Adjuvant Strategies for Lactic Acid Bacterial Mucosal Vaccines
Abstract
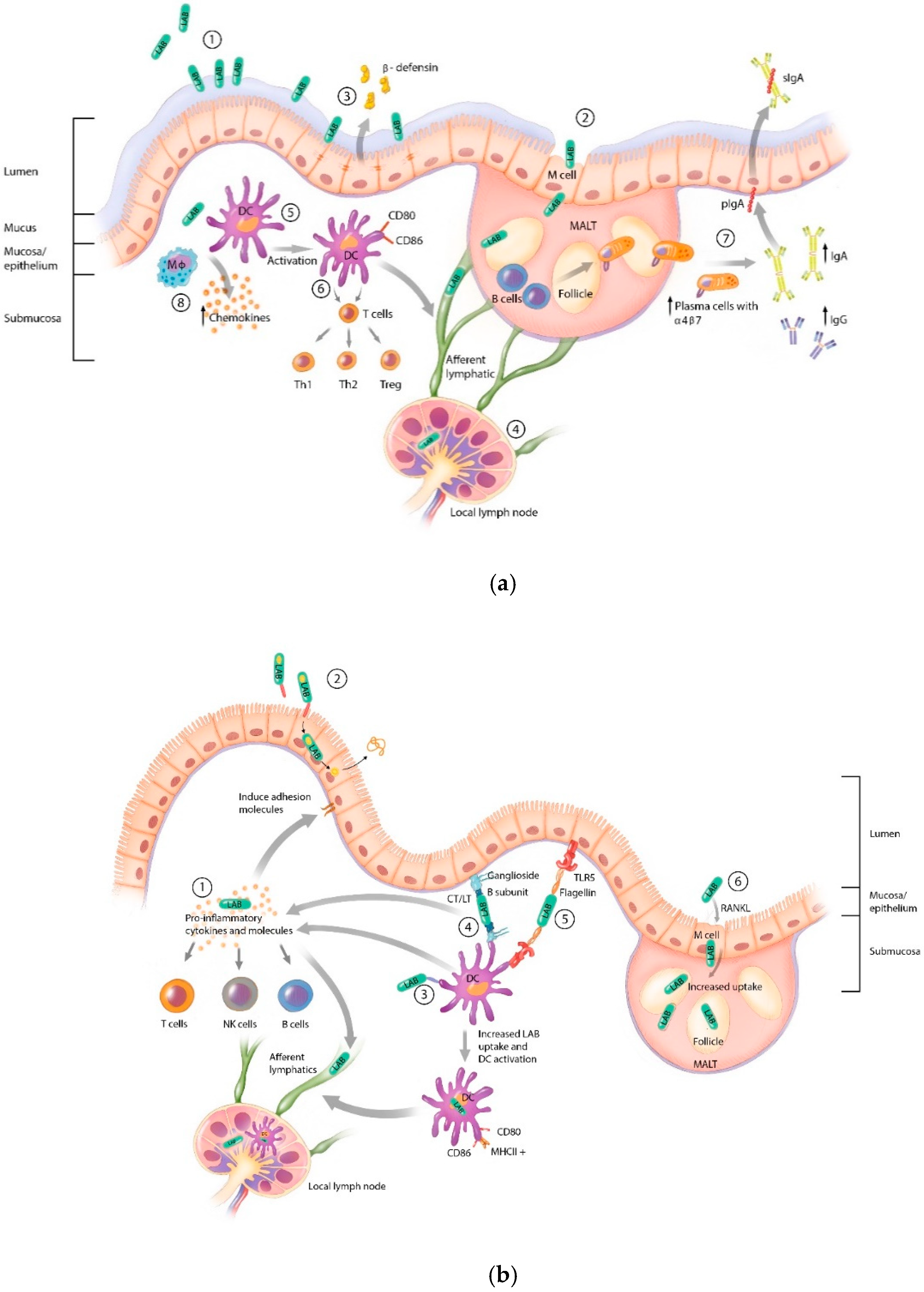
:1. Introduction
2. Lactic Acid Bacteria Mechanisms of Immune Interaction and Activation
3. Mucosal Vaccine Adjuvant Strategies
4. Lactic Acid Bacteria Adjuvant Strategies
4.1. Cytokine Secretion (Table 1)
4.1.1. IL-12
4.1.2. IL-1β
4.1.3. IL-2
4.2. Dendritic Cell (DC) Targeting Adjuvants (Table 2)
4.3. Secretion of Bacterial Toxins (Table 3)
4.4. Bacterial Derived Adjuvants (Table 4)
4.4.1. Toll-like Receptor (TLR) 5 Ligand
4.4.2. Enterocyte Cell Targeting
4.4.3. Additional Bacterial Derived Adjuvants
4.5. Other Adjuvant Strategies
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Markowiak, P.; Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Passaro, G.; Gasbarrini, A.; Landolfi, R.; Montalto, M. Modulation of microbiota as treatment for intestinal inflammatory disorders: An uptodate. World J. Gastroenterol. 2016, 22, 7186–7202. [Google Scholar] [CrossRef] [PubMed]
- Boirivant, M.; Strober, W. The mechanism of action of probiotics. Curr. Opin. Gastroenterol. 2007, 23, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Czerkinsky, C. Mucosal immunity and vaccines. Nat. Med. 2005, 11, S45–S53. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Jang, Y.S. The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants. Clin. Exp. Vaccine Res. 2017, 6, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. The influence of the intestinal microbiome on vaccine responses. Vaccine 2018, 36, 4433–4439. [Google Scholar] [CrossRef]
- Boyaka, P.N. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The influence of probiotics on vaccine responses—A systematic review. Vaccine 2018, 36, 207–213. [Google Scholar] [CrossRef]
- Wells, J.M.; Mercenier, A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 2008, 6, 349–362. [Google Scholar] [CrossRef]
- LeCureux, J.S.; Dean, G.A. Lactobacillus Mucosal Vaccine Vectors: Immune Responses against Bacterial and Viral Antigens. mSphere 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Mendoza, S.; Angulo, C.; Meza, B. Food-Grade Organisms as Vaccine Biofactories and Oral Delivery Vehicles. Trends Biotechnol. 2016, 34, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Siber, G.R. Adjuvants for human vaccines--current status, problems and future prospects. Vaccine 1995, 13, 1263–1276. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccin Immunother. 2018, 14, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef]
- Smits, H.H.; Engering, A.; van der Kleij, D.; de Jong, E.C.; Schipper, K.; van Capel, T.M.; Zaat, B.A.; Yazdanbakhsh, M.; Wierenga, E.A.; van Kooyk, Y.; et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 2005, 115, 1260–1267. [Google Scholar] [CrossRef]
- Konstantinov, S.R.; Smidt, H.; de Vos, W.M.; Bruijns, S.C.; Singh, S.K.; Valence, F.; Molle, D.; Lortal, S.; Altermann, E.; Klaenhammer, T.R.; et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. USA 2008, 105, 19474–19479. [Google Scholar] [CrossRef]
- Kawashima, T.; Ikari, N.; Watanabe, Y.; Kubota, Y.; Yoshio, S.; Kanto, T.; Motohashi, S.; Shimojo, N.; Tsuji, N.M. Double-Stranded RNA Derived from Lactic Acid Bacteria Augments Th1 Immunity via Interferon-beta from Human Dendritic Cells. Front. Immunol. 2018, 9, 27. [Google Scholar] [CrossRef]
- Ren, Y.; Pan, H.; Pan, B.; Bu, W. Identification and functional characterization of three TLR signaling pathway genes in Cyclina sinensis. Fish Shellfish Immunol. 2016, 50, 150–159. [Google Scholar] [CrossRef]
- Jounai, K.; Ikado, K.; Sugimura, T.; Ano, Y.; Braun, J.; Fujiwara, D. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS ONE 2012, 7, e32588. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.R.; Frokiaer, H.; Pestka, J.J. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 2002, 168, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.L.; Lammers, K.; Brigidi, P.; Vitali, B.; Rizzello, F.; Gionchetti, P.; Campieri, M.; Kamm, M.A.; Knight, S.C.; Stagg, A.J. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 2004, 53, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, S.; Kanaya, T.; Fukuda, S.; Nakato, G.; Hanazato, M.; Wu, X.R.; Yamamoto, N.; Ohno, H. Uromodulin-SlpA binding dictates Lactobacillus acidophilus uptake by intestinal epithelial M cells. Int. Immunol. 2017, 29, 357–363. [Google Scholar] [CrossRef]
- Mercier-Bonin, M.; Chapot-Chartier, M.P. Surface Proteins of Lactococcus lactis: Bacterial Resources for Muco-adhesion in the Gastrointestinal Tract. Front. Microbiol. 2017, 8, 2247. [Google Scholar] [CrossRef]
- Otte, J.M.; Podolsky, D.K. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G613–G626. [Google Scholar] [CrossRef] [Green Version]
- Schlee, M.; Harder, J.; Koten, B.; Stange, E.F.; Wehkamp, J.; Fellermann, K. Probiotic lactobacilli and VSL#3 induce enterocyte beta-defensin 2. Clin. Exp. Immunol. 2008, 151, 528–535. [Google Scholar] [CrossRef]
- Perdigon, G.; Maldonado Galdeano, C.; Valdez, J.C.; Medici, M. Interaction of lactic acid bacteria with the gut immune system. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 4), S21–S26. [Google Scholar] [CrossRef] [Green Version]
- Yam, K.K.; Pouliot, P.; N’Diaye, M.M.; Fournier, S.; Olivier, M.; Cousineau, B. Innate inflammatory responses to the Gram-positive bacterium Lactococcus lactis. Vaccine 2008, 26, 2689–2699. [Google Scholar] [CrossRef]
- Bermudez-Humaran, L.G.; Cortes-Perez, N.G.; Lefevre, F.; Guimaraes, V.; Rabot, S.; Alcocer-Gonzalez, J.M.; Gratadoux, J.J.; Rodriguez-Padilla, C.; Tamez-Guerra, R.S.; Corthier, G.; et al. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J. Immunol. 2005, 175, 7297–7302. [Google Scholar] [CrossRef] [PubMed]
- Kalina, W.V.; Mohamadzadeh, M. Lactobacilli as natural enhancer of cellular immune response. Discov. Med. 2005, 5, 199–203. [Google Scholar] [PubMed]
- Bermudez-Humaran, L.G. Lactococcus lactis as a live vector for mucosal delivery of therapeutic proteins. Hum. Vaccines 2009, 5, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Humaran, L.G.; Kharrat, P.; Chatel, J.M.; Langella, P. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microb. Cell Fact. 2011, 10 (Suppl. 1), S4. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.H.; Lee, S.E.; Kim, S.Y. Mucosal vaccine adjuvants update. Clin. Exp. Vaccine Res. 2012, 1, 50–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freytag, L.C.; Clements, J.D. Mucosal adjuvants. Vaccine 2005, 23, 1804–1813. [Google Scholar] [CrossRef]
- Jiang, B.; Li, Z.; Ou, B.; Duan, Q.; Zhu, G. Targeting ideal oral vaccine vectors based on probiotics: A systematical view. Appl. Microbiol. Biotechnol. 2019, 103, 3941–3953. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Liu, H.; Zhuang, S.; Yang, J.; Zhang, F. Intranasal immunization with recombinant Lactococci carrying human papillomavirus E7 protein and mouse interleukin-12 DNA induces E7-specific antitumor effects in C57BL/6 mice. Oncol. Lett. 2014, 7, 576–582. [Google Scholar] [CrossRef]
- Cortes-Perez, N.G.; Lefevre, F.; Corthier, G.; Adel-Patient, K.; Langella, P.; Bermudez-Humaran, L.G. Influence of the route of immunization and the nature of the bacterial vector on immunogenicity of mucosal vaccines based on lactic acid bacteria. Vaccine 2007, 25, 6581–6588. [Google Scholar] [CrossRef]
- Hugentobler, F.; Di Roberto, R.B.; Gillard, J.; Cousineau, B. Oral immunization using live Lactococcus lactis co-expressing LACK and IL-12 protects BALB/c mice against Leishmania major infection. Vaccine 2012, 30, 5726–5732. [Google Scholar] [CrossRef]
- Hugentobler, F.; Yam, K.K.; Gillard, J.; Mahbuba, R.; Olivier, M.; Cousineau, B. Immunization against Leishmania major infection using LACK- and IL-12-expressing Lactococcus lactis induces delay in footpad swelling. PLoS ONE 2012, 7, e30945. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.D.; Kalyanasundram, J.; Sabidi, S.; Song, A.A.; Abdullah, M.; Abdul Rahim, R.; Yusoff, K. Proof of concept in utilizing in-trans surface display system of Lactobacillus plantarum as mucosal tuberculosis vaccine via oral administration in mice. BMC Biotechnol. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, A.; Masuda, K.; Katoh, M.; Igimi, S. Adjuvant effects for oral immunization provided by recombinant Lactobacillus casei secreting biologically active murine interleukin-1{beta}. Clin. Vaccine Immunol. 2010, 17, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, A.; Zhang, L.; LaVoy, A.; Bumgardner, S.; Klaenhammer, T.R.; Dean, G.A. Mucosal Immunogenicity of Genetically Modified Lactobacillus acidophilus Expressing an HIV-1 Epitope within the Surface Layer Protein. PLoS ONE 2015, 10, e0141713. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, M.; Selvakumari Jayasurya, A.; Moochhala, S.; Huat Bay, B.; Kun Lee, Y.; Mahendran, R. Lactobacillus rhamnosus GG secreting an antigen and Interleukin-2 translocates across the gastrointestinal tract and induces an antigen specific immune response. Microbiol. Immunol. 2011, 55, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Szatraj, K.; Szczepankowska, A.K.; Saczynska, V.; Florys, K.; Gromadzka, B.; Lepek, K.; Plucienniczak, G.; Szewczyk, B.; Zagorski-Ostoja, W.; Bardowski, J. Expression of avian influenza haemagglutinin (H5) and chicken interleukin 2 (chIL-2) under control of the ptcB promoter in Lactococcus lactis. Acta Biochim. Pol. 2014, 61, 609–614. [Google Scholar] [CrossRef]
- Watford, W.T.; Moriguchi, M.; Morinobu, A.; O’Shea, J.J. The biology of IL-12: Coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003, 14, 361–368. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Conos, S.A.; Lawlor, K.E.; Vaux, D.L.; Vince, J.E.; Lindqvist, L.M. Cell death is not essential for caspase-1-mediated interleukin-1beta activation and secretion. Cell Death Differ. 2016, 23, 1827–1838. [Google Scholar] [CrossRef]
- Boucher, D.; Monteleone, M.; Coll, R.C.; Chen, K.W.; Ross, C.M.; Teo, J.L.; Gomez, G.A.; Holley, C.L.; Bierschenk, D.; Stacey, K.J.; et al. Caspase-1 self-cleavage is an intrinsic mechanism to terminate inflammasome activity. J. Exp. Med. 2018, 215, 827–840. [Google Scholar] [CrossRef]
- Staats, H.F.; Ennis, F.A., Jr. IL-1 is an effective adjuvant for mucosal and systemic immune responses when coadministered with protein immunogens. J. Immunol. 1999, 162, 6141–6147. [Google Scholar] [PubMed]
- Antoni, G.; Presentini, R.; Perin, F.; Tagliabue, A.; Ghiara, P.; Censini, S.; Volpini, G.; Villa, L.; Boraschi, D. A short synthetic peptide fragment of human interleukin 1 with immunostimulatory but not inflammatory activity. J. Immunol. 1986, 137, 3201–3204. [Google Scholar] [PubMed]
- Shornick, L.P.; De Togni, P.; Mariathasan, S.; Goellner, J.; Strauss-Schoenberger, J.; Karr, R.W.; Ferguson, T.A.; Chaplin, D.D. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J. Exp. Med. 1996, 183, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Trotta, E.; Simeonov, D.R.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Santiago, A.F.; Fernandes, R.M.; Santos, B.P.; Assis, F.A.; Oliveira, R.P.; Carvalho, C.R.; Faria, A.M. Role of mesenteric lymph nodes and aging in secretory IgA production in mice. Cell. Immunol. 2008, 253, 5–10. [Google Scholar] [CrossRef]
- Mishra, J.; Waters, C.M.; Kumar, N. Molecular mechanism of interleukin-2-induced mucosal homeostasis. Am. J. Physiol. Cell Physiol. 2012, 302, C735–C747. [Google Scholar] [CrossRef]
- Brynskov, J.; Tvede, N.; Andersen, C.B.; Vilien, M. Increased concentrations of interleukin 1 beta, interleukin-2, and soluble interleukin-2 receptors in endoscopical mucosal biopsy specimens with active inflammatory bowel disease. Gut 1992, 33, 55–58. [Google Scholar] [CrossRef]
- Pullman, W.E.; Doe, W.F. IL-2 production by intestinal lamina propria cells in normal inflamed and cancer-bearing colons. Clin. Exp. Immunol. 1992, 88, 132–137. [Google Scholar] [CrossRef]
- Chang, S.Y.; Ko, H.J.; Kweon, M.N. Mucosal dendritic cells shape mucosal immunity. Exp. Mol. Med. 2014, 46, e84. [Google Scholar] [CrossRef]
- Curiel, T.J.; Morris, C.; Brumlik, M.; Landry, S.J.; Finstad, K.; Nelson, A.; Joshi, V.; Hawkins, C.; Alarez, X.; Lackner, A.; et al. Peptides identified through phage display direct immunogenic antigen to dendritic cells. J. Immunol. 2004, 172, 7425–7431. [Google Scholar] [CrossRef]
- Yang, W.T.; Yang, G.L.; Shi, S.H.; Liu, Y.Y.; Huang, H.B.; Jiang, Y.L.; Wang, J.Z.; Shi, C.W.; Jing, Y.B.; Wang, C.F. Protection of chickens against H9N2 avian influenza virus challenge with recombinant Lactobacillus plantarum expressing conserved antigens. Appl. Microbiol. Biotechnol. 2017, 101, 4593–4603. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Jiang, X.; Jiang, Y.; Tang, L.; Xu, Y.; Qiao, X.; Min, L.; Wen, C.; Ma, G.; Li, Y. Oral Immunization against PEDV with Recombinant Lactobacillus casei Expressing Dendritic Cell-Targeting Peptide Fusing COE Protein of PEDV in Piglets. Viruses 2018, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.Y.; Yuan, M.M.; Li, D.J. Molecular adjuvant C3d3 improved the anti-hCGbeta humoral immune response in vaginal inoculation with live recombinant Lactobacillus expressing hCGbeta-C3d3 fusion protein. Vaccine 2007, 25, 6129–6139. [Google Scholar] [CrossRef] [PubMed]
- Michon, C.; Kuczkowska, K.; Langella, P.; Eijsink, V.G.; Mathiesen, G.; Chatel, J.M. Surface display of an anti-DEC-205 single chain Fv fragment in Lactobacillus plantarum increases internalization and plasmid transfer to dendritic cells in vitro and in vivo. Microb. Cell Fact. 2015, 14, 95. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Yang, G.L.; Wang, Q.; Huang, H.B.; Jiang, Y.L.; Shi, C.W.; Wang, J.Z.; Huang, K.Y.; Jin, Y.B.; Wang, C.F. Protective efficacy of Fc targeting conserved influenza virus M2e antigen expressed by Lactobacillus plantarum. Antivir. Res. 2017, 138, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Mohamadzadeh, M.; Duong, T.; Sandwick, S.J.; Hoover, T.; Klaenhammer, T.R. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc. Natl. Acad. Sci. USA 2009, 106, 4331–4336. [Google Scholar] [CrossRef]
- Mohamadzadeh, M.; Durmaz, E.; Zadeh, M.; Pakanati, K.C.; Gramarossa, M.; Cohran, V.; Klaenhammer, T.R. Targeted expression of anthrax protective antigen by Lactobacillus gasseri as an anthrax vaccine. Future Microbiol. 2010, 5, 1289–1296. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, J.; Guo, Y.; Yang, W.; Ye, L.; Shi, C.; Liu, Y.; Yang, G.; Wang, C. Construction and immunological evaluation of recombinant Lactobacillus plantarum expressing HN of Newcastle disease virus and DC- targeting peptide fusion protein. J. Biotechnol. 2015, 216, 82–89. [Google Scholar] [CrossRef]
- Shi, S.H.; Yang, W.T.; Yang, G.L.; Zhang, X.K.; Liu, Y.Y.; Zhang, L.J.; Ye, L.P.; Hu, J.T.; Xing, X.; Qi, C.; et al. Lactobacillus plantarum vaccine vector expressing hemagglutinin provides protection against H9N2 challenge infection. Virus Res. 2016, 211, 46–57. [Google Scholar] [CrossRef]
- Yang, W.T.; Shi, S.H.; Yang, G.L.; Jiang, Y.L.; Zhao, L.; Li, Y.; Wang, C.F. Cross-protective efficacy of dendritic cells targeting conserved influenza virus antigen expressed by Lactobacillus plantarum. Sci. Rep. 2016, 6, 39665. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Huang, X.; Ma, S.; Yu, M.; Shi, W.; Qiao, X.; Tang, L.; Xu, Y.; Li, Y. Oral Delivery of Probiotics Expressing Dendritic Cell-Targeting Peptide Fused with Porcine Epidemic Diarrhea Virus COE Antigen: A Promising Vaccine Strategy against PEDV. Viruses 2017, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jiang, Y.; Tong, P.; Li, C.; Yang, W.; Hu, J.; Ye, L.; Gu, W.; Shi, C.; Shan, B.; et al. Alleviation of enterotoxigenic Escherichia coli challenge by recombinant Lactobacillus plantarum expressing a FaeG- and DC-targeting peptide fusion protein. Benef. Microbes 2017, 8, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yao, J.; Yang, W.; Jiang, Y.; Du, J.; Huang, H.; Gu, W.; Hu, J.; Ye, L.; Shi, C.; et al. Construction and immunological evaluation of recombinant Lactobacillus plantarum expressing SO7 of Eimeria tenella fusion DC-targeting peptide. Vet. Parasitol. 2017, 236, 7–13. [Google Scholar] [CrossRef]
- Sahay, B.; Colliou, N.; Zadeh, M.; Ge, Y.; Gong, M.; Owen, J.L.; Valletti, M.; Jobin, C.; Mohamadzadeh, M. Dual-route targeted vaccine protects efficiently against botulinum neurotoxin A complex. Vaccine 2018, 36, 155–164. [Google Scholar] [CrossRef]
- Huang, K.Y.; Yang, G.L.; Jin, Y.B.; Liu, J.; Chen, H.L.; Wang, P.B.; Jiang, Y.L.; Shi, C.W.; Huang, H.B.; Wang, J.Z.; et al. Construction and immunogenicity analysis of Lactobacillus plantarum expressing a porcine epidemic diarrhea virus S gene fused to a DC-targeting peptide. Virus Res. 2018, 247, 84–93. [Google Scholar] [CrossRef]
- Ma, S.; Wang, L.; Huang, X.; Wang, X.; Chen, S.; Shi, W.; Qiao, X.; Jiang, Y.; Tang, L.; Xu, Y.; et al. Oral recombinant Lactobacillus vaccine targeting the intestinal microfold cells and dendritic cells for delivering the core neutralizing epitope of porcine epidemic diarrhea virus. Microb. Cell Fact. 2018, 17, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, B.; Niu, C.; Jia, S.; Sun, C.; Wang, Z.; Jiang, Y.; Cui, W.; Wang, L.; Xu, Y. Dendritic Cell Targeting of Bovine Viral Diarrhea Virus E2 Protein Expressed by Lactobacillus casei Effectively Induces Antigen-Specific Immune Responses via Oral Vaccination. Viruses 2019, 11, 575. [Google Scholar] [CrossRef]
- Liang, S.; Hajishengallis, G. Heat-labile enterotoxins as adjuvants or anti-inflammatory agents. Immunol. Investig 2010, 39, 449–467. [Google Scholar] [CrossRef]
- Petrovsky, N. Comparative Safety of Vaccine Adjuvants: A Summary of Current Evidence and Future Needs. Drug Saf. 2015, 38, 1059–1074. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Arce, S.; Gockel, C.M.; Connell, T.D.; Russell, M.W. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: Applications for oral infections. J. Dent. Res. 2005, 84, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Agren, L.; Lowenadler, B.; Lycke, N. A novel concept in mucosal adjuvanticity: The CTA1-DD adjuvant is a B cell-targeted fusion protein that incorporates the enzymatically active cholera toxin A1 subunit. Immunol. Cell Biol. 1998, 76, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Agren, L.; Sverremark, E.; Ekman, L.; Schon, K.; Lowenadler, B.; Fernandez, C.; Lycke, N. The ADP-ribosylating CTA1-DD adjuvant enhances T cell-dependent and independent responses by direct action on B cells involving anti-apoptotic Bcl-2- and germinal center-promoting effects. J. Immunol. 2000, 164, 6276–6286. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Sheng, Z.; Ding, Q.; Chen, J.; Wei, X.; Lam, D.M.; Xu, Y. Evaluation of oral immunization with recombinant avian influenza virus HA1 displayed on the Lactococcus lactis surface and combined with the mucosal adjuvant cholera toxin subunit B. Clin. Vaccine Immunol. 2011, 18, 1046–1051. [Google Scholar] [CrossRef]
- Chowdhury, M.Y.; Li, R.; Kim, J.H.; Park, M.E.; Kim, T.H.; Pathinayake, P.; Weeratunga, P.; Song, M.K.; Son, H.Y.; Hong, S.P.; et al. Mucosal vaccination with recombinant Lactobacillus casei-displayed CTA1-conjugated consensus matrix protein-2 (sM2) induces broad protection against divergent influenza subtypes in BALB/c mice. PLoS ONE 2014, 9, e94051. [Google Scholar] [CrossRef]
- Li, R.; Chowdhury, M.Y.; Kim, J.H.; Kim, T.H.; Pathinayake, P.; Koo, W.S.; Park, M.E.; Yoon, J.E.; Roh, J.B.; Hong, S.P.; et al. Mucosally administered Lactobacillus surface-displayed influenza antigens (sM2 and HA2) with cholera toxin subunit A1 (CTA1) Induce broadly protective immune responses against divergent influenza subtypes. Vet. Microbiol. 2015, 179, 250–263. [Google Scholar] [CrossRef]
- Ge, J.W.; Liu, D.Q.; Li, Y.J. Construction of recombinant lactobacilli expressing the core neutralizing epitope (COE) of porcine epidemic diarrhea virus and a fusion protein consisting of COE and Escherichia coli heat-labile enterotoxin B, and comparison of the immune responses by orogastric immunization. Can. J. Microbiol. 2012, 58, 1258–1267. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, G.; Wang, Q.; Wang, Z.; Yang, W.; Gu, W.; Shi, C.; Wang, J.; Huang, H.; Wang, C. Molecular mechanisms underlying protection against H9N2 influenza virus challenge in mice by recombinant Lactobacillus plantarum with surface displayed HA2-LTB. J. Biotechnol. 2017, 259, 6–14. [Google Scholar] [CrossRef]
- Mutsch, M.; Zhou, W.; Rhodes, P.; Bopp, M.; Chen, R.T.; Linder, T.; Spyr, C.; Steffen, R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 2004, 350, 896–903. [Google Scholar] [CrossRef]
- Colombi, D.; Oliveira, M.L.; Campos, I.B.; Monedero, V.; Perez-Martinez, G.; Ho, P.L. Haemagglutination induced by Bordetella pertussis filamentous haemagglutinin adhesin (FHA) is inhibited by antibodies produced against FHA(430-873) fragment expressed in Lactobacillus casei. Curr. Microbiol. 2006, 53, 462–466. [Google Scholar] [CrossRef]
- Okuno, T.; Kashige, N.; Satho, T.; Irie, K.; Hiramatsu, Y.; Sharmin, T.; Fukumitsu, Y.; Uyeda, S.; Yamada, S.; Harakuni, T.; et al. Expression and secretion of cholera toxin B subunit in lactobacilli. Biol. Pharm. Bull. 2013, 36, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, G.; Wang, X.; Li, X.; Liu, M.; Li, Y. Recombinant porcine rotavirus VP4 and VP4-LTB expressed in Lactobacillus casei induced mucosal and systemic antibody responses in mice. BMC Microbiol. 2009, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qi, R.; Chen, C.; Yin, J.; Ma, S.; Shi, W.; Wu, Y.; Ge, J.; Jiang, Y.; Tang, L.; et al. Immunogenicity of recombinant Lactobacillus casei-expressing F4 (K88) fimbrial adhesin FaeG in conjunction with a heat-labile enterotoxin A (LTAK63) and heat-labile enterotoxin B (LTB) of enterotoxigenic Escherichia coli as an oral adjuvant in mice. J. Appl. Microbiol. 2017, 122, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, A.; Igimi, S. Innate and acquired immune responses induced by recombinant Lactobacillus casei displaying flagellin-fusion antigen on the cell-surface. Vaccine 2010, 28, 3409–3415. [Google Scholar] [CrossRef] [PubMed]
- Stoeker, L.; Nordone, S.; Gunderson, S.; Zhang, L.; Kajikawa, A.; LaVoy, A.; Miller, M.; Klaenhammer, T.R.; Dean, G.A. Assessment of Lactobacillus gasseri as a candidate oral vaccine vector. Clin. Vaccine Immunol. 2011, 18, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, A.; Zhang, L.; Long, J.; Nordone, S.; Stoeker, L.; LaVoy, A.; Bumgardner, S.; Klaenhammer, T.; Dean, G. Construction and immunological evaluation of dual cell surface display of HIV-1 gag and Salmonella enterica serovar Typhimurium FliC in Lactobacillus acidophilus for vaccine delivery. Clin. Vaccine Immunol. 2012, 19, 1374–1381. [Google Scholar] [CrossRef]
- Guimaraes, V.D.; Gabriel, J.E.; Lefevre, F.; Cabanes, D.; Gruss, A.; Cossart, P.; Azevedo, V.; Langella, P. Internalin-expressing Lactococcus lactis is able to invade small intestine of guinea pigs and deliver DNA into mammalian epithelial cells. Microbes Infect. 2005, 7, 836–844. [Google Scholar] [CrossRef]
- de Azevedo, M.; Karczewski, J.; Lefevre, F.; Azevedo, V.; Miyoshi, A.; Wells, J.M.; Langella, P.; Chatel, J.M. In vitro and in vivo characterization of DNA delivery using recombinant Lactococcus lactis expressing a mutated form of L. monocytogenes Internalin, A. BMC Microbiol. 2012, 12, 299. [Google Scholar] [CrossRef]
- Pontes, D.; Innocentin, S.; Del Carmen, S.; Almeida, J.F.; Leblanc, J.G.; de Moreno de Leblanc, A.; Blugeon, S.; Cherbuy, C.; Lefevre, F.; Azevedo, V.; et al. Production of Fibronectin Binding Protein A at the surface of Lactococcus lactis increases plasmid transfer in vitro and in vivo. PLoS ONE 2012, 7, e44892. [Google Scholar] [CrossRef]
- Pontes, D.; Azevedo, M.; Innocentin, S.; Blugeon, S.; Lefevre, F.; Azevedo, V.; Miyoshi, A.; Courtin, P.; Chapot-Chartier, M.P.; Langella, P.; et al. Immune response elicited by DNA vaccination using Lactococcus lactis is modified by the production of surface exposed pathogenic protein. PLoS ONE 2014, 9, e84509. [Google Scholar] [CrossRef]
- Mancha-Agresti, P.; de Castro, C.P.; Dos Santos, J.S.C.; Araujo, M.A.; Pereira, V.B.; LeBlanc, J.G.; Leclercq, S.Y.; Azevedo, V. Recombinant Invasive Lactococcus lactis Carrying a DNA Vaccine Coding the Ag85A Antigen Increases INF-gamma, IL-6, and TNF-alpha Cytokines after Intranasal Immunization. Front. Microbiol. 2017, 8, 1263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yu, M.; Qiao, X.; Liu, M.; Tang, L.; Jiang, Y.; Cui, W.; Li, Y. Up-regulation of MDP and tuftsin gene expression in Th1 and Th17 cells as an adjuvant for an oral Lactobacillus casei vaccine against anti-transmissible gastroenteritis virus. Appl. Microbiol. Biotechnol. 2014, 98, 8301–8312. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.E.; Manzo, R.A.; Soto, D.A.; Barrientos, M.J.; Maldonado, A.E.; Mosqueira, M.; Avila, A.; Touma, J.; Bruce, E.; Harris, P.R.; et al. Oral administration of recombinant Neisseria meningitidis PorA genetically fused to H. pylori HpaA antigen increases antibody levels in mouse serum, suggesting that PorA behaves as a putative adjuvant. Hum. Vaccin Immunother. 2015, 11, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Quintana, I.; Espariz, M.; Villar, S.R.; Gonzalez, F.B.; Pacini, M.F.; Cabrera, G.; Bontempi, I.; Prochetto, E.; Stulke, J.; Perez, A.R.; et al. Genetic Engineering of Lactococcus lactis Co-producing Antigen and the Mucosal Adjuvant 3’ 5’- cyclic di Adenosine Monophosphate (c-di-AMP) as a Design Strategy to Develop a Mucosal Vaccine Prototype. Front. Microbiol. 2018, 9, 2100. [Google Scholar] [CrossRef]
- Wang, W.; Song, Y.; Liu, L.; Zhang, Y.; Wang, T.; Zhang, W.; Li, K.; Qi, X.; Gao, Y.; Gao, L.; et al. Neutralizing-antibody-mediated protection of chickens against infectious bursal disease via one-time vaccination with inactivated recombinant Lactococcus lactis expressing a fusion protein constructed from the RCK protein of Salmonella enterica and VP2 of infectious bursal disease virus. Microb. Cell Fact. 2019, 18, 21. [Google Scholar] [CrossRef]
- Miao, E.A.; Alpuche-Aranda, C.M.; Dors, M.; Clark, A.E.; Bader, M.W.; Miller, S.I.; Aderem, A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 2006, 7, 569–575. [Google Scholar] [CrossRef]
- Cui, B.; Liu, X.; Fang, Y.; Zhou, P.; Zhang, Y.; Wang, Y. Flagellin as a vaccine adjuvant. Expert Rev. Vaccines 2018, 17, 335–349. [Google Scholar] [CrossRef]
- Hong, S.H.; Byun, Y.H.; Nguyen, C.T.; Kim, S.Y.; Seong, B.L.; Park, S.; Woo, G.J.; Yoon, Y.; Koh, J.T.; Fujihashi, K.; et al. Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection. Vaccine 2012, 30, 466–474. [Google Scholar] [CrossRef]
- Fazeli, A.; Bruce, C.; Anumba, D.O. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum. Reprod. 2005, 20, 1372–1378. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, J.L.; Berche, P.; Frehel, C.; Gouin, E.; Cossart, P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell 1991, 65, 1127–1141. [Google Scholar] [CrossRef]
- Innocentin, S.; Guimaraes, V.; Miyoshi, A.; Azevedo, V.; Langella, P.; Chatel, J.M.; Lefevre, F. Lactococcus lactis expressing either Staphylococcus aureus fibronectin-binding protein A or Listeria monocytogenes internalin A can efficiently internalize and deliver DNA in human epithelial cells. Appl. Environ. Microbiol. 2009, 75, 4870–4878. [Google Scholar] [CrossRef] [PubMed]
- Wardowska, A.; Dzierzbicka, K.; Menderska, A.; Trzonkowski, P. New conjugates of tuftsin and muramyl dipeptide as stimulators of human monocyte-derived dendritic cells. Protein Pept. Lett. 2013, 20, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Skrnjug, I.; Rueckert, C.; Libanova, R.; Lienenklaus, S.; Weiss, S.; Guzman, C.A. The mucosal adjuvant cyclic di-AMP exerts immune stimulatory effects on dendritic cells and macrophages. PLoS ONE 2014, 9, e95728. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, E.J.; Reed, S.; Hackett, J.; Fierer, J.; Roudier, C.; Guiney, D. Mechanism of resistance to complement-mediated killing of bacteria encoded by the Salmonella typhimurium virulence plasmid gene rck. J. Clin. Investig. 1992, 90, 953–964. [Google Scholar] [CrossRef]
- Rosselin, M.; Virlogeux-Payant, I.; Roy, C.; Bottreau, E.; Sizaret, P.Y.; Mijouin, L.; Germon, P.; Caron, E.; Velge, P.; Wiedemann, A. Rck of Salmonella enterica, subspecies enterica serovar enteritidis, mediates zipper-like internalization. Cell Res. 2010, 20, 647–664. [Google Scholar] [CrossRef]
- Taguchi, A.; Kawana, K.; Yokoyama, T.; Adachi, K.; Yamashita, A.; Tomio, K.; Kojima, S.; Oda, K.; Fujii, T.; Kozuma, S. Adjuvant effect of Japanese herbal medicines on the mucosal type 1 immune responses to human papillomavirus (HPV) E7 in mice immunized orally with Lactobacillus-based therapeutic HPV vaccine in a synergistic manner. Vaccine 2012, 30, 5368–5372. [Google Scholar] [CrossRef]
- Kim, J.I.; Park, T.E.; Maharjan, S.; Li, H.S.; Lee, H.B.; Kim, I.S.; Piao, D.; Lee, J.Y.; Cho, C.S.; Bok, J.D.; et al. Soluble RANKL expression in Lactococcus lactis and investigation of its potential as an oral vaccine adjuvant. BMC Immunol. 2015, 16, 71. [Google Scholar] [CrossRef]
- Xu, Y.G.; Guan, X.T.; Liu, Z.M.; Tian, C.Y.; Cui, L.C. Immunogenicity in Swine of Orally Administered Recombinant Lactobacillus plantarum Expressing Classical Swine Fever Virus E2 Protein in Conjunction with Thymosin alpha-1 as an Adjuvant. Appl. Environ. Microbiol. 2015, 81, 3745–3752. [Google Scholar] [CrossRef]
- Underwood, J.R.; Chivers, M.; Dang, T.T.; Licciardi, P.V. Stimulation of tetanus toxoid-specific immune responses by a traditional Chinese herbal medicine. Vaccine 2009, 27, 6634–6641. [Google Scholar] [CrossRef]
- Kiyohara, H.; Nagai, T.; Munakata, K.; Nonaka, K.; Hanawa, T.; Kim, S.J.; Yamada, H. Stimulating effect of Japanese herbal (kampo) medicine, hochuekkito on upper respiratory mucosal immune system. Evid. Based Complement. Altern. Med. 2006, 3, 459–467. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Z.; Zhang, Z.; Pan, L.; Zhang, Y. Roles of M cells in infection and mucosal vaccines. Hum. Vaccin Immunother. 2014, 10, 3544–3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.F.; Ma, Z.H.; Zhao, P.W.; Pan, Y.; Liu, Y.Y.; Feng, J.Y.; Niu, J.Q. Effect of thymosin-alpha(1) on T-helper 1 cell and T-helper 2 cell cytokine synthesis in patients with hepatitis B virus e antigen-positive chronic hepatitis B. J. Int. Med. Res. 2010, 38, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Zhang, T.; Saibara, T.; Nemoto, Y.; Ono, M.; Akisawa, N.; Iwasaki, S.; Maeda, T.; Onishi, S. Thymosin alpha1 accelerates restoration of T cell-mediated neutralizing antibody response in immunocompromised hosts. Int. Immunopharmacol. 2002, 2, 39–46. [Google Scholar] [CrossRef]
- Gerritse, K.; Posno, M.; Schellekens, M.M.; Boersma, W.J.; Claassen, E. Oral administration of TNP-Lactobacillus conjugates in mice: A model for evaluation of mucosal and systemic immune responses and memory formation elicited by transformed lactobacilli. Res. Microbiol. 1990, 141, 955–962. [Google Scholar] [CrossRef]
- Marteau, P.; Rambaud, J.C. Potential of using lactic acid bacteria for therapy and immunomodulation in man. FEMS Microbiol. Rev. 1993, 12, 207–220. [Google Scholar] [CrossRef]
- Takahashi, K.; Orito, N.; Tokunoh, N.; Inoue, N. Current issues regarding the application of recombinant lactic acid bacteria to mucosal vaccine carriers. Appl. Microbiol. Biotechnol. 2019, 103, 5947–5955. [Google Scholar] [CrossRef] [PubMed]
- Apostolico Jde, S.; Lunardelli, V.A.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, Modus Operandi, and Licensing. J. Immunol. Res. 2016, 2016, 1459394. [Google Scholar] [CrossRef]
- Edelman, R. Vaccine adjuvants. Rev. Infect. Dis. 1980, 2, 370–383. [Google Scholar] [CrossRef]
- Umesaki, Y.; Setoyama, H. Structure of the intestinal flora responsible for development of the gut immune system in a rodent model. Microbes Infect. 2000, 2, 1343–1351. [Google Scholar] [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A.D. Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeostasis and immunopathology. Nutrients 2013, 5, 1869–1912. [Google Scholar] [CrossRef]
- Stout, E.; Klaenhammer, T.; Barrangou, R. CRISPR-Cas Technologies and Applications in Food Bacteria. Annu. Rev. Food Sci. Technol. 2017, 8, 413–437. [Google Scholar] [CrossRef] [PubMed]
- van Pijkeren, J.P.; Barrangou, R. Genome Editing of Food-Grade Lactobacilli to Develop Therapeutic Probiotics. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]

| Adjuvant | LAB | Expression | Antigen | Immune Response | Delivery | Species | Study |
|---|---|---|---|---|---|---|---|
| IL-12 | |||||||
| IL-12 | L. lactis | Secreted | Human Papilloma Virus (E7) | Increased BAL IgG and sIgA | Intranasal | Murine C57BL/6 | Bermudez-Humaran et al. 2005 [31] |
| Increased IFN-γ CD4+ and 8+ T cells | |||||||
| IL-12 | L. lactis L. plantarum | Secreted | Human Papilloma Virus (E7) | L. lactis, Intranasal Delivery | Intranasal Oral | Murine C57BL/6 | Cortes-Perez et al. 2007 [39] |
| Increased Serum and GAL IgG; Increased GAL IgA | |||||||
| Increased IFN-γ | |||||||
| L. plantarum, Intranasal Delivery | |||||||
| Increased IFN-γ | |||||||
| Decreased Tumor Burden | |||||||
| IL-12 | L. lactis | Secreted | Leishmania major Leishmania (Homologue of Activated C Kinase) | Subcutaneous | Subcutaneous Oral | Murine BALB/c | Hugentobler et al. 2012 [40] Hugentobler et al. 2012 [41] |
| Increased IgG1 and IgG2a | |||||||
| Increased IFN-γ | |||||||
| Decreased Parasite Load | |||||||
| Oral | |||||||
| Decreased Parasite Load | |||||||
| Increased Intestinal sIgA | |||||||
| Increased IFN-γ, IL-2 | |||||||
| IL-12 | L. lactis | Cytoplasmic (DNA) | Human Papilloma Virus (E7) | Increased IFN-γ | Intranasal | Murine C57BL/6 | Li et al. 2014 [38] |
| Decreased Tumor Volume | |||||||
| IL-12 | L. lactis L. plantarum | Secreted (by L. lactis with L. plantarum Expressing the Antigen) | Mycobacterium tuberculosis (Subunit Epitopes: Ag85B, CFP-10, ESAT-6, Rv0475, Rv2031c) | Increased IgG | Oral | Murine BALB/c | Mustafa et al. 2018 [42] |
| Increased IFN-γ, IL-2 | |||||||
| IL-1β | |||||||
| IL-1β | L. casei | Secreted | Salmonella enterica (SE) | Increased IL-6, TNF-α, TGF-β | Oral | Murine C3H/HeJ | Kajikawa et al. 2010 [43] |
| Increased IgG and Intestinal sIgA when Co-Delivered with SE | |||||||
| IL-1β | L. acidophilus | Secreted | HIV-1 (Membrane Subunit Epitope) | Increased IgG, Intestinal and Vaginal sIgA | Oral | Murine BALB/c | Kajikawa et al. 2015 [44] |
| Increased Intestinal and Vaginal Epitope-Specific IgA B cells | |||||||
| Increased IL-4 | |||||||
| IL-2 | |||||||
| IL-2 | L. rhamnosus GG | Secreted | Green Florescent Protein (GFP) | Increased Trafficking to MLN and Spleen. | Oral | MurineC57BL/6 and BALB/c | Kandasamy et al. 2011 [45] |
| Increased MLN T Cells, IgA B Cells, DCs | |||||||
| Increased GFP-Specific IgG and Fecal sIgA | |||||||
| Increased IFN-γ, IFN-α, IL-12 | |||||||
| IL-2 | L. lactis | Secreted | Avian Influenza (Haemagglutinin 5) | Increased IgG and Serum IgA | Oral | Murine BALB/c | Szatraj et al. 2014 [46] |
| Adjuvant | LAB | Expression | Antigen | Immune Response | Delivery | Species | Study |
|---|---|---|---|---|---|---|---|
| DC-peptide | |||||||
| DC-pep | L. acidophilus | Surface-Display | Bacillus anthracis (Protective Antigen) | Increased IL-12, IL-10, TNFα, MCP-1 | Oral | Murine A/J | Mohamadzadeh et al. 2009 [67] |
| Increased Survival to Challenge | |||||||
| DC-pep | L. gasseri | Surface-Display | Bacillus anthracis (Protective Antigen) | Increased IgG | Oral | Murine A/J | Mohamadzadeh et al. 2010 [68] |
| Increased IL6, MCP-1, IFN-γ, IL-12 | |||||||
| Increased Survival to Challenge | |||||||
| Increased T Cell Stimulation Following Challenge | |||||||
| DC-pep | L. plantarum | Surface-Display | Newcastle Disease Virus (Hemagglutinin-Neuraminidase) | Increased Intestinal sIgA | Oral | Chicken | Jiang et al. 2015 [69] |
| Increased Splenic and Peripheral Blood CD4+ T Cells | |||||||
| Increased Survival to Challenge | |||||||
| DC-pep | L. plantarum | Surface-Display | Avian Influenza (Hemagglutinin) | Murine | Oral | Murine BALB/c Chicken | Shi et al. 2016 [70] |
| Increased MLN and PP DC Activation (CD80+, CD86+) Increased IFN-γ Increased Survival to Challenge with Decreased Lung Viral Titer | |||||||
| Chicken | |||||||
| Increased CD3+ T Cell Proliferation and Increased CD3+CD4+/8+ PBMC Percentages Increased IFN-γ Increased BAL sIgA and Serum IgG Decreased Lung Viral Titer | |||||||
| DC-pep | L. plantarum | Surface-Display | Avian influenza (Nucleoprotein and Matrix Protein) | Increased PP and LP DC Activation (CD80+, CD86+, CD40+, MHCII+) | Oral | Murine BALB/c, C57BL/6 | Yang et al. 2016 [71] |
| Increased PP IgA+ B Cells | |||||||
| Increased Fecal and BAL sIgA Titer | |||||||
| Increased IFN-γ, TNF-α | |||||||
| Increased T Cell Proliferation | |||||||
| Increased Survival Rate to Challenge and Decreased Lesions and Virus in Lung | |||||||
| DC-pep | L. casei | Surface-Display | Porcine Epidemic Diarrhea Virus (Core Neutralizing Epitope) | Increased MLN and PP DC Activation (CD80+, CD86+, MHCII+) | Oral | Murine BALB/c | Wang et al. 2017 [72] |
| Increased IgG, Viral Neutralization, and Genital Tract and Intestinal Mucus sIgA Titer | |||||||
| Increased Lymphocyte Proliferation | |||||||
| Increased IFN-γ, IL-4 | |||||||
| DC-pep | L. plantarum | Surface-Display | Enterotoxigenic E. coli (ETEC) (FaeG of K88 Fimbriae) | Increased Adhesion to Porcine Intestinal Cells and Decreased Attachment of ETEC (In Vitro) | Oral | Murine BALB/c | Yang et al. 2017 [73] |
| Increased IgG and Intestinal sIgA | |||||||
| Increased Splenic and MLN B Cells and DCs | |||||||
| Increased TNF-α, IL-12, IL-4 Decreased Intestinal Lesions and Weight Loss Following Challenge | |||||||
| DC-pep | L. plantarum | Surface-Display | Avian Influenza (Nucleoprotein and Matrix Protein) | Oral | Oral Intranasal | Chicken | Yang et al. 2017 [61] |
| Increased Splenic CD4+ and CD8+ T Cells and T Cell Proliferation | |||||||
| Increased IgG and BAL sIgA | |||||||
| Decreased Disease and Lung Virus Intranasal | |||||||
| Increased Splenic CD8+ T Cells and T Cell Proliferation Increased BAL sIgA | |||||||
| Decreased Disease and Lung Virus | |||||||
| DC-pep | L. plantarum | Surface-Display | Eimeria tenella (SO7) | Increased IgG and Intestinal sIgA | Oral | Chicken | Yang et al. 2017 [74] |
| Decreased Oocyst Shedding and Cecum Lesion Scores Following Challenge | |||||||
| DC-pep | L. acidophilus | Surface-Display | Clostridium botulinum (Botulinum Toxin Serotype A) | Approximately 70% Protection to Challenge (Protection B cell-Mediated) | Oral | Murine BALB/c | Sahay et al. 2018 [75] |
| DC-pep | L. casei | Surface-Display | Porcine Epidemic Diarrhea Virus (Collagenase-Digested Fragment of S Protein) | Increased IgG and Intestinal sIgA | Oral | Porcine | Hou et al. 2018 [62] |
| Increased Th1/Th2 (IFN-γ/IL-4) CD4+ T Cells | |||||||
| Increased MLN TLR4, TLR9, and TGF-β and Decreased TNF-α Expression After Challenge | |||||||
| Increased Survival and Decreased Viral RNA After Challenge | |||||||
| DC-pep | L. plantarum | Surface-Display | Porcine Epidemic Diarrhea Virus (S Protein) | Increased DC Activation (CD40/CD80+) | Oral | Murine BALB/c | Huang et al. 2018 [76] |
| Increased PP IgA+ B Cells | |||||||
| Increased Serum IgG, Intestinal sIgA, and Neutralizing Antibodies (IgG/sIgA) | |||||||
| Increased MLN IFN-γ and IL-17 | |||||||
| DC-pep and M cell targeting peptide (Col) | L. casei | Surface-Display | Porcine Epidemic Diarrhea Virus (Core Neutralizing Epitope) | Increased IgG and Vaginal, Intestinal Mucus, and Fecal sIgA | Oral | Murine BALB/c | Ma et al. 2018 [77] |
| Increased Splenic Lymphocyte Proliferation | |||||||
| Increased IFN-γ, IL-4 | |||||||
| Increased Antibody-Mediated Virus Neutralization | |||||||
| DC-pep | L. casei | Surface-Display | Bovine Viral Diarrhea Virus Glycoprotein E2 | Increased PP DC Activation (CD40+) | Oral | Murine BALB/c | Wang et at. 2019 [78] |
| Increased IgG and Intestinal sIgA | |||||||
| Increased Neutralizing IgG and sIgA | |||||||
| Increased IFN-γ, IL-4 | |||||||
| Increased Splenic CD4+/CD8+ T Cells and T Cell Stimulation | |||||||
| Other | |||||||
| Complement (C3d3) | L. casei | Surface-Display | Human Chorionic Gonadotropin (hCG) | Increased Serum/Vaginal IgG and IgA with Increased Longevity of Response | Vaginal | Murine BALB/c and C57BL/6 | Yao et al. 2007 [63] |
| Increased T and B Cell Proliferation | |||||||
| Anti-CD205 | L. plantarum | Surface-Display | DNA (Plasmid) | Increased LAB DC Internalization | Oral | Murine BALB/c | Michon et al. 2015 [64] |
| Increased Delivery of Plasmid to DCs | |||||||
| Neonatal Fc receptor (FcRn) | L. plantarum | Surface-Display | Influenza (Ectodomain of Matrix 2 Protein) | Increased DC Activation (CD86+/CD80+) | Oral | Murine BALB/c | Yang et al. 2017 [65] |
| Increased Splenic and MLN IFN-γ | |||||||
| Increased Intestinal sIgA | |||||||
| Increased MLN and PP IgA+ B cells | |||||||
| Increased Survival and Decreased Viral Load Following Challenge | |||||||
| Adjuvant | LAB | Expression | Antigen | Immune Response | Delivery | Species | Study |
|---|---|---|---|---|---|---|---|
| Cholera Toxin (CT) | |||||||
| CT subunit B | L. casei | Co-administered | Bordetella pertussis (Filamentous Haemagglutinin Adhesin) | Increased IgG | Subcutaneous | Murine BALB/c | Colombi et al. 2006 [90] |
| CT subunit B | L. lactis | Co-administered | Avian Influenza (Hemagglutinin Antigen) | Increased IgG and Intestinal sIgA | Oral | Murine BALB/c | Lei et al. 2011 [84] |
| Increased IFN-γ | |||||||
| Increased Survival to Challenge | |||||||
| CT subunit B | L. casei | Secreted | None | Increased IgG | Intranasal | Murine BALB/c | Okuno et al. 2013 [91] |
| CT subunit A1 | L. casei | Surface-Display | Influenza (Matrix Protein 2) | Increased IgG and BAL sIgA | Oral Intranasal | Murine BALB/c | Chowdhury et al. 2014 [85] |
| Increased IFN-γ (Intranasal) | |||||||
| Increased Protection and Decreased Lung Viral Titer Following Challenge | |||||||
| CT subunit A1 | L. casei | Surface-Display | Influenza (Matrix Protein 2 and Hemagglutinin) | Increased IgG and BAL and Intestinal sIgA | Oral Intranasal | Murine BALB/c | Li et al. 2015 [86] |
| Increased IFN-γ (Intranasal and Oral) and IL-4 (Intranasal) | |||||||
| Increased protection and decreased lung viral titer Following challenge | |||||||
| Longer Lasting Immune Response | |||||||
| E. coli Heat-Liable Toxin (LT) | |||||||
| LT subunit B | L. casei | Surface-Display | Porcine rotavirus (VP4 capsid protein) | Increased Ocular, Vaginal, and Intestinal sIgA | Oral | Murine BALB/c | Qiao et al. 2009 [92] |
| LT subunit B | L. casei | Surface-Display Secreted | Porcine Epidemic Diarrhea Virus (Core Neutralizing Epitope) | Increased Intestinal, Vaginal, Nasal, Ocular, and Serum sIgA/IgA (Secreted Induced Highest Levels) | Oral | Murine BALB/c | Ge et al. 2012 [87] |
| Increased Neutralizing Antibodies | |||||||
| Increased IFN-γ and IL-4 | |||||||
| LT subunit B and A (LTAK63) | L. casei | Surface-display | Enterotoxigenic E. coli (F4 (K88) fimbrial adhesion FaeG) | Increased IgG and Intestinal, Vaginal, and Nasal sIgA | Oral | Murine BALB/c | Yu et al. 2016 [93] |
| Increased Splenic Lymphocyte Proliferation | |||||||
| Increased Protection to Challenge | |||||||
| LT subunit B | L. plantarum | Surface-display | Avian influenza (hemagglutinin antigen) | Increased Intestinal sIgA | Oral | Murine BALB/c | Jiang et al. 2017 [88] |
| Increased CD4+ T Cell IFN-γ (MLN), IL-4 (MLN, Splenic), IL-17 (MLN, Splenic) and CD8+ T Cell IFN-γ (MLN, Splenic) | |||||||
| Increased PP IgA+ B Cells | |||||||
| Increased Protection to Challenge | |||||||
| Adjuvant | LAB | Expression | Antigen | Immune Response | Delivery | Species | Study |
|---|---|---|---|---|---|---|---|
| Toll-like receptor 5 ligand | |||||||
| Salmonella flagellin | L. casei | Surface-Display | Salmonella enterica (SipC) | Increased IL-8 | Oral | Murine C3H/HeJ | Kajikawa et al. 2010 [94] |
| Increased IgG | |||||||
| Increased IL-2, GM-CSF, IFN-γ | |||||||
| Salmonella flagellin | L. gasseri | Surface-Display | None | Increased TLR5 Stimulation | Oral | Murine BALB/c | Stoeker et al. 2011 [95] |
| Increased DC Maturation (MHCII+CD80+CD86-) | |||||||
| Increased IL17+ Lymphocytes | |||||||
| Increased Lamina Propria Plasma Cells | |||||||
| Salmonella flagellin | L. acidophilus | Surface-Display | HIV-1 (Gag) | Increased IL-1β, IL-6 | Oral | Murine BALB/c | Kajikawa et al. 2012 [96] |
| Increased IgA-Secreting B Cells in FRT and LI | |||||||
| Decreased IFN-γ after HIV-1 In Vitro Exposure | |||||||
| Enterocyte targeting | |||||||
| Listeria monocytogenes Internalin A | L. lactis | Surface-Display | DNA (GFP) | Increased Entry into Epithelial Cells and Delivery of GFP Plasmid | Oral | Guinea pigs Hartley | Guimaraes et al. 2005 [97] |
| Internalin A | L. lactis | Surface-Display | DNA (β-Lactoglobulin Antigen) | Increased β-Lactoglobulin in Intestinal Lumen | Oral | Murine BALB/c | de Azevedo et al. 2012 [98] |
| Fibronectic-Binding Protein A | L. lactis | Surface-Display | DNA (β-Lactoglobulin Antigen) | Increased β-Lactoglobulin in Intestinal Lumen | Oral | Murine BALB/c | Pontes et al. 2012 [99] |
| Fibronectic-Binding Protein A and Internalin A | L. lactis | Surface-Display | DNA (β-Lactoglobulin Antigen) | Intranasal | Oral Intranasal | Murine BALB/c | Pontes et al. 2014 [100] |
| Increased IL-4, IL-5, Decreased IFN-γ | |||||||
| Oral | |||||||
| Increased IL-5, Decreased IFN-γ | |||||||
| Fibronectic-Binding Protein A | L. lactis | Surface-Display | DNA (Mycobacterium tuberculosis Ag85A) | Increased IFN-γ, TNF-α, IL-6 | Intranasal | Murine C57BL/6 | Mancha-Agresti et al. 2017 [101] |
| Increased Serum IgG, IgA, and BAL IgG | |||||||
| Additional bacterial derived adjuvants | |||||||
| Muramyl Dipeptide and Tuftsin | L. casei | Secreted | Transmissible Gastroenteritis Virus (D Antigenic Site of the Spike Protein) | Increased Intestinal, Serum, Nasal, Ocular, and Vaginal sIgA | Oral | Murine BALB/c | Jiang et al. 2014 [102] |
| Increased Splenic T Cell Proliferation | |||||||
| Increased Antibody-Mediated Viral Neutralization | |||||||
| Increased IL-10, TGF-β | |||||||
| Increased Th17 Cells and Decreased Treg Cells | |||||||
| Neisseria meningitidis PorA | L. lactis | Cytoplasmic | Helicobacter pylori (HpaA) | Increased IgG | Oral | Murine BALB/c | Vasquez et al. 2015 [103] |
| c-di-AMP | L. lactis | Cytoplasmic | Trypanosoma cruzi (Trans-Sialidase Enzyme) | Increased Immune Response to T. cruzi Challenge | Oral | Murine BALB/c | Quintana et al. 2018 [104] |
| Salmonella Resistance to Complement Killing | L. lactis | Surface-display | Infectious Bursal Disease (VP2) | Increased Survival and Decreased Bursal Atrophy, Following Challenge (Intramuscular > Oral) | Oral Intramuscular | Chicken | Wang et al. 2019 [105] |
| Increased Neutralizing Antibody (Intramuscular > Oral) | |||||||
| Adjuvant | LAB | Expression | Antigen | Immune Response | Delivery | Species | Study |
|---|---|---|---|---|---|---|---|
| Herbal Medicine (JTT, HET) | L. casei | Co-administered | Human Papilloma Virus (E7) | Increased IFN-γ, IL-2 Secretion | Oral | Murine C57/BL6 | Tagucki et al. 2012 [116] |
| RANKL | L. lactis | Secreted | Brachyspira hyodysenteriae (Membrane Protein B) | Increased M Cell Development | Oral | Murine BALB/c | Kim et al. 2015 [117] |
| Increased IgG and Fecal sIgA | |||||||
| Thymosin α-1 | L. plantarum | Surface-Display | Classical Swine Fever (E2 Protein) | Increased IgG and Intestinal sIgA | Oral | Porcine | Xu et al. 2015 [118] |
| Increased Virus Neutralizing Antibodies | |||||||
| Increased Cytotoxic Cells | |||||||
| Increased IFN-γ, IL-2, TNF-α | |||||||
| Increased Protection to Challenge |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilander, A.C.; Dean, G.A. Adjuvant Strategies for Lactic Acid Bacterial Mucosal Vaccines. Vaccines 2019, 7, 150. https://doi.org/10.3390/vaccines7040150
Vilander AC, Dean GA. Adjuvant Strategies for Lactic Acid Bacterial Mucosal Vaccines. Vaccines. 2019; 7(4):150. https://doi.org/10.3390/vaccines7040150
Chicago/Turabian StyleVilander, Allison C., and Gregg A. Dean. 2019. "Adjuvant Strategies for Lactic Acid Bacterial Mucosal Vaccines" Vaccines 7, no. 4: 150. https://doi.org/10.3390/vaccines7040150




