Tropomyosin: An Excretory/Secretory Protein from Haemonchus contortus Mediates the Immuno-Suppressive Potential of Goat Peripheral Blood Mononuclear Cells In Vitro
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Parasites
2.2. PBMCs Separation and Culture
2.3. RNA Isolation and Construction of cDNA Encoding H. contortus TpMy
2.4. Cloning and Expression of H. contortus TpMy Gene
2.5. Sequence Analysis of Hc-TpMy
2.6. Expression and Purification of H. contortus TpMy Protein
2.7. Antibodies Production and Immunobloting
2.8. Binding of rHc-TpMy to Goat PBMCs
2.9. ELISA Dependent Cytokines Secretion by PBMCs Treated With rHc-TpMy
2.10. Cell Proliferation Assay
2.11. Cell Migration Assay
2.12. Intracellular Nitric oxide Production
2.13. Cell Apoptosis Assay
2.14. Statistical Analysis
3. Results
3.1. Amplification, Cloning and Sequence Analysis of Hc-TpMy
3.2. Expression, Purification and Immunoblot of rHc-TpMy Protein
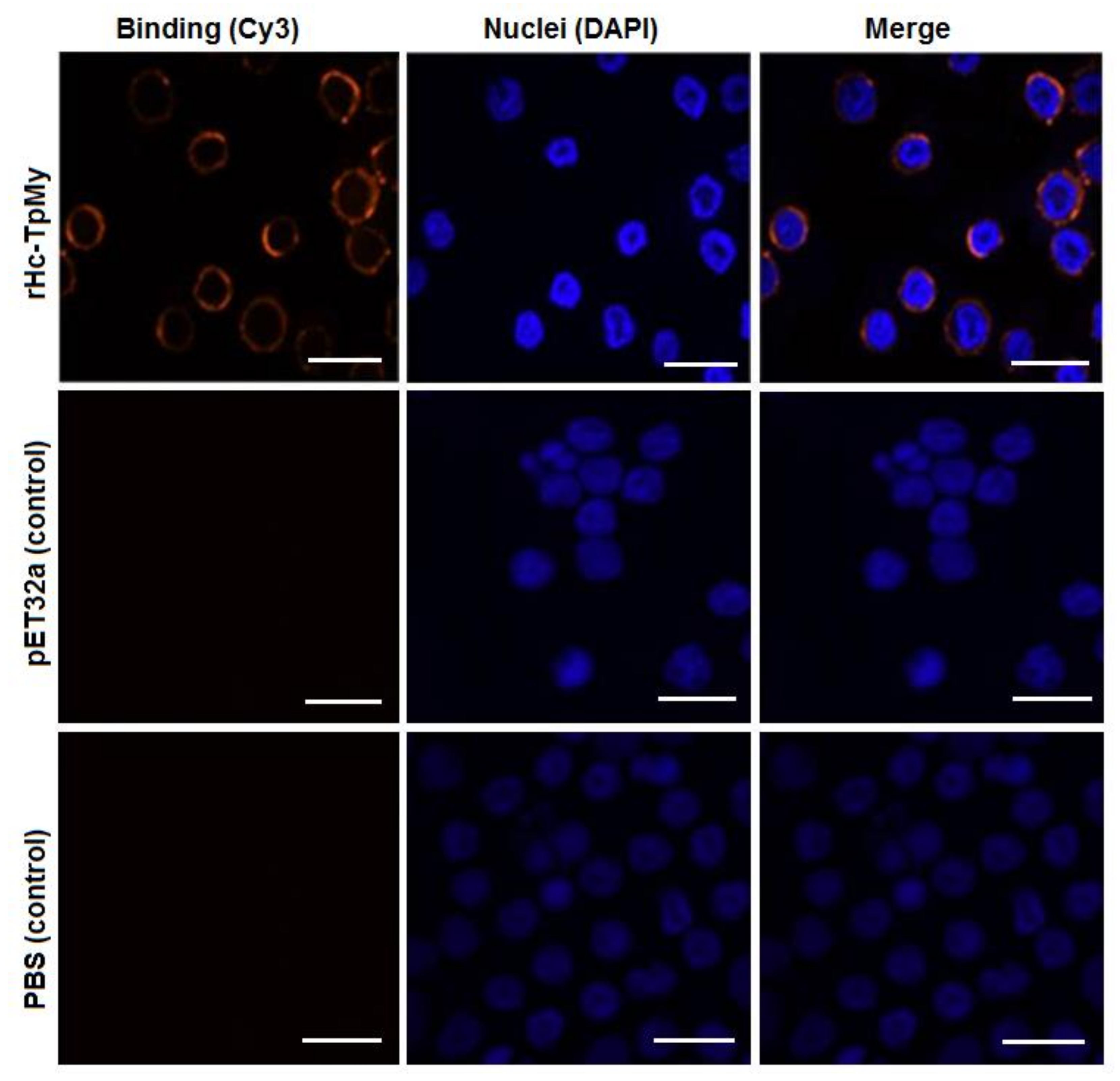
3.3. Binding Confirmation of rHc-TpMy on Surface of Goat PBMCs
3.4. Cytokines Level in PBMCs Detected by ELISA
3.5. PBMCs Proliferation Effected by rHc-TpMy
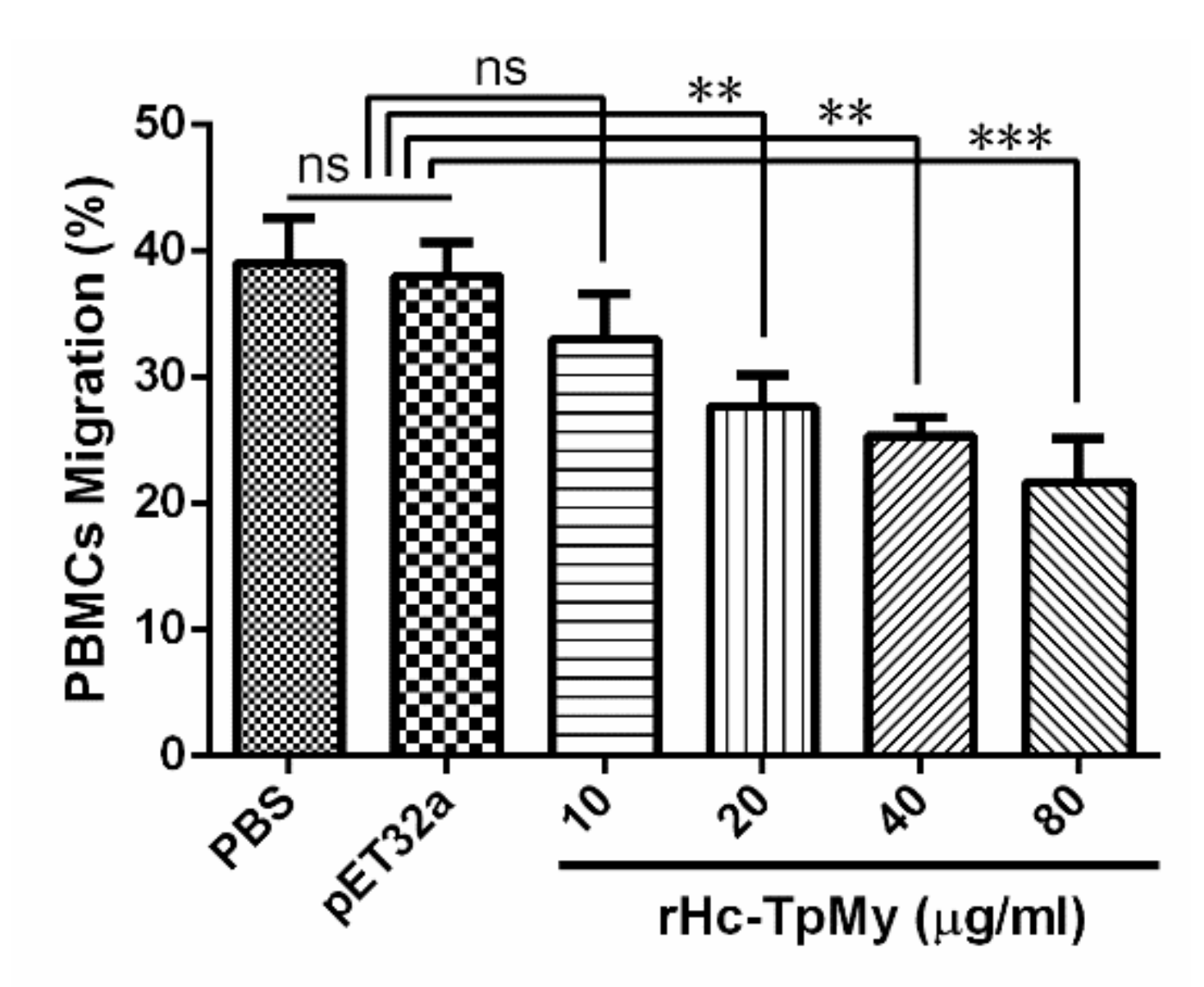
3.6. Cell Migration Assay
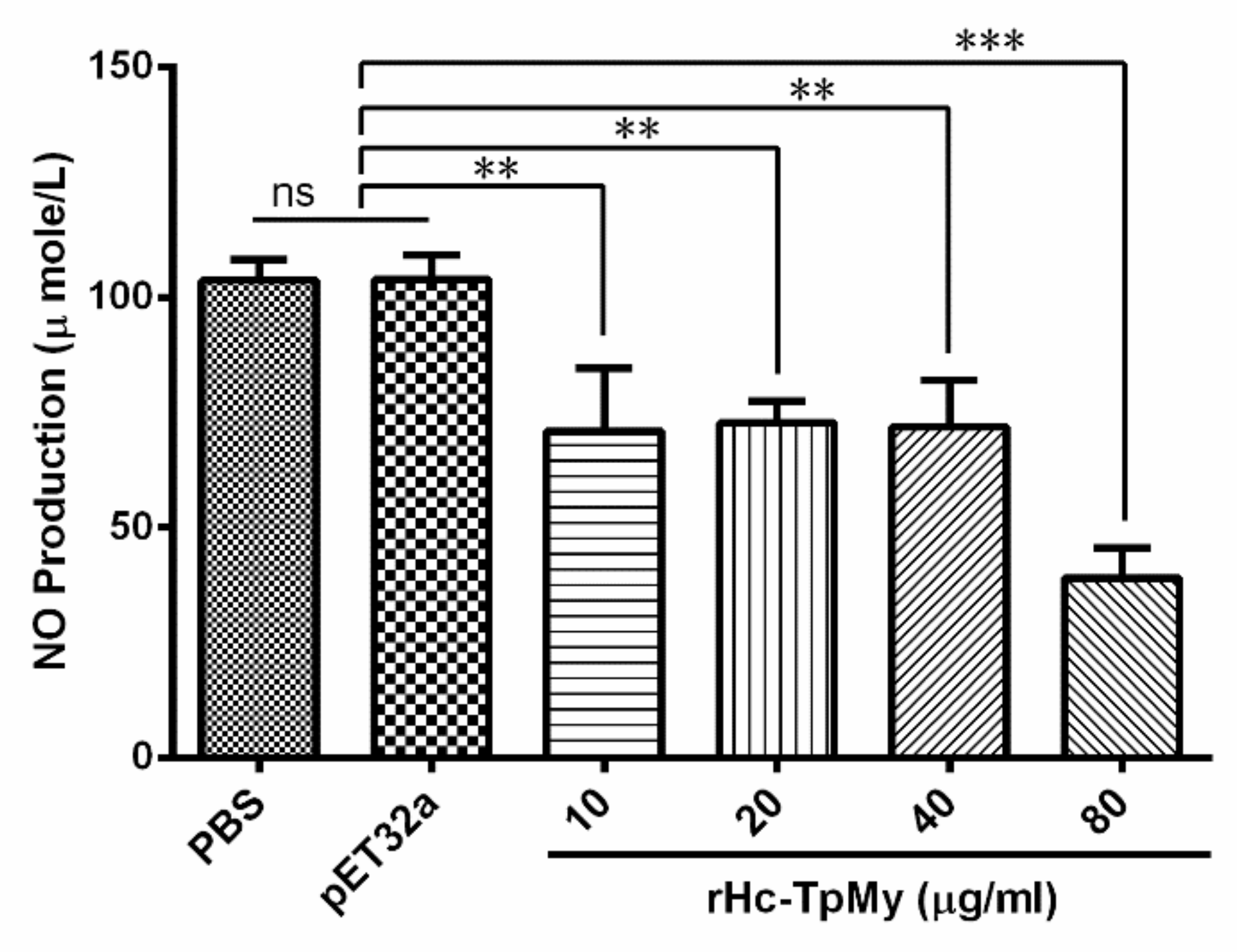
3.7. Hc-TpMy Decreased NO Production in Goat PBMCs
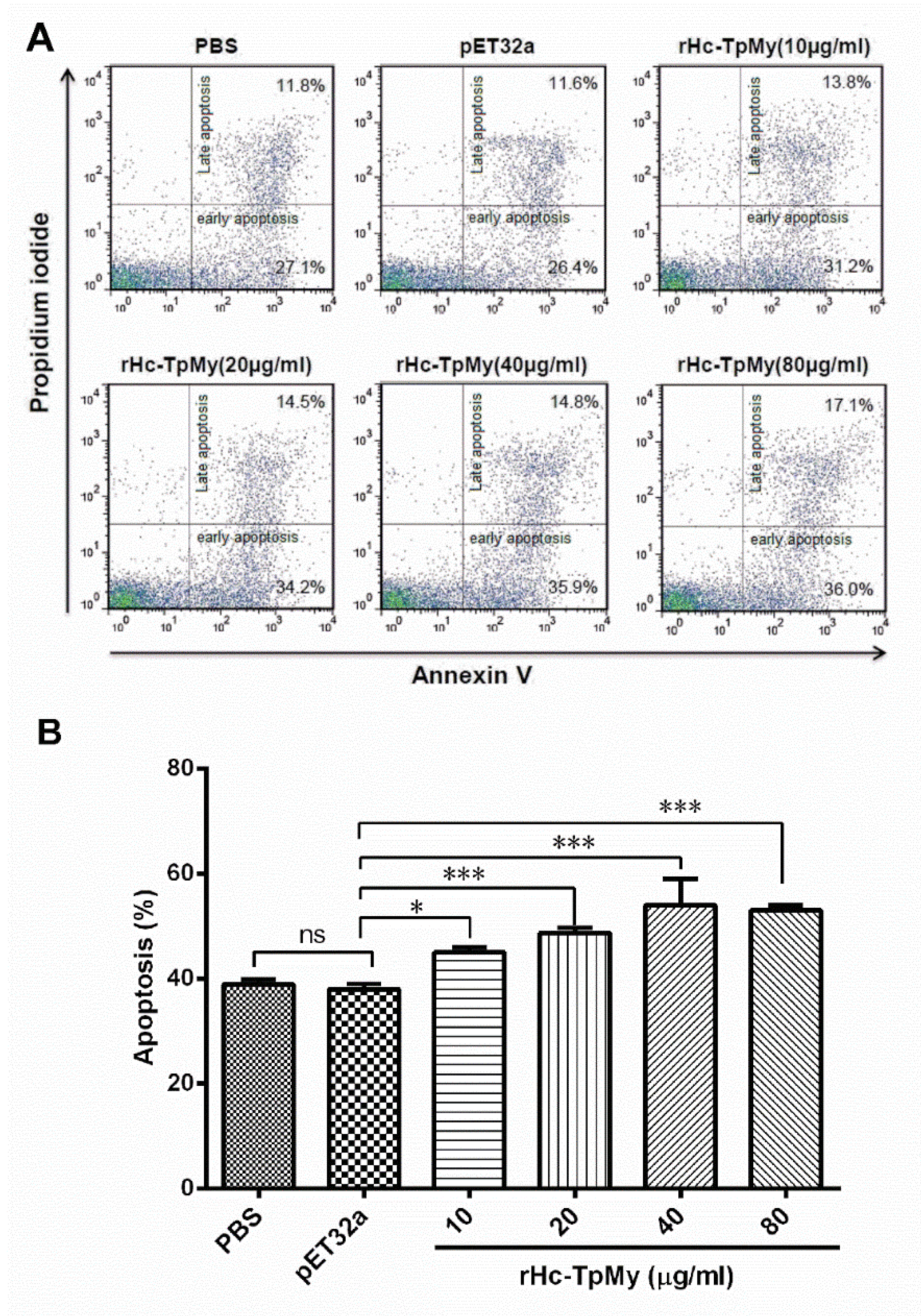
3.8. Hc-TpMy Induced Apoptosis of PBMCs
4. Discussion
5. Conclusions
6. Ethics Statement and Participation Approval
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newton, S.E.; Munn, E.A. The development of vaccines against gastrointestinal nematode parasites, particularly Haemonchus contortus. Parasitol. Today 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Waller, P.J.; Echevarria, F.; Eddi, C.; Maciel, S.; Nari, A.; Hansen, J.W. The prevalence of anthelmintic resistance in nematode parasites of sheep in Southern Latin America. Vet. Parasitol. 1996, 62, 181–187. [Google Scholar] [CrossRef]
- Van, W.J.A.; Malan, F.S.; Randles, J.L. How long before resistance makes it impossible to control some field strains of Haemonchus contortus in South Africa with any of the modern anthelmintics? Vet. Parasitol. 1997, 70, 111–122. [Google Scholar] [CrossRef]
- Maizels, R.M.; Balic, A.; Gomez-Escobar, N.; Nair, M.; Taylor, M.D.; Allen, J.E. Helminth parasites-Masters of regulation. Immunol. Rev. 2004, 201, 89–116. [Google Scholar] [CrossRef]
- Smith, T.S.; Munn, E.A.; Graham, M.; Tavernor, A.S.; Greenwood, C.A. Purification and evaluation of the integral membrane protein H11 as a protective antigen against Haemonchus contorus. Int. J. Parasitol. 1993, 23, 271–280. [Google Scholar] [CrossRef]
- Jasmer, D.P.; McGuire, T.C. Protective immunity to a blood-Feeding nematode (Haemonchus contortus) induced by parasite gut antigens. Infect. Immun. 1991, 59, 4412–4417. [Google Scholar] [CrossRef] [Green Version]
- Sereda, M.J.; Hartmann, S.; Lucius, R. Helminths and allergy: The example of tropomyosin. Trends Parasitol. 2008, 24, 272–278. [Google Scholar] [CrossRef]
- Lacroux, C.; Nguyen, T.H.; Andreoletti, O.; Prevot, F.; Grisez, C.; Bergeaud, J.P.; Gruner, L.; Brunel, J.C.; Francois, D.; Dorchies, P.; et al. Haemonchus contortus (Nematoda: Trichostrongylidae) infection in lambs elicits an unequivocal Th2 immune response. Vet. Res. 2006, 37, 607–622. [Google Scholar] [CrossRef] [Green Version]
- Shakya, K.P.; Miller, J.E.; Horohov, D.W. A Th2 type of immune response is associated with increased resistance to Haemonchus contortus in naturally infected Gulf Coast Native lambs. Vet. Parasitol. 2009, 163, 57–66. [Google Scholar] [CrossRef]
- Meeusen, E.N.; Balic, A.; Bowles, V. Cells, cytokines and other molecules associated with rejection of gastrointestinal nematode parasites. Vet. Immunol. Immunopathol. 2005, 108, 121–125. [Google Scholar] [CrossRef]
- De, L.C.C.; Bambou, J.C.; Arquet, R.; Jacquiet, P.; Mandonnet, N. Genetic analysis of the potential role of IgA and IgE responses against Haemonchus contortus in parasite resistance of Creole goats. Vet. Parasitol. 2012, 186, 337–343. [Google Scholar]
- Terefe, G.; Lacroux, C.; Prévot, F.; Grisez, C.; Bergeaud, J.P.; Bleuart, C.; Dorchies, P.; Foucras, G.; Jacquiet, P. Eosinophils in Haemonchus contortus-Infected resistant and susceptible breeds of sheep: Abomasal tissue recruitment and In Vitro functional state. Vet. Parasitol. 2009, 165, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.V. Vertebrate tropomyosin: Distribution, properties and function. J. Muscle. Res. Cell. Motil. 2001, 22, 5–49. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, R.; Lehrer, S.B.; Reese, G. Identification of continuous, allergenic regions of the major shrimp allergen Pen a 1 (tropomyosin). Int. Arch. Allergy. Immunol. 2002, 127, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Subba, R.P.V.; Rajagopal, D.; Ganesh, K.A. B- and T-Cell epitopes of tropomyosin, the major shrimp allergen. Allergy 1998, 53, 44–47. [Google Scholar]
- Ayuso, R.; Reese, G.; Leong-Kee, S.; Plante, M.; Lehrer, S.B. Molecular basis of arthropod cross-Reactivity: IgE-Binding cross-Reactive epitopes of shrimp, house dust mite and cockroach tropomyosins. Int. Arch. Allergy. Immunol. 2002, 129, 38–48. [Google Scholar] [CrossRef]
- Shuster, C.B.; Herman, I.M. The Mechanics of Vascular Cell Motility. Microcirculation 1998, 5, 239–257. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Kazzaz, J.A.; Helfman, D.M. Functional properties of non-Muscle tropomyosin isoforms. Curr. Opin. Cell. Biol. 1994, 6, 96–104. [Google Scholar] [CrossRef]
- MacDonald, N.J.; Shivers, W.Y.; Narum, D.L.; Plum, S.M.; Wingard, J.N.; Fuhrmann, S.R.; Liang, H.; Holland-Linn, J.; Chen, D.H.; Sim, B.K. Endostatin binds tropomyosin. A potential modulator of the antitumor activity of endostatin. J. Biol. Chem. 2001, 276, 25190–25196. [Google Scholar] [CrossRef] [Green Version]
- Gadahi, J.A.; Wang, S.; Bo, G.; Ehsan, M.; Yan, R.; Song, X.; Xu, L.; Li, X. Proteomic Analysis of the Excretory and Secretory Proteins of Haemonchus contortus (HcESP) Binding to Goat PBMCs In Vivo Revealed Stage-Specific Binding Profiles. PLoS ONE 2016, 11, 0159796. [Google Scholar] [CrossRef] [Green Version]
- Gadahi, J.A.; Yongqian, B.; Ehsan, M.; Zhang, Z.C.; Wang, S.; Yan, R.F.; Song, X.K.; Xu, L.X.; Li, X.R. Haemonchus contortus excretory and secretory proteins (HcESPs) suppress functions of goat PBMCs In Vitro. Oncotarget 2016, 7, 35670–35679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naqvi, M.A.; Jamil, T.; Naqvi, S.Z.; Memon, M.A.; Aimulajiang, K.; Aleem, M.T.; Ehsan, M.; Xu, L.; Song, X.; Li, X.; et al. Immunodiagnostic potential of recombinant tropomyosin during prepatent Haemonchus contortus infection in goat. Res. Vet. Sci. 2020, 128, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, S.; Zhang, H.; Yuan, C.; Yan, R.; Song, X.; Xu, L.; Li, X. Galectin Hco-gal-m from Haemonchus contortus modulates goat monocytes and T cell function in different patterns. Parasites Vectors 2014, 7, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehsan, M.; Gao, W.; Gadahi, J.A.; Lu, M.; Liu, X.; Wang, Y.; Yan, R.; Xu, L.; Song, X.; Li, X. Arginine kinase from Haemonchus contortus decreased the proliferation and increased the apoptosis of goat PBMCs In Vitro. Parasites Vectors 2017, 10, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubert, J.; Kerboeuf, D. A microlarval development assay for the detection of anthelmintic resistance in sheep nematodes. Vet. Rec. 1992, 130, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Sommerville, R.I. Development of Haemonchus contortus In Vitro and the stimulus from the host. J. Prasitol. 1977, 63, 344–347. [Google Scholar] [CrossRef]
- Nicholson, I.C.; Mavrangelos, C.; Fung, K.; Ayhan, M.; Levichkin, I.; Johnston, A.; Zola, H.; Hoogenraad, N.J. Characterisation of the protein composition of peripheral blood mononuclear cell microsomes by SDS-PAGE and mass spectrometry. J. Immunol. Methods 2005, 305, 84–93. [Google Scholar] [CrossRef]
- Chen, W.J.; Niu, D.S.; Zhang, X.Y.; Chen, M.L.; Cui, H.; Wei, W.J.; Wen, B.H.; Chen, X.R. Recombinant 56-Kilodalton major outer membrane protein antigen of Orientia tsutsugamushi Shanxi and its antigenicity. Infect. Immun. 2003, 71, 4772–4779. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, C.; Wang, S.; Song, X.; Xu, L.; Yan, R.; Hasson, I.A.; Li, X. Transcriptional and proteomic analysis reveal recombinant galectins of Haemonchus contortus down-regulated functions of goat PBMC and modulation of several signaling cascades In Vitro. J. Proteom. 2014, 98, 123–137. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Q.; Sun, X.; Lu, M.; Ehsan, M.; Hasan, M.W.; Xu, L.; Yan, R.; Song, X.; Li, X. Effects of Recombinant Toxoplasma gondii Citrate Synthase I on the Cellular Functions of Murine Macrophages In Vitro. Front. Microbiol. 2017, 8, 1376. [Google Scholar] [CrossRef] [PubMed]
- Gadahi, J.A.; Ehsan, M.; Wang, S.; Zhang, Z.; Yan, R.; Song, X.; Xu, L.; Li, X. Recombinant protein of Haemonchus contortus small GTPase ADP-ribosylation factor 1 (HcARF1) modulate the cell mediated immune response In Vitro. Oncotarget 2017, 8, 112211–112221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadahi, J.A.; Li, B.; Ehsan, M.; Wang, S.; Zhang, Z.; Wang, Y.; Hasan, M.W.; Yan, R.; Song, X.; Xu, L. Recombinant Haemonchus contortus 24 kDa excretory/secretory protein (rHcES-24) modulate the immune functions of goat PBMCs In Vitro. Oncotarget 2016, 7, 83926–83937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehsan, M.; Wang, W.; Gadahi, J.A.; Hasan, M.W.; Lu, M.; Wang, Y.; Liu, X.; Haseeb, M.; Yan, R.; Xu, L.; et al. The Serine/Threonine-Protein Phosphatase 1 From Haemonchus contortus Is Actively Involved in Suppressive Regulatory Roles on Immune Functions of Goat Peripheral Blood Mononuclear Cells. Front. Immunol. 2018, 9, 1627. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Zhang, H.; Wang, W.; Li, Y.; Yan, R.; Xu, L.; Song, X.; Li, X. Transmembrane protein 63A is a partner protein of Haemonchus contortus galectin in the regulation of goat peripheral blood mononuclear cells. Parasites Vectors 2015, 8, 211. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yuan, C.; Wang, L.; Lu, M.; Wang, Y.; Wen, Y.; Yan, R.; Xu, L.; Song, X.; Li, X. Transmembrane protein 147 (TMEM147): Another partner protein of Haemonchus contortus galectin on the goat peripheral blood mononuclear cells (PBMC). Parasites Vectors 2016, 9, 355. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, S.; Scott, I.; Simpson, H.V. Neutrophil and eosinophil chemotactic factors in the excretory/secretory products of sheep abomasal nematode parasites. Parasitol. Res. 2011, 109, 627–635. [Google Scholar] [CrossRef]
- Jenkins, R.E.; Taylor, M.J.; Gilvary, N.J.; Bianco, A.E. Tropomyosin implicated in host protective responses to microfilariae in onchocerciasis. PNAS 1998, 95, 7550–7555. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Jise, Q.; Zheng, W.; Ren, Y.; Nong, X.; Wu, X.; Gu, X.; Wang, S.; Peng, X.; Lai, S. Characterization and evaluation of a Sarcoptes scabiei allergen as a candidate vaccine. Parasites Vectors 2012, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.B.R.; Rocha, G.M.; Oliver, C.; Ferriani, V.P.; Lima, R.C.; Palma, M.S.; Sales, V.S.; Aalberse, R.C.; Chapman, M.D.; Arruda, L.K. Cross-Reactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J. Allergy Clin. Immunol. 2008, 121, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Asturias, J.A.; Eraso, E.; Moneo, I.; Martínez, A. Is tropomyosin an allergen in Anisakis? Allergy 2000, 55, 898. [Google Scholar] [CrossRef] [PubMed]
- Van, D.; Biggelaar, A.H.; Van, R.R.; Rodrigues, L.C.; Lell, B.; Deelder, A.M.; Kremsner, P.G.; Yazdanbakhsh, M. Decreased atopy in children infected with Schistosoma haematobium: A role for parasite-Induced interleukin-10. Lancet 2000, 356, 1723–1727. [Google Scholar]
- Anbu, K.; Joshi, P. Identification of a 55 kDa Haemonchus contortus excretory/secretory glycoprotein as a neutrophil inhibitory factor. Parasite Immunol. 2008, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.R.; Sommers, K.N.; Zajac, A.M.; Notter, D.R.; Bowdridge, S.A. Early IL-4 gene expression in abomasum is associated with resistance to Haemonchus contortus in hair and wool sheep breeds. Parasite Immunol. 2016, 38, 333–339. [Google Scholar] [CrossRef]
- Moreau, E.; Chauvin, A. Immunity against Helminths: Interactions with the Host and the Intercurrent Infections. J. Biomed. Biotechnol. 2010, 2010, 428593. [Google Scholar] [CrossRef] [Green Version]
- Lendner, M. Functional Analysis of Tropomyosin of Parasitic Nematodes; Humboldt-Universität zu Berlin, Mathematisch-Naturwissenschaftliche Fakultät I: Berlin, Germany, 2010. [Google Scholar]
- Cope, A.; Le, F.G.; Cardone, J.; Kemper, C. The Th1 life cycle: Molecular control of IFN-γ to IL-10 switching. Trends Immunol. 2011, 32, 278–286. [Google Scholar] [CrossRef]
- Gadahi, J.A.; Ehsan, M.; Wang, S.; Zhang, Z.; Wang, Y.; Yan, R.; Song, X.; Xu, L.; Li, X. Recombinant protein of Haemonchus contortus 14-3-3 isoform 2 (rHcftt-2) decreased the production of IL-4 and suppressed the proliferation of goat PBMCs In Vitro. Exp. Parasitol. 2016, 171, 57–66. [Google Scholar] [CrossRef]
- Grimbaldeston, M.A.; Nakae, S.; Kalesnikoff, J.; Tsai, M.; Galli, S.J. Mast cell-Derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat. Immunol. 2007, 8, 1095–1104. [Google Scholar] [CrossRef]
- Wilson, M.S.; Maizels, R.M. Regulation of allergy and autoimmunity in helminth infection. Clin. Rev. Allergy Immunol. 2004, 26, 35–50. [Google Scholar] [CrossRef]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-Beta signaling in tumor suppression and cancer progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-Beta regulation of immune responses. Ann. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, G.; Yang, R. Th17 cell induction and immune regulatory effects. J. Cell. Physiol. 2007, 211, 273–278. [Google Scholar] [CrossRef]
- Katawa, G.; Layland, L.E.; Debrah, A.Y.; Von, H.C.; Batsa, L.; Kwarteng, A.; Arriens, S.; David, W.T.; Specht, S.; Hoerauf, A.; et al. Hyperreactive onchocerciasis is characterized by a combination of Th17-Th2 immune responses and reduced regulatory T cells. PLoS Negl. Trop. Dis. 2015, 9, 3414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, X.; Gao, H.; Qian, Y. Th17 differentiation and their pro-inflammation function. Adv. Exp. Med. Biol. 2014, 841, 99–151. [Google Scholar] [PubMed]
- Leger, J.; Bouveret, P.; Lompre, A.-M.; Schwartz, K. Species-Dependent immunological differences between various mammalian cardiac tropomyosins. Biochimica. Biophysica. Acta 1979, 576, 314–321. [Google Scholar] [CrossRef]
- Loke, P.; MacDonald, A.S.; Robb, A.; Maizels, R.M.; Allen, J.E. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell to cell contact. Eur. J. Immunol. 2000, 30, 2669–2678. [Google Scholar] [CrossRef]
- Wang, S.; Delgado, J.C.; Ravkov, E.; Eckels, D.D.; Georgelas, A.; Pavlov, I.Y.; Cusick, M.; Sebastian, K.; Gleich, G.J.; Wagner, L.A. Penaeus monodon tropomyosin induces CD4 T-Cell proliferation in shrimp-Allergic patients. Hum. Immunol. 2012, 73, 426–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGovern, K.E.; Wilson, E.H. Role of chemokines and trafficking of immune cells in parasitic infections. Curr. Immunol. Rev. 2013, 9, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, S.L. Role of nitric oxide in parasitic infections. Microbiol. Rev. 1995, 59, 533–547. [Google Scholar] [CrossRef]
- Donskow-Schmelter, K.; Doligalska, M. Apoptosis, a protective mechanism for pathogens and their hosts. Wiad. Parazytol. 2005, 51, 271–280. [Google Scholar] [PubMed]
- Hsu, D.K.; Yang, R.Y.; Saegusa, J.; Liu, F.T. Analysis of the intracellular role of galectins in cell growth and apoptosis. Methods Mol. Biol. 2015, 1207, 451–463. [Google Scholar] [PubMed] [Green Version]
- Sturm, A.; Lensch, M.; Andre, S.; Kaltner, H.; Wiedenmann, B.; Rosewicz, S.; Dignass, A.U.; Gabius, H.J. Human galectin-2: Novel inducer of T cell apoptosis with distinct profile of caspase activation. J. Immunol. 2004, 173, 3825–3837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Yan, R.; Muleke, C.I.; Zhao, G.; Xu, L.; Li, X. Recombinant Galectins of Haemonchus contortus Parasite Induces Apoptosis in the Peripheral Blood Lymphocytes of Goat. Int. J. Pept. Res. Ther. 2007, 13, 387–392. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehsan, M.; Haseeb, M.; Hu, R.; Ali, H.; Memon, M.A.; Yan, R.; Xu, L.; Song, X.; Zhu, X.; Li, X. Tropomyosin: An Excretory/Secretory Protein from Haemonchus contortus Mediates the Immuno-Suppressive Potential of Goat Peripheral Blood Mononuclear Cells In Vitro. Vaccines 2020, 8, 109. https://doi.org/10.3390/vaccines8010109
Ehsan M, Haseeb M, Hu R, Ali H, Memon MA, Yan R, Xu L, Song X, Zhu X, Li X. Tropomyosin: An Excretory/Secretory Protein from Haemonchus contortus Mediates the Immuno-Suppressive Potential of Goat Peripheral Blood Mononuclear Cells In Vitro. Vaccines. 2020; 8(1):109. https://doi.org/10.3390/vaccines8010109
Chicago/Turabian StyleEhsan, Muhammad, Muhammad Haseeb, Ruisi Hu, Haider Ali, Muhammad Ali Memon, Ruofeng Yan, Lixin Xu, Xiaokai Song, Xingquan Zhu, and Xiangrui Li. 2020. "Tropomyosin: An Excretory/Secretory Protein from Haemonchus contortus Mediates the Immuno-Suppressive Potential of Goat Peripheral Blood Mononuclear Cells In Vitro" Vaccines 8, no. 1: 109. https://doi.org/10.3390/vaccines8010109
APA StyleEhsan, M., Haseeb, M., Hu, R., Ali, H., Memon, M. A., Yan, R., Xu, L., Song, X., Zhu, X., & Li, X. (2020). Tropomyosin: An Excretory/Secretory Protein from Haemonchus contortus Mediates the Immuno-Suppressive Potential of Goat Peripheral Blood Mononuclear Cells In Vitro. Vaccines, 8(1), 109. https://doi.org/10.3390/vaccines8010109







