Synthetic Peptides Elicit Strong Cellular Immunity in Visceral Leishmaniasis Natural Reservoir and Contribute to Long-Lasting Polyfunctional T-Cells in BALB/c Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Design
2.3. L. infantum Naturally Infected Dog’s Selection for Peptide Screening
2.4. Dogs’ Sample Collection
2.5. Parasites
2.6. Peptides
2.7. In Vitro Peptide Screening—Lymphoproliferation and Intracellular IFN-γ Production
2.8. In Vivo Peptide Screening—Reverse Antigen Screening (RAS)
2.9. Morphometrical and mRNA Expression Analyses of Dogs’ Skin Biopsies
2.10. Immunization Regimens and Vaccine Efficacy in the Mice Model
2.11. Polyfunctional T-Cell Analyses
2.12. Proliferation Assay and Intracellular Cytokine Stain
2.13. Analyses of T-Cell Memory Phenotypes through Flow Cytometry
2.14. Statistical Analysis
3. Results
3.1. In Silico Analysis of L. infantum Predicted Proteome
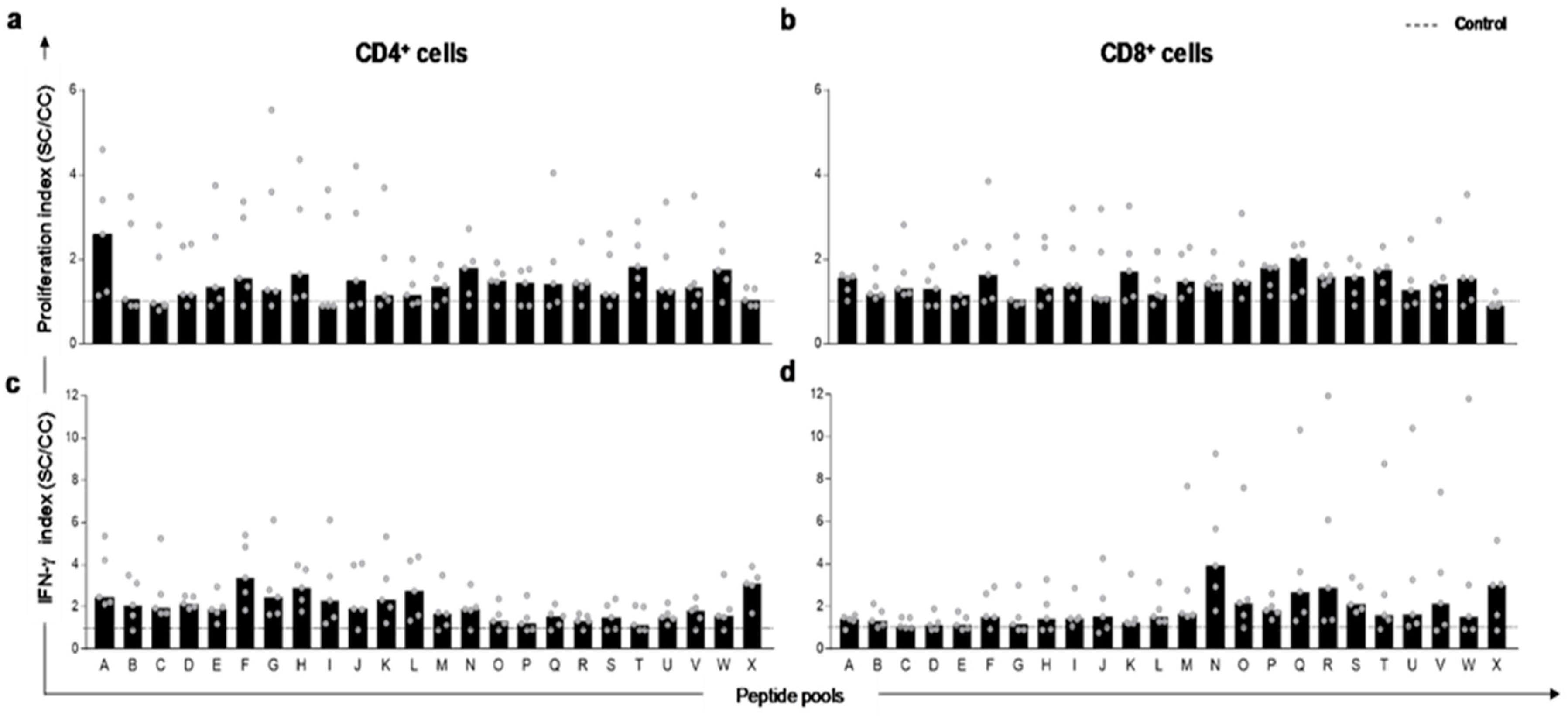
3.2. Peptides Selected In Silico Trigger In Vitro Proliferation and IFN-γ Production by T-Lymphocytes in PBMC of L. infantum Naturally Infected Dogs
3.3. Peptides Selected In Silico Lead to a Cellular Response in Skin of L. infantum Naturally Infected Dogs
3.4. Peptide Cocktail Vaccine—Cockt-1 Enhances Frequency of Polyfunctional T-Cells in the Spleen of Immunized Mice
3.5. Peptide Cocktail Vaccine Cockt-1 Promoted Significant T-Cell Proliferation in Spleen of Immunized and Challenged Mice
3.6. The Cocktail 1 of Peptide-Based Vaccine Promoted a Reduction in Parasite Load in the Spleen of Vaccinated Mice and this Correlates with the Development of Central Memory and Effector Memory T-Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iborra, S.; Solana, J.C.; Requena, J.M.; Soto, M. Vaccine candidates against leishmania under current research. Expert Rev. Vaccines 2018, 17, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Bambini, S.; Rappuoli, R. The use of genomics in microbial vaccine development. Drug Discov. Today 2009, 14, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Dikhit, M.R.; Vijayamahantesh; Kumar, A.; Amit, A.; Dehury, B.; Nathsharma, Y.P.; Ansari, M.Y.; Ali, V.; Topno, R.K.; Das, V.; et al. Mining the Proteome of Leishmania donovani for the Development of Novel MHC Class I Restricted Epitope for the Control of Visceral Leishmaniasis. J. Cell. Biochem. 2018, 119, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Mahantesh, V.; Amit, A.; Dikhit, M.R.; Singh, A.K.; Venkateshwaran, T.; Das, V.N.R.; Das, P.; Bimal, S. Immuno-informatics based approaches to identify CD8+ T cell epitopes within the Leishmania donovani 3-ectonucleotidase in cured visceral leishmaniasis subjects. Microbes Infect. Inst. Pasteur 2017, 19, 358–369. [Google Scholar]
- Agallou, M.; Athanasiou, E.; Koutsoni, O.; Dotsika, E.; Karagouni, E. Experimental Validation of Multi-Epitope Peptides Including Promising MHC Class I- and II-Restricted Epitopes of Four Known Leishmania infantum Proteins. Front. Immunol. 2014, 5, 268. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.B.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.C.F.; Guimarães, F.G.; Velloso, J.P.L.; Corrêa-Oliveira, R.; Ruiz, J.C.; Reis, A.B.; Resende, D.M. Immunoinformatics Features Linked to Leishmania Vaccine Development: Data Integration of Experimental and In Silico Studies. Int. J. Mol. Sci. 2017, 18, 371. [Google Scholar] [CrossRef]
- Reis, A.B.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Giunchetti, R.C.; Carneiro, C.M.; Mayrink, W.; Tafuri, W.L.; Correa-Oliveira, R. Systemic and compartmentalized immune response in canine visceral leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 87–95. [Google Scholar] [CrossRef]
- Reis, A.B.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; Carvalho, M.G.; Mayrink, W.; França-Silva, J.C.; Giunchetti, R.C.; Genaro, O.; Correa-Oliveira, R. Parasite density and impaired biochemical/hematological status are associated with severe clinical aspects of canine visceral leishmaniasis. Res. Vet. Sci. 2006, 81, 68–75. [Google Scholar] [CrossRef]
- Moreira, N.; Vitoriano-Souza, J.; Roatt, B.M.; Vieira, P.M.; Ker, H.G.; de Oliveira Cardoso, J.M.; Giunchetti, R.C.; Carneiro, C.M.; de Lana, M.; Reis, A.B. Parasite burden in hamsters infected with two different strains of leishmania (Leishmania) infantum: “Leishman Donovan units” versus real-time PCR. PLoS ONE 2012, 7, e47907. [Google Scholar] [CrossRef][Green Version]
- Reis, A.B.; Teixeira-Carvalho, A.; Vale, A.M.; Marques, M.J.; Giunchetti, R.C.; Mayrink, W.; Guerra, L.L.; Andrade, R.A.; Correa-Oliveira, R.; Martins-Filho, O.A. Isotype patterns of immunoglobulins: Hallmarks for clinical status and tissue parasite density in Brazilian dogs naturally infected by Leishmania (Leishmania) chagasi. Vet. Immunol. Immunopathol. 2006, 112, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Neto, F.; Sousa, J.; Rodrigues, M.; Cabral, M. Use of a leishmanin skin test in the detection of canine Leishmania-specific cellular immunity. Vet. Parasitol. 1998, 79, 213–220. [Google Scholar] [CrossRef]
- Franzoni, G.; Kurkure, N.V.; Essler, S.E.; Pedrera, M.; Everett, H.E.; Bodman-Smith, K.B.; Crooke, H.R.; Graham, S.P. Proteome-wide screening reveals immunodominance in the CD8 T cell response against classical swine fever virus with antigen-specificity dependent on MHC class I haplotype expression. PLoS ONE 2013, 8, e84246. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; Aguiar-Soares, R.D.D.O.; Reis, L.E.S.; Cardoso, J.M.D.O.; Mathias, F.A.S.; De Brito, R.C.F.; Da Silva, S.M.; Gontijo, N.D.F.; Ferreira, S.D.A.; Valenzuela, J.G.; et al. A Vaccine Therapy for Canine Visceral Leishmaniasis Promoted Significant Improvement of Clinical and Immune Status with Reduction in Parasite Burden. Front. Immunol. 2017, 8, 217. [Google Scholar] [CrossRef]
- IEDB Epitope Conservancy Tool. Available online: http://tools.iedb.org/conservancy/ (accessed on 20 February 2019).
- Cardoso, J.M.O.; Ker, H.G.; Aguiar-Soares, R.D.O.; Moreira, N.D.D.; Mathias, F.A.S.; Reis, L.E.S.; Roatt, B.M.; Vieira, P.M.A.; Coura-Vital, W.; Carneiro, C.M.; et al. Association between masT-cells, tissue remodelation and parasite burden in the skin of dogs with visceral leishmaniasis. Vet. Parasitol. 2017, 243, 260–266. [Google Scholar] [CrossRef]
- Roatt, B.M.; Aguiar-Soares, R.D.; Vitoriano-Souza, J.; Coura-Vital, W.; Braga, S.L.; Correa-Oliveira, R.; Martins-Filho, O.A.; Teixeira-Carvalho, A.; de Lana, M.; Figueiredo Gontijo, N.; et al. Performance of LBSap vaccine after intradermal challenge with L. infantum and saliva of Lu. longipalpis: Immunogenicity and parasitological evaluation. PLoS ONE 2012, 7, e49780. [Google Scholar] [CrossRef]
- Reis, L.E.S.; Brito, R.C.F.; Cardoso, J.M.O.; Mathias, F.A.S.; Aguiar Soares, R.D.O.; Carneiro, C.M.; de Abreu Vieira, P.M.; Ramos, G.S.; Frezard, F.J.G.; Roatt, B.M.; et al. Mixed Formulation of Conventional and Pegylated Meglumine Antimoniate-Containing Liposomes Reduces Inflammatory Process and Parasite Burden in Leishmania infantum-Infected BALB/c Mice. Antimicrob. Agents Chemother. 2017, 61, e00962-17. [Google Scholar] [CrossRef]
- Agallou, M.; Margaroni, M.; Karagouni, E. Cellular vaccination with bone marrow-derived dendritic cells pulsed with a peptide of Leishmania infantum KMP-11 and CpG oligonucleotides induces protection in a murine model of visceral leishmaniasis. Vaccine 2011, 29, 5053–5064. [Google Scholar] [CrossRef]
- Lundegaard, C.; Lund, O.; Keşmir, C.; Brunak, S.; Nielsen, M. Modeling the adaptive immune system: Predictions and simulations. Bioinformatics 2007, 23, 3265–3275. [Google Scholar] [CrossRef]
- Herrera-Najera, C.; Piña-Aguilar, R.; Xacur-Garcia, F.; Ramirez-Sierra, M.J.; Dumonteil, E. Mining the Leishmania genome for novel antigens and vaccine candidates. Proteomics 2009, 9, 1293–1301. [Google Scholar] [CrossRef]
- Ferreira, L.F.G.R.; Hernandes, M.Z.; de Brito, M.E.F.; de Oliveira, B.C.; da Silva, A.A.; de-Melo-Neto, O.P.; Rezende, A.M.; Pereira, V.R.A. Combination of in silico methods in the search for potential CD4(+) and CD8(+) T cell epitopes in the proteome of Leishmania braziliensis. Front. Immunol. 2016, 7, 327. [Google Scholar]
- Debenham, S.L.; Hart, E.A.; Ashurst, J.L.; Howe, K.L.; Quail, M.A.; Ollier, W.E.; Binns, M.M. Genomic sequence of the class II region of the canine MHC: Comparison with the MHC of other mammalian species. Genomics 2005, 85, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.M.; Schreitmuller, C.M.; Proehl, F.; Oehl, K.; Lumpp, L.M.; Kowalewski, D.J.; Di Marco, M.; Sturm, T.; Backert, L.; Schuster, H.; et al. Characterization of the Canine MHC Class I DLA-88*50101 Peptide Binding Motif as a Prerequisite for Canine T Cell Immunotherapy. PLoS ONE 2016, 11, e0167017. [Google Scholar] [CrossRef] [PubMed]
- Badaró, R.; Carvalho, E.M.; Orge, M.D.L.G.O.; Teixeira, R.S.; Rocha, H. Imunidade humoral e celular em indivíduos curados de leishmaniose visceral. Rev. Da Soc. Bras. De Med. Trop. 1985, 18, 77–83. [Google Scholar] [CrossRef]
- Pinelli, E.; Killick-Kendrick, R.; Wagenaar, J.; Bernadina, W.; del Real, G.; Ruitenberg, J. Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect. Immun. 1994, 62, 229–235. [Google Scholar] [PubMed]
- Carcelen, J.; Iniesta, V.; Fernández-Cotrina, J.; Serrano, F.; Parejo, J.C.; Corraliza, I.; Gallardo-Soler, A.; Marañón, F.; Soto, M.; Alonso, C.; et al. The Chimerical Multi-Component Q protein from Leishmania in the absence of adjuvant protects dogs against an experimental Leishmania infantum infection. Vaccine 2009, 27, 5964–5973. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.B.; Teixeira-Carvalho, A.; Giunchetti, R.C.; Guerra, L.L.; Carvalho, M.G.; Mayrink, W.; Genaro, O.; Correa-Oliveira, R.; Martins-Filho, O.A. Phenotypic features of circulating leucocytes as immunological markers for clinical status and bone marrow parasite density in dogs naturally infected by Leishmania chagasi. Clin. Exp. Immunol. 2006, 146, 303–311. [Google Scholar] [CrossRef]
- Reis, A.B.; Giunchetti, R.C.; Carrillo, E.; Martins-Filho, O.A.; Moreno, J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends Parasitol. 2010, 26, 341–349. [Google Scholar] [CrossRef]
- Martins, V.T.; Duarte, M.C.; Lage, D.P.; Costa, L.E.; Carvalho, A.M.; Mendes, T.A.; Roatt, B.M.; Menezes-Souza, D.; Soto, M.; Coelho, E.A. A recombinant chimeric protein composed of human and mice-specific CD4(+) and CD8(+) T-cell epitopes protects against visceral leishmaniasis. Parasite Immunol. 2017, 39, e112359. [Google Scholar] [CrossRef]
- Collin, N.; Gomes, R.; Teixeira, C.; Cheng, L.; Laughinghouse, A.; Ward, J.M.; Elnaiem, D.E.; Fischer, L.; Valenzuela, J.G.; Kamhawi, S. Sand fly salivary proteins induce strong cellular immunity in a natural reservoir of visceral leishmaniasis with adverse consequences for Leishmania. PLoS Pathog. 2009, 5, e1000441. [Google Scholar] [CrossRef]
- Nico, D.; Gomes, D.C.; Alves-Silva, M.V.; Freitas, E.O.; Morrot, A.; Bahia, D.; Palatnik, M.; Rodrigues, M.M.; Palatnik-de-Sousa, C.B. Cross-protective immunity to Leishmania amazonensis is mediated by CD4+ and CD8+ epitopes of Leishmania donovani nucleoside hydrolase terminal domains. Front. Immunol. 2014, 5, 189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nico, D.; Claser, C.; Borja-Cabrera, G.P.; Travassos, L.R.; Palatnik, M.; Soares, I.S.; Rodrigues, M.M.; Palatnik-de-Sousa, C.B. Adaptive immunity against Leishmania nucleoside hydrolase maps its c-terminal domain as the target of the CD4+ T cell-driven protective response. PLoS Negl. Trop. Dis. 2010, 4, e866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dikhit, M.R.; Kumar, A.; Das, S.; Dehury, B.; Rout, A.K.; Jamal, F.; Sahoo, G.C.; Topno, R.K.; Pandey, K.; Das, V.N.R.; et al. Identification of Potential MHC Class-II-Restricted Epitopes Derived from Leishmania donovani Antigens by Reverse Vaccinology and Evaluation of Their CD4+ T-Cell Responsiveness against Visceral Leishmaniasis. Front. Immunol. 2017, 8, 1763. [Google Scholar] [CrossRef] [PubMed]
- Sabur, A.; Bhowmick, S.; Chhajer, R.; Ejazi, S.A.; Didwania, N.; Asad, M.; Bhattacharyya, A.; Sinha, U.; Ali, N. Liposomal Elongation Factor-1alpha Triggers Effector CD4 and CD8 T-cells for Induction of Long-Lasting Protective Immunity against Visceral Leishmaniasis. Front. Immunol. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Nico, D.; Martins Almeida, F.; Maria Motta, J.; Soares Dos Santos Cardoso, F.; Freire-de-Lima, C.G.; Freire-de-Lima, L.; de Luca, P.M.; Maria Blanco Martinez, A.; Morrot, A.; Palatnik-de-Sousa, C.B. NH36 and F3 Antigen-Primed Dendritic Cells Show Preserved Migrating Capabilities and CCR7 Expression and F3 Is Effective in Immunotherapy of Visceral Leishmaniasis. Front. Immunol. 2018, 9, 967. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sampedro, L.; Gomez, C.E.; Mejias-Perez, E.; Sorzano, C.O.; Esteban, M. High quality long-term CD4+ and CD8+ effector memory populations stimulated by DNA-LACK/MVA-LACK regimen in Leishmania major BALB/c model of infection. PLoS ONE 2012, 7, e38859. [Google Scholar] [CrossRef]
- Dey, R.; Dagur, P.K.; Selvapandiyan, A.; McCoy, J.P.; Salotra, P.; Duncan, R.; Nakhasi, H.L. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. J. Immunol. 2013, 190, 2138–2149. [Google Scholar] [CrossRef]
- Seder, R.A.; Darrah, P.A.; Roederer, M. T-cell quality in memory and protection: Implications for vaccine design. Nat. Rev. Immunol. 2008, 8, 247–258. [Google Scholar] [CrossRef]
- Alves-Silva, M.V.; Nico, D.; Morrot, A.; Palatnik, M.; Palatnik-de-Sousa, C.B. A chimera containing CD4+ and CD8+ T-cell epitopes of the Leishmania donovani nucleoside hydrolase (NH36) optimizes cross-protection against Leishmania amazonesis infection. Front. Immunol. 2017, 8, 100. [Google Scholar] [CrossRef]
- Selvapandiyan, A.; Dey, R.; Nylén, S.; Duncan, R.; Sacks, D.; Nakhasi, H.L. Intracellular Replication-Deficient Leishmania donovani Induces Long Lasting Protective Immunity against Visceral Leishmaniasis. J. Immunol. 2009, 183, 1813–1820. [Google Scholar] [CrossRef]
- Margaroni, M.; Agallou, M.; Athanasiou, E.; Kammona, O.; Kiparissides, C.; Gaitanaki, C.; Karagouni, E. Vaccination with poly(D,L-lactide-co-glycolide) nanoparticles loaded with soluble Leishmania antigens and modified with a TNFalpha-mimicking peptide or monophosphoryl lipid A confers protection against experimental visceral leishmaniasis. Int. J. Nanomed. 2017, 12, 6169–6184. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Margaroni, M.; Athanasiou, E.; Toubanaki, D.K.; Kontonikola, K.; Karidi, K.; Kammona, O.; Kiparissides, C.; Karagouni, E. Identification of BALB/c Immune Markers Correlated with a Partial Protection to Leishmania infantum after Vaccination with a Rationally Designed Multi-epitope Cysteine Protease A Peptide-Based Nanovaccine. PLoS Negl. Trop. Dis. 2017, 11, e0005311. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, E.; Agallou, M.; Tastsoglou, S.; Kammona, O.; Hatzigeorgiou, A.; Kiparissides, C.; Karagouni, E. A poly(Lactic-co-Glycolic) acid nanovaccine based on chimeric peptides from different Leishmania infantum proteins induces dendritic cells maturation and promotes peptide-specific IFN gamma-producing CD8(+) T-cells essential for the protection against experimental visceral leishmaniasis. Front. Immunol. 2017, 8, 684. [Google Scholar] [PubMed]
- Martins, V.T.; Chavez-Fumagalli, M.A.; Lage, D.P.; Duarte, M.C.; Garde, E.; Costa, L.E.; da Silva, V.G.; Oliveira, J.S.; Magalhaes-Soares, D.F.; Teixeira, S.M.; et al. Antigenicity, Immunogenicity and Protective Efficacy of Three Proteins Expressed in the Promastigote and Amastigote Stages of Leishmania infantum against Visceral Leishmaniasis. PLoS ONE 2015, 10, e0137683. [Google Scholar]
- Ramos, F.F.; Costa, L.E.; Dias, D.S.; Santos, T.T.O.; Rodrigues, M.R.; Lage, D.P.; Salles, B.C.S.; Martins, V.T.; Ribeiro, P.A.F.; Chávez-Fumagalli, M.A.; et al. Selection strategy of phage-displayed immunogens based on an in vitro evaluation of the Th1 response of PBMCs and their potential use as a vaccine against Leishmania infantum infection. Parasites Vectors 2017, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bhattacharya, P.; Dagur, P.K.; Karmakar, S.; Ismail, N.; Joshi, A.B.; Akue, A.D.; KuKuruga, M.; McCoy, J.P., Jr.; Dey, R.; et al. Live Attenuated Leishmania donovani Centrin Gene-Deleted Parasites Induce IL-23-Dependent IL-17-Protective Immune Response against Visceral Leishmaniasis in a Murine Model. J. Immunol. 2018, 200, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gomes, G.; Rodrigues, A.; Teixeira, F.; Carreira, J.; Alexandre-Pires, G.; Carvalho, S.; Santos-Mateus, D.; Martins, C.; Vale-Gato, I.; Marques, C.; et al. Immunization with the Leishmania infantum recombinant cyclophilin protein 1 confers partial protection to subsequent parasite infection and generates specific memory T cells. Vaccine 2014, 32, 1247–1253. [Google Scholar] [CrossRef]
- Farber, D.L.; Yudanin, N.A.; Restifo, N.P. Human memory T-cells: Generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014, 14, 24–35. [Google Scholar] [CrossRef]




| Peptide ID | Sequence | % of Conservancy in Leishmania Species | |||
|---|---|---|---|---|---|
| L. donovani | L. major | L. braziliennsis | L. amazonensis | ||
| PEP4 | QMVYNQDEI | 100 | 100 | 100 | 88.98 |
| PEP12 | RLCPRGHSL | 100 | 100 | 88.98 | 88.98 |
| PEP13 | QSGHNSGCL | 100 | 100 | 88.98 | 100 |
| PEP15 | FALKRLSSL | 100 | 100 | 66.67 | 100 |
| PEP17 | SVIHNATVV | 100 | 100 | 100 | 100 |
| PEP25 | GGHFFFYVPPSPILF | 93 | 80 | 80 | 80 |
| PEP30 | KGTTYPTTPNGLPSV | 100 | 100 | 86.76 | 86.76 |
| PEP33 | IRQGFESFPPTPKTS | 100 | 100 | 86.76 | 100 |
| PEP34 | GFESFPPTPKTSMM | 100 | 100 | 86.76 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Brito, R.C.F.; Cardoso, J.M.d.O.; Reis, L.E.S.; Mathias, F.A.S.; Aguiar-Soares, R.D.d.O.; Teixeira-Carvalho, A.; Roatt, B.M.; Corrêa-Oliveira, R.; Ruiz, J.C.; Resende, D.d.M.; et al. Synthetic Peptides Elicit Strong Cellular Immunity in Visceral Leishmaniasis Natural Reservoir and Contribute to Long-Lasting Polyfunctional T-Cells in BALB/c Mice. Vaccines 2019, 7, 162. https://doi.org/10.3390/vaccines7040162
De Brito RCF, Cardoso JMdO, Reis LES, Mathias FAS, Aguiar-Soares RDdO, Teixeira-Carvalho A, Roatt BM, Corrêa-Oliveira R, Ruiz JC, Resende DdM, et al. Synthetic Peptides Elicit Strong Cellular Immunity in Visceral Leishmaniasis Natural Reservoir and Contribute to Long-Lasting Polyfunctional T-Cells in BALB/c Mice. Vaccines. 2019; 7(4):162. https://doi.org/10.3390/vaccines7040162
Chicago/Turabian StyleDe Brito, Rory Cristiane Fortes, Jamille Mirelle de Oliveira Cardoso, Levi Eduardo Soares Reis, Fernando Augusto Siqueira Mathias, Rodrigo Dian de Oliveira Aguiar-Soares, Andréa Teixeira-Carvalho, Bruno Mendes Roatt, Rodrigo Corrêa-Oliveira, Jeronimo Conceição Ruiz, Daniela de Melo Resende, and et al. 2019. "Synthetic Peptides Elicit Strong Cellular Immunity in Visceral Leishmaniasis Natural Reservoir and Contribute to Long-Lasting Polyfunctional T-Cells in BALB/c Mice" Vaccines 7, no. 4: 162. https://doi.org/10.3390/vaccines7040162
APA StyleDe Brito, R. C. F., Cardoso, J. M. d. O., Reis, L. E. S., Mathias, F. A. S., Aguiar-Soares, R. D. d. O., Teixeira-Carvalho, A., Roatt, B. M., Corrêa-Oliveira, R., Ruiz, J. C., Resende, D. d. M., & Reis, A. B. (2019). Synthetic Peptides Elicit Strong Cellular Immunity in Visceral Leishmaniasis Natural Reservoir and Contribute to Long-Lasting Polyfunctional T-Cells in BALB/c Mice. Vaccines, 7(4), 162. https://doi.org/10.3390/vaccines7040162






