Immune Therapy Targeting E6/E7 Oncogenes of Human Papillomavirus Type 6 (HPV-6) Reduces or Eliminates the Need for Surgical Intervention in the Treatment of HPV-6 Associated Recurrent Respiratory Papillomatosis
Abstract
1. Introduction
2. Materials and Methods
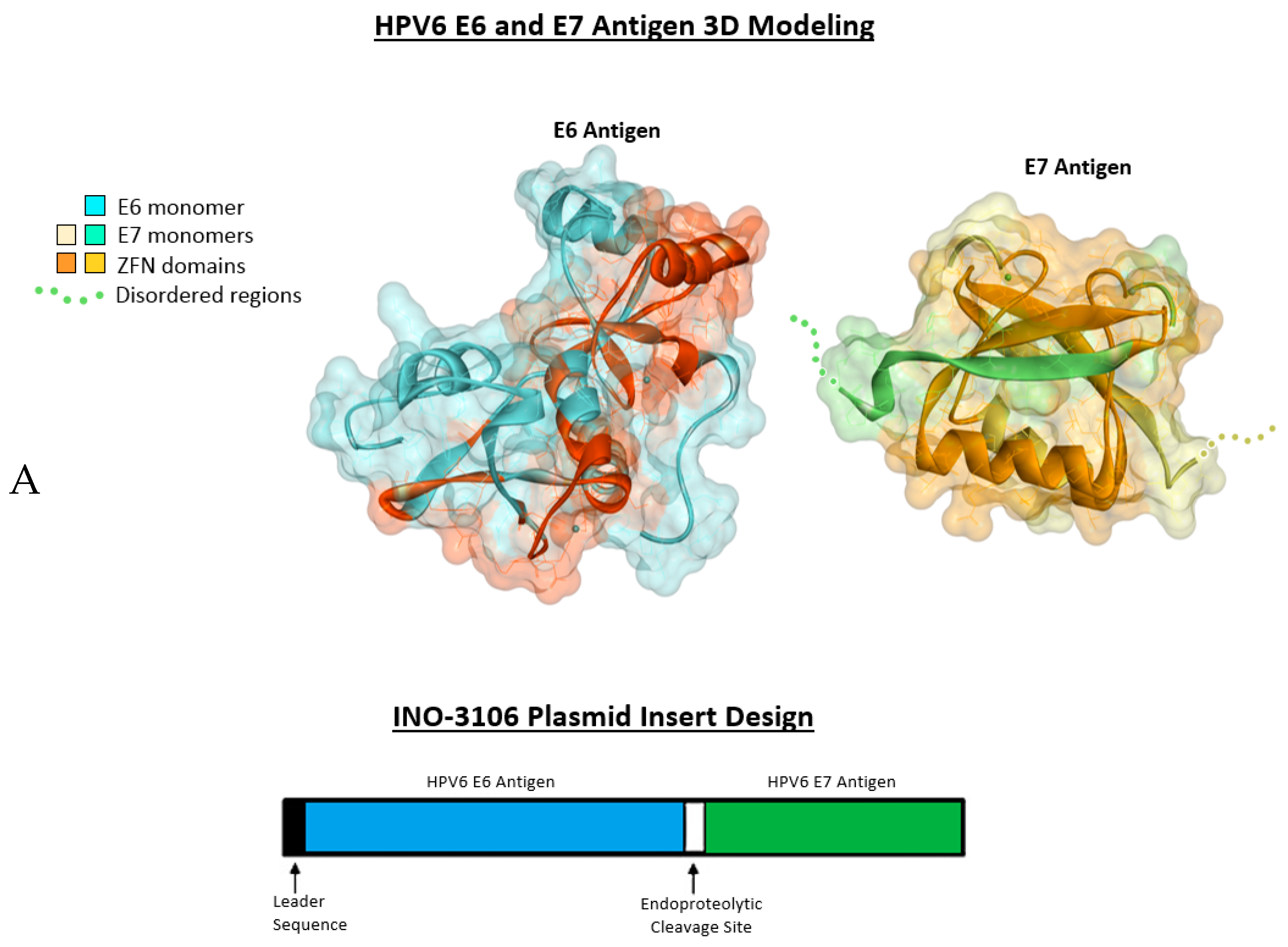
2.1. Antigenic 3D Modeling
2.2. Immunization of Mice
2.3. IFN-γ ELISpot Assay
2.4. CD8+ T-Cell Depletion of Mouse Samples
3. Clinical Study
3.1. Clinical Study Population
3.2. Immunotherapy and Electroporation Using CELLECTRA® Device
3.3. Study Design
3.4. Safety Assessments
3.5. HPV6 Specific ELISA
3.6. HPV6 Specific Flow Cytometry
3.7. HPV6 Specific PBMC Stimulation for Gene Expression Analysis
3.8. Multiplexed Gene Expression Analysis
3.9. Statistical Methods
4. Results
4.1. INO-3106 Displays Immunogenicity in Animal Models
4.2. INO-3106 Is Tolerable, Immunogenic and Potentially Effective in Patients with RRP
4.2.1. Patient Characteristics and Disposition
4.2.2. Safety and Tolerability of INO-3106 and INO-9012 with Electroporation (EP)
4.3. INO-3106 Induces Humoral Immune Reactivity in Patients with RRP
4.4. INO-3106 Induces the Expression of Activation Markers and Lytic Proteins in T Cells from Treated Patients with RRP
4.5. INO-3106 Changes Immune Transcriptional Profiles of T Cells in RRP Patients
4.6. INO-3106 Reduces the Need for Surgical Intervention for the Treatment of RRP
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gradishar, W.J.; Anderson, B.O.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H.; Goldstein, L.J.; et al. Breast cancer version 3.2014. JNCCN. 2014, 12, 542–590. [Google Scholar] [CrossRef] [PubMed]
- Mounts, P.; Shah, K.V.; Kashima, H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc. Natl. Acad. Sci. USA 1982, 79, 5425–5429. [Google Scholar] [CrossRef] [PubMed]
- Gissmann, L.; Wolnik, L.; Ikenberg, H.; Koldovsky, U.; Schnurch, H.G.; zur Hausen, H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc. Natl. Acad. Sci. USA 1983, 80, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Bonagura, V.R.; Hatam, L.J.; Rosenthal, D.W.; de Voti, J.A.; Lam, F.; Steinberg, B.M.; Abramson, A.L. Recurrent respiratory papillomatosis: A complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS 2010, 118, 455–470. [Google Scholar] [CrossRef]
- Winton, T.; Livingston, R.; Johnson, D.; Rigas, J.; Johnston, M.; Butts, C.; Cormier, Y.; Goss, G.; Inculet, R.; Vallieres, E.; et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N. Engl. J. Med. 2005, 352, 2589–2597. [Google Scholar] [CrossRef]
- Omland, T.; Lie, K.A.; Akre, H.; Sandlie, L.E.; Jebsen, P.; Sandvik, L.; Nymoen, D.A.; Bzhalava, D.; Dillner, J.; Brondbo, K. Recurrent respiratory papillomatosis: HPV genotypes and risk of high-grade laryngeal neoplasia. PLoS ONE 2014, 9, e99114. [Google Scholar] [CrossRef]
- Derkay, C.S.; Bluher, A.E. Update on recurrent respiratory papillomatosis. Otolaryngol. Clin. N. Am. 2019, 52, 669–679. [Google Scholar] [CrossRef]
- Richey, L.M.; Shores, C.G.; George, J.; Lee, S.; Couch, M.J.; Sutton, D.K.; Weissler, M.C. The effectiveness of salvage surgery after the failure of primary concomitant chemoradiation in head and neck cancer. Otolaryngol. Head Neck Surg. 2007, 136, 98–103. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Mendenhall, C.M.; Malyapa, R.S.; Palta, J.R.; Mendenhall, N.P. Re-irradiation of head and neck carcinoma. Am. J. Clin. Oncol. 2008, 31, 393–398. [Google Scholar] [CrossRef]
- Lin, K.; Roosinovich, E.; Ma, B.; Hung, C.F.; Wu, T.C. Therapeutic HPV DNA vaccines. Immunol. Res. 2010, 47, 86–112. [Google Scholar] [CrossRef]
- Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Shen, X.; Amante, D.; Sakata, L.; Parker, L.; Yan, J.; Boyer, J.; Roh, C.; et al. Augmentation of cellular and humoral immune responses to HPV16 and HPV18 E6 and E7 antigens by VGX-3100. Mol. Ther. Oncolytics. 2016, 3, 16025. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Knoblock, D.M.; Bauml, J.M.; Weinstein, G.S.; Lin, A.; Boyer, J.; et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin. Cancer Res. 2019, 25, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Killian, S.; Donofrio, R.S. DNA vaccines: A mini review. Recent Pat. DNA Gene Seq. 2011, 5, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.I.; Friedman, A.D.; Franco, R.; Muniappan, A.; Park, J.C.; Campbell, N.P.; Krantz, S.B.; Song, P.C.; Bove, M.; Carroll, T.L.; et al. A phase II study of pembrolizumab for HPV-associated papilloma patients with laryngeal, tracheal, and/or pulmonary involvement. J. Clin. Oncol. 2019, 37, 2502. [Google Scholar] [CrossRef]
- Bagarazzi, M.L.; Yan, J.; Morrow, M.P.; Shen, X.; Parker, R.L.; Lee, J.C.; Giffear, M.; Pankhong, P.; Khan, A.S.; Broderick, K.E.; et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012, 4, 155ra38. [Google Scholar] [CrossRef]
- Chattergoon, M.A.; Saulino, V.; Shames, J.P.; Stein, J.; Montaner, L.J.; Weiner, D.B. Co-immunization with plasmid IL-12 generates a strong T-cell memory response in mice. Vaccine 2004, 22, 1744–1750. [Google Scholar] [CrossRef]
- Hanlon, L.; Argyle, D.; Bain, D.; Nicolson, L.; Dunham, S.; Golder, M.C.; McDonald, M.; McGillivray, C.; Jarrett, O.; Neil, J.C.; et al. Feline leukemia virus DNA vaccine efficacy is enhanced by coadministration with interleukin-12 (IL-12) and IL-18 expression vectors. J. Virol. 2001, 75, 8424–8433. [Google Scholar] [CrossRef]
- Kim, J.J.; Maguire, H.C., Jr.; Nottingham, L.K.; Morrison, L.D.; Tsai, A.; Sin, J.I.; Chalian, A.A.; Weiner, D.B. Coadministration of IL-12 or IL-10 expression cassettes drives immune responses toward a Th1 phenotype. J. Interferon. Cytokine Res. 1998, 18, 537–547. [Google Scholar] [CrossRef]
- Kim, J.J.; Trivedi, N.N.; Nottingham, L.K.; Morrison, L.; Tsai, A.; Hu, Y.; Mahalingam, S.; Dang, K.; Ahn, L.; Doyle, N.K.; et al. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur. J. Immunol. 1998, 28, 1089–1103. [Google Scholar] [CrossRef]
- Operschall, E.; Schuh, T.; Heinzerling, L.; Pavlovic, J.; Moelling, K. Enhanced protection against viral infection by co-administration of plasmid DNA coding for viral antigen and cytokines in mice. J. Clin. Virol. 1999, 13, 17–27. [Google Scholar] [CrossRef]
- Kalams, S.A.; Parker, S.D.; Elizaga, M.; Metch, B.; Edupuganti, S.; Hural, J.; de Rosa, S.; Carter, D.K.; Rybczyk, K.; Frank, I.; et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J. Infect. Dis. 2013, 208, 818–829. [Google Scholar] [CrossRef]
- Tebas, P.; Kraynyak, K.A.; Patel, A.; Maslow, J.N.; Morrow, M.P.; Sylvester, A.J.; Knoblock, D.; Gillespie, E.; Amante, D.; Racine, T.; et al. Intradermal syncon(R) ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and humoral immunogenicity advantages in healthy volunteers. J. Infect. Dis. 2019, 220, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.L.; Kudchodkar, S.B.; Zaidi, F.I.; White, S.; Khan, A.S.; Racine, T.; Choi, H.; et al. Safety and immunogenicity of an Anti-Zika virus DNA vaccine- prelinary report. N. Engl. J. Med. 2017. [Google Scholar] [CrossRef]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef]
- Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Dallas, M.; Knoblock, D.; Boyer, J.D.; Yan, J.; Vang, R.; Khan, A.S.; Humeau, L.; et al. Clinical and immunologic biomarkers for histologic regression of high-grade cervical dysplasia and clearance of HPV16 and HPV18 after immunotherapy. Clin. Cancer Res. 2018, 24, 276–294. [Google Scholar] [CrossRef]
- Morrow, M.P.; Tebas, P.; Yan, J.; Ramirez, L.; Slager, A.; Kraynyak, K.; Diehl, M.; Shah, D.; Khan, A.; Lee, J.; et al. Synthetic consensus HIV-1 DNA induces potent cellular immune responses and synthesis of granzyme B, perforin in HIV infected individuals. Mol. Ther. 2015, 23, 591–601. [Google Scholar] [CrossRef]
- Shin, T.H.; Pankhong, P.; Yan, J.; Khan, A.S.; Sardesai, N.Y.; Weiner, D.B. Induction of robust cellular immunity against HPV6 and HPV11 in mice by DNA vaccine encoding for E6/E7 antigen. Hum. Vaccin. Immunother. 2012, 8, 470–478. [Google Scholar] [CrossRef]
- Yan, J.; Harris, K.; Khan, A.S.; Draghia-Akli, R.; Sewell, D.A.; Weiner, D.B. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine 2008, 26, 5210–5215. [Google Scholar] [CrossRef]
- Yan, J.; Reichenbach, D.K.; Corbitt, N.; Hokey, D.A.; Ramanathan, M.P.; McKinney, K.A.; Weiner, D.B.; Sewell, D. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine 2009, 27, 431–440. [Google Scholar] [CrossRef]
- Migueles, S.A.; Rood, J.E.; Berkley, A.M.; Guo, T.; Mendoza, D.; Patamawenu, A.; Hallahan, C.W.; Cogliano, N.A.; Frahm, N.; Duerr, A.; et al. Trivalent adenovirus type 5 HIV recombinant vaccine primes for modest cytotoxic capacity that is greatest in humans with protective HLA class I alleles. PLoS Pathog. 2011, 7, e1002002. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, N.; Julg, B.; Yamanaka, Y.J.; Chen, H.; Ogunniyi, A.O.; McAndrew, E.; Porter, L.C.; Piechocka-Trocha, A.; Hill, B.J.; Douek, D.C.; et al. A high-throughput single-cell analysis of human CD8 (+) T cell functions reveals discordance for cytokine secretion and cytolysis. J. Clin. Investig. 2011, 121, 4322–4331. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.T.; Broker, T.R.; Steinberg, B.M. The natural history of human papillomavirus infections of the mucosal epithelia. APMIS. 2010, 118, 422–449. [Google Scholar] [CrossRef]
- Makiyama, K.; Hirai, R.; Matsuzaki, H. Gardasil vaccination for recurrent laryngeal papillomatosis in adult men: First report: Changes in hpv antibody titer. J. Voice. 2017, 31, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Bishop, J.A.; Roden, R.B.S.; Allen, C.T.; Best, S.R.A. The PD-1 and PD-L1 pathway in recurrent respiratory papillomatosis. Laryngoscope 2018, 128, E27–E32. [Google Scholar] [CrossRef] [PubMed]
- Duhen, T.; Duhen, R.; Montler, R.; Moses, J.; Moudgil, T.; de Miranda, N.F.; Goodall, C.P.; Blair, T.C.; Fox, B.A.; McDermott, J.E.; et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 2018, 13, 2724. [Google Scholar] [CrossRef] [PubMed]





| Markers Co-Expressed on CD8+ T Cells: | Before INO-3106 (%) | After INO-3106 (%) |
|---|---|---|
| CD38+Ki67+ | 0.49 | 3.95 |
| Ki67+ | 0.45 | 3.88 |
| CD38+Ki67+Prf+ | 0.53 | 3.93 |
| CD38+Ki67+GrzA+Prf+ | 0.58 | 3.95 |
| Ki67+GrzA+Prf+ | 0.53 | 3.91 |
| Ki67+Prf+ | 0.48 | 3.84 |
| Ki67+GrzB+Prf+ | 0.50 | 3.66 |
| CD38+Ki67+GrzA+GrzB+Prf+ | 0.51 | 3.66 |
| CD38+Ki67+GrzB+Prf+ | 0.51 | 3.66 |
| Ki67+GrzA+GrzB+Prf+ | 0.51 | 3.66 |
| Markers Co-Expressed on CD8+ T Cells: | Before INO-3106 (%) | After INO-3106 (%) |
|---|---|---|
| CD38+CD137+Ki67+GrzB+Prf+ | 0.35 | 0.51 |
| CD38+CD69+CD137+ Ki67+GrzB+Prf+ | 0.35 | 0.48 |
| CD137+Ki67+GrzB+Prf+ | 0.36 | 0.48 |
| CD38+CD137+Ki67+ GrzA+GrzB+Prf+ | 0.36 | 0.48 |
| CD69+CD137+Ki67+GrzB+Prf+ | 0.36 | 0.47 |
| CD38+CD69+CD137+Ki67+ GrzA+GrzB+Prf+ | 0.36 | 0.46 |
| CD137+Ki67+GrzA+GrzB+Prf+ | 0.37 | 0.46 |
| CD38+CD137+GrzB+Prf+ | 0.41 | 0.49 |
| CD38+Ki67+Gnly+GrzA+Prf+ | 0.00 | 0.08 |
| CD38+Gnly+ | 0.00 | 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Cheung, J.; Dickerson, K.; Schulten, V.; Knoblock, D.; Gillespie, E.; et al. Immune Therapy Targeting E6/E7 Oncogenes of Human Papillomavirus Type 6 (HPV-6) Reduces or Eliminates the Need for Surgical Intervention in the Treatment of HPV-6 Associated Recurrent Respiratory Papillomatosis. Vaccines 2020, 8, 56. https://doi.org/10.3390/vaccines8010056
Aggarwal C, Cohen RB, Morrow MP, Kraynyak KA, Sylvester AJ, Cheung J, Dickerson K, Schulten V, Knoblock D, Gillespie E, et al. Immune Therapy Targeting E6/E7 Oncogenes of Human Papillomavirus Type 6 (HPV-6) Reduces or Eliminates the Need for Surgical Intervention in the Treatment of HPV-6 Associated Recurrent Respiratory Papillomatosis. Vaccines. 2020; 8(1):56. https://doi.org/10.3390/vaccines8010056
Chicago/Turabian StyleAggarwal, Charu, Roger B. Cohen, Matthew P. Morrow, Kimberly A. Kraynyak, Albert J. Sylvester, Jocelyn Cheung, Kelsie Dickerson, Veronique Schulten, Dawson Knoblock, Elisabeth Gillespie, and et al. 2020. "Immune Therapy Targeting E6/E7 Oncogenes of Human Papillomavirus Type 6 (HPV-6) Reduces or Eliminates the Need for Surgical Intervention in the Treatment of HPV-6 Associated Recurrent Respiratory Papillomatosis" Vaccines 8, no. 1: 56. https://doi.org/10.3390/vaccines8010056
APA StyleAggarwal, C., Cohen, R. B., Morrow, M. P., Kraynyak, K. A., Sylvester, A. J., Cheung, J., Dickerson, K., Schulten, V., Knoblock, D., Gillespie, E., Bauml, J. M., Yan, J., Diehl, M., Boyer, J., Dallas, M., Kim, J. J., Weiner, D. B., & Skolnik, J. M. (2020). Immune Therapy Targeting E6/E7 Oncogenes of Human Papillomavirus Type 6 (HPV-6) Reduces or Eliminates the Need for Surgical Intervention in the Treatment of HPV-6 Associated Recurrent Respiratory Papillomatosis. Vaccines, 8(1), 56. https://doi.org/10.3390/vaccines8010056





