Measles Virus Infection and Immunity in a Suboptimal Vaccination Coverage Setting
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Seroprevalence Study
2.1.2. Measles-Specific Antibody Titers in Vaccinees
2.1.3. Measles Cases
2.1.4. Measles Vaccine Cross-Neutralization Study
2.2. Laboratory Procedures
2.3. Evolutionary and Phylogenetic Analyses
2.4. Statistical Analysis
3. Results
3.1. Measles Vaccination Uptake and Seroprevalence
3.2. Description of Measles Outbreaks
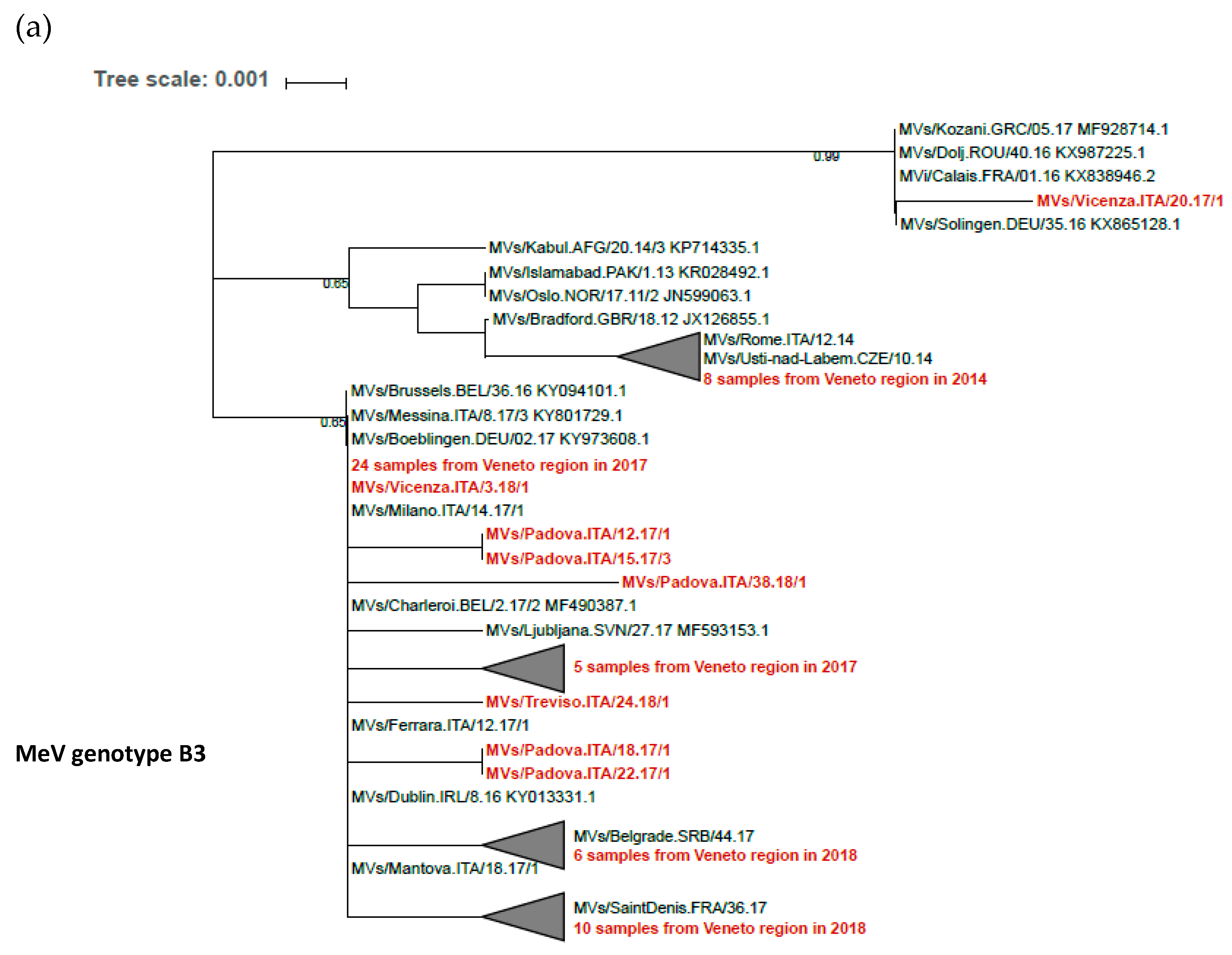
3.3. MeV Genome Diversity and Evolution in Transmission Clusters
3.4. Vaccine-Induced Cross-Neutralizing Antibodies against Epidemic MeV Strains
3.5. Measles in Previously Immunized Subjects
3.6. Persistence of Measles Vaccine-Induced Immunity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moss, W.J. Measles. Lancet 2017, 390, 2490–2502. [Google Scholar] [CrossRef]
- World Health Organization. Global Measles and Rubella Update. November 2018. Available online: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/GlobaG_MR_Update_November_2018.pdf?ua=1 (accessed on 2 November 2019).
- European Centre for Disease Prevention and Control. Monthly Measles and Rubella Monitoring Report, December 2018. ECDC: Stockholm, Sweden, 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/monthly-measles-and-rubella-monitoring-report-december-2018 (accessed on 2 November 2019).
- Holt, E. 41 000 measles cases in Europe since the beginning of 2018. Lancet 2018, 392, 724. [Google Scholar] [CrossRef]
- Burki, T. Measles in Europe. Lancet Infect. Dis. 2018, 18, 1070–1071. [Google Scholar] [CrossRef]
- Trentini, F.; Poletti, P.; Merler, S.; Melegaro, A. Measles immunity gaps and the progress toward elimination: A multi-country modelling analysis. Lancet Infect. Dis. 2017, 17, 1089–1097. [Google Scholar] [CrossRef]
- Melenotte, C.; Zandotti, C.; Gautret, P.; Parola, P.; Raoult, D. Measles: Is a new vaccine approach needed? Lancet Infect. Dis. 2018, 18, 1060–1061. [Google Scholar] [CrossRef]
- Rota, P.A.; Brown, K.; Mankertz, A.; Santibanez, S.; Shulga, S.; Muller, C.P.; Hübschen, J.M.; Siqueira, M.; Beirnes, J.; Ahmed, H.; et al. Global distribution of measles genotypes and measles molecular epidemiology. J. Infect. Dis. 2011, 204 (Suppl. 1), 514–523. [Google Scholar] [CrossRef] [PubMed]
- Ackley, S.F.; Hacker, J.K.; Enanoria, W.T.A.; Worden, L.; Blumberg, S.; Porco, T.C.; Zipprich, J. Genotype-specific measles transmissibility: A branching process analysis. Clin. Infect. Dis. 2018, 66, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- El Mubarak, H.S.; Yüksel, S.; van Amerongen, G.; Mulder, P.G.; Mukhtar, M.M.; Osterhaus, A.D.; de Swart, R.L. Infection of cynomolgus macaques (Macaca fascicularis) and rhesus macaques (Macaca mulatta) with different wild-type measles viruses. J. Gen. Virol. 2007, 88, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Fatemi Nasab, G.S.; Salimi, V.; Abbasi, S.; Adjami Nezhad Fard, F.; Mokhtari Azad, T. Comparison of neutralizing antibody titers against outbreak-associated measles genotypes (D4, H1 and B3) in Iran. Pathog. Dis. 2016, 74. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Measles & Rubella Strategic Plan 2012–2020. Geneva. 2012. Available online: http://apps.who.int/iris/bitstream/10665/44855/1/9789241503396_eng.pdf (accessed on 2 November 2019).
- Toniolo, F.; Mantoan, D.; Maresso, A. Veneto Region, Italy: Health system review. Health Syst. Transit. 2012, 14, 1–138. [Google Scholar]
- Anello, P.; Cestari, L.; Baldovin, T.; Simonato, L.; Frasca, G.; Caranci, N.; Pascucci, M.G.; Valent, F.; Canova, C. Socioeconomic factors influencing childhood vaccination in two northern Italian regions. Vaccine 2017, 35, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; Odone, A. Four Italian experiences on vaccination policies: Results and lessons. Ann. Ig. 2019, 31, 36–44. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Manual for the Laboratory Diagnosis of Measles and Rubella Virus Infection. Geneva. 2007. Available online: https://www.who.int/ihr/elibrary/manual_diagn_lab_mea_rub_en.pdf (accessed on 2 November 2019).
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Tavaré, S. Some probabilistic and statistical problems in the analysis of DNA sequences. Lect. Math. Life Sci. 1986, 17, 57–86. [Google Scholar]
- Weaver, S.; Shank, S.D.; Spielman, S.J.; Li, M.; Muse, S.V.; Kosakovsky Pond, S.L. Datamonkey 2.0: A modern web application for characterizing selective and other evolutionary processes. Mol. Biol. Evol. 2018, 35, 773–777. [Google Scholar] [CrossRef]
- Kosakovsky Pond, S.L.; Frost, S.D. Not so different after all: A comparison of methods for detecting amino acid sites under selection. Mol. Biol. Evol. 2005, 22, 1208–1222. [Google Scholar] [CrossRef]
- Murrell, B.; Moola, S.; Mabona, A.; Weighill, T.; Sheward, D.; Kosakovsky Pond, S.L.; Scheffler, K. FUBAR: A fast, unconstrained bayesian approximation for inferring selection. Mol. Biol. Evol. 2013, 30, 1196–1205. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Ministero della Salute. Vaccinazioni dell’età pediatrica e dell’adolescente—Coperture vaccinali. Available online: http://www.salute.gov.it/portale/documentazione/p6_2_8_3_1.jsp?lingua=italiano&id=20 (accessed on 1 November 2019).
- Regione del Veneto. Sistema Vaccinale Regionale. Available online: https://www.regione.veneto.it/web/sanita/sistema-vaccinale-regionale (accessed on 1 November 2019).
- Signorelli, C.; Odone, A.; Cella, P.; Iannazzo, S. Childhood vaccine coverage in Italy after the new law on mandatory immunization. Ann. Ig. 2018, 30 (Suppl. 1), 1–10. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità. EpiCentro. Measles. Archives. Available online: https://www.epicentro.iss.it/morbillo/archivio. (accessed on 2 November 2019).
- Pomeroy, L.W.; Bjørnstad, O.N.; Holmes, E.C. The evolutionary and epidemiological dynamics of the Paramyxoviridae. J. Mol. Evol. 2008, 66, 98–106. [Google Scholar] [CrossRef] [PubMed]
- De Swart, R.L.; Yüksel, S.; Osterhaus, A.D. Relative contributions of measles virus hemagglutinin- and fusion protein-specific serum antibodies to virus neutralization. J. Virol. 2005, 79, 11547–11551. [Google Scholar] [CrossRef] [PubMed]
- Dine, M.S.; Hutchins, S.S.; Thomas, A.; Williams, I.; Bellini, W.J.; Redd, S.C. Persistence of vaccine-induced antibody to measles 26–33 year after vaccination. J. Infect. Dis. 2004, 189 (Suppl. 1), 123–130. [Google Scholar] [CrossRef]
- Wendorf, K.A.; Winter, K.; Zipprich, J.; Schechter, R.; Hacker, J.K.; Preas, C.; Cherry, J.D.; Glaser, C.; Harriman, K. Subacute sclerosing panencephalitis: The devastating measles complication that might be more common than previously estimated. Clin. Infect. Dis. 2017, 65, 226–232. [Google Scholar] [CrossRef]
- Hoes, J.; Knol, M.J.; Mollema, L.; Buisman, A.; de Melker, H.E.; van der Klis, F.R.M. Comparison of antibody response between boys and girls after infant and childhood vaccinations in the Netherlands. Vaccine 2019, 37, 4504–4510. [Google Scholar] [CrossRef]
- Gardy, L.J.; Maus, M.; Amlani, A.; Chung, W.; Kim, H.; Tan, M.; Severini, A.; Krajden, M.; Puddicombe, D.; Sahni, V.; et al. Whole-genome sequencing of measles virus genotypes H1 and D8 during outbreaks of infection following the 2010 Olympic Winter Games reveals viral transmission routes. J. Infect. Dis. 2015, 212, 1574–1578. [Google Scholar] [CrossRef]
- Rosen, J.B.; Rota, J.S.; Hickman, C.J.; Sowers, S.B.; Mercader, S.; Rota, P.A.; Bellini, W.J.; Huang, A.J.; Doll, M.K.; Zucker, J.R.; et al. Outbreak of measles among persons with prior evidence of immunity, New York City, 2011. Clin. Infect. Dis. 2014, 58, 1205–1210. [Google Scholar] [CrossRef]
- Cherry, J.D.; Zahn, M. Clinical characteristics of measles in previously vaccinated and unvaccinated patients in California. Clin. Infect. Dis. 2018, 67, 1315–1319. [Google Scholar] [CrossRef]
- Bernadou, A.; Astrugue, C.; Méchain, M.; Le Galliard, V.; Verdun-Esquer, C.; Dupuy, F.; Dina, J.; Aït-Belghiti, F.; Antona, D.; Vandentorren, S. Measles outbreak linked to insufficient vaccination coverage in Nouvelle-Aquitaine Region, France, October 2017 to July 2018. Euro. Surveill. 2018, 23. [Google Scholar] [CrossRef]
- Gibney, K.B.; Attwood, L.O.; Nicholson, S.; Tran, T.; Druce, J.; Healy, J.; Strachan, J.; Franklin, L.; Hall, R.; Cross, G.B. Emergence of attenuated measles illness among IgG positive/IgM negative measles cases, Victoria, Australia 2008–2017. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef]
- Munoz-Alia, M.A.; Muller, C.P.; Russell, S.J. Antigenic drift defines a new D4 subgenotype of measles virus. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, T.; Barbezange, C.; Francart, A.; Hamouda, A.; Litzroth, A.; Hutse, V.; Martens, L.; Vandermarliere, E.; Van Gucht, S. Sera from different age cohorts in Belgium show limited cross-neutralisation between the mumps vaccine and outbreak strains. Clin. Microbiol. Infect. 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- Gouma, S.; Ten Hulscher, H.I.; Schurink-van ‘t Klooster, T.M.; de Melker, H.E.; Boland, G.J.; Kaaijk, P.; van Els, C.A.C.M.; Koopmans, M.P.G.; van Binnendijk, R.S. Mumps-specific cross-neutralization by MMR vaccine-induced antibodies predicts protection against mumps virus infection. Vaccine 2016, 34, 4166–4171. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Bürckert, J.P.; Kanou, K.; Maenaka, K.; Muller, C.P.; Takeda, M. Measles virus hemagglutinin protein epitopes: The basis of antigenic stability. Viruses 2016, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Gidding, H.F.; Quinn, H.E.; Hueston, L.; Dwyer, D.E.; McIntyre, P.B. Declining measles antibodies in the era of elimination: Australia’s experience. Vaccine 2018, 36, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Haralambieva, I.H.; Ovsyannikova, I.G.; Kennedy, R.B.; Vierkant, R.A.; Pankratz, V.S.; Jacobson, R.M.; Poland, G.A. Associations between single nucleotide polymorphisms and haplotypes in cytokine and cytokine receptor genes and immunity to measles vaccination. Vaccine 2011, 29, 7883–7895. [Google Scholar] [CrossRef]
- Haralambieva, I.H.; Kennedy, R.B.; Ovsyannikova, I.G.; Schaid, D.J.; Poland, G.A. Current perspectives in assessing humoral immunity after measles vaccination. Expert Rev. Vaccin. 2019, 18, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, N.; Bucciol, G.; Moens, L.; Le Pen, J.; Shahrooei, M.; Goudouris, E.; Shirkani, A.; Changi-Ashtiani, M.; Rokni-Zadeh, H.; Sayar, E.H.; et al. Inherited IFNAR1 deficiency in otherwise healthy patients with adverse reaction to measles and yellow fever live vaccines. J. Exp. Med. 2019, 216, 2057–2070. [Google Scholar] [CrossRef]
- Duncan, C.J.; Mohamad, S.M.; Young, D.F.; Skelton, A.J.; Leahy, T.R.; Munday, D.C.; Butler, K.M.; Morfopoulou, S.; Brown, J.R.; Hubank, M.; et al. Human IFNAR2 deficiency: Lessons for antiviral immunity. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef]
- Burns, C.; Cheung, A.; Stark, Z.; Choo, S.; Downie, L.; White, S.; Conyers, R.; Cole, T. A novel presentation of homozygous loss-of-function STAT-1 mutation in an infant with hyperinflammation—A case report and review of the literature. J. Allergy Clin. Immunol. Pract. 2016, 4, 777–779. [Google Scholar] [CrossRef]
- Hambleton, S.; Goodbourn, S.; Young, D.F.; Dickinson, P.; Mohamad, S.M.B.; Valappil, M.; McGovern, N.; Cant, A.J.; Hackett, S.J.; Ghazal, P.; et al. STAT2 deficiency and susceptibility to viral illness in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 3053–3058. [Google Scholar] [CrossRef] [PubMed]
- Moens, L.; Van Eyck, L.; Jochmans, D.; Mitera, T.; Frans, G.; Bossuyt, X.; Matthys, P.; Neyts, J.; Ciancanelli, M.; Zhang, S.-Y.; et al. A novel kindred with inherited STAT2 deficiency and severe viral illness. J. Allergy Clin. Immunol. 2017, 139, 1995–1997. [Google Scholar] [CrossRef] [PubMed]
- Durrheim, D.N.; Orenstein, W.A.; Schluter, W.W. Assessing population immunity for measles elimination—The promise and peril of serosurveys. Vaccine 2018, 36, 4001–4003. [Google Scholar] [CrossRef] [PubMed]
- Hahné, S.J.; Nic Lochlainn, L.M.; van Burgel, N.D.; Kerkhof, J.; Sane, J.; Yap, K.B.; van Binnendijk, R.S. Measles outbreak among previously immunized healthcare workers, the Netherlands, 2014. J. Infect. Dis. 2016, 214, 1980–1986. [Google Scholar] [CrossRef]






| Gene | dN/dS | Negatively Selected Sites (Amino Acid Position) |
|---|---|---|
| Genotype B3 (25 samples) | ||
| L | 0.249 | 1695 |
| N | 0.107 | 220 |
| P | 0 | - |
| Genotype D8 (5 samples) | ||
| H | 0.643 | - |
| L | 0.318 | 56 |
| N | 0.839 | - |
| Measles Vaccine Doses | Age (Years) | Years since Vaccination | Days from Rash | MeV IgM EIA Finding | MeV IgG EIA (IU/L) | MeV IgG avidity (%) | MeV RNA Pharyngeal Swab (CT) | MeV RNA Urine (CT) | MeV Strain |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 26 | 22 | 4 | positive | 15,000 | 84 | ND | 39 | MVs/Padova.ITA/14.17/9 [B3] |
| 2 | 30 | 26 | 0 | negative | 2100 | 58 | 24 | 30 | MVs/Venezia.ITA/16.17/1 [B3] |
| 2 | 26 | 22 | 1 | borderline | 12,000 | 82 | 27 | 28 | MVs/Verona.ITA/20.17/1 [D8] |
| 2 | 22 | 18 | 2 | borderline | 5000 | 81 | 30 | ND | MVs/Padova.ITA/13.18/1 [B3] |
| 2 | 13 | 9 | 1 | negative | 1460 | 69 | 38 | 39 | ND |
| unknown | 36 | - | 1 | negative | 2200 | 74 | 23 | 30 | MVs/Padova.ITA/13.17/3 [B3] |
| unknown | 42 | - | 1 | negative | 23,000 | 79 | 27 | ND | MVs/Padova.ITA/17.17/4 [D8] |
| unknown | 39 | - | 1 | negative | 5700 | 68 | 28 | 28 | MVs/Padova.ITA/16.17/8 [B3] |
| unknown | 68 | - | 1 | negative | 13,000 | 86 | ND | 27 | MVs/Venezia.ITA/16.17/1 [B3] |
| unknown | 29 | - | 1 | borderline | 21,000 | 81 | 22 | 18 | MVs/Venezia.ITA/10.17/2 [B3] |
| unknown | 52 | - | 2 | positive | 15,000 | 84 | 29 | 30 | MVs/Venezia.ITA/16.17/2 [B3] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacenti, M.; Maione, N.; Lavezzo, E.; Franchin, E.; Dal Bello, F.; Gottardello, L.; Barzon, L. Measles Virus Infection and Immunity in a Suboptimal Vaccination Coverage Setting. Vaccines 2019, 7, 199. https://doi.org/10.3390/vaccines7040199
Pacenti M, Maione N, Lavezzo E, Franchin E, Dal Bello F, Gottardello L, Barzon L. Measles Virus Infection and Immunity in a Suboptimal Vaccination Coverage Setting. Vaccines. 2019; 7(4):199. https://doi.org/10.3390/vaccines7040199
Chicago/Turabian StylePacenti, Monia, Nataskya Maione, Enrico Lavezzo, Elisa Franchin, Federico Dal Bello, Lorena Gottardello, and Luisa Barzon. 2019. "Measles Virus Infection and Immunity in a Suboptimal Vaccination Coverage Setting" Vaccines 7, no. 4: 199. https://doi.org/10.3390/vaccines7040199
APA StylePacenti, M., Maione, N., Lavezzo, E., Franchin, E., Dal Bello, F., Gottardello, L., & Barzon, L. (2019). Measles Virus Infection and Immunity in a Suboptimal Vaccination Coverage Setting. Vaccines, 7(4), 199. https://doi.org/10.3390/vaccines7040199







