol. 2018, 221, 8–12. [Google Scholar] [CrossRef]
- Quattrocchi, V.; Molinari, P.; Langellotti, C.; Gnazzo, V.; Taboga, O.; Zamorano, P. Co-inoculation of baculovirus and FMDV vaccine in mice, elicits very early protection against foot and mouth disease virus without interfering with long lasting immunity. Vaccine 2013, 31, 2713–2718. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sharma, R.; Chhabra, R. Interleukin-2 potentiates foot-and-mouth disease vaccinal immune responses in mice. Vaccine 2005, 23, 3005–3009. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, V.; John, L.; Bharatiraja, S.; Dechamma, H.J.; Reddy, G.R. Adjuvantation of inactivated Foot and Mouth Disease Virus vaccine with IL-15 expressing plasmid improves the immune response in Guinea Pigs. Biologicals 2017, 49, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Brito, B.P.; Mohapatra, J.K.; Subramaniam, S.; Pattnaik, B.; Rodriguez, L.L.; Moore, B.R.; Perez, A.M. Dynamics of widespread foot-and-mouth disease virus serotypes A, O and Asia 1 in southern Asia: A Bayesian phylogenetic perspective. Transbound. Emerg. Dis. 2018, 65, 696–710. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Mohapatra, J.K.; Sharma, G.K.; Biswal, J.K.; Ranjan, R.; Rout, M.; Das, B.; Dash, B.B.; Sanyal, A.; Pattnaik, B. Evolutionary dynamics of foot-and-mouth disease virus O/ME-SA/Ind2001 lineage. Vet. Microbiol. 2015, 178, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, J.K.; Das, B.; Rout, M.; Sreenivasa, B.P.; Subramaniam, S.; Sanyal, A.; Pattnaik, B. Alternate vaccine strain selection in the wake of emerging foot-and-mouth disease virus serotype A antigenic variants in India. Vaccine 2018, 36, 3191–3194. [Google Scholar] [CrossRef] [PubMed]
- Rudreshappa, A.G.; Sanyal, A.; Mohapatra, J.K.; Subramaniam, S.; De, A.; Das, B.; Singanallur, N.B.; Jangam, A.K.; Muthukrishnan, M.; Villuppanoor, S.A.; et al. Emergence of antigenic variants with in serotype A foot and mouth disease virus in India and evaluation of a new vaccine candidate panel. Vet. Microbiol. 2012, 158, 405–409. [Google Scholar] [CrossRef]
- Das, B.; Mohapatra, J.K.; Pande, V.; Subramaniam, S.; Sanyal, A. Evolution of foot-and-mouth disease virus serotype A capsid coding (P1) region on a timescale of three decades in an endemic context. Infect. Genet. Evol. 2016, 41, 36–46. [Google Scholar] [CrossRef]
- Subramaniam, S.; Mohapatra, J.K.; Sharma, G.K.; Das, B.; Dash, B.B.; Sanyal, A.; Pattnaik, B. Phylogeny and genetic diversity of foot and mouth disease virus serotype Asia 1 in India during 1964–2012. Vet. Microbiol. 2013, 167, 280–288. [Google Scholar] [CrossRef]
- Sanyal, A.; Subramaniam, S.; Mohapatra, J.K.; Tamilselvan, R.P.; Singh, N.K.; Hemadri, D.; Pattnaik, B. Phylogenetic analysis of Indian serotype Asia 1 foot-and-mouth-disease virus isolates revealed emergence and reemergence of different genetic lineages. Vet. Microbiol. 2010, 144, 198–202. [Google Scholar] [CrossRef]
- Ullah, H.; Siddique, M.A.; Amin, M.A.; Das, B.C.; Sultana, M.; Hossain, M.A. Reemergence of circulatory foot-and-mouth disease virus serotypes Asia 1 in Bangladesh and VP1 protein heterogeneity with vaccine strain IND 63/72. Lett. Appl. Microbiol. 2015, 60, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.K.; Mahajan, S.; Matura, R.; Subramaniam, S.; Ranjan, R.; Biswal, J.; Rout, M.; Mohapatra, J.K.; Dash, B.B.; Sanyal, A.; et al. Diagnostic assays developed for the control of foot-and-mouth disease in India. World J. Virol. 2015, 4, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, J.K.; Pandey, L.K.; Sanyal, A.; Pattnaik, B. Recombinant non-structural polyprotein 3AB-based serodiagnostic strategy for FMD surveillance in bovines irrespective of vaccination. J. Virol. Methods 2011, 177, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Kitching, P.; Hammond, J.; Jeggo, M.; Charleston, B.; Paton, D.; Rodriguez, L.; Heckert, R. Global FMD control-Is it an option. Vaccine 2007, 25, 5660–5664. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, T.J.; Bulut, A.N.; Gubbins, S.; Stärk, K.D.; Pfeiffer, D.U.; Sumption, K.J.; Paton, D.J. Retrospective evaluation of foot-and-mouth disease vaccine effectiveness in Turkey. Vaccine 2014, 32, 1848–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Los Santos, T.; Diaz-San Segundo, F.; Rodriguez, L.L. The need for improved vaccines against foot-and-mouth disease. Curr. Opin. Virol. 2018, 29, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Rweyemamu, M.M.; Booth, J.C.; Head, M.; Pay, T.W.F. Microneutralization tests for serological typing and subtyping of foot-and-mouth disease virus strains. J. Hyg. (Lond.) 1978, 81, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Doel, T.R. FMD vaccines. Virus Res. 2003, 91, 81–99. [Google Scholar] [CrossRef]
- World Organization for Animal Health (OIE). Foot and Mouth Disease. In Terrestrial Animal Health Code, 22nd ed.; World Organisation for Animal Health (OIE) 12: Paris, France, 2013; Volume II, pp. 414–434. [Google Scholar]
- Pega, J.; Bucafusco, D.; Di Giacomo, S.; Schammas, J.M.; Malacari, D.; Capozzo, A.V.; Arzt, J.; Pérez-Beascoechea, C.; Maradei, E.; Rodríguez, L.L.; et al. Early adaptive immune responses in the respiratory tract of foot-and-mouth disease virus-infected cattle. J. Virol. 2013, 87, 2489–2495. [Google Scholar] [CrossRef]
- Pega, J.; Di Giacomo, S.; Bucafusco, D.; Schammas, J.M.; Malacari, D.; Barrionuevo, F.; Capozzo, A.V.; Rodríguez, L.L.; Borca, M.V.; Pérez-Filgueira, M. Systemic Foot-and-Mouth Disease Vaccination in Cattle Promotes Specific Antibody-Secreting Cells at the Respiratory Tract and Triggers Local Anamnestic Responses upon Aerosol Infection. J. Virol. 2015, 89, 9581–9590. [Google Scholar] [CrossRef] [Green Version]
- Lannes, N.; Python, S.; Summerfield, A. Interplay of foot-and-mouth disease virus, antibodies and plasmacytoid dendritic cells: Virus opsonization under non-neutralizing conditions results in enhanced interferon-alpha responses. Vet. Res. 2012, 43, 64. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Windsor, M.; McLaughlin, K.; Hope, J.; Jackson, T.; Charleston, B. Foot-and-mouth disease virus exhibits an altered tropism in the presence of specific immunoglobulins, enabling productive infection and killing of dendritic cells. J. Virol. 2011, 85, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Langellotti, C.; Quattrocchi, V.; Alvarez, C.; Ostrowski, M.; Gnazzo, V.; Zamorano, P.; Vermeulen, M. Foot-and-mouth disease virus causes a decrease in spleen dendritic cells and the early release of IFN-α in the plasma of mice. Differences between infectious and inactivated virus. Antivir. Res. 2012, 94, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Guzman, E.; Taylor, G.; Charleston, B.; Skinner, M.A.; Ellis, S.A. An MHC-restricted CD8+ T-cell response is induced in cattle by foot-and-mouth disease virus (FMDV) infection and also following vaccination with inactivated FMDV. J. Gen. Virol. 2008, 89, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Juleff, N.; Windsor, M.; Lefevre, E.A.; Gubbins, S.; Hamblin, P.; Reid, E.; McLaughlin, K.; Beverley, P.C.; Morrison, I.W.; Charleston, B. Foot-and-mouth disease virus can induce a specific and rapid CD4+ T-cell-independent neutralizing and isotype class-switched antibody response in naïve cattle. J. Virol. 2009, 83, 3626–3636. [Google Scholar] [CrossRef] [PubMed]
- Patch, J.R.; Kenney, M.; Pacheco, J.M.; Grubman, M.J.; Golde, W.T. Characterization of cytotoxic T lymphocyte function after foot-and-mouth disease virus infection and vaccination. Viral Immunol. 2013, 26, 239–429. [Google Scholar] [CrossRef]
- Knight-Jones, T.J.; Bulut, A.N.; Gubbins, S.; Stärk, K.D.; Pfeiffer, D.U.; Sumption, K.J.; Paton, D.J. Randomised field trial to evaluate serological response after foot-and-mouth disease vaccination in Turkey. Vaccine 2015, 33, 805–811. [Google Scholar] [CrossRef]
- Doel, T.R. Natural and vaccine-induced immunity to foot and mouth disease: The prospects for improved vaccines. Rev. Sci. Tech. Off. Int. Epiz. 1996, 15, 883–911. [Google Scholar] [CrossRef]
- León, E.A.; Perez, A.M.; Stevenson, M.A.; Robiolo, B.; Mattion, N.; Seki, C.; La Torre, J.; Torres, A.; Cosentino, B.; Duffy, S.J. Effectiveness of systematic foot and mouth disease mass vaccination campaigns in Argentina. Rev. Sci. Tech. 2014, 33, 917–926. [Google Scholar] [CrossRef]
- Leforban, Y.; Gerbier, G. Review of the status of foot and mouth disease and approach to control/eradication in Europe and Central Asia. Rev. Sci. Tech. 2002, 21, 477–492. [Google Scholar] [CrossRef]
- Naranjo, J.; Cosivi, O. Elimination of foot-and-mouth disease in South America: Lessons and challenges. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120381. [Google Scholar] [CrossRef] [PubMed]
- Nyaguthii, D.M.; Armson, B.; Kitala, P.M.; Sanz-Bernardo, B.; Di Nardo, A.; Lyons, N.A. Knowledge and risk factors for foot-and-mouth disease among small-scale dairy farmers in an endemic setting. Vet. Res. 2019, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- McVey, D.S.; Shi, J. Vaccination Strategies for Emerging Disease Epidemics of Livestock. Vet. Clin. Food Anim. Pract. 2010, 26, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.; Eames, K.; Heymann, D.L. “Herd immunity”: A rough guide. Clin. Infect. Dis. 2011, 52, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Infectious Diseases of Humans: Dynamics and Control; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Heesterbeek, J.A.P. A brief history of Ro and a recipe for its calculation. Acta Biotheor. 2002, 50, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, T.J.; Gubbins, S.; Bulut, A.N.; Stärk, K.D.; Pfeiffer, D.U.; Sumption, K.J.; Paton, D.J. Mass vaccination, immunity and coverage: Modelling population protection against foot-and-mouth disease in Turkish cattle. Sci. Rep. 2016, 26, 22121. [Google Scholar] [CrossRef] [PubMed]
- Dekker, A.; Eblé, P.; Stockhofe, N.; Chénard, G. Intratypic heterologous vaccination of calves can induce an antibody response in presence of maternal antibodies against foot-and-mouth disease virus. BMC Vet. Res. 2014, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Bucafusco, D.; Giacomo, S.D.; Pega, J.; Juncos, M.S.; Schammas, J.M.; Perez-Filgueira, M.; Capozzo, A.V. Influence of antibodies transferred by colostrum in the immune responses of calves to current foot-and-mouth disease vaccines. Vaccine 2014, 32, 6576–6582. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.K.; Sajjanar, C.M.; Natarajan, C.; Bayry, J. Neutralizing antibody responses to foot-and-mouth disease quadrivalent (type O, A, C and Asia 1) vaccines in growing calves with pre-existing maternal antibodies. Vet. Microbiol. 2014, 169, 233–235. [Google Scholar] [CrossRef]
- Wesley, R.D.; Lager, K.M. Overcoming maternal antibody interference by vaccination with human adenovirus 5 recombinant viruses expressing the hemagglutinin and the nucleoprotein of swine influenza virus. Vet. Microbiol. 2006, 118, 67–75. [Google Scholar] [CrossRef]
- OIE. Foot-and-Mouth Disease. In OIE Terrestrial Manual; OIE (World Organization for Animal Health): Paris, France, 2012; Chapter 2.1.5. [Google Scholar]
- Stenfeldt, C.; Heegaard, P.M.; Stockmarr, A.; Tjornehoj, K.; Belsham, G.J. Analysis of the acute phase responses of serum amyloid a, haptoglobin and type 1 interferon in cattle experimentally infected with foot-and-mouth disease virus serotype O. Vet. Res. 2011, 42, 66. [Google Scholar] [CrossRef] [PubMed]
- Eschbaumer, M.; Stenfeldt, C.; Smoliga, G.R.; Pacheco, J.M.; Rodriguez, L.L.; Li, R.W.; Zhu, J.; Arzt, J. Transcriptomic Analysis of Persistent Infection with Foot-and-Mouth Disease Virus in Cattle Suggests Impairment of Apoptosis and Cell-Mediated Immunity in the Nasopharynx. PLoS ONE 2016, 11, e0162750. [Google Scholar] [CrossRef] [PubMed]
- Hayer, S.S.; Ranjan, R.; Biswal, J.K.; Subramaniam, S.; Mohapatra, J.K.; Sharma, G.K.; Rout, M.; Dash, B.B.; Das, B.; Prusty, B.R.; et al. Quantitative characteristics of the foot-and-mouth disease carrier state under natural conditions in India. Transbound. Emerg. Dis. 2018, 65, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Cortey, M.; Ferretti, L.; Pérez-Martín, E.; Zhang, F.; de Klerk-Lorist, L.M.; Scott, K.; Freimanis, G.; Seago, J.; Ribeca, P.; van Schalkwyk, L.; et al. Persistent infection of African buffalo (Synceruscaffer) with Foot-and-Mouth Disease Virus: Limited viral evolution and no evidence of antibody neutralization escape. J. Virol. 2019, 93, 00563-19. [Google Scholar] [CrossRef] [PubMed]
- Moonen, P.; Schrijver, R. Carriers of foot-and-mouth disease virus: A review. Vet. Q. 2000, 22, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Salt, J.S. Persistence of Foot-and-Mouth Disease. In Foot and Mouth Disease: Current Perspectives; Sobrino, F., Domingo, E., Eds.; Horizon Bioscience: Norfolk, UK, 2004; pp. 103–143. [Google Scholar]
- Davies, G. The foot-and-mouth disease (FMD) epidemic in the United Kingdom 2001. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 331–343. [Google Scholar] [CrossRef]
- Miguel, E.; Grosbois, V.; Caron, A.; Boulinier, T.; Fritz, H.; Cornélis, D.; Foggin, C.; Makaya Pious, V.; Tshabalala Priscillia, T.; de Garine-Wichatitsky, M. Contacts and foot and mouth disease transmission from wild to domestic bovines in Africa. Ecosphere 2013, 4, 51. [Google Scholar] [CrossRef]
- Hemadri, D.; Sanyal, A.; Tosh, C.; Rasool, T.J.; Bhattacharya, S.; Pan, T.S.; Chattaopadhyay, A.P.; Bandyopadhyay, A.G.; Chakravarthy, J.L.; Negi, A.B.; et al. FMD in the Andaman and Nicobar Islands. Vet. Rec. 2006, 158, 347–348. [Google Scholar] [CrossRef]
- Parthiban, A.B.R.; Mahapatra, M.; Gubbins, S.; Parida, S. Virus Excretion from Foot-And-Mouth Disease Virus Carrier Cattle and Their Potential Role in Causing New Outbreaks. PLoS ONE 2015, 10, e0128815. [Google Scholar] [CrossRef]
- Alexandersen, S.; Zhang, Z.; Reid, S.M.; Hutchings, G.H.; Donaldson, A.I. Quantities of infectious virus and viral RNA recovered from sheep and cattle experimentally infected with foot-and-mouth disease virus O UK 2001. J. Gen. Virol. 2002, 83, 1915–1923. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Brito, B.; Hartwig, E.; Smoliga, G.R.; Perez, A.; Arzt, J.; Rodriguez, L.L. Early Detection of Foot-And-Mouth Disease Virus from Infected Cattle Using A Dry Filter Air Sampling System. Transbound. Emerg. Dis. 2017, 64, 564–573. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.K.; Sharma, G.K.; Mahajan, S.; Dhama, K.; Basagoudanavar, S.H.; Hosamani, M.; Sreenivasa, B.P.; Chaicumpa, W.; Gupta, V.K.; Sanyal, A. Foot-and-Mouth Disease Virus: Immunobiology, Advances in Vaccines and Vaccination Strategies Addressing Vaccine Failures—An Indian Perspective. Vaccines 2019, 7, 90. https://doi.org/10.3390/vaccines7030090
Singh RK, Sharma GK, Mahajan S, Dhama K, Basagoudanavar SH, Hosamani M, Sreenivasa BP, Chaicumpa W, Gupta VK, Sanyal A. Foot-and-Mouth Disease Virus: Immunobiology, Advances in Vaccines and Vaccination Strategies Addressing Vaccine Failures—An Indian Perspective. Vaccines. 2019; 7(3):90. https://doi.org/10.3390/vaccines7030090
Chicago/Turabian StyleSingh, Raj Kumar, Gaurav Kumar Sharma, Sonalika Mahajan, Kuldeep Dhama, Suresh H. Basagoudanavar, Madhusudan Hosamani, B P Sreenivasa, Wanpen Chaicumpa, Vivek Kumar Gupta, and Aniket Sanyal. 2019. "Foot-and-Mouth Disease Virus: Immunobiology, Advances in Vaccines and Vaccination Strategies Addressing Vaccine Failures—An Indian Perspective" Vaccines 7, no. 3: 90. https://doi.org/10.3390/vaccines7030090
APA StyleSingh, R. K., Sharma, G. K., Mahajan, S., Dhama, K., Basagoudanavar, S. H., Hosamani, M., Sreenivasa, B. P., Chaicumpa, W., Gupta, V. K., & Sanyal, A. (2019). Foot-and-Mouth Disease Virus: Immunobiology, Advances in Vaccines and Vaccination Strategies Addressing Vaccine Failures—An Indian Perspective. Vaccines, 7(3), 90. https://doi.org/10.3390/vaccines7030090





