Simple Nanoparticles from the Assembly of Cationic Polymer and Antigen as Immunoadjuvants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Stock Solutions
2.2. Preparation of the PDDA/OVA Nanoparticles (NPs)
2.3. Determination of Physical Properties and Colloidal Stability for NPs Dispersions from Dynamic Light Scattering (DLS), Turbidimetry, and Scanning Electron Microscopy (SEM)
2.4. Cell Culture, Preparation of PDDA, OVA, or PDDA/OVA Solutions for Interaction with Cells, and Determination of Cell Viability in the Interaction Mixtures
2.5. Determination of Cells Morphology in the Presence of PDDA by Scanning Electron Microscopy (SEM)
2.6. Immunization Scheme
2.7. Determination of OVA-Specific IgG1 and IgG2a Antibodies
2.8. Determination of Delayed-Type Hypersensitivity Reaction (DTH)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of PDDA/OVA Dispersions Regarding Formation of Nanoparticles, Their Size, Surface Potential, Polydispersity, Morphology, and Colloidal Stability.
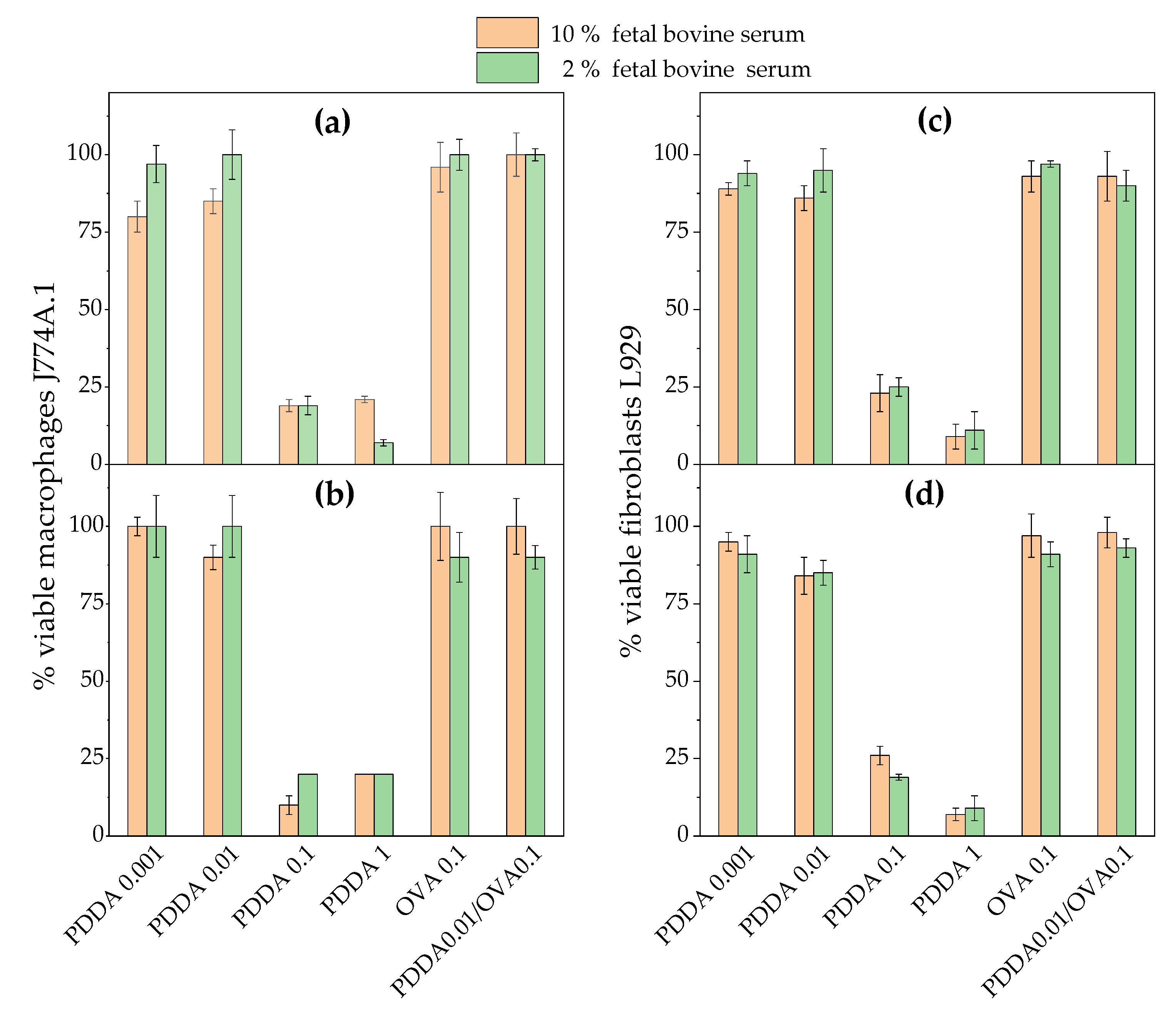
3.2. Cytotoxicity of PDDA/OVA NPs Against Mammalian Cells in Culture
3.3. Immunoadjuvant Properties of PDDA/OVA NPs.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Bergmann-Leitner, E.S.; Leitner, W.W. Adjuvants in the driver’s seat: How magnitude, type, fine specificity and longevity of immune responses are driven by distinct classes of immune potentiators. Vaccines 2014, 2, 252–296. [Google Scholar] [CrossRef] [Green Version]
- Pasquale, A.; Preiss, S.; Silva, F.; Garçon, N. Vaccine adjuvants: From 1920 to 2015 and beyond. Vaccines 2015, 3, 320–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. Npj Vaccines 2018, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Martiñón, S.; Cisneros, A.; Villicaña, S.; Hernández-Miramontes, R.; Mixcoha, E.; Calderón-Vargas, P. Chemical and immunological characteristics of aluminum-based, oil-water emulsion, and bacterial-origin adjuvants. J. Immunol. Res. 2019, 2019, 3974127. [Google Scholar] [CrossRef] [Green Version]
- Apostólico, J.d.S.; Lunardelli, V.A.S.; Coirada, F.C.; Boscardin, S.B.; Rosa, D.S. Adjuvants: Classification, modus operandi, and licensing. J. Immunol. Res. 2016, 2016, 1459394. [Google Scholar]
- Lincopan, N.; Espindola, N.M.; Vaz, A.J.; da Costa, M.H.B.; Faquim-Mauro, E.; Carmona-Ribeiro, A.M. Novel immunoadjuvants based on cationic lipid: Preparation, characterization and activity in vivo. Vaccine 2009, 27, 5760–5771. [Google Scholar] [CrossRef]
- Lincopan, N.; Espindola, N.M.; Vaz, A.J.; Carmona-Ribeiro, A.M. Cationic supported lipid bilayers for antigen presentation. Int. J. Pharm. 2007, 340, 216–222. [Google Scholar] [CrossRef]
- Nawwab Al-Deen, F.M.; Selomulya, C.; Kong, Y.Y.; Xiang, S.D.; Ma, C.; Coppel, R.L.; Plebanski, M. Design of magnetic polyplexes taken up efficiently by dendritic cell for enhanced DNA vaccine delivery. Gene Ther. 2014, 21, 212–218. [Google Scholar] [CrossRef]
- Rose, F.; Wern, J.E.; Gavins, F.; Andersen, P.; Follmann, F.; Foged, C. A strong adjuvant based on glycol-chitosan-coated lipid-polymer hybrid nanoparticles potentiates mucosal immune responses against the recombinant Chlamydia trachomatis fusion antigen CTH522. J. Control. Release 2018, 271, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, L.R.; Quintilio, W.; Costa, M.H.; Carmona-Ribeiro, A.M. Interactions between cationic liposomes and an antigenic protein: The physical chemistry of the immunoadjuvant action. J. Lipid Res. 1997, 38, 2003–2011. [Google Scholar] [PubMed]
- Davidsen, J.; Rosenkrands, I.; Christensen, D.; Vangala, A.; Kirby, D.; Perrie, Y.; Agger, E.M.; Andersen, P. Characterization of cationic liposomes based on dimethyldioctadecylammonium and synthetic cord factor from M. tuberculosis (trehalose 6,6′-dibehenate)-a novel adjuvant inducing both strong CMI and antibody responses. Biochim. Biophys. Acta 2005, 1718, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riehl, M.; Harms, M.; Hanefeld, A.; Baleeiro, R.B.; Walden, P.; Mader, K. Combining R-DOTAP and a particulate antigen delivery platform to trigger dendritic cell activation: Formulation development and in-vitro interaction studies. Int. J. Pharm. 2017, 532, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, J.H.K.; Silva, S.R.; Raneia, P.A.; Faquim-Mauro, E.; Carmona-Ribeiro, A.M. Stable assemblies of cationic bilayer fragments and CpG oligonucleotide with enhanced immunoadjuvant activity in vivo. J. Control. Release 2012, 160, 367–373. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.F.; De Gaspari, E. Dioctadecyldimethylammonium bromide (DODAB-BF) as a new adjuvant for maternal-fetal immunization in mice against Neisseria meningitidis: Evaluation of humoral response. Pathog. Dis. 2018, 76, ftx128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lincopan, N.; Santana, M.R.; Faquim-Mauro, E.; da Costa, M.H.B.; Carmona-Ribeiro, A.M. Silica-based cationic bilayers as immunoadjuvants. BMC Biotechnol. 2009, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Nawwab Al-Deen, F.; Ma, C.; Xiang, S.D.; Selomulya, C.; Plebanski, M.; Coppel, R.L. On the efficacy of malaria DNA vaccination with magnetic gene vectors. J. Control. Release 2013, 168, 10–17. [Google Scholar] [CrossRef]
- Xiang, S.D.; Scholzen, A.; Minigo, G.; David, C.; Apostolopoulos, V.; Mottram, P.L.; Plebanski, M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods 2006, 40, 1–9. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Cationic Nanostructures for Vaccines. In Immune Response Activation; Duc, G.H.T., Ed.; InTech: Rijeka, Croatia, 2014; pp. 1–45. ISBN 978-953-51-1374-4. [Google Scholar]
- Pelkmans, L. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim. Biophys. Acta 2005, 1746, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.C.; Plebanski, M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hagan, D.T.; MacKichan, M.L.; Singh, M. Recent developments in adjuvants for vaccines against infectious diseases. Biomol. Eng. 2001, 18, 69–85. [Google Scholar] [CrossRef]
- Denis-Mize, K.S.; Dupuis, M.; MacKichan, M.L.; Singh, M.; Doe, B.; O’Hagan, D.; Ulmer, J.B.; Donnelly, J.J.; McDonald, D.M.; Ott, G. Plasmid DNA adsorbed onto cationic microparticles mediates target gene expression and antigen presentation by dendritic cells. Gene Ther. 2000, 7, 2105–2112. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M. Nanomaterials based on lipids for vaccine development. In Micro and Nano Technologies; Skwarczynski, M., Toth, I.B.T.-M., Eds.; Elsevier: Oxford, UK; Cambridge, MA, USA, 2017; pp. 241–257. ISBN 978-0-323-39981-4. [Google Scholar]
- Carmona-Ribeiro, A.M. The versatile dioctadecyldimethylammonium bromide. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: Rijeka, Croatia, 2017; pp. 157–182. ISBN 978-953-51-3326-1. [Google Scholar]
- Holten-Andersen, L.; Doherty, T.M.; Korsholm, K.S.; Andersen, P. Combination of the cationic surfactant dimethyl dioctadecyl ammonium bromide and synthetic mycobacterial cord factor as an efficient adjuvant for tuberculosis subunit vaccines. Infect. Immun. 2004, 72, 1608–1617. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Santos, F.A.; Lincopan, N.; De Gaspari, E. Evaluation of intranasal and subcutaneous route of immunization in neonatal mice using DODAB-BF as adjuvant with outer membrane vesicles of Neisseria meningitis B. Immunobiology 2018, 223, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Monnery, B.D.; Wright, M.; Cavill, R.; Hoogenboom, R.; Shaunak, S.; Steinke, J.H.G.; Thanou, M. Cytotoxicity of polycations: Relationship of molecular weight and the hydrolytic theory of the mechanism of toxicity. Int. J. Pharm. 2017, 521, 249–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, J.S.; Keller, T.C.S.; Schlenoff, J.B. Cytotoxicity of free versus multilayered polyelectrolytes. Biomacromolecules 2011, 12, 4063–4070. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, L.D.d.M.; Sampaio, J.L.M.; Carmona-Ribeiro, A.M. Supramolecular cationic assemblies against multidrug-resistant microorganisms: Activity and mechanism of action. Int. J. Mol. Sci. 2015, 16, 6337–6352. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.; Hwang, J.; Lee, H.; Hammond, P.T.; Choi, J.; Hong, J. In vitro blood cell viability profiling of polymers used in molecular assembly. Sci. Rep. 2017, 7, 9481. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, K.; Si, Y.; Guo, X.; Xu, Y. Protein-polyelectrolyte interaction: Thermodynamic analysis based on the titration method (†). Polymers 2019, 11, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wusiman, A.; Gu, P.; Liu, Z.; Xu, S.; Zhang, Y.; Hu, Y.; Liu, J.; Wang, D.; Huang, X. Cationic polymer modified PLGA nanoparticles encapsulating Alhagi honey polysaccharides as a vaccine delivery system for ovalbumin to improve immune responses. Int. J. Nanomed. 2019, 14, 3221–3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegmann, F.; Gartlan, K.H.; Harandi, A.M.; Brinckmann, S.A.; Coccia, M.; Hillson, W.R.; Kok, W.L.; Cole, S.; Ho, L.-P.; Lambe, T.; et al. Polyethyleneimine is a potent mucosal adjuvant for viral glycoprotein antigens. Nat. Biotechnol. 2012, 30, 883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheppard, N.C.; Brinckmann, S.A.; Gartlan, K.H.; Puthia, M.; Svanborg, C.; Krashias, G.; Eisenbarth, S.C.; Flavell, R.A.; Sattentau, Q.J.; Wegmann, F. Polyethyleneimine is a potent systemic adjuvant for glycoprotein antigens. Int. Immunol. 2014, 26, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic nanoparticles for delivery of amphotericin B: Preparation, characterization and activity in vitro. J. Nano Biotechnol. 2008, 6, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, L.D.; Mamizuka, E.M.; Carmona-Ribeiro, A.M. Antimicrobial particles from cationic lipid and polyelectrolytes. Langmuir 2010, 26, 12300–12306. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M.; de Melo Carrasco, L.D. Cationic antimicrobial polymers and their assemblies. Int. J. Mol. Sci. 2013, 14, 9906–9946. [Google Scholar] [CrossRef] [Green Version]
- Bohidar, H.; Dubin, P.L.; Majhi, P.R.; Tribet, C.; Jaeger, W. Effects of protein-polyelectrolyte affinity and polyelectrolyte molecular weight on dynamic properties of bovine serum albumin-poly(diallyldimethylammonium chloride) coacervates. Biomacromolecules 2005, 6, 1573–1585. [Google Scholar] [CrossRef]
- Kayitmazer, A.B.; Strand, S.P.; Tribet, C.; Jaeger, W.; Dubin, P.L. Effect of polyelectrolyte structure on protein-polyelectrolyte coacervates: Coacervates of bovine serum albumin with poly(diallyldimethylammonium chloride) versus chitosan. Biomacromolecules 2007, 8, 3568–3577. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Liu, Y.; Wu, X.; Ji, Y.; Zhao, Y.; et al. Surface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012, 12, 2003–2012. [Google Scholar] [CrossRef]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 1994, 32, 5–8. [Google Scholar] [PubMed]
- Grabowski, E.; Morrison, I. Particle size distribution from analysis of quasi-elastic light scattering data. In Measurement of Suspended Particles by Quasi-Elastic Light Scattering; John Wiley & Sons: New York, NY, USA, 1983; pp. 199–236. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Niu, F.; Su, Y.; Liu, Y.; Wang, G.; Zhang, Y.; Yang, Y. Ovalbumin–gum arabic interactions: Effect of pH, temperature, salt, biopolymers ratio and total concentration. Colloids Surf. B Biointerfaces 2014, 113, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Dubin, P.L. Potentiometric studies of the interaction of bovine serum albumin and poly(dimethyldiallylammonium chloride). Macromolecules 1997, 30, 7856–7861. [Google Scholar] [CrossRef] [Green Version]
- Ianeselli, L.; Zhang, F.; Skoda, M.W.A.; Jacobs, R.M.J.; Martin, R.A.; Callow, S.; Prevost, S.; Schreiber, F. Protein-protein interactions in ovalbumin solutions studied by small-angle scattering: Effect of ionic strength and the chemical nature of cations. J. Phys. Chem. B 2010, 114, 3776–3783. [Google Scholar] [CrossRef]
- Carmona-Ribeiro, A.M.; Chaimovich, H. Salt-induced aggregation and fusion of dioctadecyldimethylammonium chloride and sodium dihexadecylphosphate vesicles. Biophys. J. 1986, 50, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Strutt, J.W. On the scattering of light by shells. Proc. R. Soc. Lond. A Math. Phys. Sci. 1918, 94, 296–300. [Google Scholar]
- Karibyants, N.; Dautzenberg, H.; Cölfen, H. Characterization of PSS/PDADMAC-co-AA polyelectrolyte complexes and their stoichiometry using analytical ultracentrifugation. Macromolecules 1997, 30, 7803–7809. [Google Scholar] [CrossRef]
- Sanches, L.M.; Petri, D.F.S.; de Melo Carrasco, L.D.; Carmona-Ribeiro, A.M. The antimicrobial activity of free and immobilized poly (diallyldimethylammonium) chloride in nanoparticles of poly (methylmethacrylate). J. Nanobiotechnol. 2015, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Lindblad, E.B. Aluminium adjuvants—In retrospect and prospect. Vaccine 2004, 22, 3658–3668. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.T.; Galvão, C.N.; Betancourt, Y.P.; Mathiazzi, B.I.; Carmona-Ribeiro, A.M. Microbicidal dispersions and coatings from hybrid nanoparticles of poly (methyl methacrylate), poly (diallyl dimethyl ammonium) chloride, lipids, and surfactants. Int. J. Mol. Sci. 2019, 20, 6150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvão, C.N.; Sanches, L.M.; Mathiazzi, B.I.; Ribeiro, R.T.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Antimicrobial coatings from hybrid nanoparticles of biocompatible and antimicrobial polymers. Int. J. Mol. Sci. 2018, 19, 2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, E.M.A.; Kosaka, P.M.; Rosa, H.; Vieira, D.B.; Kawano, Y.; Petri, D.F.S.; Carmona-Ribeiro, A.M. Hybrid materials from intermolecular associations between cationic lipid and polymers. J. Phys. Chem. B 2008, 112, 9301–9310. [Google Scholar] [CrossRef]
- Melo, L.D.; Palombo, R.R.; Petri, D.F.S.; Bruns, M.; Pereira, E.M.A.; Carmona-Ribeiro, A.M. Structure-activity relationship for quaternary ammonium compounds hybridized with poly(methyl methacrylate). ACS Appl. Mater. Interfaces 2011, 3, 1933–1939. [Google Scholar] [CrossRef]
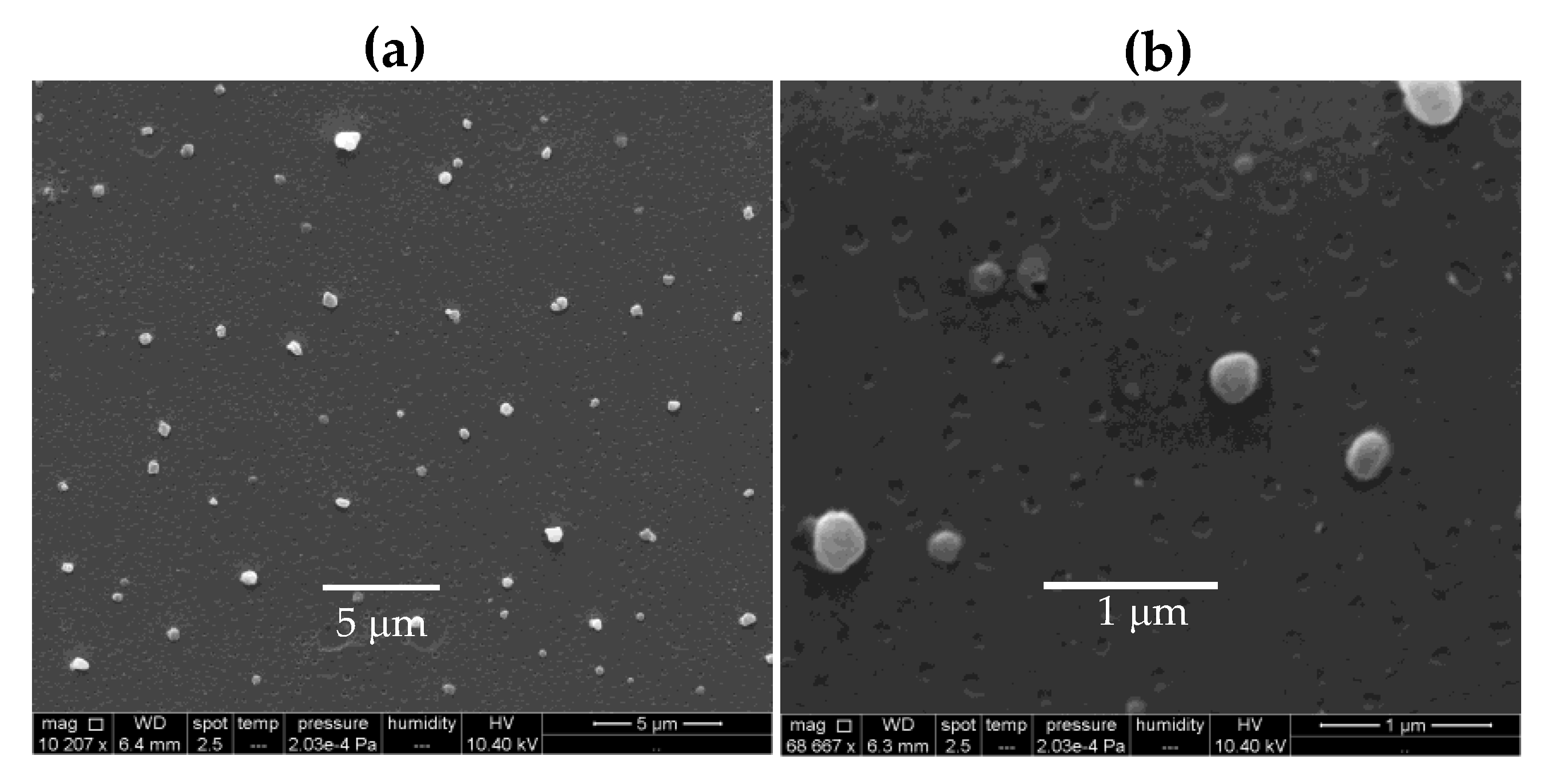






| Group | Solution or Dispersion | [OVA] mg·mL−1 | [Al(OH)3] mg·mL−1 | [PDDA] mg·mL−1 |
|---|---|---|---|---|
| 1 | Milli-Q water | 0 | 0 | 0 |
| 2 | OVA | 0.1 | 0 | 0 |
| 3 | Al(OH)3/OVA | 0.1 | 0.1 | 0 |
| 4 | PDDA/OVA | 0.1 | 0 | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Betancourt, Y.; Távora, B.d.C.L.F.; Colombini, M.; Faquim-Mauro, E.L.; Carmona-Ribeiro, A.M. Simple Nanoparticles from the Assembly of Cationic Polymer and Antigen as Immunoadjuvants. Vaccines 2020, 8, 105. https://doi.org/10.3390/vaccines8010105
Pérez-Betancourt Y, Távora BdCLF, Colombini M, Faquim-Mauro EL, Carmona-Ribeiro AM. Simple Nanoparticles from the Assembly of Cationic Polymer and Antigen as Immunoadjuvants. Vaccines. 2020; 8(1):105. https://doi.org/10.3390/vaccines8010105
Chicago/Turabian StylePérez-Betancourt, Yunys, Bianca de Carvalho Lins Fernandes Távora, Mônica Colombini, Eliana L. Faquim-Mauro, and Ana Maria Carmona-Ribeiro. 2020. "Simple Nanoparticles from the Assembly of Cationic Polymer and Antigen as Immunoadjuvants" Vaccines 8, no. 1: 105. https://doi.org/10.3390/vaccines8010105
APA StylePérez-Betancourt, Y., Távora, B. d. C. L. F., Colombini, M., Faquim-Mauro, E. L., & Carmona-Ribeiro, A. M. (2020). Simple Nanoparticles from the Assembly of Cationic Polymer and Antigen as Immunoadjuvants. Vaccines, 8(1), 105. https://doi.org/10.3390/vaccines8010105






